Abstract
Objective
To analyze factors associated with the development of early symptomatic hydrocephalus following posterior fossa tumor (PFT) surgery in children.
Methods
In this retrospective study, data from 148 children (age < 18 years) who underwent primary resection of their PFTs without preoperative permanent CSF diversion procedures were collected. The incidence of symptomatic hydrocephalus within 30 days of tumor resection was studied and its association with various demographic, tumor-related, and surgery-related risk factors was analyzed.
Results
At presentation, 131 (89%) of the 148 patients had symptomatic hydrocephalus. There were 99 males and 49 females (mean age 8.7 years; range 1 to 17 years). Postoperatively, 14 (9.4%) patients required shunt placement for symptomatic hydrocephalus. The indications for shunt surgery were persistent symptoms of raised intracranial pressure (n = 6, 43%), CSF leak from the wound (n = 7, 50%), and tense pseudomeningocele (n = 1, 7%). On multivariate analysis, age < 6 years (OR 5.9, 95% CI 1.6–22.6, p = 0.009) and the presence of intraventricular blood (IVB) on postoperative CT (OR 6.4, 95% CI 1.7–23.7, p = 0.006) were independent risk factors for developing symptomatic hydrocephalus.
Conclusions
The incidence of postoperative symptomatic hydrocephalus in our series (9.4%) is lower than that reported in most previous studies. Age < 6 years and the presence of postoperative IVB were independent risk factors for developing symptomatic hydrocephalus. Of these, postoperative IVB is probably the only modifiable risk factor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Up to 30% of children undergoing surgery for posterior fossa tumors (PFTs) develop postoperative symptomatic hydrocephalus requiring permanent cerebrospinal fluid (CSF) diversion either by ventriculoperitoneal (VP) shunt or endoscopic third ventriculostomy (ETV) [1, 2]. Some of the studies reporting rates of shunt requirement have failed to differentiate between early and late postoperative hydrocephalus [3,4,5]. While the former, which forms the vast majority, is often related to either tumor-related or surgery-related factors; late postoperative hydrocephalus is usually a result of either tumor recurrence or radiation-induced changes. A recent systematic review addressing management of post-PFT resection hydrocephalus with either ETV or VP shunt has not stated the time interval between PFT resection and the CSF diversion procedure [1].
Others, who have reported the incidence of early postoperative hydrocephalus, have included patients who developed hydrocephalus as late as 6 weeks after surgery by which time the children might have begun radiation therapy. Also, most authors have not separately analyzed factors that may predict the development of early and late postoperative hydrocephalus [6,7,8,9,10]. Furthermore, some of the series predate the microsurgical era and hence might have lower rates of gross total resection of the tumor [3, 10]. Reported risk factors for hydrocephalus include young age, shorter symptom duration, midline tumors, more severe preoperative hydrocephalus, incomplete tumor resection, aggressive tumor pathology, cerebral metastases, CSF infection, and prolonged external ventricular drainage [11].
Our study was designed to overcome some of the shortcomings of the previous studies. We defined early postoperative hydrocephalus as that occurring within 30 days of the surgery. We aimed to determine the incidence of early symptomatic postoperative hydrocephalus and study the possible risk factors associated with its development in children undergoing excision of PFTs at our institution.
Materials and methods
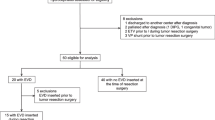
We initially reviewed the inpatient and outpatient medical records of 200 consecutive children (< 18 years of age) who underwent PFT resection in a single neurosurgical unit at our institution between January 2005 and September 2018. Patients undergoing surgery for brainstem tumors and extra-axial tumors were excluded.
Of the 200 patients, VP shunt had been inserted preoperatively in 42 (21%) of them—25 in our institution and 17 elsewhere. Of the remaining158 patients, 10 were lost to follow-up after surgery and were excluded. Data from the 148 children (99 males and 49 females) who had a follow-up of 30 days or more were analyzed to study the incidence and risk factors for development of symptomatic hydrocephalus. The median age at presentation was 8 (IQR 5–12) years.
Surgical procedure and extent of resection
All patients underwent tumor resection via suboccipital craniectomy. External ventricular drain (EVD) was inserted prior to surgery only in children who presented with persistent bradycardia or altered sensorium. In all patients, an attempt was made to achieve a radical excision of the tumor in order to restore the normal CSF pathways. The extent of resection (EOR) of the tumor was categorized as gross total resection (GTR; no visible residue), sub-total resection (STR; < 10% residue and CSF pathways opened intraoperatively), and partial resection (PR; > 10% residue or < 10% residue but CSF pathways not opened intraoperatively) based on postoperative contrast computed tomography (CT) done within 2 weeks of tumor resection. GTR was performed in 99 (67%) patients, STR in 25 (17%), and the remaining 24 (16%) underwent PR.
Hydrocephalus
The presence of hydrocephalus was determined using Evan’s ratio on preoperative and postoperative CT or magnetic resonance imaging (MRI) [12]. An Evan’s ratio of more than 0.3 was considered as ventriculomegaly.
Pathology
Tumors were classified as either midline or lateral, according to their location in the vermis/fourth ventricle or cerebellar hemisphere respectively. Pilocytic astrocytoma and choroid plexus papilloma were classified as benign tumors, whereas medulloblastoma, ependymoma, and high-grade gliomas were classified as aggressive.
Risk factors for CSF diversion
The variables studied were age at presentation, gender, location of the tumor, perioperative EVD, EOR, pathological diagnosis, occurrence of postoperative meningitis (bacterial if CSF culture showed growth or aseptic if CSF was sterile) [13] and the presence of intraventricular blood (IVB) on early postoperative CT (done 1 day to 14 days after surgery; mean 4.6 ± 2.4 days; available in 144 of the 148 patients).
Data analysis
Statistical analysis was done using SPSS (version 16.0; SPSS, Chicago IL, USA). For univariate analysis, chi-square test was used for categorical variables and Student t test or non-parametric test (Mann-Whitney) was used for continuous variables as required. Multivariate logistic regression was done on variables which had p values < 0.2 on univariate analysis, to determine independent risk factors for early postoperative shunt placement. Agreement statistics (kappa) between categorical variables and comparison of the regression coefficients with the standard error ruled out collinearity of these variables. A p value of < 0.05 was considered significant.
Results
Pathology
Table 1 summarizes the pathological diagnoses in our patient cohort. The most common tumors in our series were medulloblastoma (n = 76, 51.3%) and pilocytic astrocytoma (n = 53, 35.8%). Overall, 55 (37.2%) of the148 tumors were classified as benign while 93 (62.8%) were classified as aggressive.
Early postoperative hydrocephalus
At presentation, 141 (95.3%) of the 148 patients had hydrocephalus on preoperative CT/MRI and 131 out of 141 (92.9%) were symptomatic for the same. Fourteen of the 148 patients (9.4%) required shunt placement (one of whom declined consent for the same) within 30 days following tumor excision. The median time from tumor excision to placement of shunt was 14 (IQR 6.7–17.2) days. The indications for shunt placement were persistent symptoms of raised intracranial pressure (n = 6, 43%), CSF leak from the wound (n = 7, 50%), and tense pseudomeningocele (n = 1, 7%). The details of the patients who underwent early postoperative shunt placement within 30 days of tumor excision are summarized in Table 2.
Perioperative EVD and lumbar subarachnoid drain
Preoperative EVD was performed in 10 (9.4%) of the 148 patients, of whom two required postoperative shunt placement. Three patients who did not have preoperative EVD insertion had postoperative EVD placement, two of whom required subsequent shunt surgery. In 4 of the 17 patients with CSF leak from the operative wound, lumbar subarachnoid drain was inserted and none of these four patients required a permanent CSF diversion. Lumbar drains were inserted only if there was no hydrocephalus on the postoperative CT scan.
Factors predicting early postoperative shunt requirement
On univariate analysis, age < 6 years (p = 0.02), postoperative meningitis (p = 0.008), and the presence of IVB on early postoperative CT (p = 0.001) were significantly associated with early postoperative shunt requirement. However, on multivariate analysis, the only independent predictors of early postoperative shunt requirement were age < 6 years (OR 5.9, 95% CI 1.6–22.6, p = 0.009) and the presence of IVB (OR 6.4, 95% CI 1.7–23.7, p = 0.006). Gender, tumor location, placement of preoperative EVD, degree of tumor resection, and tumor pathology were not significantly associated with requirement of postoperative permanent CSF diversion procedure. The results of univariate and multivariate analysis are summarized in Table 3. The management and outcomes of selected patients are depicted in Figs. 1 and 2.
a Axial post-gadolinium MR images showing a contrast-enhancing tumor in the fourth ventricle with hydrocephalus in a 7-year-old boy. He underwent subtotal resection of the medulloblastoma but free flow of CSF could be seen from the aqueduct by the end of surgery. b Contrast-enhanced CT done on the third postoperative day showing residual tumor along the floor of the fourth ventricle with persistent hydrocephalus. Since he had no symptoms of raised intracranial pressure, a shunt was not performed. c Axial post-gadolinium MR images 6 months after craniospinal irradiation and chemotherapy showing stable tumor residue with persistent but asymptomatic hydrocephalus
a Axial post-gadolinium MR images showing a vermian medulloblastoma with mild hydrocephalus in a 5-year-old boy. He underwent gross total resection of the tumor. b Contrast-enhanced CT done 3 days after surgery showing no enhancing tumor residue. There was no change in ventricular size and there was blood in the third and lateral ventricles. c Non-contrast CT done on the 15th postoperative day when he was readmitted with symptoms of raised intracranial pressure CT showing marked worsening of hydrocephalus
Comparison between patients who had pre-resection shunts and those who underwent direct tumor resection
Table 4 compares the 42 patients who underwent pre-resection shunts and the 158 patients who underwent direct tumor resection. Patient factors such as age, tumor location, and tumor pathology were analyzed and no statistically significant difference could be found between both groups.
Discussion
Preoperative management of hydrocephalus
In our cohort of children with PFTs, 141 (95.3%) patients had hydrocephalus at presentation which is consistent with the rate of 75 to 95% reported in literature [5, 6, 8]. Preoperative hydrocephalus in children with PFTs is largely attributable to obstruction of the CSF flow at the aqueduct or fourth ventricular outlet but may occasionally be caused by dysfunctional absorption of CSF due to subarachnoid spread of tumor, hemorrhage, or chronic inflammation [11, 14]. The high prevalence of hydrocephalus at presentation is probably related to the lack of symptoms in children when these tumors are small and the non-specific nature of symptoms like vomiting.
More than 50 years ago, Abraham and Chandy [15] from our institution documented for the first time the efficacy of preoperative ventriculoatrial shunt in reducing the postoperative mortality in children with PFTs. They reported a reduction in the postoperative mortality in these children from a historical figure of around 40 to 8%. Subsequent to this report, other authors also advocated preoperative shunt or ETV to improve the operative conditions at the time of tumor surgery and reduce mortality [8, 16,17,18,19,20].
A preoperative shunt was a useful strategy when children with PFTs presented late and in a moribund condition with poor nutritional status due to incessant vomiting. The reduction of the raised intracranial pressure and the associated vomiting meant that these children’s nutritional status could be improved and thus they were better able to tolerate tumor surgery.
However, with modern imaging, children PFTs are being diagnosed earlier and present in a relatively better condition. This has led to most surgeons adopting a strategy of direct tumor resection without the benefit of a preoperative shunt. Proponents of direct tumor resection have also cited the occurrence of complications such as cerebellar herniation and intratumoral hemorrhage following pre-resection shunt insertion [21,22,23]. Some even believe that PFT surgery following a VP shunt can sometimes be more technically challenging and hazardous due to migration of the tumor closer to the brainstem [24].
Pre-resection VP shunts also largely fell out of favor following the finding of several authors that the rate of persistent hydrocephalus following tumor resection was less than 40% [2, 4,5,6, 14, 16, 17]. Hence, > 60% of children with PFTs and hydrocephalus do not need a shunt after the tumor surgery.
We prefer to operate on the tumor directly without a preoperative shunt. We perform an EVD for a short period (1 to 3 days) before excision of the tumor only when a child presents with clinical features of impending herniation. Some authors recommend routine perioperative EVD in PFT surgery [25].
Early postoperative hydrocephalus
Although the goals of tumor resection for PFTs in children include a safe radical excision of the tumor with restoration of normal CSF pathways, up to 18–40% of them have been reported to need some form of CSF diversion in the postoperative period [2]. Cerebellar swelling and edema after tumor resection, adhesions at the surgery site, and postoperative aseptic/bacterial meningitis may contribute to persistence of hydrocephalus after surgery. The literature available on early post-resection hydrocephalus requiring permanent CSF diversion in children with PFT ranges from 7 to 32% [4, 6,7,8, 10, 14, 26,27,28]. In our patient cohort, only 9.4% of patients required permanent CSF diversion, which is lower than the rate reported in most of the largest series in literature (Table 5) [2, 4, 6, 14, 17, 25, 26, 28,29,30]. This could possibly be attributed to the use of suboccipital craniectomies. We also achieved a relatively high rate of GTR (67%) in our patients, comparable to the 57 to 73% reported by other large series [1, 4, 10, 14, 26].
The definition of the early postoperative period in different reports has varied between 4 and 6 weeks after surgery and factors associated with early and late postoperative shunt requirement have not been analyzed separately [4, 6, 7, 14]. The requirement for delayed postoperative shunts are mostly related to factors such as tumor recurrence and radiation-induced scarring of fourth ventricular outflow tracts and may not necessarily reflect the failure of the primary surgery in restoring the normal CSF pathways.
Risk factors for early postoperative hydrocephalus
Younger age has been consistently reported in literature (Table 6) to be a risk factor for needing CSF diversion after resection of PFTs [2, 4, 6, 9, 10, 14, 27], except in the series reported by Gopalakrishnan et al. [15]. However, the threshold age below which the risk increases has been found to range from 2 to 10 years. In our patient cohort, age < 6 years was an independent risk factor for postoperative VP shunt after tumor resection. This cutoff was chosen based on the age distribution of our study population. The Canadian Preoperative Prediction Rule for Hydrocephalus (CPPRH) proposed by Riva-Cambrin et al. [2] and its subsequent modification (mCPPRH) [29] were predictive scores based on preoperative clinical features, demographics, and radiological findings. In the CPPRH, maximum weightage was given to age < 2 years, presence of preoperative moderate/severe hydrocephalus, and presence of cerebral metastases while tumor histology was given a lower weightage. However, this scoring system does not factor in the extent of tumor excision which may be a major determinant for the persistence of symptomatic hydrocephalus in the postoperative period.
Failure of restoration of physiological CSF flow through the aqueduct should theoretically place this subgroup of patients at a high risk for needing a VP shunt post-tumor resection. GTR and near-total resection (NTR) of PFTs have been reported to be associated with lower post-resection CSF diversion rates in a number of series [4, 14, 26]. However, in our patient cohort, partial tumor failed to show a significant association with postoperative shunt requirement. A few other authors also were unable to find any association between EOR and shunt insertion rate [3, 6]. Interestingly, an older series on patients with cerebellar astrocytomas found a significantly higher requirement of postoperative VP shunts amongst patients who had GTR [31].
In our study, postoperative meningitis (bacterial/aseptic) was a risk factor for postoperative VP shunt placement on univariate analysis but failed to attain statistical significance on multivariate analysis. Other authors have reported a clear association between postoperative meningitis and the requirement for permanent CSF diversion [8, 14]. Shunt placement may be mandated in patients with postoperative meningitis due to increased CSF production resulting in raised intracranial pressure or obstruction of CSF outflow pathways due to inflammation of the meninges [32].
Although midline PFTs have been reported to have a higher rate of post-resection CSF diversion requirement by some authors [8, 10, 14, 27], the location of the tumor was not a risk factor for symptomatic postoperative hydrocephalus in our patient cohort. Our findings agree with others who also did not report tumor location as a risk factor [3, 6, 33].
Tumor histology—medulloblastoma/ependymoma—has been proven to be associated with increased risk for postoperative VP shunt placement [4, 8, 34]. However, in this study, an association between aggressive tumor pathology and early postoperative hydrocephalus could not be found.
Culley et al. [14] were the first to suggest that a longer duration of perioperative EVD insertion could increase the likelihood of persistent postoperative hydrocephalus. Subsequently, Bognár et al. [6] proposed that children with preoperative or postoperative EVD placement had significantly higher shunt insertion rates than those who did not, particularly if the EVD was kept for more than 8 days. Contrary to this, insertion of preoperative EVD was not a risk factor for VP shunt placement in our cohort. Others have reported a similar experience [3, 4].
Modifiable risk factors
Most risk factors for early postoperative symptomatic hydrocephalus in children with PFT are not modifiable. EOR is a possible modifiable risk factor but the aim of surgery for PFT is to achieve as radical a resection of the tumor without additional neurological morbidity. EVD placement is another possible modifiable risk factor but in some instances such as when the patient is acutely symptomatic for raised intracranial pressure, it may not be possible to avoid this form of CSF diversion prior to tumor surgery. The duration of EVD could, however, be kept to the minimum.
Our finding that IVB on postoperative CT is an independent risk factor also seems to be one of the modifiable risk factors for early postoperative symptomatic hydrocephalus. No other study on children with PFTs has reported this association. It is well known that intraventricular hemorrhage predisposes a patient to develop hydrocephalus [35]. The most widely accepted cause for this is direct blockage of CSF drainage pathways by blood clots [36]. Another established mechanism is the impaired absorption of CSF due to inflammation (via transforming growth factors TGFβ1 and TGFβ1) and fibrosis of the arachnoid granulations, caused by blood degradation products [37]. The role of free iron and thrombin in damage to the ependymal lining of the ventricles and periventricular blood-brain barrier causing hydrocephalus has also been described [38, 39]. Surgeons should therefore take measures to avoid spillage of blood into the ventricular system by layering the ventral aspect of the tumor and opened fourth ventricle with cottonoids and gelatin sponges. These measures might reduce the incidence of early postoperative symptomatic hydrocephalus in children with PFTs.
The limitations of this study need to be highlighted. The first is its retrospective nature, due to which adequate follow-up could not be obtained for 10 patients. Second, the relatively small number of patients who developed shunt-dependent hydrocephalus following surgery may have decreased the overall strength of the risk factor analysis. Third, it is well known that MRI and not CT is the gold standard to determine degree of resection. However, it was not our routine practice to perform MRI in the early postoperative period for children because it can be challenging and often necessitates administration of anesthesia. Lastly, although the vast majority of our patients’ postoperative CT scans were done within 1 week following surgery, 16 had them done in the second week; by which time, any IVB, if present, may have disappeared. Nonetheless, ours is the first study to study the association between postoperative IVB and shunt requirement following PFT resection in children and will hopefully pave the way for more research by other authors.
Conclusions
The early postoperative shunt requirement rate of 9.4% in our children with PFTs is one of the lowest reported. Age < 6 years and the presence of postoperative IVB were independent risk factors for the requirement of a VP shunt in the early postoperative period. Surgeons should take measures to avoid entry of blood in to the ventricular system as it is a modifiable risk factor for the development of early postoperative symptomatic hydrocephalus.
References
Dewan MC, Lim J, Shannon CN, Wellons JC (2017) The durability of endoscopic third ventriculostomy and ventriculoperitoneal shunts in children with hydrocephalus following posterior fossa tumor resection: a systematic review and time-to-failure analysis. J Neurosurg Pediatr 19:578–584. https://doi.org/10.3171/2017.1.PEDS16536
Riva-Cambrin J, Detsky AS, Lamberti-Pasculli M, Sargent MA, Armstrong D, Moineddin R, Cochrane DD, Drake JM (2009) Predicting postresection hydrocephalus in pediatric patients with posterior fossa tumors. J Neurosurg Pediatr 3:378–385. https://doi.org/10.3171/2009.1.PEDS08298
Dias MS, Albright AL (1989) Management of hydrocephalus complicating childhood posterior fossa tumors. Pediatr Neurosci 15:283–290
Kumar V, Phipps K, Harkness W, Hayward RD (1996) Ventriculo-peritoneal shunt requirement in children with posterior fossa tumours: an 11-year audit. Br J Neurosurg 10:467–470
Raimondi AJ, Tomita T (1981) Hydrocephalus and infratentorial tumors. Incidence, clinical picture, and treatment. J Neurosurg 55:174–182. https://doi.org/10.3171/jns.1981.55.2.0174
Bognár L, Borgulya G, Benke P, Madarassy G (2003) Analysis of CSF shunting procedure requirement in children with posterior fossa tumors. Childs Nerv Syst 19:332–336. https://doi.org/10.1007/s00381-003-0745-x
Due-Tønnessen BJ, Helseth E (2007) Management of hydrocephalus in children with posterior fossa tumors: role of tumor surgery. Pediatr Neurosurg 43:92–96. https://doi.org/10.1159/000098379
Gopalakrishnan CV, Dhakoji A, Menon G, Nair S (2012) Factors predicting the need for cerebrospinal fluid diversion following posterior fossa tumor surgery in children. Pediatr Neurosurg 48:93–101. https://doi.org/10.1159/000343009
Lee M, Wisoff JH, Abbott R, Freed D, Epstein FJ (1994) Management of hydrocephalus in children with medulloblastoma: prognostic factors for shunting. Pediatr Neurosurg 20:240–247. https://doi.org/10.1159/000120797
Papo I, Caruselli G, Luongo A (1982) External ventricular drainage in the management of posterior fossa tumors in children and adolescents. Neurosurgery 10:13–15
Lin C-T, Riva-Cambrin JK (2015) Management of posterior fossa tumors and hydrocephalus in children: a review. Childs Nerv Syst 31:1781–1789. https://doi.org/10.1007/s00381-015-2781-8
Evans WA Jr (1942) An encephalographic ratio for estimating ventricular enlargement and cerebral atrophy. Arch Neurol Psychiatr 47:931–937. https://doi.org/10.1001/archneurpsyc.1942.02290060069004
Moorthy RK, Sarkar H, Rajshekhar V (2013) Conservative antibiotic policy in patients undergoing non-trauma cranial surgery does not result in higher rates of postoperative meningitis: an audit of nine years of narrow-spectrum prophylaxis. Br J Neurosurg 27:497–502. https://doi.org/10.3109/02688697.2013.771138
Culley DJ, Berger MS, Shaw D, Geyer R (1994) An analysis of factors determining the need for ventriculoperitoneal shunts after posterior fossa tumor surgery in children. Neurosurgery 34:402–408
Abraham J, Chandy J (1963) Ventriculo-atrial shunt in the management of posterior-fossa tumours: preliminary report. J Neurosurg 20:252–253. https://doi.org/10.3171/jns.1963.20.3.0252
Albright L, Reigel DH (1977) Management of hydrocephalus secondary to posterior fossa tumors. J Neurosurg 46:52–55. https://doi.org/10.3171/jns.1977.46.1.0052
Sainte-Rose C, Cinalli G, Roux FE et al (2001) Management of hydrocephalus in pediatric patients with posterior fossa tumors: the role of endoscopic third ventriculostomy. J Neurosurg 95:791–797. https://doi.org/10.3171/jns.2001.95.5.0791
Ruggiero C, Cinalli G, Spennato P et al (2004) Endoscopic third ventriculostomy in the treatment of hydrocephalus in posterior fossa tumors in children. Childs Nerv Syst 20:828–833. https://doi.org/10.1007/s00381-004-0938-y
Bhatia R, Tahir M, Chandler CL (2009) The management of hydrocephalus in children with posterior fossa tumours: the role of pre-resectional endoscopic third ventriculostomy. Pediatr Neurosurg 45:186–191. https://doi.org/10.1159/000222668
El Beltagy MA, Kamal HM, Taha H, Awad M, El Khateeb N (2010) Endoscopic third ventriculostomy before tumor surgery in children with posterior fossa tumors, CCHE experience. Childs Nerv Syst 26:1699–1704. https://doi.org/10.1007/s00381-010-1180-4
Epstein F, Murali R (1978) Pediatric posterior fossa tumors: hazards of the “preoperative” shunt. Neurosurgery 3:348–350
El-Gaidi MA, El-Nasr AHA, Eissa EM (2015) Infratentorial complications following preresection CSF diversion in children with posterior fossa tumors. J Neurosurg Pediatr 15:4–11. https://doi.org/10.3171/2014.8.PEDS14146
Santhanam R, Balasubramaniam A, Chandramouli BA (2009) Fatalintratumoral hemorrhage in posterior fossa tumors following ventriculoperitoneal shunt. J Clin Neurosci 16:135–137. https://doi.org/10.1016/j.jocn.2008.02.016
Goel A (1993) Whither preoperative shunts for posterior fossa tumours? Br J Neurosurg 7:395–399
Rappaport ZH, Shalit MN (1989) Perioperative external ventricular drainage in obstructive hydrocephalus secondary to infratentorial brain tumours. Acta Neurochir 96:118–121
Gnanalingham KK, Lafuente J, Thompson D, Harkness W, Hayward R (2003) The natural history of ventriculomegaly and tonsillar herniation in children with posterior fossa tumours – an MRI study. Pediatr Neurosurg 39:246–253. https://doi.org/10.1159/000072869
Santos de Oliveira R, Barros Jucá CE, Valera ET, Machado HR (2008) Hydrocephalus in posterior fossa tumors in children. Are there factors that determine a need for permanent cerebrospinal fluid diversion? Childs Nerv Syst 24:1397–1403. https://doi.org/10.1007/s00381-008-0649-x
Tamburrini G, Pettorini BL, Massimi L, Caldarelli M, Di Rocco C (2008) Endoscopic third ventriculostomy: the best option in the treatment of persistent hydrocephalus after posterior cranial fossa tumour removal? Childs Nerv Syst 24:1405–1412. https://doi.org/10.1007/s00381-008-0699-0
Foreman P, McClugage S, Naftel R, Griessenauer CJ, Ditty BJ, Agee BS, Riva-Cambrin J, Wellons J (2013) Validation and modification of a predictive model of postresection hydrocephalus in pediatric patients with posterior fossa tumors. J Neurosurg Pediatr 12:220–226. https://doi.org/10.3171/2013.5.PEDS1371
Schneider C, Ramaswamy V, Kulkarni AV et al (2015) Clinical implications of medulloblastoma subgroups: incidence of CSF diversion surgery. J Neurosurg Pediatr 15:236–242. https://doi.org/10.3171/2014.9.PEDS14280
Stein BM, Tenner MS, Fraser RA (1972) Hydrocephalus following removal of cerebellar astrocytomas in children. J Neurosurg 36:763–768. https://doi.org/10.3171/jns.1972.36.6.0763
Dubey AK, Rao KL (1997) Pathology of post meningitic hydrocephalus. Indian J Pediatr 64:30–33
Schmid UD, Seiler RW (1986) Management of obstructive hydrocephalus secondary to posterior fossa tumors by steroids and subcutaneous ventricular catheter reservoir. J Neurosurg 65:649–653. https://doi.org/10.3171/jns.1986.65.5.0649
Morelli D, Pirotte B, Lubansu A, Detemmerman D, Aeby A, Fricx C, Berré J, David P, Brotchi J (2005) Persistent hydrocephalus after early surgical management of posterior fossa tumors in children: is routine preoperative endoscopic third ventriculostomy justified? J Neurosurg 103:247–252. https://doi.org/10.3171/ped.2005.103.3.0247
Bu Y, Chen M, Gao T, Wang X, Li X, Gao F (2016) Mechanisms of hydrocephalus after intraventricular haemorrhage in adults. Stroke Vasc Neurol 1:23–27. https://doi.org/10.1136/svn-2015-000003
Strahle J, Garton HJL, Maher CO, Muraszko KM, Keep RF, Xi G (2012) Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl Stroke Res 3:25–38. https://doi.org/10.1007/s12975-012-0182-9
Kaestner S, Dimitriou I (2013) TGF beta1 and TGF beta2 and their role in posthemorrhagic hydrocephalus following SAH and IVH. J Neurol Surg A Cent Eur Neurosurg 74:279–284. https://doi.org/10.1055/s-0033-1342929
Gao C, Du H, Hua Y, Keep RF, Strahle J, Xi G (2014) Role of red blood cell lysis and iron in hydrocephalus after intraventricular hemorrhage. J Cereb Blood Flow Metab 34:1070–1075. https://doi.org/10.1038/jcbfm.2014.56
Gao F, Liu F, Chen Z, Hua Y, Keep RF, Xi G (2014) Hydrocephalus after intraventricular hemorrhage: the role of thrombin. J Cereb Blood Flow Metab 34:489–494. https://doi.org/10.1038/jcbfm.2013.225
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest. The datasets generated for the study are available from the corresponding author on reasonable request.
Additional information
A portion of this study was presented as a podium presentation at the 29th Annual Conference of the Indian Society for Pediatric Neurosurgery in February, 2018.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abraham, A.P., Moorthy, R.K., Jeyaseelan, L. et al. Postoperative intraventricular blood: a new modifiable risk factor for early postoperative symptomatic hydrocephalus in children with posterior fossa tumors. Childs Nerv Syst 35, 1137–1146 (2019). https://doi.org/10.1007/s00381-019-04195-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-019-04195-z