Abstract
Purpose
The aim of the study was to evaluate established risk factors and define new inflammation-associated factors associated with postoperative ventriculoperitoneal shunt placement.
Methods
The electronic medical records of children who underwent surgery for a tumor in the posterior fossa between January 2009 and January 2018 were retrospectively analyzed. Factors evaluated include age, clinical symptoms, tumor type, extent of surgical tumor resection, treatment with EVD and/or ETV, radiological findings, postoperative serum CRP, and leucocyte levels. Tumor tissue was stained immunohistochemically with antibodies against CD3, and leucocyte counts were performed. Patients with pre- or postoperative signs of infection or confirmation of a concurrent infection were excluded from some analyses.
Results
Seventy patients ages 0.4–20.8 years (median, 8.2) were included. Forty-five of 70 (65.3%) presented postoperative radiological signs of hydrocephalus. Fifteen of 70 (21.4%) patients required shunt placement postoperatively. Shunt placement was significantly associated with age < 3 years at diagnosis (p = 0.013), perioperative EVD placement (p < 0.001), signs of hydrocephalus in postoperative imaging (p = 0.047), a frontooccipital horn ratio (FOHR) > 0.46 within the first 72 h postoperatively (p < 0.001), and the presence of intraventricular blood postoperatively (p = 0.007). Six patients who underwent shunting had serum CRP levels > 40 mg/l (p = 0.030) within the first 48 h postoperatively. Tumor type or extent of resection did not correlate with shunt placement.
Conclusions
Several established and new factors associated with shunt placement after posterior fossa tumor surgery could be identified. Additional studies are needed to explore the aseptic inflammation pathways involved with increased CRP levels and shunt placement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although CSF passage should no longer be blocked after (gross) tumor resection, approximately 30% [3, 7, 11, 12, 15, 24, 26, 27] of all pediatric patients with infratentorial neoplasia develop persisting hydrocephalus over the postoperative course and require consequent operative CSF diversion.
A number of factors that predict permanent CSF diversion have been described before such as young age at surgery, preoperative hydrocephalus, the presence of metastases, tumor type and location, subtotal tumor resection, insertion of an external ventricular drain, and postresection complications such as pseudomeningocele [3, 7, 15, 27, 32, 35].
The fact that many patients require CSF diversion despite gross total resection shows us that the pathomechanism associated with the development of postoperative hydrocephalus is not fully understood. It is imperative to better understand the process in order to avoid unnecessary shunting because shunts are associated with lifelong complications, such as shunt failure in a form of mechanical obstruction [22], infection [20], or shunt-associated slit-ventricle syndrome due to over-drainage [31].
Under the assumption that hydrocephalus is not only the result of mechanical CSF blockage, we theorized that factors related to inflammatory pathways that block microcirculation of CSF due to aseptic inflammation could be the cause of postoperative communicating hydrocephalus.
In the retrospective setting, serum levels of C-reactive protein (CRP) are the only values related to inflammatory pathways available for the present cohort. Although CRP is a rather unspecific marker, it was hypothesized that CRP levels were significantly elevated postoperatively in children requiring permanent CSF diversion.
The aim of the study was therefore to evaluate established and define new perioperative factors associated with postoperative ventriculoperitoneal shunt placement, especially those related to inflammation.
Methods
After ethics approval (File Nr. WF-12/17), patients ages 0–21 who underwent surgery on a neoplasm located in the posterior fossa between January 2009 and January 2018 at the University Medical Center Hamburg Eppendorf in Hamburg, Germany, were included. Patients with isolated brain stem lesions were not included.
The electronic medical records were retrospectively analyzed. The following factors were assessed: gender, age at surgery, and pre- and postoperative clinical symptoms including disturbance of consciousness, impaired speech, movement disorders (ataxia and focal weakness), nausea and vomiting, headaches, anisocoria, papilledema, and impaired vision. In addition, postoperative signs of meningitis and the presence of a pseudomeningocele, CSF leakage, and/or wound healing disturbance were included.
All surgical procedures, including endoscopic third ventriculostomy (ETV) and perioperative placement of an extraventricular drain (EVD), were documented. Further, perioperative administration of steroids and antibiotics beyond the intraoperative single-shot standard was included.
The extent of tumor resection according to both the surgical and radiological reports as well as the tumor histology was noted, including molecular subtypes, if applicable, otherwise stated as “not classified.” All tumor surgeries were performed by the same three surgeons. Surgical resection was classified as subtotal or total.
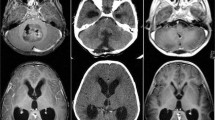
Radiological findings included hydrocephalus, transependymal edema, the presence of blood inside the ventricular system (postoperatively) as well as cerebral and/or spinal metastases. These findings were determined by using the first available magnetic resonance imaging (MRI) scans preoperatively and the first within 72 h postoperatively. Further, the frontooccipital horn ratio (FOHR) was calculated for all pre- and early postoperative scans (see Fig. 1). A value equal or less than 0.37 is considered to be physiological and independent of age, as described previously [29].
Frontooccipital-horn-ratio (FOHR). The FOHR was calculated according to the following formula: (maximum frontal horn diameter + maximum occipital horn diameter) divided by (2 × the biparietal diameter). The FOHR of the non-shunt group was 0.43 (median), whereas a higher value could be identified within the shunt group (mean 0.48, median 0.47), measured preoperatively. An increased postoperative FOHR could be shown for patients who required shunt placement (mean 0.50, median 0.52) compared with the preoperative values, whereas patients who did not undergo shunt placement showed decreased FOHR levels postoperatively (mean 0.41, median 0.40)
The serum levels of CRP and leucocytes recorded preoperatively and within the first 48 h postoperatively were analyzed. With respect to CRP and leucocytes, patients with pre- or postoperative confirmation of a concurrent infection were excluded for these further analyses.
In addition, leucocyte counts were performed within the tumor tissue specimens. In order to determine the infiltration of leucocytes within the tumor tissue, the histological specimens were stained immunohistochemically with antibodies against CD3 which is characteristically found on T cells. Subsequently, the marked leucocytes were counted single-blinded under the use of ImageJ software (Version 2.0.0) in randomly chosen sections with a total area of 1 mm2.
Statistical analyses were performed with IBM SPSS Statistics (Version 24). Chi-square and Fisher’s exact test were used to calculate correlations for nominal data. p values less than or equal to 0.05 were considered significant.
Receiver operating characteristic curves (ROC curves) were used to define the limit values for serum CRP levels, leucocytes, and FOHR related to postoperative shunt placement, whereby importance was placed on high specificity. The cut-off value for CRP was thus defined as > 40 mg/l (specificity 79%, sensitivity 54%), while the cut-off value for the FOHR was defined as > 0.46 (specificity 85%, sensitivity 33%). A determination for serum leucocytes was not possible due to the distribution of values within the curve.
Results
A total of 70 patients were included in the present collective. There were 41 (58.6%) male and 29 (41.4%) female patients, ages 0.4–20.8 years (mean, 8.5; median, 8.2). Fifteen of the 70 patients (21.4%) required shunt placement over the postoperative course. Shunt placement occurred at an average of 16.6 days after tumor resection (median, 16 days; range, 7–27 days).
Age at surgery
Patients in the shunt group (n = 15; mean, 5.4; median, 3.0) were younger than in the non-shunt group (n = 55; mean, 9.3; median, 9.0). An age < 3 years at surgery was significantly associated with postoperative shunt placement (p = 0.013). Patients with medulloblastoma (mean, 9.7 years; median, 9.0 years) and astrocytoma (mean, 10.2 years; median, 10.9 years) were older than patients with ependymoma (mean, 3.1 years; median, 2.0 years).
Clinical symptoms
The median duration between the onset of symptoms and hospital admission was 3 weeks within the non-shunt group (range, 0.9–78 weeks), whereas the shunt group showed a duration of symptoms of 2 weeks (range, 0.9–20 weeks). Specific symptoms are detailed in Table 1.
Meningitis was diagnosed in 3/70 patients over the postoperative clinical course, two of whom required shunt placement. There was no significant correlation found between postoperative meningitis and shunt requirement (p = 0.12). A pseudomeningocele was documented in 10/55 patients (18.2%) of the non-shunt group and in 7/15 patients (47.7%) within the shunt group, while a CSF leak was documented in seven (12.7%) patients of the non-shunt- and in 11 (73.3%) patients of the shunt group. Patients with pseudomeningocele were treated nonsurgically with tight compression bandaging. Wound healing disturbances appeared in four out of 70 patients (5.7%); three of them required a VP shunt. The presence of a CSF leak (p < 0.001) as well as wound healing disturbances (p = 0.03) was significantly associated with postoperative shunt placement. There was no significant relationship found between the presence of a pseudomeningocele and shunt requirement (p = 0.16).
Surgical procedures
According to the postoperative radiological report, 32 (45.7%) patients underwent gross total surgical resection, while 36/70 (51.4%) underwent subtotal resection. Two cases were not documented.
Within the shunt group, gross total resection was achieved in nine (60%) and a subtotal resection in 6/15 patients (40%). There was no significance found between postoperative shunt placement and gross total tumor resection according to the postoperative neuroradiological report (p = 0.38).
Endoscopic third ventriculostomy (ETV) was implemented in 9/70 children (12.9%). Five of these patients underwent shunt placement over the perioperative course, due to clinical and radiological signs of persistent hydrocephalus (p = 0.018). Twenty-two of 70 patients (31.4%) were treated with an external ventricular drain (EVD) perioperatively (see Table 2). Overall, perioperative EVD placement was significantly associated with postoperative shunt placement (p < 0.001).
Radiological findings
In preoperative imaging, 51/70 patients (72.9%) presented with hydrocephalus, while 10 (14.3%) children showed no radiological signs of elevated intracranial pressure (9: not documented). Twenty-seven (38.6%) developed transependymal edema, and 28 (40%) were not documented. Fourteen patients with preoperative hydrocephalus required VP shunting (93.3% within the shunt group, not documented in 1).
After surgical tumor resection, fewer children overall presented with radiological signs of hydrocephalus (45; 65.3%) and transependymal edema (15; 21.4%) compared with preoperative resection. Fourteen (93.3%) of 15 patients within the shunt group had radiological signs of hydrocephalus postoperatively. Transependymal edema was found in six of 15 patients (40%). Signs of hydrocephalus on postoperative imaging were significantly associated with shunt placement (p = 0.047), whereas preoperative radiological signs of hydrocephalus did not correlate significantly with postoperative shunt requirement (p = 0.098).
A difference between pre- and postoperative values for the FOHR could be found (Fig. 1). A postoperative FOHR over 0.46 measured within the first 72 h was significantly associated with shunt placement (p < 0.001).
Postoperative imaging revealed the presence of intraventricular blood in 35/70 cases (53.8%): 22/55 (44%) patients in the non-shunt group and 13/15 (86.7%) within the shunt group. Five of 13 patients had also postoperative serum CRP levels above 40 mg/l. The presence of intraventricular blood postoperatively correlated significantly with shunt placement (p = 0.007); furthermore, it was found to correlate significantly with a FOHR above 0.46 (p = 0.002) as well as with a postoperative serum CRP level above 40 mg/l (p = 0.02).
Tumor type and molecular pathology
There was no significant correlation between postoperative shunt placement and tumor type for the three most frequent diagnoses: medulloblastoma (p = 0.37), astrocytoma (p = 0.76), and ependymoma (p = 0.07). There was no prevalent histological or molecular subgroup within the shunt- or the non-shunt group (see Fig. 2).
Distribution of Tumor histology and molecular subtypes. Medulloblastoma (24/55; 4/15) and astrocytoma (18/55; 4/15) were the major tumor histologies within the non-shunt group, while ependymoma (9/55; 6/15) was the prevalent tumor type in patients requiring a postoperative VP shunt. Each one out of 55 without shunt was classified as hemangioblastoma and atypical teratoid rhabdoid tumor (ATRT), two were classified as glioblastoma. There was one glioblastoma within the shunt-group. The molecular pathological classification revealed the following subgroups in total (n = 70): Six medulloblastomas showed a molecular pathology of a wingless (WNT)-subgroup tumor, five could be classified as sonic-hedgehog (SHH), eight as non-SHH/non-WNT, two as group 3, and two as group 4 subgroup tumor. There were 21 pilocytic and one anaplastic astrocytomas. Thirteen of 15 ependymomas were classified as PF-A subgroup, whereas two could not be further specified
Inflammation markers
Whereas, preoperatively, no difference for leucocytes and CRP between the groups was found, CRP levels on postoperative day 2 were higher within the shunt group compared with the non-shunt group. Postoperative serum leucocytes were not significantly different between the groups (see Fig. 3). Within the first 48 h after tumor resection, 6/14 cases with following shunt placement and 7/49 patients without shunting showed non-infection-associated serum CRP levels > 40 mg/l. A serum CRP level > 40 mg/l postoperatively was significantly associated with shunt placement (p = 0.03).
Median serum CRP and leucocyte level pre- and postoperatively. There was no significant difference for median serum CRP level (a) and leucocyte level (b) compared between the groups preoperatively (non-shunt group CRP 4.9 mg/l, leucocytes 8.6 109/l; shunt-group CRP 4.9 mg/l, leucocytes 8.1 109/l). Median serum CRP levels on postoperative day 2 were higher within the shunt group compared with the non-shunt group; a postoperative serum CRP level > 40 mg/l was significantly associated with shunt placement. No significant difference was found for postoperative serum leucocytes (non-shunt group CRP 16.5 mg/l, leucocytes 12.2 109/L; shunt-group CRP 28.5 mg/l, leucocytes 10.1 109/L)
Tumor leucocyte infiltration
The infiltration of leucocytes inside the tumor tissue was quantified in 48 of 70 patients. In 22 cases, there was not enough tumor tissue left for analysis. A median value over 100/mm2 within the collective- (median, 107/mm2) and non-shunt group (median, 118/mm2; missing, 21) and a median value of 90/mm2 within the shunt group (missing, 1) was found. Medulloblastomas (median, 78/mm2; missing, 13) and ependymomas (median, 76/mm2; missing, 2) showed an approximately equal median density of infiltration, whereas the median density in astrocytomas (median, 138/mm2; missing, 4) was higher.
Discussion
Age at surgery
As described in several studies [3, 7, 15, 25, 27, 30, 32, 35], children who required a shunt are on average younger than children without shunt placement. In this study, age < 3 years at surgery was significantly associated with postoperative shunt placement. Riva-Cambrin et al. [32] described a significant correlation between age under 2 years at surgery and postoperative shunting, whereas others found no significant correlation with young age and shunt placement [20, 26]. Patients with medulloblastoma and astrocytoma were older than patients with ependymoma in this study, similar to other studies published [12]. Thus, it is difficult to ascertain whether age itself or tumor pathology is the factor that predisposes to shunt placement.
Surgical procedures
In this series, there was no significant correlation between postoperative shunt placement and the extent of tumor resection per radiological report. This is in agreement with the results of other groups [3, 7, 15, 35]; as many as one-third of cases with complete tumor resection have manifested persistent hydrocephalus [10]. Though this finding is not new, it seems counterintuitive that CSF diversion is required when the tumor has been completely removed. This prompted us to identify further factors that may be responsible for inhibiting CSF flow on the microcirculation level, i.e., via aseptic activation of inflammation pathways.
In the present study, nine patients underwent ETV treatment; five of these patients underwent shunt placement (see Tables 2 and 3). Previous studies have demonstrated the rates of ETV failure with subsequent persistent hydrocephalus in 10–42% of cases [2, 3, 14, 23, 27, 32, 33, 38, 39]. El Beltagy and colleagues found ETV success to be related to tumor type [13]. Dewan et al. [9] compared both the rate and time of failure between ETV and VP shunting. They showed that there is no significant statistical difference between the cumulative failure rate of ETV (21%) and VP shunt (29%), similar to others [8]. However, Sainte-Rose et al. found preresection ETV to significantly reduce the rate of VP shunting [34]. Further studies are needed to determine the best CSF diversion strategy for these patients. One expert review recommended preresection ETV in patients with high risk of persisting postoperative hydrocephalus and/or in patients in which tumor surgery is delayed [10]. At our institution, similar to others [39], preoperative ETV is not favored in order to avoid an unnecessary procedure.
Radiological findings
Approximately 71–92% [2, 3, 7, 12, 27, 35, 37] of patients with posterior fossa tumors show signs of hydrocephalus in preoperative imaging studies. In the present study, 51 patients presented with hydrocephalus preoperatively, 14 of whom required VP shunting postoperatively. Considering the measurement of preoperative ventriculomegaly via the FOHR method, the values were higher in patients in the shunt groups. However, there was no statistically significant relationship found between the presence or extent of preoperative hydrocephalus and postoperative shunt insertion. Whereas previous authors found similar results [3, 7, 35, 37], other studies confirmed a significant correlation between the presence or extent of preoperative ventriculomegaly and postoperative persistent hydrocephalus by using Evan’s index or FOHR [15, 27, 32, 39].
Within the first 72 h after surgical tumor resection, 65% of the children presented with radiological signs of hydrocephalus in this series. Thirty-one percent of them developed a persistent hydrocephalus and required shunt placement. Other studies have reported that 15–36% of patients demonstrated persistent hydrocephalus [3, 7, 14, 35, 37]. Nevertheless, the extent of ventriculomegaly might be a more reliable predictor. A postoperative FOHR > 0.46 was highly and significantly associated with shunt placement in accordance with Gopalakrishnan et al. [15].
Intraventricular hemorrhage (IVH) with consequent post-hemorrhagic hydrocephalus is common in premature neonates [1, 4, 22]. The most accepted assumption is that post-hemorrhagic hydrocephalus results from a decreased CSF absorption due to an obstructed intraventricular CSF flow and/or arachnoid granulation dysfunction as the result of intraventricular blood and metabolic products [6, 19]. Karimy et al. [19] recently demonstrated in a rat model that induced intraventricular hemorrhage causes a Toll-like receptor 4 (TLR4-) and NF-B-dependent inflammatory response in the choroid plexus epithelia with consequent hypersecretion and post-hemorrhagic communicating hydrocephalus within 48 h after induction. This is similar to the findings of Gram et al., who observed an upregulation of genes related to the activation of TLR-4 and other pathways with induction of NF-κβ in the choroid plexus following IVH, which leads to an upregulation of pro-inflammatory molecules [16]. As a result, the authors concluded that extracellular hemoglobin following IVH is an inducer of inflammation in the immature brain.
Inflammation markers
There are no other studies on CRP levels in children with hydrocephalus after tumor resection to date. However, several groups [5, 17, 18, 21, 28, 36, 40] determined the serum CRP levels or IL-6 concentration in CSF in patients with subarachnoid hemorrhage (SAH) or post-hemorrhagic hydrocephalus. Juvela et al. measured the CRP on admission, on postoperative day 1, and on discharge in adults with SAH. They found that CRP levels were significantly higher in patients with SAH and poor outcome compared with those with favorable outcome according to the Glasgow Outcome Scale at 3 months after SAH [18]. This is in agreement with other studies that found a significant increase of IL-6 in the CSF of SAH patients [21, 40]. Chaudhry et al. reported significantly increased serum IL-6 levels in patients with aneurysmal SAH, who presented with additional intraventricular or intracerebral bleeding, or both. Furthermore, serum IL-6 levels were also significantly higher in patients who developed persistent hydrocephalus and required VP shunt placement [5]. Savman et al. evaluated the CSF concentration of inflammatory cytokines in infants with post-hemorrhagic hydrocephalus due to IVH. They found an intense and prolonged inflammatory reaction with highly significant levels of TNFα, IL-1β, IL-6, and IL-8 in infants with post-hemorrhagic hydrocephalus compared with controls [36]. Similar findings were reported by others [17, 28].
CRP levels on postoperative day 2 were higher in patients of the shunt group compared with the non-shunt group and were almost equal to the postoperative findings of Juvela et al. [18]. A serum CRP over 40 mg/l 48 h postoperatively was significantly associated with shunt placement. The presence of intraventricular blood was also significantly correlated with a postoperative serum CRP level over 40 mg/l within the first 48 h postoperatively. Five of six patients with a CRP over 40 mg/l in the shunt group had blood within the ventricular system. Further, not every patient with blood showed an increased serum CRP level and vice versa. These findings remain unclear and need further evaluation.
Limitations
This study has several limitations. It is a retrospective, single-center study with the use of electronical record review. Thus, underreporting of complications, missing specimens, etc. cannot be ruled out. There is a bias concerning the indication of shunt placement, as only three senior surgeons managed these 70 cases. However, this single institution setting can also be seen as an advantage. Further, although 70 patients are a relatively large sample size for a pediatric cohort, the statistical power was limited for the variables tested, so that the results can formally be viewed as hypothesis-generating. Finally, it would have been ideal to investigate the inflammation pathways further, i.e., the concentration of inflammation markers in the CSF which were unavailable due to the retrospective setting.
Conclusions
We were able to identify several factors associated with shunt placement (Table 4) after posterior fossa tumor surgery in children. The data suggest that avoiding blood leakage into the ventricular system intraoperatively may be a factor the surgeon can influence in order to reduce the rate of shunt placements. Future studies are needed to explore the relationship between intraventricular blood and shunt placement in the setting of multivariate analysis including known shunt risk factors. Moreover, the aseptic inflammation pathways behind increased CRP levels and shunt requirement also need to be explored further.
References
Ahmann PA, Lazzara A, Dykes FD, Brann AW Jr, Schwartz JF (1980) Intraventricular hemorrhage in the high-risk preterm infant: incidence and outcome. Ann Neurol 7:118–124. https://doi.org/10.1002/ana.410070205
Bhatia R, Tahir M, Chandler CL (2009) The management of hydrocephalus in children with posterior fossa tumours: the role of pre-resectional endoscopic third ventriculostomy. Pediatr Neurosurg 45:186–191. https://doi.org/10.1159/000222668
Bognar L, Borgulya G, Benke P, Madarassy G (2003) Analysis of CSF shunting procedure requirement in children with posterior fossa tumors. Childs Nerv Syst 19:332–336. https://doi.org/10.1007/s00381-003-0745-x
Chaplin ER, Goldstein GW, Myerberg DZ, Hunt JV, Tooley WH (1980) Posthemorrhagic hydrocephalus in the preterm infant. Pediatrics 65:901–909
Chaudhry SR, Stoffel-Wagner B, Kinfe TM, Guresir E, Vatter H, Dietrich D, Lamprecht A, Muhammad S (2017) Elevated systemic IL-6 levels in patients with aneurysmal subarachnoid hemorrhage is an unspecific marker for post-SAH complications. Int J Mol Sci 18. https://doi.org/10.3390/ijms18122580
Chen Q, Feng Z, Tan Q, Guo J, Tang J, Tan L, Feng H, Chen Z (2017) Post-hemorrhagic hydrocephalus: recent advances and new therapeutic insights. J Neurol Sci 375:220–230. https://doi.org/10.1016/j.jns.2017.01.072
Culley DJ, Berger MS, Shaw D, Geyer R (1994) An analysis of factors determining the need for ventriculoperitoneal shunts after posterior fossa tumor surgery in children. Neurosurgery 34:402–407 discussion 407-408
de Ribaupierre S, Rilliet B, Vernet O, Regli L, Villemure JG (2007) Third ventriculostomy vs ventriculoperitoneal shunt in pediatric obstructive hydrocephalus: results from a Swiss series and literature review. Childs Nerv Syst 23:527–533. https://doi.org/10.1007/s00381-006-0283-4
Dewan MC, Lim J, Shannon CN, Wellons JC 3rd (2017) The durability of endoscopic third ventriculostomy and ventriculoperitoneal shunts in children with hydrocephalus following posterior fossa tumor resection: a systematic review and time-to-failure analysis. J Neurosurg Pediatr 19:1–7. https://doi.org/10.3171/2017.1.peds16536
Di Rocco F, Jucá CE, Zerah M, Sainte-Rose C (2013) Endoscopic third ventriculostomy and posterior fossa tumors. World Neurosurg 79(2 Suppl):15–19
Dias MS, Albright AL (1989) Management of hydrocephalus complicating childhood posterior fossa tumors. Pediatr Neurosci 15:283–289; discussion 290
Due-Tonnessen BJ, Helseth E (2007) Management of hydrocephalus in children with posterior fossa tumors: role of tumor surgery. Pediatr Neurosurg 43:92–96. https://doi.org/10.1159/000098379
El Beltagy MA, Kamal HM, Taha H, Awad M, El Khateeb N (2010) Endoscopic third ventriculostomy before tumor surgery in children with posterior fossa tumors, CCHE experience. Childs Nerv Syst 26:1699–1704
Foreman P, Mc-Clugage S 3rd, Naftel R, Griessenauer CJ, Ditty BJ, Agee BS, Riva-Cambrin J, Wellons J 3rd (2013) Validation and modification of a predictive model of postresection hydrocephalus in pediatric patients with posterior fossa tumors. J Neurosurg Pediatr 12:220–226. https://doi.org/10.3171/2013.5.peds1371
Gopalakrishnan CV, Dhakoji A, Menon G, Nair S (2012) Factors predicting the need for cerebrospinal fluid diversion following posterior fossa tumor surgery in children. Pediatr Neurosurg 48:93–101. https://doi.org/10.1159/000343009
Gram M, Sveinsdottir S, Cinthio M, Sveinsdottir K, Hansson SR, Morgelin M, Akerstrom B, Ley D (2014) Extracellular hemoglobin - mediator of inflammation and cell death in the choroid plexus following preterm intraventricular hemorrhage. J Neuroinflammation 11:200. https://doi.org/10.1186/s12974-014-0200-9
Habiyaremye G, Morales DM, Morgan CD, McAllister JP, CreveCoeur TS, Han RH, Gabir M, Baksh B, Mercer D, Limbrick DD Jr (2017) Chemokine and cytokine levels in the lumbar cerebrospinal fluid of preterm infants with post-hemorrhagic hydrocephalus. Fluids Barriers CNS 14:35. https://doi.org/10.1186/s12987-017-0083-0
Juvela S, Kuhmonen J, Siironen J (2012) C-reactive protein as predictor for poor outcome after aneurysmal subarachnoid haemorrhage. Acta Neurochir 154:397–404. https://doi.org/10.1007/s00701-011-1243-7
Karimy JK, Zhang J, Kurland DB, Theriault BC, Duran D, Stokum JA, Furey CG, Zhou X, Mansuri MS, Montejo J, Vera A, DiLuna ML, Delpire E, Alper SL, Gunel M, Gerzanich V, Medzhitov R, Simard JM, Kahle KT (2017) Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat Med 23:997–1003. https://doi.org/10.1038/nm.4361
Kestle JR, Riva-Cambrin J, Wellons JC 3rd, Kulkarni AV, Whitehead WE, Walker ML, Oakes WJ, Drake JM, Luerssen TG, Simon TD, Holubkov R, Hydrocephalus Clinical Research N (2011) A standardized protocol to reduce cerebrospinal fluid shunt infection: the Hydrocephalus Clinical Research Network Quality Improvement Initiative. J Neurosurg Pediatr 8:22–29. https://doi.org/10.3171/2011.4.PEDS10551
Killer M, Arthur A, Al-Schameri AR, Barr J, Elbert D, Ladurner G, Shum J, Cruise G (2010) Cytokine and growth factor concentration in cerebrospinal fluid from patients with hydrocephalus following endovascular embolization of unruptured aneurysms in comparison with other types of hydrocephalus. Neurochem Res 35:1652–1658. https://doi.org/10.1007/s11064-010-0226-z
Kulkarni AV, Riva-Cambrin J, Butler J, Browd SR, Drake JM, Holubkov R, Kestle JR, Limbrick DD, Simon TD, Tamber MS, Wellons JC 3rd, Whitehead WE, Hydrocephalus Clinical Research N (2013) Outcomes of CSF shunting in children: comparison of Hydrocephalus Clinical Research Network cohort with historical controls. clinical article J Neurosurg Pediatr 12:334–338. https://doi.org/10.3171/2013.7.PEDS12637
Kulkarni AV, Riva-Cambrin J, Holubkov R, Browd SR, Cochrane DD, Drake JM, Limbrick DD, Rozzelle CJ, Simon TD, Tamber MS, Wellons JC 3rd, Whitehead WE, Kestle JR (2016) Endoscopic third ventriculostomy in children: prospective, multicenter results from the Hydrocephalus Clinical Research Network. J Neurosurg Pediatr 18:423–429. https://doi.org/10.3171/2016.4.peds163
Kumar V, Phipps K, Harkness W, Hayward RD (1996) Ventriculo-peritoneal shunt requirement in children with posterior fossa tumours: an 11-year audit. Br J Neurosurg 10:467–470
Lee M, Wisoff JH, Abbott R, Freed D, Epstein FJ (1994) Management of hydrocephalus in children with medulloblastoma: prognostic factors for shunting. Pediatr Neurosurg 20:240–247. https://doi.org/10.1159/000120797
Merchant TE, Fouladi M (2005) Ependymoma: new therapeutic approaches including radiation and chemotherapy. J Neuro-Oncol 75:287–299. https://doi.org/10.1007/s11060-005-6753-9
Morelli D, Pirotte B, Lubansu A, Detemmerman D, Aeby A, Fricx C, Berre J, David P, Brotchi J (2005) Persistent hydrocephalus after early surgical management of posterior fossa tumors in children: is routine preoperative endoscopic third ventriculostomy justified? J Neurosurg 103:247–252. https://doi.org/10.3171/ped.2005.103.3.0247
Naureen I, Waheed Kh A, Rathore AW, Victor S, Mallucci C, Goodden JR, Chohan SN, Miyan JA (2014) Fingerprint changes in CSF composition associated with different aetiologies in human neonatal hydrocephalus: inflammatory cytokines. Childs Nerv Syst 30:1155–1164. https://doi.org/10.1007/s00381-014-2415-6
O’Hayon BB, Drake JM, Ossip MG, Tuli S, Clarke M (1998) Frontal and occipital horn ratio: a linear estimate of ventricular size for multiple imaging modalities in pediatric hydrocephalus. Pediatr Neurosurg 29:245–249 doi:28730
Papo I, Caruselli G, Luongo A (1982) External ventricular drainage in the management of posterior fossa tumors in children and adolescents. Neurosurgery 10:13–15
Rekate HL (2008) Shunt-related headaches: the slit ventricle syndromes. Childs Nerv Syst 24:423–430. https://doi.org/10.1007/s00381-008-0579-7
Riva-Cambrin J, Detsky AS, Lamberti-Pasculli M, Sargent MA, Armstrong D, Moineddin R, Cochrane DD, Drake JM (2009) Predicting postresection hydrocephalus in pediatric patients with posterior fossa tumors. J Neurosurg Pediatr 3:378–385. https://doi.org/10.3171/2009.1.PEDS08298
Ruggiero C, Cinalli G, Spennato P, Aliberti F, Cianciulli E, Trischitta V, Maggi G (2004) Endoscopic third ventriculostomy in the treatment of hydrocephalus in posterior fossa tumors in children. Childs Nerv Syst 20:828–833. https://doi.org/10.1007/s00381-004-0938-y
Sainte-Rose C, Cinalli G, Roux FE, Maixner R, Chumas PD, Mansour M, Carpentier A, Bourgeois M, Zerah M, Pierre-Kahn A, Renier D (2001) Management of hydrocephalus in pediatric patients with posterior fossa tumors: the role of endoscopic third ventriculostomy. J Neurosurg 95:791–797
Santos de Oliveira R, Barros Juca CE, Valera ET, Machado HR (2008) Hydrocephalus in posterior fossa tumors in children. Are there factors that determine a need for permanent cerebrospinal fluid diversion? Childs Nerv Syst 24:1397–1403. https://doi.org/10.1007/s00381-008-0649-x
Savman K, Blennow M, Hagberg H, Tarkowski E, Thoresen M, Whitelaw A (2002) Cytokine response in cerebrospinal fluid from preterm infants with posthaemorrhagic ventricular dilatation. Acta Paediatr 91:1357–1363
Schmid UD, Seiler RW (1986) Management of obstructive hydrocephalus secondary to posterior fossa tumors by steroids and subcutaneous ventricular catheter reservoir. J Neurosurg 65:649–653. https://doi.org/10.3171/jns.1986.65.5.0649
Schneider C, Ramaswamy V, Kulkarni AV, Rutka JT, Remke M, Tabori U, Hawkins C, Bouffet E, Taylor MD (2015) Clinical implications of medulloblastoma subgroups: incidence of CSF diversion surgery. J Neurosurg Pediatr 15:236–242. https://doi.org/10.3171/2014.9.PEDS14280
Tamburrini G, Pettorini BL, Massimi L, Caldarelli M, Di Rocco C (2008) Endoscopic third ventriculostomy: the best option in the treatment of persistent hydrocephalus after posterior cranial fossa tumour removal? Childs Nerv Syst 24:1405–1412. https://doi.org/10.1007/s00381-008-0699-0
Wang L, Gao Z (2018) Expression of MMP-9 and IL-6 in patients with subarachnoid hemorrhage and the clinical significance. Exp Ther Med 15:1510–1514. https://doi.org/10.3892/etm.2017.5553
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Helmbold, L.J., Kammler, G., Regelsberger, J. et al. Predictive factors associated with ventriculoperitoneal shunting after posterior fossa tumor surgery in children. Childs Nerv Syst 35, 779–788 (2019). https://doi.org/10.1007/s00381-019-04136-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-019-04136-w