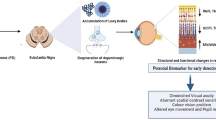
Abstract
Visual disorder is one of the non-motor symptoms found in Parkinson’s disease (PD). It can be easily identified in the early stages even before the spread of pathological conditions to the brain parts. Studies have revealed that loss of dopamine (DA) cells in retinal layers is a prime cause for both retinal disturbance and pathological conditions of PD. This reduction of DA in retina is due to the aggregation of phosphorylated α-synuclein (aSyn) in the intra-retinal region, which eventually results in visual impairment in PD. Until now, very limited studies have been focused on the mechanism of aSyn influence and DA depletion as a cause for both retinal layer dysfunction and PD. Thus, more research is warranted to provide the missing connection between the exact role of DA and aSyn as a risk factor for visual problems in PD. Hence, the current review’s focus is on the function and effects of DA degeneration in retinal cells of PD. Further, we suggest that iron plays a major role in regulating the aggregation of aSyn in the DA cells of retina and brain in PD. The study finds that the unidentified pathophysiological role of retinal degeneration in PD is an essential biomarker that needs further investigation to use it as a novel therapy in treating retinal dysfunctions in PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Retina is popularly called the “window to the brain”. Any abnormalities in the brain affects the normal functions of the retinal layers and its parts. Disorders in the brain and retina are associated with many conditions including Parkinson’s disease (PD), Alzheimer’s disease and glaucoma. PD is characterized by the degeneration of dopaminergic neurons (DA) in the substantia nigra region of the brain. Although motor impairment is the major drawback seen in PD, one of the non-motor manifestations observed in PD is the visual dysfunction. Many studies have observed that PD patients display various forms of visual impairments including colour discrimination, visual acuity, contrast sensitivity, blurred image, motion perception and loss of vision [1, 2]. This visual dysfunction is mainly caused due to the depletion of DA levels in amacrine and inner plexiform cells of the retina in PD patients [3].
DA is a very important biochemical molecule necessary for the regulation of the brain and to the retina for retinal development, visual signalling and refractive development [4]. It has been proved that abnormal changes in DA and depletion of amacrine cell of retina lead to alterations in the receptive properties of ganglion cells, which eventually results in dysfunctional visual processing in PD patients [5]. In a recent functional study, it was observed that aSyn was found to be aggregated in the inner nuclear, inner plexiform layer and ganglion cell layer of the intra-retinal region [6]. Thus, it could be assumed that irreversible loss of DA in both the brain and retina of the PD patients is merely due to the accumulation of the phosphorylated aSyn [7]. Moreover it was found that iron plays a pivotal role in the aggregation of aSyn and depletion of DA neurons in the retinal cells of PD patients [8]. These studies suggest that identification of specific retinal biomarkers in PD would be a stepping stone to detect this neurodegenerative disease in the early stages. Thus, in this review we highlight the normal and irregular functions of DA and aggregation of aSyn in the retinal layers as a causative agent for visual dysfunctions associated with PD. Further, we also suggest that retina is a potential early biomarker in PD and more research that would unravel its mechanism will fill the gap and emerge as a promising remedial route for retinal dysfunctions in PD.
Visual dysfunctions in Parkinson’s disease
Among the non-motor symptoms of PD, visual abnormalities are frequently observed during the early stages of the disease. Some of the visual symptoms found in the PD are described below.
Visual contrast acuity
The ability of the eye to resolve even the small details of stimulus is known as visual acuity. According to a study, it was reported that dysfunction in visual acuity might be a prime factor in causing chronic hallucinations in PD patients [9]. It has been explored that loss of amacrine cells and aggregation of α synuclein in the retina could be the reason for visual contrast acuity in PD patients. In 2015, Lin et al. [10] reported the use of a new iPad application to measure low contrast acuity in PD patients; it could serve as a quick screening tool to complement more formal testing of patients with PD and other neurologic disorders.
Colour vision
Colour vision is cone medicated and is processed by two pathways such as parvocellular and koniocellular visual pathways. Further, the achromatic information is transmitted through the magnocellular pathway. Earlier it has been reported that in PD, there is occurrence of chromatic and achromatic sensitivity changes due to the parvocellular, koniocellular and magnocellular pathways [11]. It has been reported that in PD patients, the colour vision impairment correlates with the severity of clinical symptoms and PD disease progression [12]. Colour vision dysfunction in PD may be associated with significant loss of cells in the ganglion cell layer [13], but studies related to dopamine deficiency and severity of colour vision impairment still remain unanswered.
Eye movement
Eye movement problems are important aspects of PD conditions [9]. There are three kinds of eye movements, saccadic eye movements which directs us to gaze at a specific object or to read lines in prints; pursuit eye movement which allows us to follow moving objects; and vergence eye movement that allows us to move our eyes in different directions [14]. In a functional study, it has been reported that electrooculography (EOG) responses are normal for PD patients when the eyes are in a resting condition; however, 75% of PD patients reported abnormalities in saccadic and smooth pursuit eye movements [9]. Elmar et al. [15] reported that dopaminergic dysfunction might not be a prime reason for impairment of saccadic eye movement in PD, but basal ganglia dysfunction might be the reason for the difficulty to rapidly execute alternating voluntary gaze shift (AVGS).
Pupil reactivity
Larger pupil diameter and unequal pupil sizes after light adaptation have been observed in PD patients [16]. Longer light reflex latencies and constriction times have also been observed while contraction amplitudes may be reduced, suggesting early involvement of the parasympathetic system in PD [9]. In PD, the contraction ability of the iris muscle is maximum when compared to controls; this suggests that the muscle might have acquired adaptive sensitivity changes [17].
VEP and ERG
Retinal responses to visual stimuli generate electrical activity in the eye, as does the transmission of these response to the primary visual cortex [18]. Measurement of the amplitude and latency of such electrical responses provide information on the functional integrity of the visual pathway and both electroretinograms (ERG) and visual-evoked potentials (VEP) which has been extensively studied in Parkinson’s disease [9]. In PD patients, the ERG amplitude is reduced in a variety of light conditions and this indicates the defect in visual processing involving dopamine neurons. VEP response to coloured stimuli, especially blue-yellow horizontal gratings, are affected in PD. VEP latency and ERG wave alterations are evident in PD patients when compared to control and delay visual processing at one or more stages of the visual system [9].
Mechanism of DA in normal and PD retina
The retina and brain are associated over a range of neurological disorders such as Alzheimer’s disease (AD), Parkinson’s disease (PD) and glaucoma. Many experimental evidences have suggested that PD patients exhibit various forms of visual dysfunctions such as visual acuity, contrast sensitivity, colour discrimination and motion perception [1, 2]. In PD patients, dopaminergic cell degeneration is not only limited inside the brain, but also in the retinal layer. Dopamine (DA) is an essential neurotransmitter found in the retina and is involved in various functions of the retina such as retinal development, visual signalling and refractive development [4]. Thus, the deficiency of DA, associated with loss of amacrine cells in retina, would result in altered visual processing by changing the input of ganglion cells [5].
In normal retina cells, the DA acts through the G-protein coupled receptor protein (GPCR) by regulating the cyclic adenosine monophosphate (cAMP). The activation of rod and cone photoreceptors are inhibited by D2—receptor family of DA, while bipolar, horizontal, RGCs and amacrine cells are excited by D1—receptors of DA. Thus in a normal cell, DA is released into retinal cells by using the gap junction permeability concept, where first the interaction between the rod and cone with horizontal cells are initiated followed by AII:AII and AII:cone bipolar communication. Finally, there is a reduction in the gap junction permeability with simultaneous rise in DA concentration (Fig. 1) [19,20,21,22]. Thus, DA acts both in the outer and inner retinal layer which results in alterations in flow of visual information in a complicated manner. In other words, dopamine is a chemical messenger for light adaptation, promoting the flow of information through cone circuits while diminishing that through rod circuits.
Normal and abnormal flow of DA in retina: the figure depicts the normal and abnormal flow of dopamine (DA) inside and outside the retina. DA is a chemical messenger for light adaptation, promoting the flow of information through cone circuits while diminishing that through rod circuits. This abnormal flow could be a reason for the visual problems faced by Parkinson’s disease patients. In the figure sign depicts the flow of DA, whereas the sign—resembles the inhibition of DA flow
The neurological evidence for deficiency of DA in human retina was first reported with reduction of tyrosine hydroxylase (TH) in PD patients [23]. DA depletion can be found majorly in the three different layers of retina. Degeneration of retinal DA neurons and their postsynaptic AII amacrine cells was allied with loss of TH immune-reactivity in the inner plexiform layer of PD retina [24]. According to another study, reduction of DA levels in basal ganglia and frontal cortex decreased the superior colliculus region that has an essential role in producing saccadic eye movements in PD patients [25]. In PD, the inner retinal layer thinning is found especially in the GCL and IPL of the inferior sector with the loss of dopamine transporter (DAT), which proved the loss of retinal dopaminergic cell degeneration in PD. Thus, DA is involved in controlling the overall retinal visual system, and any impairment in DA and its metabolites would result in retinal degeneration associated with PD.
Some of the studies have presented the mechanism of dopaminergic neurons in the retina of PD subjects. MPTP (1-methyl, 4-phenyl, 1-2-3-6-tetrahydropyridine) in PD animal models causes visual impairment due to retinal DA morphological changes or absence of retinal DA along with loss of retinal amacrine cells [24]. In rotenone-treated rat model, inhibition of complex I was noted with a depletion of DA in the striatum and substantia nigra as well as in the retinal amacrine cells [26]. As a therapeutic approach, in a rat model of hemi-parkinsonism, dopaminergic neurons were replaced through direct differentiation of limbus-derived neural progenitors that act as a potential stem cell approach in PD [27]. Limited studies have been conducted in animal models and observed for retinal alterations as depicted in Table 1 [28,29,30,31,32,33].
Indistinct mechanism of aSyn and iron in normal and Parkinson’s retinal cells
The relation of iron with aSyn and retina has a major role in PD-associated visual impairment. Iron is a cofactor for the synthesis of neurotransmitters and plays an important role in visual phototransduction cascade. In a previous study, it was shown that iron deposition alters the expression of aSyn and results in aSyn aggregation and toxicity in PD patients [34]. Post-translational modifications of aSyn affect iron and dopamine-dependent oxidative stress, thereby increasing the tendency of aSyn aggregation. In retina, the iron uptake is mediated by transferrin/transferrin receptor pathway and it is exported by ferroportin along with ceruloplasmin and hephaestin. Iron uptake occurs in the RPE through aSyn which has an effective role in iron levels due to its presence on the C-terminal end [35, 36]. During translation, it is alleged that iron modulates aSyn synthesis through an iron-responsive element on the 5′-UTR region. Also, more iron content aggregates aSyn and alters dopamine to a toxic compound that ends up in the poor activity of both aSyn and dopamine [8, 37]. This highlights the interconnection between aSyn, dopamine and iron in PD [38].
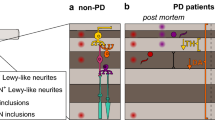
As reported earlier, aSyn is a highly conserved protein where its function remains unclear [39, 40]. The deposits of aSyn in Lewy bodies and Lewy neurites are connected with abnormal phosphorylated aSyn [41], whereas the unphosphorylated state is seen in retinal cell layers [42]. In contrast, a confirmative study on phosphorylated deposition of aSyn at serine-129 in retina was explained by [6, 43]. The pathophysiology of aSyn in retina has not been elucidated, though studies have stated its presence in the inner plexiform layer (IPL) of mouse and human retinas [44, 45]. The presence of aSyn in healthy retinas is found to be in four retinal layers such as inner nuclear layer (INL) and IPL, ganglion cell layer (GCL) and photoreceptor layer (PL) [46, 47]. In addition, colocalization of aSyn and synaptophysin constitute phagocytic structures which were observed in RPE [47]. For the first time, in PD, Bodis-Wollner et al. confirmed the presence of aSyn aggregates in INL, IPL and GCL. In PD retinal cells, phosphorylated aSyn, Lewy-like inclusions and neurites were found to be in INL and GCL [48]. Moreover, decreased dopamine and TH immunoreactivity were seen in IPL and INL [46]. Hence, the localization aSyn along with iron interaction in retinal layers might have a mechanistic behaviour on visual impairment in PD (Fig. 2).
Depicts the probable mechanism of α-synuclein in Parkinson’s retinal cells: the probable mechanism explains when iron uptake occurs in retinal epithelial cells through α-synuclein and iron modulates α-synuclein synthesis, thereby increasing α-synuclein accumulation and aggregation in retinal cells and thus causing dopamine level to decrease and act as a toxic compound in retinal cells
Retina as a candidate biomarker in Parkinson’s disease
Early diagnosis and disease-modifying therapies in PD would be beneficial in understanding the disease mechanism and identifying specific biomarkers [49]. Though numerous biomarkers have been explored, retina has shown optical properties and ease of access to non-invasive approaches for early detection of neurodegenerative diseases [50]. Imaging techniques are a useful diagnostic tool for identifying the close relationship between the eye and brain; hence, the retina is a candidate biomarker in neurodegenerative diseases [6]. Retinas can be potentially assessed for the presence of synucleinopathy using optical coherence tomography (OCT), eye fundus and angiography. These techniques will permit to visualize the retina and its changes [6].
Though varied standard measuring protocols have been analyzed for specific diseases, there is no particular method reliable for evaluating retina in PD. Lewy-type α-synucleinopathy (LTS) can be detected in retina using fluorescent dyes through intravitreal injection, where it results in minimal complications in clinical ophthalmology. Based on retinal LTS fluorescent ligands, intraocular injection and retinal imaging analysis are used to perceive PD progression [6]. Visual dysfunctions such as reduced electroretinogram (ERG) responses and visual-evoked potentials have been reported in PD patients and animal models [51, 52]. ERG has been used for early detection in two untreated PD patients, where one study revealed no ERG changes with significant reduction in b-wave amplitude, whereas another study exhibited decreased a-wave and b-wave amplitude [53].
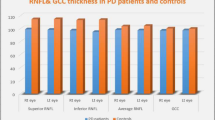
Significant differences in retinal nerve fibre layer (RNFL) thickness with varied OCT measurements have been detected in PD. Peripapillary nerve fibre layer thickness in PD has been detected in 17 studies, of which 12 studies correlated with thinning in RNFL as well as inferotemporal [54], temporal [55, 56], nasal [57], diffuse area [58], inferior nasal and temporal [59], both inferotemporal and superotemporal and inferior regions [60]. In contrast, five studies did not show RNFL thinning in PD [61, 62]. The discrepancy in these studies is due to sample size and different measurement protocol and devices. In addition, age and disease duration with severity is a significant factor in RNFL thickness in PD [41]. Fourier-domain devices are consistent in identifying axonal atrophy in RNFL of PD [63]. RNFL thickness correlated with PD and the pronounced retinal alterations were detected by OCT [64].
OCT imaging technique has been utilized in patients to identify the thinning of ganglion cell layer, inner plexiform layer, and inner nuclear layer [65, 66]. By OCT imaging,structural alterations in retinal layers such as retinal dopamine loss and foveal dysfunction in PD can be detected [41]. Several PD studies used automated OCT measures in which three studies were excluded, since the thickness of vertical retinal layers was identified manuallyfrom OCT images or with software [41, 67]. Another study showed the changes in the foveal region using advanced method [68]. The thickness of retinal vertical layers was determined in four studies, in which two studies detected the thinning in the inner layer [69, 70] and one in interocular differences [68], and the other study did not show any difference between PD and controls [71]. The inner layers in the foveal pit was identified with thinning in PD. Retinal imaging using OCT and DARC (detection of apoptosing retinal cells) was carried out in rotenone-induced PD rat model, where the study found an increased ganglion cell apoptosis and swelling of the retinal layers. In addition, a follow-up study showed neurodegenerative changes in the substantia nigra and striatum which concludes that retinal changes are early manifestations in PD [72]. DARC has been applied in various animal models of retinal neurodegeneration, where it has been shown to successfully detect ganglionic cell apoptosis [73]. While various studies have evaluated the retinal changes in PD, till date there have been incomplete clinical trial studies conducted using OCT in PD.
Immunohistological studies show the presence of aSyn and Lewy bodies in the inner plexiform layer and RNFL with retinal thinning using OCT, and ERG responses were altered effects with decreased dopamine levels observed in the retina [43, 48]. In a study, 49 eyes from PD patients were analyzed for retinal alterations using spectral-domain optical coherence tomography (SD-OCT) which showed decreased retinal microvascular density [74]. In one group, the presence of aSyn showed thickening of the outer plexiform layer in PD [75]; conversely, the other group showed thinning of the outer plexiform layer [76].
Visual hallucination (VH) is one of the features in PD, where decreased visual perception and decreased contrast sensitivity as well as colour vision are related to VH in PD [10, 41, 51]. RNFL has been associated with VH and PD severity [41]. Visualizing the retinal layers would be a beneficial biomarker and forecaster of VH in PD [41]. Low contrast sensitivity was detected in PD with larger acuity reduction [10]. The colour vision is used for disease staging [77], whereas visual field is used as an adjunct in PD [53, 78].
Thus, the mechanisms of retinal thinning and the defect in visual information processing in PD are yet to be identified. It is stated that the retina acts as a window that reflects the brain pathology and serves as a PD biomarker. Hence, from these findings, it is inferred that more research has to be conducted in creating a track for therapeutic strategies.
Future perspectives
In the research of ophthalmic condition associated with Parkinson’s disease, conflicting results appear regarding the study of retinal thinning using OCT and the exact role of phosphorylated aSyn in the retina. There were no exact results correlating the severity and duration of disease with structural changes on OCT and accumulation of phosphorylated aSyn. Relatively low number of sample size and quality of post-mortem retinas and different study protocols for retinal studies on PD do not allow making a final conclusion about the specificity and importance of aSyn in PD. Future studies should include patients with different stages of disease and it should be age matched to assess the involvement of aSyn in PD progression. Most importantly in future, there is also a need to develop non-invasive, high-resolution, preferably label-free, αSYN-imaging techniques to visualize and quantify aSyn reactivity in the retina of PD patients.
Conclusion
PD results in progressive retinal degeneration and accumulation of phosphorylated aSyn in the retinal layers. Retinal layer thinning can be seen in the early stages of disease and it could be measured by non-invasive SD-OCT technique and DARC (Detection of Apoptotic Retinal Cells) for evaluating retinal cell apoptosis. The mechanism of dopamine regulation by phosphorylated aSyn in the retinal layers might cause retinal neurodegeneration as in the brain. Neuropathological, structural and electrophysiological alterations in the PD retina and also phosphorylated aSyn aggregation in PD retina give some evidences for suggesting that the retina can be used as a biomarker for PD. However, we need some strong research proof to confirm this idea.
Abbreviations
- PD:
-
Parkinson’s disease
- AD:
-
Alzhiemer’s disease
- DA:
-
Dopaminergic neurons
- aSYN:
-
α-Synuclein
- OCT:
-
Optical coherence tomography
- mfERG:
-
Multifocal ERG
- ERG:
-
Electroretinograms
- VEP:
-
Visual-evoked potentials
- TH:
-
Tyrosine hydroxylase
- MPTP:
-
1-Methyl, 4-phenyl, 1-2-3-6-tetrahydropyridine
- PERG:
-
Pattern electroretinogram
- STF:
-
Spatial tuning function
- RNFL:
-
Retinal nerve fiber layer
- RPE:
-
Retinal epithelial cells
- IPL:
-
Inner plexiform layer
- INL:
-
Inner nuclear layer
- GCL:
-
Ganglion cell layer
- PL:
-
Photoreceptor layer
- GPCR:
-
G-protein coupled receptor protein
- cAMP:
-
Cycline adenosine monophosphate
- DAT:
-
Dopamine transporter
- LTS:
-
Lewy-type α-synucleinopathy
- DARC:
-
Detection of apoptosing retinal cells
- SD-OCT:
-
Spectral-domain optical coherence tomography
- VH:
-
Visual hallucination
References
Archibald NK, Clarke MP, Mosimann UP, Burn DJ (2009) The retina in Parkinson’s disease. Brain 132:1128–1145
Bodis-Wollner I (2009) Retinopathy in Parkinson disease. J Neural Transm Suppl 116:1493–1501
Aydin TS, Umit D, Nur OM, Fatih U, Asena K, Nefse OY, Serpil Y (2018) Optical coherence tomography findings in Parkinson’s disease. Kaohsiung J Med Sci 34:166–171
Zhou Z, Chen T, Wang M, Jin L, Zhao Y, Chen S, Wang C, Zhang G, Wang Q, Deng Q, Liu Y, Morgan IG, He M, Liu Y, Congdon N (2017) Pilot study of a novel classroom designed to prevent myopia by increasing children’s exposure to outdoor light. PLoS One 12:e0181772
Djamgoz MB, Hankins MW, Hirano J, Archer SN (1997) Neurobiology of retinal dopamine in relation to degenerative states of the tissue. Vision Res 37:3509–3529
Ortuño-Lizarán I, Beach TG, Serrano GE, Walker DG, Adler CH, Cuenca N (2018) Phosphorylated α-synuclein in the retina is a biomarker of Parkinson's disease pathology severity. Mov Disord 33:1315–1324
Mammadova N, Summers CM, Kokemuller RD, He Q, Ding S, Baron T, Yu C, Valentine RJ, Sakaguchi DS, Kanthasamy AG, Greenlee JJ (2019) Accelerated accumulation of retinal α-synuclein (pSer129) and tau, neuroinflammation, and autophagic dysregulation in a seeded mouse model of Parkinson's disease. Neurobiol Dis 121:1–6
Funke C, Schneider SA, Berg D, Kell DB (2013) Genetics and iron in the systems biology of Parkinson's disease and some related disorders. Neurochem Int 62:637–652
Armstrong RA (2008) Visual signs and symptoms of Parkinson's disease. Clin Exp Optom 91:129–138
Lin TP, Rigby H, Adler JS, Hentz JG, Balcer LJ, Galetta SL, Devick S, Cronin R, Adler CH (2015) Abnormal visual contrast acuity in Parkinson’s disease. J Parkinsons Dis 5:125–130
Oh YS, Kim JS, Chung SW, Song IU, Kim YD, Kim YI, Lee KS (2011) Color vision in Parkinson’s disease and essential tremor. Eur J Neurol 18:577–583
Diederich NJ, Raman R, Leurgans S, Goetz CG (2002) Progressive worsening of spatial and chromatic processing deficits in Parkinson disease. Arch Neurol 59:1249–1252
Polo V, Satue M, Rodrigo MJ, Otin S, Alarcia R, Bambo MP, Fuertes MI, Larrosa JM, Pablo LE, Garcia-Martin E (2016) Visual dysfunction and its correlation with retinal changes in patients with Parkinson’s disease: an observational cross-sectional study. BMJ Open 6:e009658
Shibasaki H, Tsuji S, Kuroiwa Y (1979) Oculomotor abnormalities in Parkinson’s disease. Arch Neurol 36:360–364
Elmar HP, Reinhart J, Dorothée L, Johanna H, Albert CL, Wolfgang B, Jan K (2012) Eye movement impairments in Parkinson’s disease: possible role of extradopaminergic mechanisms. BMC Neurol 12:5
Manohar SG, Husain M (2015) Reduced pupillary reward sensitivity in Parkinson's disease. NPJ Parkinson’s Dis 1:15026
Armstrong RA (2015) Oculo-visual dysfunction in Parkinson’s disease. J Parkinsons Dis 5:715–726
Armstrong RA (2017) Visual dysfunction in Parkinson's disease. Int Rev Neurobiol 134:921–946
Veruki ML (1997) Dopaminergic neurons in the rat retina express dopamine D2/3 receptors. Eur J Neurosci 9:1096–1100
He S, Weiler R, Vaney DI (2000) Endogenous dopaminergic regulation of horizontal cell coupling in the mammalian retina. J Comp Neurol 418:33–40
Xia XB, Mills SL (2004) Gap junctional regulatory mechanisms in the AII amacrine cell of the rabbit retina. Vis Neurosci 21:791–805
Ribelayga C, Cao Y, Mangel SC (2008) The circadian clock in the retina controls rod-cone coupling. Neuron 59:790–801
Nguyen-Legros J (1988) Functional neuroarchitecture of the retina: hypothesis on the dysfunction of retinal dopaminergic circuitry in Parkinson’s disease. Surg Radiol Anat 10:137–144
Cuenca N, Herrero MT, Angulo A, De Juan E, Martınez- Navarrete GC, Lopez S, Barcia C, Martín-Nieto J (2005) Morphological impairments in retinal neurons of the scotopic visual pathway in a monkey model of Parkinson’s disease. J Comp Neurol 493:261–273
Crawford TJ, Goodrich S, Henderson L, Kennard C (1989) Predictive responses in PD: manual keypresses and saccadic eye movements to regular stimulus events. J Neurol Neurosurg Psychiatry 52:1033–1042
Biehlmaier O, Alam M, Schmidt WJ (2007) A rat model of Parkinsonism shows depletion of dopamine in the retina. Neurochem Int 50:189–195
Ahmad I, Zhao X, Parameswaran S, Destache CJ, Rodriguez-Sierra J, Thoreson WB, Ahmad H, Sorrentino J, Balasubramanian S (2015) Direct differentiation of adult ocular progenitors into striatal dopaminergic neurons. Int J Stem Cells 8:106
Stutz B, da Conceição FS, Santos LE, Cadilhe DV, Fleming RL, Acquarone M, Gardino PF, de Melo Reis RA, Dickson PW, Dunkley PR, Rehen S (2014) Murine dopaminergic Müller cells restore motor function in a model of Parkinson's disease. J Neurochem 128(6):829–840
Ghilardi F, Chung M, Bodis-Wollner E, Dvorzniak IM, Glover A, Onofrj M (1988) Systemic 1-methyl, 4-phenyl, 1-2-3-6-tetrahydropyridine (MPTP) administration decreases retinal dopamine content in primates. Life Sci 43(3):255–262
Bodis-Wollner I (1990) Visual deficits related to dopamine deficiency in experimental animals and Parkinson’s disease patients. Trends Neurosci 13(7):296–302
Tatton WG, Kwan MM, Verrier MC, Seniuk NA, Theriault E (1990) MPTP produces reversible disappearance of tyrosine hydroxylase-containing retinal amacrine cells. Brain Res 527(1):21–31
Dyer RS, Howell WE, MacPhail RC (1981) Dopamine depletion slows retinal transmission. Exp Neurol 71(2):326–340
Onofrj M, Bodis-Wollner I (1982) Dopaminergic deficiency causes delayed visual evoked potentials in rats. Ann Neurol 11(5):484–490
Febbraro F, Giorgi M, Caldarola S, Loreni F, Romero-Ramos M (2012) Alpha-synuclein expression is modulated at the translational level by iron. NeuroReport 23:576–580
Lu Y, Prudent M, Fauvet B, Lashuel HA, Girault HH (2011) Phosphorylation of alpha-synuclein at Y125 and S129 alters its metal binding properties: implications for understanding the role of alpha-synuclein in the pathogenesis of Parkinson’s disease and related disorders. ACS Chem Neurosci 2:667–675
Davies P, Moualla D, Brown DR (2011) Alpha-synuclein is a cellular ferrireductase. PLoS One 6:e15814
Paris I, Martinez-Alvarado P, Cardenas S, Perez-Pastene C, Graumann R, Fuentes P, Olea-Azar C, Caviedes P, Segura-Aguilar J (2005) Dopamine-dependent iron toxicity in cells derived from rat hypothalamus. Chem Res Toxicol 18:415–419
Duce JA, Wong BX, Durham H, Devedjian JC, Smith DP, Devos D (2017) Post translational changes to α-synuclein control iron and dopamine trafficking; a concept for neuron vulnerability in Parkinson’s disease. Mol Neurodegener 12:45
Gallegos S, Pacheco C, Peters C, Opazo C, Aguayo LG (2015) Features of alpha-synuclein that could explain the progression and irreversibility of Parkinson’s disease. Front Neurosci 9:1–11
Lotharius J, Brundin P (2002) Pathogenesis of Parkinson’s disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurol 3:932–942
Lee JY, Ahn J, Kim TW, Jeon BS (2014) Optical coherence tomography in Parkinson's disease: is the retina a biomarker? J Parkinson's Dis 4:197–204
Bodis-Wollner I, Miri S, Glazman S (2014) Venturing into the no-man’s land of the retina in Parkinson’s disease. Mov Disord 29:15–22
Beach TG, Carew J, Serrano G, Adler CH, Shill HA, Sue LI, Sabbagh MN, Akiyama H, Cuenca N (2014) Phosphorylated alpha-synuclein-immunoreactive retinal neuronal elements in Parkinson’s disease subjects. Neurosci Lett 571:34–38
Surguchov A, McMahan B, Masliah E, Surgucheva I (2001) Synucleins in ocular tissues. J Neurosci Res 65:68–77
Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC (2005) Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell 123:383–396
Veys L, Vandenabeele M, Ortuño-Lizarán I, Baekelandt V, Cuenca N, Moons L, De Groef L (2019) Retinal α-synuclein deposits in Parkinson’s disease patients and animal models. Acta Neuropathol 5:1–7
Martınez-Navarrete GC, Martın-Nieto J, Esteve RJ, Angulo A, Cuenca N (2007) Alpha synuclein gene expression profile in the retina of vertebrates. Mol Vis 13:949–961
Bodis-Wollner I, Kozlowski PB, Glazman S, Miri S (2014) α-Synuclein in the inner retina in Parkinson disease. Ann Neurol 75:964–966
Le W, Dong J, Li S, Korczyn AD (2017) Can biomarkers help the early diagnosis of Parkinson’s disease? Neurosci Bull 33:535–542
Weil RS, Schrag AE, Warren JD, Crutch SJ, Lees AJ, Morris HR (2016) Visual dysfunction in Parkinson’s disease. Brain 139:2827–2843
Cuenca N, Fernandez-Sanchez L, Campello L, Maneu V, De la Villa P, Lax P, Pinilla I (2014) Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog Retin Eye Res 43:17–75
Miri S, Glazman S, Mylin L, Bodis-Wollner I (2016) A combination of retinal morphology and visual electrophysiology testing increases diagnostic yield in Parkinson’s disease. Parkinsonism Relat Disord 22:S134–S137
Nowacka B, Lubinski W, Honczarenko K, Potemkowski A, Safranow K (2015) Bioelectrical function and structural assessment of the retina in patients with early stages of Parkinson's disease (PD). Doc Ophthalmol 131:95–104
Inzelberg R, Ramirez JA, Nisipeanu P, Ophir A (2004) Retinal nerve fiber layer thinning in Parkinson disease. Vis Res 44:2793–2797
Kirbas S, Turkyilmaz K, Tufekci A, Durmus M (2013) Retinal nerve fiber layer thickness in Parkinson disease. J Neuroophthalmol 33:62–65
La Morgia C, Barboni P, Rizzo G, Carbonelli M, Savini G, Scaglione C, Capellari S, Bonazza S, Giannoccaro MP, Calandra BG, Liguori R (2013) Loss of temporal retinal nerve fibers in Parkinson disease: a mitochondrial pattern? Eur J Neurol 20:198–201
Altintas O, Iseri P, Ozkan B, Caglar Y (2008) Correlation between retinal morphological and functional findings and clinical severity in Parkinson’s disease. Doc Ophthalmol 116:137–146
Moreno-Ramos T, Benito-Le´on J, Villarejo A, Bermejo-Pareja F (2013) Retinal nerve fiber layer thinning in dementia associated with Parkinson’s disease, dementia with Lewy bodies, and Alzheimer’s disease. J Alzheimer’s Dis 34:659–694
Garcia-Martin E, Satue M, Otin S, Fuertes I, Alarcia R, Larrosa JM, Polo V, Pablo LE (2013) Retina measurements for diagnosis of Parkinson disease. Retina 34:971–980
Satue M, Garcia-Martin E, Fuertes I, Otin S, Alarcia R, Herrero R, Bambo MP, Pablo LE, Fernandez FJ (2013) Use of Fourier-domain OCT to detect retinal nerve fiber layer degeneration in Parkinson’s disease patients. Eye 27:507–514
Albrecht P, Muller AK, Sudmeyer M, Ferrea S, Ringelstein M, Cohn E, Aktas O, Dietlein T, Lappas A, Foerster A, Hartung HP, Schnitzler A, Methner A (2012) Optical coherence tomography in Parkinsonian syndromes. PLoS One 7:e34891
Cubo E, L´opez Pe˜na MJ, Diez-Feijo VE, P´erez GO, Garcia GP, Araus GE, Prieto TR, Mariscal PN, Armesto D (2014) Lack of association of morphologic and functional retinal changes with motor and non-motor symptoms severity in Parkinson’s disease. J Neural Transm 121:139–145
Garcia-Martin E, Satue M, Fuertes I, Otin S, Alarcia R, Herrero R, Bambo MP, Fernandez J, Pablo LE (2012) Ability and reproducibility of Fourier-domain optical coherence tomography to detect retinal nerve fiber layer atrophy in Parkinson’s disease. Ophthalmology 119:2161–2167
Ma LJ, Xu LL, Mao CJ, Fu YT, Ji XY, Shen Y, Chen J, Yang YP, Liu CF (2018) progressive changes in the retinal structure of patients with Parkinson’s disease. J Parkinson's Dis 8:85–92
Satue M, Rodrigo MJ, Obis J, Vilades E, Gracia H, Otin S, Fuertes MI, Alarcia R, Crespo JA, Polo V, Larrosa JM, Pablo LE, Garcia-Martin E (2017) Evaluation of progressive visual dysfunction and retinal degeneration in patients with Parkinson’s disease. Investig Ophthalmol Vis Sci 58:1151–1157
Garcia-Martin E, Larrosa JM, Polo V, Satue M, Marques ML, Alarcia R, Seral M, Fuertes I, Otin S, Pablo LE (2014) Distribution of retinal layer atrophy in patients with Parkinson disease and association with disease severity and duration. Am J Ophthalmol 157:470–478
Schneider M, M¨uller HP, Lauda F, Tumani H, Ludolph AC, Kassubek J, Pinkhardt EH (2014) Retinal single-layer analysis in Parkinsonian syndromes: an optical coherence tomography study. J Neural Transm 121:41–47
Schrier EM, Adam CR, Spund B, Glazman S, Bodis-Wollner I (2012) Interocular asymmetry of foveal thickness in Parkinson’s disease. J Ophthalmol 2012:728457
Hajee ME, March WF, Lazzaro DR, Wolintz AH, Shrier EM, Glazman S, Bodis-Wollner IG (2009) Inner retinal layer thinning in Parkinson’s disease. Arch Ophthalmol 127:737–741
Adam CR, Shrier E, Ding Y, Glazman S, Bodis-Wollner I (2013) Correlation of inner retinal thickness evaluated by spectral-domain optical coherence tomography and contrast sensitivity in Parkinson disease. J Neuroophthalmol 33:137–142
Aaker GD, Myung JS, Ehrlich JR, Mohammed M, Henchcliffe C, Kiss S (2010) Detection of retinal changes in Parkinson’s disease with spectral-domain optical coherence tomography. Clin Ophthalmol 4:1427–1432
Normando EM, Davis BM, De Groef L, Nizari S, Turner LA, Ravindran N, Pahlitzsch M, Brenton J, Malaguarnera G, Guo L, Somavarapu S (2016) The retina as an early biomarker of neurodegeneration in a rotenone-induced model of Parkinson’s disease: evidence for a neuroprotective effect of rosiglitazone in the eye and brain. Acta Neuropathol Commun 4:86
Cordeiro MF, Guo L, Coxon KM, Duggan J, Nizari S, Normando EM, Sensi SL, Sillito AM, Fitzke FW, Salt TE, Moss SE (2010) Imaging multiple phases of neurodegeneration: a novel approach to assessing cell death in vivo. Cell Death Dis 1:e3
Kwapong WR, Ye H, Peng C, Zhuang X, Wang J, Shen M, Lu F (2018) Retinal microvascular impairment in the early stages of Parkinson's disease. Investig Ophthalmol Vis Sci 59:4115–4122
Chorostecki J, Seraji-Bozorgzad N, Shah A, Bao F, Bao G, George E, Gorden V, Caon C, Frohman E, Bhatti MT, Khan O (2015) Characterization of retinal architecture in Parkinson's disease. J Neurol Sci 355:44–48
Garcia-Martin E, Rodriguez-Mena D, Satue M, Almarcegui C, Dolz I, Alarcia R, Seral M, Polo V, Larrosa JM, Pablo LE (2014) Electrophysiology and optical coherence tomography to evaluate Parkinson disease severity. Investig Ophthalmol Vis Sci 55:696–705
Veselá O, Růžička E, Jech R, Roth J, Štěpánková K, Mečíř P, Solano Z, Preclíková E (2001) Colour discrimination impairment is not a reliable early marker of Parkinson's disease. J Neurol 248:975–978
Sun L, Zhang H, Gu ZQ, Cao M, Li DW, Chan P (2014) Stereopsis impairment is associated with decreased color perception and worse motor performance in Parkinson’s disease. Eur J Med Res 19:29
Acknowledgement
I thank Sankara Nethralaya, Chennai, Avinashilingam Institute for Home Science and Higher Education for Women, and Bharathiar University, Coimbatore, for providing the necessary help to carry out the article review process.
Funding
No funding was obtained to carry out this review article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SMD, IM, APN, DV and VB declare that they have no conflict of interest.
Research involving human participants and/or animals
This is a review article; thus, it does not contain any studies with human participants performed by any of the authors.
Informed consent
This is a review article; thus it does not need any consent form.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohana Devi, S., Mahalaxmi, I., Aswathy, N.P. et al. Does retina play a role in Parkinson's Disease?. Acta Neurol Belg 120, 257–265 (2020). https://doi.org/10.1007/s13760-020-01274-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-020-01274-w