Abstract
Background
Aneurysmal subarachnoid haemorrhage (SAH) is a severe disease with high case-fatality and morbidity rates. After SAH, the value of C-reactive protein (CRP)—an acute phase sensitive inflammatory marker—as a prognostic factor has been poorly studied, with conflicting results. In this prospective study, we tested whether increased CRP levels increase independently the risk for cerebral infarct and poor outcome.
Methods
Previous diseases as well as clinical, laboratory and radiological variables were recorded for 178 patients with SAH admitted within 48 h and with aneurysms occluded within 60 h after bleeding. Plasma CRP was measured, as well as computed tomography (CT) scans routinely obtained on admission, in the morning after aneurysm occlusion, and at discharge during second week after SAH. Factors predicting occurrence of cerebral infarct and poor outcome at 3 months after SAH were tested with multiple logistic regression.
Results
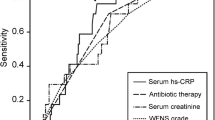
CRP levels increased significantly (p < 0.001) between hospital admission (mean ± SD, 11.4 ± 21.3 mg/l) and the postoperative morning (27.0 ± 31.0 mg/l) and then decreased (p < 0.001) during the the second week (19.8 ± 25.0 mg/l). Admission (18.0 ± 35.7 vs 8.5 ± 8.4 mg/l) and postoperative (41.0 ± 40.2 vs 21.1 ± 24.1 mg/l) CRP levels were higher (p < 0.001) in those with a poor outcome than in those with a favourable outcome, but CRP values did not predict delayed cerebral ischaemia or cerebral infarction. CRP levels did not independently predict outcome, since these correlated with admission clinical grade and occurrence of intraventricular haemorrhage. Higher increase in CRP level between admission and postoperative morning, however, independently predicted poor outcome (p = 0.004). Part of this increased risk was likely due to an appearance of early postoperative cerebral infarction.
Conclusions
CRP levels correlate with outcome but do not seem to predict delayed cerebral ischaemia or infarction after SAH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aneurysmal subarachnoid haemorrhage (SAH) is a serious disease with high case fatality and morbidity rates [12, 14, 15, 22, 24]. According to population-based studies during the last few decades, outcome after SAH has shown only modest improvement [12, 22, 24]. Outcome is determined principally by a severity of initial bleeding and by occurrence of rebleeding or cerebral infarction.
C-reactive protein (CRP) is an acute phase sensitive, non-specific inflammatory marker and initiating factor of inflammation and infection. Previous large studies including meta-analyses indicate that elevated CRP levels increase modestly and independent of confounding factors risk for coronary heart disease as well as for both vascular and non-vascular mortality [3, 6]. CRP levels predict also risk for ischaemic stroke but no more after adjustment for risk factors (hypertension, cigarette smoking, diabetes and heart disease), since CRP levels correlate with these risk factors [6].
Aneurysmal SAH leads to a relatively high risk for cerebral infarct within 2 week after aneurysm rupture. This yields a possibility to study prospectively the appearance of cerebral infarct. Among survivors with clipping of a ruptured intracranial aneurysm, a high rate of hypodense lesions consistent with cerebral infarctions (40–60%) has been evident on follow-up computed tomography (CT) scanning at 3 months [9, 10, 18, 29]. Although most of these lesions are small (median volume, 10 ml) [9, 10, 29], their presence correlates highly with outcome of survivors. Cerebral infarction within 24 h after early aneurysm surgery may be even more harmful for outcome than delayed cerebral ischaemia (DCI) or late infarction [16, 27].
Despite extensive investigation, the pathogenesis of DCI is poorly understood and its treatment is not satisfactory [1, 28]. This may partly be due to inconsistency of definition of DCI [28]. Recently, inflammation after SAH has obtained increasing attention in this process [1]. CRP concentrations have been investigated appropriately after SAH only in a few mostly retrospective studies with relative small sample sizes [8, 19, 20, 25]. These results have suggested that CRP values correlate with outcome and might predict DCI, suggesting inflammatory involvement being an important factor in the pathogenesis.
CRP can increase within few hours up to 1,000-fold after onset of stimulus such as infection, tissue necrosis, trauma, cancer or various inflammatory diseases. This response reaches a peak at 48 h after the initial stimulus and falls to baseline levels within 7–12 days once the inflammatory stimulus is disappeared [23, 25]. It has wide variety of biological properties and functions, and it activates complement proteins [8, 20, 23, 25].
In this study, we tested whether CRP or increase in its levels could independent of confounding factors predict poor outcome or appearance cerebral infarct after SAH.
Methods
Patient population
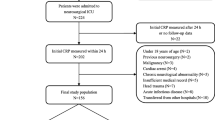
This study was approved by the Ethics Committee of the Helsinki and Uusimaa Hospital District. Included were 178 SAH patients (90 women; mean ± SD age, 50.3 ± 12.6 years; mean body mass index, 25.6 ± 4.4 kg/m2) admitted within 48 h and whose aneurysms were occluded within 60 h after bleeding between February 1998 and March 2001 at the Department of Neurosurgery, Helsinki University Hospital, which is the only neurosurgical unit serving a population of 1.8 million. Of the 178 patients of the present post hoc analysis, 170 were included in a prospective, randomised, double-blind, placebo-controlled enoxaparin trial testing the drug’s effect on clinical outcome after SAH [26]. Enoxaparin (a low-molecular weight heparin) had no effect on clinical outcome (primary endpoint) of 170 randomised patients [26], on occurrence, size, number or cause of ischemic lesions in 156 survivors [18], or on CRP levels [11]. Of 178 patients of the present study, eight were not included in the randomised trial because of either pre-operative or postoperative intracerebral haematoma (ICH) size exceeded 20 mm, being between 20 and 30 mm in diameter (n = 4), or other individual reasons.
We excluded moribund patients who died before or soon after hospital admission because of poor clinical condition and thus without any aneurysm occlusion. We also excluded those with aneurysms operated on as an emergency because of space-occupying ICH, since the causes of cerebral ischaemic lesions later would have been difficult to be estimated (lesion in previous location of ICH, or lesions due to increased intracranial pressure with/without brain herniation, temporary or permanent artery occlusion during surgery or delayed cerebral ischaemia). In addition, those admitted later than 48 hs after bleeding were also excluded because of missing early phase CRP measurements and because of a difficulty to distinguish early infarct from a late one as well as treatment-associated infarct from infarct caused by delayed ischaemia.
After admission, patients and family members were interviewed with a structured questionnaire for previous diseases, medication, and health habits [17, 18, 26]. Of the 59 (33%) patients with a history of hypertension (pre-SAH blood pressure readings >140/90 mm Hg or use of antihypertensive medication), 27 (15%) used antihypertensive medication and 14 (8%) had values >160/95 mm Hg. Of 120 (67%) ever regular smokers, 102 (57%) were current smokers. Prior to SAH, two (1%) patients had diabetes mellitus, nine (5%) had coronary heart disease, 40 (22%) had used non-steroidal anti-inflammatory drugs, and 19 (11%) were heavy alcohol drinkers (≥300 g of ethanol per week).
Clinical monitoring, treatment, and outcome
Each patient’s clinical condition on admission was scored according to the World Federation of Neurological Societies (WFNS) Grading Scale [5]. The ruptured aneurysms were occluded within a median 22 h after bleeding (range 5–60 h) by open surgery (173 patients) or by endovascular coiling (five patients). Cases of aneurysm coiling were few, since the principal aneurysm occlusion method is open aneurysm surgery in our hospital. During surgery, 46 patients underwent temporary occlusion of the proximal artery (in 13 patients, >10 min). Permanent artery occlusion was visible on angiography in 12 (7%) of 178 patients: clipping of the aneurysm together with the artery (in six), trapping or proximal clipping of a fusiform aneurysm (in five) and after endovascular coiling (in one). During surgery, a routine single prophylactic intravenous dose of vancomycin (1 g) was used to prevent infection. Patients who underwent surgery routinely received betamethasone, 4 mg every 6 h, starting before surgery and continuing in diminishing doses until the sixth postoperative day.
Neurological examinations took place daily after admission. Delayed cerebral ischaemia (RIND = reversible ischaemic neurological deficit, FIND = fixed ischaemic neurological deficit) was determined as a gradual development of focal neurological signs, or deterioration in consciousness after ruling out other causes of symptoms. Causes of poor clinical condition were determined by routine repeated CT scanning, postoperative angiograms, autopsies or laboratory investigations [28].
CT scans were routinely performed on admission (178 patients), on the first postoperative day (mean 1.8, range 0.7-3.0 days after SAH; 178 patients), at discharge (mean 10.2, range 7-13 days; 171 patients), and at 3 months (158 patients, 16 had died before a planned CT); scans were repeated if clinical deterioration occurred. The amount of subarachnoid blood on the CT scan on admission was categorised according to Fisher et al. [7]. Intraventricular haemorrhage (IVH) was categorised to be slight (small amount of blood in occipital horns or in the third or fourth ventricle; 70 patients), moderate (blood occupying most of one lateral ventricle with or without blood in the third or fourth ventricle; 17 patients), or severe (major IVH with blood filling all ventricles and frequently distending the ventricular system; four patients).
The ischaemic lesions, none of which were visible on the CT scan on admission, appeared on follow-up scans in 109 (61%) of 178 patients. Lesions in 59 (33%) patients were seen already on the CT of the first postoperative day (early ischaemic lesions). In addition, 50 (28%) had lesions appearing later than on the 1st postoperative day within 3 months after SAH (late ischaemic lesions). Of them, 45 had already lesions visible on CT scan at hospital discharge. Small hypodense lesions in location of previous ICH were not considered ischaemic lesions [28]. All neuroimaging studies were evaluated together by experienced neuroradiologists (Matti Porras and Kristiina Poussa), who were blinded to the case history of each patient, except for time of surgery [18, 27]. Outcome was assessed at 3 months after SAH according to the Glasgow Outcome Scale (GOS) [13].
CRP analysis
Blood samples for CRP analysis were obtained at least three times: (1) on admission before aneurysm treatment (mean ± SD, 22 ± 13 h after SAH), (2) after the patient had fasted, on the first postoperative morning after surgery (44 ± 15 h after SAH) and (3) after fasting at discharge 10.2 ± 1.3 days after SAH. CRP values obtained during diagnosed bacterial infection were not recorded in this study. CRP was analysed with an immunochemical kinetic turbidimetric method within an hour of sampling (normal values <10 mg/l; Roche-Diagnostic, Hitachi).
Statistical analysis
Data were analysed with the SPSS for Windows (release 17.0.2.2009, SPSS, Chicago, IL, USA). Categorical variables were compared by Fisher’s exact two-tailed test, the Pearson chi-square test, or the test for linear trend. Continuous variables were compared between groups by the Mann–Whitney U test, t-tests, or by analysis of variance (ANOVA) with corrected multiple pairwise comparisons based on either Dunnett’s or Bonferroni’s method. The effect of elapsed time on CRP values was tested by the Wilcoxon signed-rank or Friedman test. Univariate association of continuous variables was tested by Spearman rank (r s) correlation coefficients.
In patients without missing samples, the association of elapsed time after SAH (within factor), different grouping variables (between factors) and their interactions with CRP values (expressed as mean ± SD) were compared by repeated measures ANOVA and analysis of covariance. For this analysis, values for CRP were analysed after logarithmic transformation to obtain the normal distribution and equality of variances between different groups.
Univariate and multivariate odds ratios (ORs) and 95% confidence intervals (CIs) of risk factors, including their interactions, for poor outcome and for ischaemic lesions visible on the CT scan were analysed by unconditional logistic regression. Maximum-likelihood stepwise forward elimination procedures were used, with selection of variables based on the magnitude of their probability values (<0.05). A two-tailed p value <0.05 was considered significant.
Results
Of 178 patients, 124 (70%) had a favourable outcome (independent state; moderate disability or better outcome) and 54 (30%) had a poor outcome (dependent state; severe disability or worse outcome) at 3 months after SAH. Baseline characteristics, previous diseases, medications or health habits did not correlate significantly with CRP levels. Plasma CRP values according to clinical and radiological variables assessing severity of bleeding and outcome are shown in Table 1.
CRP values on admission, on the first postoperative day, and at discharge correlated very significantly with each other (r s ranged from 0.337 to 0.551, p < 0.001). The increase in CRP values between admission and postoperative day was very significant (p < 0.001), as was also a decline in the levels between postoperative day and hospital discharge. Time between aneurysm rupture and sampling correlated very significantly with CRP values on admission (r s = 0.415, p = 0.003) but no more with those of later samples. This suggests that CRP increase occurs soon after aneurysm rupture and early aneurysm surgery to maximum within 1–2 days.
Those with severe disability at 3 months had higher values on admission and after surgery compared with those with a favourable outcome, while those who died had higher values at discharge. Those who died of later infection had similar CRP values to those obtained from others during sampling times. No association was found between CRP values and duration of temporary artery clipping during surgery. Those with open aneurysm surgery had similar CRP values to those with aneurysm coiling but the number of patients in the latter group was small. CRP values were increased after surgery among those with early cerebral infarction compared with those without infarct (p = 0.041) (Table 1). CRP values according to early infarction did not, however, reach significance when values obtained both on admission and after surgery were taken into account.
CRP values in those with poor outcome at 3 months (18.0 ± 35.7 mg/l on admission, 41.0 ± 40.2 mg/l on the first postoperative day, and 25.0 ± 31.1 mg/l at discharge) were significantly (p < 0.001) higher than corresponding values in those with favourable outcome (8.5 ± 8.4, 21.1 ± 24.1 and 17.6 ± 21.7 mg/l, respectively). Increase in CRP values between admission and postoperative day was also significant (p < 0.001), and it was more prominent in those with poor outcome than in those with favourable outcome (interaction between outcome groups and time of sampling, p = 0.004). Correspondingly, decrease in CRP values between postoperative day and discharge from hospital was significant (p < 0.001) and values were higher in those with poor outcome (p < 0.001), but the decrease only tended to be more prominent (p = 0.083) in those with poor outcome compared with those with favourable outcome. This suggests that those with a poor outcome have a relatively rapid and short-time increase in CRP values soon after SAH and aneurysm surgery.
After adjustment for presence of IVH (no/yes) and WFNS (grade I vs others), CRP levels still were significantly higher in those with a poor outcome (p = 0.014). In this analysis, IVH (p = 0.081) and WFNS (p = 0.073), as well as their interactions with elapsed time between admission and postoperative day (p = 0.057 and p = 0.74, respectively), had no significant confounding effect on the significant association between outcome and CRP levels. Increase in CRP levels between admission and postoperative day in those with poor outcome was still almost significant (interaction, p = 0.061). Additional adjustment for hyperglycaemia did not change results.
Significant univariate risk factors for poor outcome at 3 months after SAH are shown in Table 2 and a multivariate logistic regression model in Table 3. Early infarction was the most significant risk factor for poor outcome, followed by patient age and occurrence of IVH or early rebleeding, but CRP level after surgery did not independently predict outcome (Table 3). Independent risk factors for early infarction compared with those without any infarction are shown in Table 4. Independent risk factors were amount of subrachnoid blood, plasma glucose level on admission and, most significantly, duration of temporary artery clip application during surgery. In univariate analysis, postoperative CRP values also associated with appearance of early infarction but no more in multivariate analysis since postoperative CRP values correlated with admission glucose levels (r s = 0.266, p < 0.001).
Discussion
On the basis of this study, elevated plasma CRP values after aneurysm rupture—and particularly if values increase after early aneurysm surgery—predict the risk for poor outcome. Part of this prediction is due to association of elevated CRP values with the patient’s poor clinical condition and the occurrence of both IVH, hyperglycaemia and early infarction. On the other hand, CRP values do not seem to predict occurrence of either DCI or late cerebral infarct.
Several large studies have shown that elevated CRP levels may increase risk for myocardial or cerebral infarction as well as for both vascular and non-vascular mortality [3, 6]. CRP values may also correlate with several cardiovascular risk factors (hypertension, cigarette smoking, diabetes and coronary heart disease) [6]. In our study, we found no significant association between cardiovascular risk factors and CRP values since aneurysm rupture itself elevated significantly CRP levels. Furthermore, CRP levels could have been even higher on the first postoperative day since corticosteroids are known to decrease CRP levels [2].
CRP levels correlate with severity and outcome of several diseases [3, 6, 23]. CRP is mainly synthesised in the liver after induction by cytokines, particularly by interleukin-6, and it activates the complement system contributing to natural immunity [23]. Elevated CRP levels have been estimated to be a marker for the extent of atherosclerosis or inflammatory activity and for vulnerability of atherosclerotic plaques, and thus an epiphenomenon. CRP could also directly contribute towards the development of ischaemic cardiovascular or cerebrovascular disease. This may suggest that inflammatory mechanisms could contribute to secondary neuronal injury after cerebral ischaemia, e.g. after SAH. However, recent studies preferably suggest that elevated CRP levels do not cause cerebral ischaemia, and it is unknown whether anti-inflammatory drugs could prevent ischaemic diseases and complications [6, 23].
Only few appropriate studies on CRP after SAH, but without multivariate analyses, have been done [8, 19, 20, 25]. The independent role of elevated CRP values as risk factors for impaired outcome as well as for occurrence of infarction and ischaemic symptoms have, thus, remained unknown. In addition, the results have been contradictory since plasma CRP levels have increased after SAH concomitantly with time profile of delayed ischaemia in some studies [19, 20, 25], while in one study CRP levels in both plasma and cerebrospinal fluid (levels higher than in plasma) were increased already to peak levels before angiographic vasospasm [8]. Similarly, early phase CRP levels after SAH have predicted delayed ischaemia or angiographic vasospasm in two studies [8, 20], while not in others [19, 25].
CRP levels during the second week after SAH were associated with DCI in three studies [8, 20, 25], but not in one [19]. This kind of positive association can at least partly be owing to a publication bias favouring small positive studies versus small negative ones to be published or to the criteria of definition of DCI used in different studies [28]. It can also be an epiphenomenon of clinical condition since patients with DCI are in worse condition than those without DCI. We found no significant association between CRP levels and DCI, amount of subarachnoid blood or late cerebral infarct. However, we did not record CRP values between 4–7 days, when levels have been highest in some previous studies [19, 20, 25]. On the other hand, patients with an infection had significantly elevated CRP levels only during that phase in one study [25], when the effect of corticosteroids in reducing of CRP levels in our study would have been expected to be most obvious [2].
CRP as a continuous variable has been shown to be associated with outcome [8, 25]. This association seems to be caused by high correlation between CRP values and several variables assessing severity of bleeding including stress hyperglycaemia. However, CRP levels may, independent of severity of bleeding, predict outcome if elevated CRP values after aneurysm rupture further increase markedly after surgery. This may partly be a marker of appearance of perioperative early cerebral infarct which seems to be an important risk factor for poor outcome [16, 27]. Surgical stress or trauma could also increase CRP levels. We could not analyse whether patients who underwent surgical aneurysm occlusion have higher CRP levels than those with endovascular aneurysm coiling, since we had only few patients in the latter group. Previously, no significant difference in CRP values between these treatment modalities was found, but the number of patients with endovascular treatment was also small [8].
Admission plasma glucose values correlated with CRP levels. Admission blood glucose values have been shown in prospective studies to predict patient’s outcome after SAH and also to correlate with clinical and radiological severity of bleeding [4, 17, 21]. In two studies [17, 21], admission hyperglycaemia or plasma glucose values were associated, independent of severity of bleeding and other confounding factors, with outcome but not with delayed ischaemia or late ischaemic lesions on follow-up CT scan, i.e. in somewhat similar way as do CRP values. CPR levels increase somewhat later after SAH than plasma glucose values do. So, CPR likely seems to be more a reactive response to initial bleeding and surgery.
Our study also has limitations. We have excluded moribund patients with an emergency surgery as well as those who were admitted late and thus with a favourable prognosis. Inclusion of them into the study would have caused more confounding factors to be statistically controlled for and less precise and reliable estimation of cause of cerebral infarction. The present patient population likely represents a subgroup of SAH patients in whom improvement of outcome can be expected by more active, safe and early treatment modalities.
CRP seems to be a sensitive but non-specific marker for several stimuli. Although, there is likely no causal relationship between CRP levels and outcome after SAH, elevated CRP level can be used as a marker of a risk factor for poor outcome after SAH, particularly in sedated patients whose clinical condition is difficult to be estimated. Furthermore, our results suggest that inflammation is not likely a very important initiating factor in pathogenesis of DCI or cerebral infarct after SAH.
References
Al-Tamimi YZ, Orsi NM, Quinn AC, Homer-Vanniasinkam S, Ross SA (2010) A review of delayed ischemic neurologic deficit following aneurysmal subarachnoid hemorrhage: historical overview, current treatment, and pathophysiology. World Neurosurg 73:654–667
Brotman DJ, Girod JP, Garcia MJ, Patel JV, Gupta M, Posch A, Saunders S, Lip GY, Worley S, Reddy S (2005) Effects of short-term glucocorticoids on cardiovascular biomarkers. J Clin Endocrinol Metab 90:3202–3208
Buckley DI, Fu R, Freeman M, Rogers K, Helfand M (2009) C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. preventive services task force. Ann Intern Med 151:483–495
Dorhout Mees SMD, van Dijk GW, Algra A, Kempink DR, Rinkel GJ (2003) Glucose levels and outcome after subarachnoid hemorrhage. Neurology 61:1132–1133
Drake CG (1988) Report of world federation of neurological surgeons committee on a universal subarachnoid hemorrhage grading scale. J Neurosurg 68:985–986
Elkind MS, Luna JM, Moon YP, Liu KM, Spitalnik SL, Paik MC, Sacco RL (2009) High-sensitivity C-reactive protein predicts mortality but not stroke: the Northern Manhattan study. Neurology 73:1300–1307
Fisher CM, Kistler JP, Davis JM (1980) Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 6:1–9
Fountas KN, Tasiou A, Kapsalaki EZ, Paterakis KN, Grigorian AA, Lee GP, Robinson JS Jr (2009) Serum and cerebrospinal fluid C-reactive protein levels as predictors of vasospasm in aneurysmal subarachnoid hemorrhage. Clinical article. Neurosurg Focus 26:E22
Haley EC Jr, Kassell NF, Torner JC (1993) A randomized controlled trial of high-dose intravenous nicardipine in aneurysmal subarachnoid hemorrhage. A report of the cooperative aneurysm study. J Neurosurg 78:537–547
Haley EC Jr, Kassell NF, Torner JC, Truskowski LL, Germanson TP (1994) A randomized trial of two doses of nicardipine in aneurismal subarachnoid hemorrhage. A report of the cooperative aneurysm study. J Neurosurg 80:788–796
Ilveskero S, Juvela S, Siironen J, Lassila R (2005) D-dimer predicts outcome after aneurysmal subarachnoid hemorrhage: no effect of thromboprophylaxis on coagulation activity. Neurosurgery 57:16–24
Inagawa T (2001) Trends in incidence and case fatality rates of aneurysmal subarachnoid hemorrhage in Izumo City, Japan, between 1980–1989 and 1990–1998. Stroke 32:1499–1507
Jennett B, Bond M (1975) Assessment of outcome after severe brain damage. Lancet 1:480–484
Juvela S (2002) Natural history of unruptured intracranial aneurysms: risks for aneurysm formation, growth, and rupture. Acta Neurochir Suppl 82:27–30
Juvela S (2003) Prehemorrhage risk factors for fatal intracranial aneurysm rupture. Stroke 34:1852–1858
Juvela S, Siironen J (2012) Early cerebral infarction as a risk factor for poor outcome after aneurysmal subarachnoid haemorrhage. Eur J Neurol. doi:10.1111/j.1468-1331.2011.03523.x
Juvela S, Siironen J, Kuhmonen J (2005) Hyperglycemia, excess weight, and history of hypertension as risk factors for poor outcome and cerebral infarction after aneurysmal subarachnoid hemorrhage. J Neurosurg 102:998–1003
Juvela S, Siironen J, Varis J, Poussa K, Porras M (2005) Risk factors for ischemic lesions following aneurysmal subarachnoid hemorrhage. J Neurosurg 102:194–201
Kasius KM, Frijns CJM, Algra A, Rinkel GJE (2010) Association of platelet and leukocyte counts with delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. Cerebrovasc Dis 29:576–583
Kubo Y, Ogasawara K, Kakino S, Kashimura H, Tomitsuka N, Sugawara A, Ogawa A (2008) Serum inflammatory adhesion molecules and high-sensitivity C-reactive protein correlates with delayed ischemic neurologic deficits after subarachnoid hemorrhage. Surg Neurol 69:592–596
Lanzino G, Kassell NF, Germanson T, Truskowski L, Alves W (1993) Plasma glucose levels and outcome after aneurysmal subarachnoid hemorrhage. J Neurosurg 79:885–891
Nieuwkamp DJ, Setz LE, Algra A, Linn FHH, de Rooij NK, Rinkel GJE (2009) Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol 8:635–642
Nordestgaard BG, Zacho J (2009) Lipids, atherosclerosis and CVD risk: is CRP an innocent bystander? Nutr Metab Cardiovas 19:521–524
Numminen H, Kotila M, Waltimo O, Aho K, Kaste M (1996) Declining incidence and mortality rates of stroke in Finland from 1972 to 1991: results of three population-based stroke registers. Stroke 27:1487–1491
Rothoerl RD, Axmann C, Pina AL, Woertgen C, Brawanski A (2006) Possible role of the C-reactive protein and white blood cell count in the pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol 18:68–672
Siironen J, Juvela S, Varis J, Porras M, Poussa K, Ilveskero S, Hernesniemi J, Lassila R (2003) No effect of enoxaparin on outcome of aneurysmal subarachnoid hemorrhage: a randomized, double-blind, placebo-controlled clinical trial. J Neurosurg 99:953–959
Siironen J, Porras M, Varis J, Poussa K, Hernesniemi J, Juvela S (2007) Early ischemic lesion on computed tomography: predictor of poor outcome among survivors of aneurysmal subarachnoid hemorrhage. J Neurosurg 107:1074–1079
Vergouwen MDI, Vermeulen M, Gijn J, Rinkel GJE, Wijdicks EF, Muizelaar JP, Mendelow AD, Juvela S, Yonas H, Terbrugge KG, Macdonald RL, Diringer MN, Broderick JP, Dreier JP, Roos EBWEM (2010) Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies—proposal of a multidisciplinary research group. Stroke 41:2391–2395
Vilkki JS, Juvela S, Siironen J, Ilvonen T, Varis J, Porras M (2004) Relationship of local infarctions to cognitive and psychosocial impairments after aneurysmal subarachnoid hemorrhage. Neurosurgery 55:790–803
Acknowledgements
This study was supported in part by research grants from the Paavo Nurmi Foundation, the Maire Taponen Foundation and the Paulo Foundation.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Juvela, S., Kuhmonen, J. & Siironen, J. C-reactive protein as predictor for poor outcome after aneurysmal subarachnoid haemorrhage. Acta Neurochir 154, 397–404 (2012). https://doi.org/10.1007/s00701-011-1243-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-011-1243-7