Abstract
Previous research on the biological and toxic effects of nano-TiO2 particles on animals only limit to a single dose. However, the toxicity caused by single dose nano-TiO2 does not truly represent ecological and health effects of nano-TiO2 retained in the environment. In order to further evaluate the toxicity of nano-TiO2 particles, nano-anatase TiO2 (5 nm) was injected into the abdominal cavity of ICR mice everyday for 14 days and the coefficients of organs and serum biochemical parameters were investigated. The results showed that, with increasing doses of nano-anatase TiO2, the coefficients of liver, kidney, and spleen increased gradually, while the coefficients of lung and brain decreased gradually, and the coefficient of heart had little change. The order of the titanium accumulation in the organs was liver > kidneys > spleen > lung > brain > heart. The serum biochemical parameters with lower dose of nano-anatase TiO2 showed little difference compared with the control mice, while with higher dose of nano-anatase TiO2, the indicators of liver function, such as alkaline phosphatase, alanine aminotransferase, leucine acid peptide, pseudocholinesterase, total protein, and albumin level, were enhanced significantly; the indicators of kidney function, such as uric acid and blood urea nitrogen, were decreased; the activities of aspartate aminotransferase, creatine kinase, lactate dehydrogenase, and alpha-hydroxybutyrate dehydrogenase, indicator of the myocardium function, were increased. The contents of triglycerides, glucose, and high-density lipoprotein cholesterol were significantly elevated. Taken together, nano-anatase TiO2 in higher dose caused serious damage to the liver, kidney, and myocardium of mice and disturbed the balance of blood sugar and lipid in mice. The accumulation of titanium in the organs might be closely related to the coefficients of organs and the inflammatory responses of mice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Titanium dioxide (TiO2), a natural nonsilicate mineral oxide, exists in different forms (such as anatase, rutile, and brookite) and is widely used in the cosmetics, pharmaceutical, and paint industries as a coloring material because of its high stability, anticorrosion, and photocatalysis [1]. More nanoparticles are released into the environment with the increasing development of nanotechnology. The small size and large surface areas endow them with an active group or intrinsic toxicity. The impacts of nanoparticles on human and the environment have been concerned recently by some scientists and organizations [2–13].
In the study of toxicity of nano-TiO2 (<30 m, 2 g each) to rats by bronchial injection, Afaq et al. found that the number of alveolar macrophage increased, the activities of glutathione peroxidase, glutathione reductase, 6-phosphate glucose dehydrogenase, and glutathione S-transferase were significantly elevated. However, the production of lipid peroxidation and hydrogen peroxide radicals was not altered with increased activities of these enzymes, suggesting that nano-TiO2 could induce the generation of antioxidant enzymes in animals [14]. Oberdörster et al. showed that the nano-TiO2 (50 g/l, 20 m) induced the increase of the total protein (TP) of bronchoalveolar lavage fluid and the activities of lactate dehydrogenase (LDH) and acid-glucosidase in rats and mice [15]. The toxic effects of nano-TiO2 in adult mice have been accomplished, suggesting that 5 g/kg body weight (BW) nano-TiO2 (25 and 80 m) increased the ratio of alanine aminotransferase (ALT) to aspartate aminotransferase (AST), the activity of lactate dehydrogenase and the liver weight, and caused the hepatocyte necrosis; the indicators for kidney function such as urea nitrogen and creatinine (Cr) levels increased, but there were no obvious toxicity to heart, lung, spleen, and testicle by nano-TiO2 treatment [11]. Fabian et al. reported that rats exposed to nano-TiO2 in lower dose (5 g/kg BW, 20–0 m) showed no damage to all of the organs and considered that nano-TiO2 in the dose could be used safely [16].
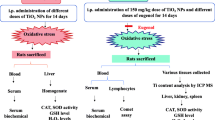
Most studies mentioned above on the biological and toxic effects of nano-TiO2 particles limit to only single dose in animals. However, the toxic effects caused by single dose of nano-TiO2 do not truly reflect the potential ecological and health effects of nano-TiO2 in the environment in general. Thus, the toxicity of nano-TiO2 should be evaluated similar to naturally occurring conditions. The aim of this study was to further investigate the biological and toxic effects of various doses of nano-anatase TiO2 (5 m) with injection into abdominal cavity of mice everyday for 14 days, involving the organ body coefficients of the liver, kidney, spleen, heart, lung, brain, and serum biochemical parameters.
Materials and Methods
Chemicals and Preparation
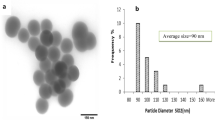
Nano-anatase TiO2 was prepared via controlled hydrolysis of titanium tetrabutoxide. The details of the synthesis are as follows [17]: Colloidal titanium dioxide was prepared via controlled hydrolysis of titanium tetrabutoxide. In a typical experiment, 1 ml of Ti(OC4H9)4 dissolved in 20 ml of anhydrous isopropanol was added dropwise to 50 ml of double-distilled water adjusted to pH 1.5 with nitric acid under vigorous stirring at room temperature. Then, the temperature was raised to 60°C and kept 6 h for better crystallization of nano-TiO2 particles. The resulting translucent colloidal suspension was evaporated using a rotary evaporator yielding a nanocrystalline powder. The obtained powder was washed three times with isopropanol and dried at 50°C until complete evaporation of the solvent. The average grain size calculated from broadening of the (101) X-ray diffraction peak of anatase (Fig. 1) using Scherrer’s equation was approximately 5 nm. The Ti2+ content in the nano-anatase was measured by inductively coupled plasma mass spectroscopy (ICP-MS) and O, C, and H contents in the nano-anatase were assayed by Elementar Analysensysteme Gmbh, showing that Ti, O, C, and H contents in the nano-anatase were 58.114%, 40.683%, 0.232%, and 0.136%, respectively. Bulk TiO2 (rutile) was purchased from Shanghai Chem. Co. and the average grain size was 10–15 μm.
Animals and Treatment
CD-1 (ICR) mice of 70 females (20 ± 2 g) were purchased from Animal Center of Soowchow University. Animals were housed in stainless steel cages in a ventilated animal room. Room temperature was maintained at 20 ± 2°C, relative humidity at 60 ± 10%, and a 12-h light/dark cycle. Distilled water and sterilized food for mice were available ad libitum. They were acclimated to this environment for 5 days prior to dosing. All procedures used in animal experiments were in compliance with the local ethics committee. Animals were randomly divided into seven groups: control group (treated with sterile saline) and six experimental groups (5, 10, 50, 100, and 150 mg/kg BW nano-anatase TiO2 and 150 mg/kg BW bulk TiO2). Experimental groups were injected into abdominal cavity with nano-anatase TiO2 (5, 10, 50, 100, and 150 mg/kg BW) and with bulk TiO2 (150 mg/kg BW) everyday for 14 days, respectively (in acute toxicity testing, we found that LD50 was 150 mg/kg BW for acute abdominal cavity TiO2 toxicity). The control group was treated with equivalent sterile saline. The symptom and mortality were observed and recorded carefully everyday for 14 days. After 14 days, the body weight of all animals were weighed accurately and sacrificed after being anesthetized by ether. Blood samples were collected from the eye vein by removing the eyeball quickly. Serum was harvested by centrifuging blood at 2,500 rpm for 10 min. The tissues and organs, such as liver, spleen, kidneys, lung, heart, and brain, were excised and weighed accurately.
Coefficients of Organs
After weighing the body and tissues, the coefficients of liver, spleen, kidneys, lung, heart, and brain to body weight were calculated as the ratio of tissues (wet weight, mg) to body weight (g).
Titanium Content Analysis of Organs
Tissues were taken out and thawed. About 0.1–0.3 g of each tissue were weighed, digested, and analyzed for titanium content. Briefly, prior to elemental analysis, the tissues of interest were digested in nitric acid (ultrapure grade) overnight. After adding 0.5 ml of H2O2, the mixed solutions were heated at about 160°C using high-pressure reaction container in an oven chamber until the samples were completely digested. Then, the solutions were heated at 120°C to remove the remaining nitric acid until the solutions were colorless and clear. At last, the remaining solutions were diluted to 3 ml with 2% nitric acid. ICP-MS (Thermo Elemental X7, Thermo Electron Co.) was used to analyze the titanium concentration in the samples. Of indium, 20 ng/ml was chosen as an internal standard element. The detection limit of titanium was 0.076 ng/ml. Data are expressed as nanograms per gram of fresh tissue.
Biochemical Analysis of Serum Contents
In the present study, liver function was evaluated with serum levels of alkaline phosphatase (ALP), alanine aminotransferase, leucine acid peptide (LAP), pseudocholinesterase (ChE), total protein, albumin (ALB), total bilirubin level (TBIL), total bile acid, and aspartate aminotransferase. Nephrotoxicity was determined by uric acid (UA), blood urea nitrogen (BUN), creatinine, calcium (Ca), and phosphonium (P). The activities of aspartate aminotransferase, creatine kinase (CK), lactate dehydrogenase, and alpha-hydroxybutyrate dehydrogenase (HBDH) were assayed for evaluating cardiac damage. Total cholesterol (TCHO), triglycerides (TG), glucose (GLU), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were determined for assessing blood sugar and lipid level using a Biochemical Autoanalyzer (Type 7170, Hitachi, Japan).
Statistical Analysis
Results were analyzed statistically by analysis of variance. When analyzing the variance treatment effect (p ≤ 0.05), the least standard deviation test was applied to make a comparison between means at the 0.05 levels of significance.
Results
The Enhancement of Body Weight and the Coefficients of Organs
After 14 days, the mice were sacrificed, various organs were collected, and the body weight was measured. Table 1 shows the coefficients of the liver, kidney, spleen, lung, heart, and brain to body weight which were expressed as milligrams (wet weight of tissues)/grams (body weight). No obvious differences were found in the body weight of seven groups. The significant differences were not observed in the coefficients of the liver, kidney, spleen, lung, heart, and brain in the 5 and 10 mg/kg BW nano-anatase TiO2 groups (p > 0.05). However, the coefficients of the liver, kidney, and spleen in the 50, 100, and 150 mg/kg BW nano-anatase TiO2 groups and 150 mg/kg BW bulk TiO2 group were significantly higher (p < 0.05 or p < 0.01) than the control, suggesting that the inflammation might be induced in mice after injection of higher dose of nano-anatase TiO2 and bulk TiO2. It can be seen from Table 1 that with the dose increased, the coefficients of the lung and brain were decreased gradually, and those of 50, 100, and 150 mg/kg BW nano-anatase TiO2-treated groups and 150 mg/kg BW bulk TiO2-treated group were lower than the control (p < 0.05 or p < 0.01), while the coefficients of the heart have no obvious changes in the various animal groups, indicating that higher dose of nano-anatase TiO2 and bulk TiO2 might cause the damage of the lung and brain but did not cause the heart damage of mice.
Titanium Content Analysis
The contents of titanium in each organ of female mice during 14 days daily injection of various doses of nano-anatase TiO2 and 150 mg/kg BW bulk TiO2 into abdominal cavity are shown in Table 2. In the experimental groups, the order of the titanium accumulation in the organs was liver > kidneys > spleen > lung > brain > heart (p < 0.05), and the titanium was mainly accumulated in the liver, kidneys, and spleen. This phenomenon showed that the accumulation of titanium in the organs was closely related to the coefficients of organs of mice. However, the contents of titanium of the organs in 150 mg/kg BW bulk TiO2 group was lower than those of 150 mg/kg BW nano-anatase TiO2 group (p < 0.05), suggesting that nano-anatase TiO2 (5 nm) entered organs of mice more easily than the bulk TiO2 (15–20 μm) did.
Biochemical Parameters in Serum of Liver
Table 3 exhibits the changes of biochemical parameters in serum of mice liver after nano-anatase TiO2 suspension was injected into abdominal cavity for 14 days. In lower doses (5 and 10 mg/kg BW, there were no significant changes for all the parameters compared with the control group (p > 0.05). In higher dose of nano-anatase TiO2 (50, 100, and 150 mg/kg BW) groups, however, the activities of ALT and ALP were significantly higher than the control group (p < 0.05 or p < 0.01), and the obvious enhancement of LAP, ChE, TP, ALB levels, and the reduction of TBIL levels were observed in comparison with the control group (p < 0.05). In the 150 mg/kg BW bulk TiO2 group, there were only ALT, ALP, and ALB higher than the control (p < 0.05), and the other parameters have no obvious difference from the control group (p > 0.05), indicating that nano-anatase TiO2 in lower dose had little influence on liver function of mice, but higher dose could cause serious damage to liver.
Biochemical Parameters in Serum of Kidney
The changes of biochemical parameters in the blood serum of mice kidney after nano-anatase TiO2 suspension was injected into abdominal cavity are presented in Table 4. With the nano-anatase TiO2 dose increased, the contents of Cr, Ca and P of kidney function parameters were increased gradually, although there was no statistically differences compared with the control group (p > 0.05). However, the UA and BUN were decreased gradually, with the higher nano-anatase TiO2 dosage groups being lower than the control group (p < 0.05 or p < 0.01). In the 150 mg/kg BW bulk TiO2 group, the UA and BUN were significantly lower than the control (p < 0.05), demonstrating that higher dose of nano-anatase TiO2 had serious toxicity to mice kidney.
Biochemical Parameters in Serum of Myocardium
As seen in Table 5, the activities of AST, CK, LDH, and alpha-HBDH in 5 and 10 mg/kg BW nano-anatase TiO2 groups showed no obvious differences from the control group (p > 0.05). In the 50, 100, and 150 mg/kg BW nano-anatase TiO2 groups, the activities of CK and LDH were significantly higher than the control group (p < 0.05), and the activities of AST and HBDH were also increased significantly (p < 0.01). In the 150 mg/kg BW bulk TiO2 group, the four parameters were also increased significantly (p < 0.05). These results indicate that nano-anatase TiO2 in higher dose make serious damage to myocardium of mice.
Blood Glucose and Fat Contents in Mice
Figures 2 and 3 show that the contents of GLU, TG, TCHO, and HDL-C from 100 to 150 mg/kg BW groups were higher than the control group (p < 0.05). The LDL-C contents from nano-anatase TiO2-treated groups had no obvious differences from the control group (p > 0.05). In the 150 mg/kg BW bulk TiO2 group, the contents of GLU, THCO, and HDL-C were higher than the control group (p < 0.05), but the contents of TG and LDL-C was not significantly different from the control group (p > 0.05). These results indicate that nano-anatase TiO2 in higher dose caused metabolism imbalance of blood sugars and lipids in mice.
Change of glucose content in the blood of mice after nano-anatase suspension was injected into abdominal cavity. Bars marked with an asterisk or double asterisks are significantly different from the control (no nano-anatase or bulk TiO2) at the 5% or 1% confidence level, respectively. Values represent means ± SE, n = 10
Discussion
In this study, the ICR mice were injected with 150 mg/kg BW bulk TiO2 (15–20 μm) and various doses of nano-anatase TiO2 (5 nm) into abdominal cavity everyday for 14 days. In 50, 100, and 150 mg/kg BW nano-anatase TiO2-treated groups and 150 mg/kg BW bulk TiO2-treated group, the higher coefficients of the liver, kidney, and spleen (p < 0.05 or p < 0.01) and the lower coefficients of the lung and brain were observed (p < 0.05 or p < 0.01). We did not find obvious differences for the coefficient of heart of seven groups. Wang et al. reported that when a fixed large dose of 5 g/kg BW of TiO2 suspensions (25 and 80 nm) was administrated by a single oral gavage, the coefficient of liver after 2 weeks was significantly increased, while the coefficients of spleen and kidney changed a little [12]. The discrepancy between our study and others is most likely attributed to differences in the sizes or the types of nanoparticles or the treatment methods. Nevertheless, all studies did demonstrate that nano-TiO2 in higher dose had serious toxicity to mice liver.
Our studies showed that the order of the titanium accumulation in the organs of mice was liver > kidneys > spleen > lung > brain > heart (p < 0.05). The accumulation of titanium in the organs might be closely related to the coefficients of organs of mice. In addition, the accumulation of titanium of the organs in 150 mg/kg BW nano-anatase TiO2 group was higher than those of 150 mg/kg BW bulk TiO2 group (p < 0.05). Compared with bulk TiO2, smaller grain size of nano-anatase TiO2 (5 nm) would allow easier entry to mouse cells and its higher surface makes its intake to the organs of mice easier. Combination of both resulted in the enhancement of the titanium in the organs.
It is well known that LDH is an important isoenzyme in glycolysis and glyconeogenesis and widely exists in the heart, liver, lung, and many other tissues. When the tissues are subjected to injure, LDH would leak into the serum of blood from organs or cells, which resulted in the increase of LDH activity and its isoenzyme in the corresponding organs. Most of HBDH are contained in myocardium. ALP mainly distributed in the liver, bone, and in bile duct, and ALT and AST exist in the liver, heart, and other organs. When the organs injured, the activities of HBDH, ALP, ALT, and AST in serum would increase. In order to further study the biochemical mechanism of nano-anatase TiO2 particles, the parameters for the damages of the liver, kidney, myocardium function, the glucose, and lipid contents in the blood were determined. The results showed that, in the 50, 100, and 150 mg/kg BW groups, the parameters for liver function including ALT, ALP, LAP, ChE, TP, ALB, and TBIL increased greatly (p < 0.05 or p < 0.01); the parameters for kidney function including UA and BUN decreased significantly (p < 0.05 or 0.01); the parameters for myocardium function, the activities of CK, LDH, AST, and HBDH, increased notably (p < 0.05 or p < 0.01); the contents of GLU, TG, THCO, and HDL-C in blood increased obviously (p < 0.05). However, the parameters mentioned above from the 5 and 10 mg/kg BW groups were not significantly different from the control group. It was concluded that nano-anatase TiO2 in higher dose had serious toxicity to the liver, kidney, and myocardium of mice and caused inflammatory response of the liver, kidney, and myocardium and the metabolism imbalance of blood sugar and lipid. Wang et al. showed that after a single oral gavage of dose of 5 g/kg BW of TiO2 suspensions (25 and 80 nm), TBIL, ALT, BUN, LDH, and HBDH in serum had statistical significance compared with the control mice (p < 0.05), others had not (p > 0.05) [12]. We hypothesize that the discrepancy may be resulted from the sizes, the types of nano-TiO2, and the treatment methods to mice. However, the mechanisms of inflammatory response of mice caused by nano-anatase TiO2 require further investigation.
References
Crabtree RH (1998) A new type of hydrogen bond. Science 282:2000–2001
Service RF (2003) American Chemical Society meeting: nanomaterials show signs of toxicity. Science 300:243
Brumfiel GA (2003) Little knowledge. Nature 424(17):246
Zhang WX (2003) Environmental technologies at the nanoscale. Environ Sci Technol 37(5):103–108
Kelly KL (2004) Nanotechnology grows up. Science 304:1732–1734
Sayes CM, Wahi R, Kurian PA, Liu YP, West JL, Ausman KD, Warheit DB, Colvin VL (2006) Correlating nanoscale titania structure with toxicity: a cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicol Sci 92:174–185
Lu L, Ma M, Zhang Y, Tang M, Gu N (2004) Development of study on the biosafety of nanomaterials. J Southeast Univ (Nat Sci) 34(5):711–715 (in Chinese)
Wang B, Feng WY, Zhao YL, Xing GM, Chai ZF, Wang H, Jia G (2005) Status of study on biological and toxicological effects of nanoscale materials. Sci China Ser B 48(5):385–394
Zhu RR, Wang SL, Yao SD (2005) Effects nano-TiO2 on biology. Life Chem 25(4):344–346 (in Chinese)
Wang B, Feng WY, Wang TC, Jia G, Wang M, Shi JW, Zhang F, Zhao YL, Chai ZF (2006) Acute toxicity of nano- and micro-scale zinc powder in healthy adult mice. Toxicol Lett 161:115–123
Chen Z, Meng H, Xing GM, Chen CY, Zhao YL, Jia G, Wang TC, Yuan H, Ye C, Zhao F, Chai ZF, Zhu CF, Fang XH, Ma BC, Wan LJ (2006) Acute toxicological effects of copper nanoparticles in vivo. Toxicol Lett 163:109–120
Wang JX, Zhou GQ, Chen CY, Yu HW, Wang TC, Ma YM, Jia G, Gao YX, Li B, Sun J, Li YF, Jia G, Zhao YL, Chai ZF (2007) Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett 168:176–185
Baan R, Straif K, Grosse Y, Secretan B, Ghissassi FEl, Cogliano V (2006) WHO International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of carbon black, titanium dioxide, and talc. Lancet Oncol 7:295–296
Afaq F, Abidi P, Matin R, Rahman Q (1998) Cytotoxicity, pro-oxidant effects and antioxidant depletion in rat lung alveolar macrophages exposed to ultrafine titanium dioxide. J Appl Toxicol 18:307–312
Oberdörster G, Finkelstein JN, Johnston C (2000) Acute pulmonary effects of ultrafine particles in rats and mice. Res Rep Health Eff Inst 96:5–74
Fabian E, Landsiedel R, Ma-Hock L, Wiench K, Wohlleben W, van Ravenzwaay B (2008) Tissue distribution and toxicity of intravenously administered titanium dioxide nanoparticles in rats. Arch Toxicol 82(3):151–157
Yang P, Lu C, Hua N, Du Y (2002) Titanium dioxide nanoparticles co-doped with Fe3+ and Eu3+ ions for photocatalysis. Mater Lett 57:794–801
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no. 20671067) and by the Medical Development Foundation of Suzhou University (grant no. EE120701) and by the National Innovation Foundation of Student (grant no. 57315427).
Author information
Authors and Affiliations
Corresponding author
Additional information
Huiting Liu, Linglan Ma, Jinfang Zhao, and Jie Liu contributed equally to this work.
An erratum to this article is available at http://dx.doi.org/10.1007/s12011-014-0011-y.
Rights and permissions
About this article
Cite this article
Liu, H., Ma, L., Zhao, J. et al. Biochemical Toxicity of Nano-anatase TiO2 Particles in Mice. Biol Trace Elem Res 129, 170–180 (2009). https://doi.org/10.1007/s12011-008-8285-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-008-8285-6