Abstract
Purpose
The treatment of deep-seeded pediatric brain arteriovenous malformations (AVMs) remains a challenging task. We describe our experience using a new detachable tip microcatheter in the embolization of brain arteriovenous malformations, pial arteriovenous fistulas, and vein of Galen malformations. We describe the safety and efficacy using a new detachable tip microcatheter in the treatment of pediatric deep brain arteriovenous malformations, pial malformations, and vein of Galen malformations.
Methods
During a period of 9 months from March 2013 through January 2014, 11 pediatric patients in 14 procedures with 27 total injections were selected for treatment with a detachable tip under Food and Drug Administration (FDA) compassionate use exemption and were admitted to our department for treatment of their brain AVM using a liquid embolic agent and a detachable tip microcatheter. The ages of the patients ranged from 3 months to 18 years old.
Results
Of the 27 total injections done, the tip detached in seven cases. For the 16 n-BCA injections, the tip detached six times (37.5 %), and for the 11 Onyx injections, the tip detached one time (9 %). There were no cases of premature microcatheter detachment during normal vessel navigation.
Conclusions
The introduction of these detachable tip microcatheters allows for a safe and relaxed injection that permits a true circumferential occlusion, and may further permit filling a larger amount of angioarchitecture without the risk of distal migration, or vessel damage during the usual rapid removal of non detachable micocatheters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arteriovenous malformations (AVMs) remain the most common cause of intracranial hemorrhage in children [7, 21]. Deep location lesions have a higher risk of intracranial hemorrhage, and when they hemorrhage, they are more likely to have devastating morbidity and mortality [4–6, 9, 12, 13, 20, 23, 29, 31, 33, 35, 40]. The treatment of deep and eloquent region brain arteriovenous malformations in the pediatric population remains a challenging task. AVMs in early childhood often present with cardiac failure or hydrocephalus due to their extremely high-flow nature. While surgery can be definitive, these lesions are usually not safely surgically accessible. Stereotactic radiosurgery can also be effective for lesions that are smaller in size, but there is still risk for hemorrhage before they are obliterated [3, 14]. The length of time from radiosurgery to obliteration can take up to 10 years, and the rate of successful obliteration is up to only 72 % [10]. The efficacy of radiosurgery in high-flow arteriovenous fistulas (AVFs) is not well documented. Also, the long-term consequences of radiosurgery in children are still not completely understood. Staged embolization procedures can be curative in arteriovenous fistulas (AVFs) with certain angioarchitectures in a very high number [2]. Cure rates in nidal malformations is lower with a range between 5.6 and 53.9 % [11, 19, 25–28, 30, 32, 36, 38, 39] and also comes with the risks of radiation exposure, and treatment associated hemorrhage [8, 15–17, 22, 37]. At the moment the goals of embolization in AVM with a nidus angioarchitecture are to reduce the risk of bleeding, symptomatic improvement of cardiac failure and venous hypertension.
Although the newer hydrophilic coated microcatheters have improved navigation, are safer to embolize with, and have a decreased the risk of catheter gluing in the cerebral arteries, this concern is still a limiting factor in embolization with either n-BCA or Onyx. The feared risk of retention of the microcatheter by trapping of the distal catheter by the embolic agent within the vessel, or rupturing the artery while pulling the microcatheter limits the amount of embolic agent used with each embolization [22]. Because of this, the entire technique for injection of n-BCA involves a short injection time with removal of the catheter prior to polymerization that can frequently result in incomplete circumferential occlusion. In the case of cyanoacrylates, the problem is that of adherence of the catheter to the glue. The present technique available in the USA for injection with Onyx usually involves creation of a “plug” proximal to the tip of the catheter to prevent significant reflux. Detachable tip catheters have been developed to circumvent these limitations, allowing for longer injections and better ability to penetrate the lesion. The second-generation detachable tip catheter, the Apollo (Ev3-Covidien, Irvine, CA), improves on deliverability and is compatible with both n-BCA and Onyx. We present a case series safely using the Apollo microcatheter for pediatric and young adult brain and pial AVMs with both Onyx and n-BCA.
Methods
Patients
During 9 months from March 2013 through January 2014, 11 pediatric patients in 14 procedures with 27 total injections were selected for treatment with a detachable tip under an Food and Drug Administration (FDA) compassionate use exemption and were admitted to our department for treatment of their brain AVM using a liquid embolic agent and a detachable tip microcatheter (Table 1). The ages of the patients ranged from 3 months to 18 years old. In all cases, magnetic resonance imaging (MRI)/MR angiography and catheter angiography were used as a diagnostic tool in planning for treatment. All cases were discussed in a combined neurosurgery, neurointerventional radiology, neurology, radiation oncology conference and agreed upon to undergo staged embolization procedures, either as the primary treatment, or in preparation for microsurgical resection or radiosurgery. The device was used with approval by both the FDA and institutional International Review Board (IRB) as a compassionate use device. The follow-up ranged from 1 to 10 months.
Embolization materials
There were 27 total injections performed (Table 1). Onyx-18 was the embolic agent used for ten injections and Onyx-34 was used for one injection. n-BCA was used for 16 injections.
Microcatheters/microwire
In every case, the Apollo microcatheter was used to gain direct access to each arterial feeder, at or close to the nidus and/or fistula site. Either a 0.008-inch-diameter microwire (Mirage .008, Ev3-Covidien) or a 0.010-inch-diameter microwire (Silverspeed 10, Ev3-Covidien) was used to aid in the navigation process.
Procedure
All procedures were done using a Siemens Artis zee biplane suite (Siemens, Erlangen, Germany). All cases were performed using general anesthesia with neurophysiologic [somatosensory evoked potentials (SSEPs) and and motor evoked potentials (MEPs)] monitoring. Transfemoral access was obtained in each case with ultrasound guidance. Depending on the age and size of the patient a 4 French or 5 French guide sheath was placed with a 4 French or 5 French guide catheter. For Onyx injections, the catheter was flushed with the appropriate amount of dimethyl sulfoxide (DMSO) to fill the dead space of the catheter (0.24 ml). For n-BCA injections, the catheter was flushed with 5 % dextrose in water. For each n-BCA injection, there were different ratios of n-BCA; tantalum and ethiodol were used, depending on the flow and territory to be occluded.
Results
Of the 27 total injections done, the tip detached in seven cases. For the nine n-BCA injections the tip detached six times (37.5 %), and for the 11 Onyx injections the tip detached one time (9 %; Table 1). There were no cases of premature microcatheter detachment during normal vessel navigation despite the fact that, in every instance, a microguidewire (either Mirage 008 or Silverspeed 10, Ev3-Covidien) was used, removed and reintroduced in situ, in tortuous anatomy. There were no leaks of contrast material or embolic agent at the detachment zone on any injection.
Success and complications
In all catheterizations, we were able to reach our target, and there were no complications, either immediate hemorrhagic or ischemic complications associated with the use of this device based on clinical and radiologic findings [MRI or Dyna computed tomography (CT) when appropriate]. Within the follow-up period, there was no evidence or suggestion of catheter tip migration in the seven cases in which the tip detached. Within the range of 1 to 10 months of follow-up, there were no long-term complications seen either.
Case example 1
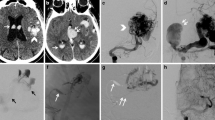
Patient #7 is a 4.5-month-old boy with a sister with a known multiple pial AVF, and RASA-1 mutation. This malformation was diagnosed in utero. When he was born, he was also found to have a RASA-1 mutation, in addition to hemophilia B. On serial follow-up, at age 4 months, the child congestive heart failure and cardiomegaly worsen, with failure to thrive and meet milestones. The angiographic study (Fig. 1a and b) revealed an enlarged left vertebral artery with a dilated and very tortuous course. The vertebral artery ended in a left posterior inferior cerebellar artery which was enlarged and it supplied multiple branches that ended in a multifocal high-flow fistule in the posterolateral part of the cerebellum. The venous drainage from this fistulous location was into a dilated venous sac that drained medially and superiorly into the torcular region. From here, the venous drainage was into both the transverse and sigmoid sinuses as well as through the occipital sinuses into the jugular veins on both sides. The basilar artery was minimally visible initially, as there was flow reversal in the basilar artery because of the high demand of the fistule. Figure 1c and d are post embolization images after four total n-BCA injections through feeders from the left posterior inferior cerebellar arteries. Note the increased flow into the basilar artery now that the fistula has been partially closed. Figure 2a and b illustrates the catheter position prior to the second n-BCA embolization. Figure 2c and d shows the native image post embolization with the detached catheter tip inside the glue cast. Figure 3 shows native images of the embolic material after the second stage of the procedure 1 month after the first procedure. There were a total of four microcatheter embolizations with n-BCA. Note in these images the initial detached catheter remains in its original position and has not migrated, and there is another detached microcatheter from the second-stage embolization. Given the initial high flow of this fistula, and the age of this patient, we elected to stage this procedures. The main advantage of the detachable tip microcatheter is the ability to inject 90 % N-butyl cyanoacrylate (NBCA) in a large vessel and be able to seal it circumferentially in a very controlled and safe manner, without the need for rapid removal, incomplete occlusion, and risk of vessel damage (Fig. 3a and b) . (Fig. 4)
a and b: Plain films in PA and lateral views showing the cast of the first NBCA deposition, note the circumferential cast of the pedicle, and the catheter position prior to the next embolization. c and d: plain films in PA and lateral views showing the detached catheter tip, after n-BCA embolization. In images c and d, the single arrow shows the catheter tip, and double arrow shows the distal end of the detached catheter
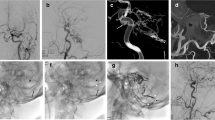
a and b: PA and lateral right common carotid artery injection with enlarged right middle cerebral artery with drainage into a fistulous sac adjacent to the superior sagittal sinus then into the sinus itself. c and d: PA and lateral left common carotid artery injection with enlarged right anterior cerebral artery supplying drainage into the fistulous sac. e and f: PA and lateral left vertebral artery injection with supply to the fistula from an enlarged right posterior cerebral artery, entering the sac in a diferent location
Case example 2
Patient #5 is an 18-month-old girl who was noticed to have asymmetry of her head by her parents with an abnormal bulge on the left parietal region at 3 months of age. Her parents also noticed a decreased abillity to grasp with her left hand. During her workup, she was found to have multiple pial AVFs in the right parietal region. She was also found to have a RASA-1 mutation. She had three embolization procedures prior to the availability of the Apollo catheter. In her last procedure prior to cure, the Apollo catheter was used for two embolizations (Fig. 5), one with n-BCA and the second with Onyx-34. During both injections the catheter tip did not detach. She had a follow-up angiogram 2 months after final embolization that showed complete cure of the multiple pial AVFs (Fig. 6).
a and b: PA and lateral native films after final embolization at 2 months follow-up. c and d: PA and lateral left vertebral artery injection showing no residual shunting. e and f: PA and Lateral right common carotid artery injections showing no residual shunting. g and h: PA and lateral left common carotid artery injections showing no residual shunting
Discussion
The first report addresing the problem of catheter entrapment using n-BCA was during the use of a detachable balloon with a calibrated leak in 1979 [1]. The use of a detachable tip microcatheter for the treatment of brain arteriovenous malformations (AVMs) with Onyx was first reported in 2008 [24]. There has since been two reports of its use in adults revealing that this microcatheter can be used safely and efficatiously [18, 34]. The Sonic catheter (Balt, Montmorency, France) was the first generation of this type of catheter. This catheter has been used safely with both Onyx (Ev3-Covidien) [18, 34] and n-BCA (Trufill, Codman-DePuy, Raynham, Massachussets) [24] for intracranial AVMs. The appeal of this type of microcatheter is that one can use embolic agents to more deeply penetrate fistulas and malformations with permissible reflux based on length of the detachable tip and less concern for having a retained microcatheter. Another concern with the use of traditional DMSO compatible catheters is the risk of vessel perforation because they are somewhat stiffer. These detachable tip microcatheters are less stiff, with better trackability and are designed with the hope to help reduce this risk. The concern with using a detachable microcatheter is that the detachable tip could deploy while still maneuvering the catheter in a normal arterial vessel, the potential for embolic material leakage at the detachable junction and that, in the long term, the detached segment may migrate.
The Sonic microcatheter (Balt) is not FDA approved in the USA. A new generation detachable tip microcatheter, the Apollo microcatheter (Ev3-Covidien), that is now being used in Europe, is in the process of getting FDA approval. This catheter is compatible with n-BCA and Onyx (DMSO). The traditional prior DMSO-compatible catheters are stiffer with a less flexible tip that require more micro-guidewire manipulation for navigation; all factors that decrease trackability and presumably increase the risk for perforation and hemorrhage. Another concern with using embolic agents is having these agents reflux and embolize inadvertently to normal parenchymal supply. The new detachable tip microcatheter addresses both of these issues, making it safer to embolize these lesions with an extra 1.5–3.0 cm safety valve of reflux allowing for a more controlled, greater penetration into abnormal fistulas, and reduced concern for permanent adherence of microcatheter to the embolic cast. This degree of permissable reflux can improve flow control and improve the amount of forward embolization. In addition, the most proximal marker at the detachable segment facilitates the assessment of the reflux. While the detachable tip catheter has been more commonly used with Onyx, it appears in our series that this catheter may be even more beneficial when using n-BCA because of its faster polymerization in the blood and greater concern for catheter adherence to the glue cast compared to the slower, more controlled precipitation of Onyx. In our review, the rate of tip detachment was higher with n-BCA (37.5 %) embolizations than Onyx embolization (9 %). It, in fact, can transform n-BCA embolization into a more controlled Onyx-type embolization procedure, allowing repetitive injection of material within the same catheter.
Conclusion
The introduction of these catheters represents a “game changer” in liquid embolization, mostly with n-BCA, where it can permit a very controlled, relaxed injection, allow for a true circumferential occlusion, and may further permit filling a larger amount of angioarchitecture without the risk of distal migration, or vessel damage during the usual rapid removal of nondetachable micocatheters. With the detachable tip microcatheter, once the injection is done, one can gently remove the catheter, and if the tip is adherent or glued, it can be simply detached in a very atraumatic, controlled manner. In all our present experience, the microcatheter was able to reach its target. All in all, it performed as designed. A liquid embolic injection was possible in all cases and utilized even better than originally intended. In all cases, the catheter could be withdrawn without any difficulty. The distal tip, as expected, had to be detached more frequently when using NBCA than when using Onyx. This catheter is particularly useful for embolization in pediatric patients with high-flow fistulas. You can use this catheter to allow for permissable reflux to decrease the high-flow state and then have a more controlled forward injection, without the concern of gluing the catheter in position. In our experience, the one drawback in the use of this catheter is that you will sometimes get a false sense of security during embolization as to the amount of reflux one should allow. Care must be taken to note that, in certain cases, the amount of reflux allowed may not always be at the proximal marker as there may be functional vessels more distal to the marker that supply normal brain. When injecting embolic agents, careful analysis of superselective angiography should define how much reflux should be permited. As experience is being gained, further technical improvements in liquid embolization will follow.
References
Berenstein A, Kricheff II (1979) Catheter and material selection for transarterial embolization: technical considerations: II. Mater Radiol 132:631–639
Berenstein A, Ortiz R, Niimi Y, Elijovich L, Fifi J, Madrid M, Ghatan S, Molofsky W (2010) Endovascular management of arteriovenous malformations and other intracranial arteriovenous shunts in neonates, infants, and children. Childs Nerv Syst 26:1345–1358
Buis DR, Dirven CM, Lagerwaard FJ, Mandl ES, Lycklama ANGJ, Eshghi DS, van den Berg R, Baayen JC, Meijer OW, Slotman BJ, Vandertop WP (2008) Radiosurgery of brain arteriovenous malformations in children. J Neurol 255:551–560
da Costa L, Wallace MC, Ter Brugge KG, O’Kelly C, Willinsky RA, Tymianski M (2009) The natural history and predictive features of hemorrhage from brain arteriovenous malformations. Stroke 40:100–105
Fleetwood IG, Marcellus ML, Levy RP, Marks MP, Steinberg GK (2003) Deep arteriovenous malformations of the basal ganglia and thalamus: natural history. J Neurosurg 98:747–750
Hernesniemi JA, Dashti R, Juvela S, Vaart K, Niemela M, Laakso A (2008) Natural history of brain arteriovenous malformations: a long-term follow-up study of risk of hemorrhage in 238 patients. Neurosurgery 63:823–829, discussion 829–831
Hladky JP, Lejeune JP, Blond S, Pruvo JP, Dhellemmes P (1994) Cerebral arteriovenous malformations in children: report on 62 cases. Childs Nerv Syst 10:328–333
Jafar JJ, Davis AJ, Berenstein A, Choi IS, Kupersmith MJ (1993) The effect of embolization with N-butyl cyanoacrylate prior to surgical resection of cerebral arteriovenous malformations. J Neurosurg 78:60–69
Kader A, Young WL, Pile-Spellman J, Mast H, Sciacca RR, Mohr JP, Stein BM (1994) The influence of hemodynamic and anatomic factors on hemorrhage from cerebral arteriovenous malformations. Neurosurgery 34:801–807, discussion 807–808
Kano H, Kondziolka D, Flickinger JC, Yang HC, Flannery TJ, Awan NR, Niranjan A, Novotny J, Lunsford LD (2012) Stereotactic radiosurgery for arteriovenous malformations, part 2: management of pediatric patients. J Neurosurg Pediatr 9:1–10
Katsaridis V, Papagiannaki C, Aimar E (2008) Curative embolization of cerebral arteriovenous malformations (AVMs) with Onyx in 101 patients. Neuroradiology 50:589–597
Khaw AV, Mohr JP, Sciacca RR, Schumacher HC, Hartmann A, Pile-Spellman J, Mast H, Stapf C (2004) Association of infratentorial brain arteriovenous malformations with hemorrhage at initial presentation. Stroke 35:660–663
Kondziolka D, Humphreys RP, Hoffman HJ, Hendrick EB, Drake JM (1992) Arteriovenous malformations of the brain in children: a forty year experience. Can J Neurol Sci 19:40–45
Kondziolka D, Kano H, Yang HC, Flickinger JC, Lunsford L (2010) Radiosurgical management of pediatric arteriovenous malformations. Childs Nerv Syst 26:1359–1366
Li TL, Fang B, He XY, Duan CZ, Wang QJ, Zhao QP, Huan QY (2005) Complication analysis of 469 brain arteriovenous malformations treated with N-butyl cyanoacrylate. Interv Neuroradiol 11:141–148
Lv X, Wu Z, Jiang C, Li Y, Yang X, Zhang Y, Zhang N (2011) Complication risk of endovascular embolization for cerebral arteriovenous malformation. Eur J Radiol 80:776–779
Lv X, Wu Z, Li Y, Yang X, Jiang C (2012) Hemorrhage risk after partial endovascular NBCA and ONYX embolization for brain arteriovenous malformation. Neurol Res 34:552–556
Maimon S, Strauss I, Frolov V, Margalit N, Ram Z (2010) Brain arteriovenous malformation treatment using a combination of Onyx and a new detachable tip microcatheter, SONIC: short-term results. AJNR Am J Neuroradiol 31:947–954
Mounayer C, Hammami N, Piotin M, Spelle L, Benndorf G, Kessler I, Moret J (2007) Nidal embolization of brain arteriovenous malformations using Onyx in 94 patients. AJNR Am J Neuroradiol 28:518–523
Nagy G, Major O, Rowe JG, Radatz MW, Hodgson TJ, Coley SC, Kemeny AA (2012) Stereotactic radiosurgery for arteriovenous malformations located in deep critical regions. Neurosurgery 70:1458–1469, discussion 1469–1471
Nicolato A, Foroni R, Seghedoni A, Martines V, Lupidi F, Zampieri P, Sandri MF, Ricci U, Mazza C, Beltramello A, Gerosa M, Bricolo A (2005) Leksell gamma knife radiosurgery for cerebral arteriovenous malformations in pediatric patients. Childs Nerv Syst 21:301–307, discussion 308
Niimi Y, Berenstein A, Setton A (2003) Complications and their management during NBCA embolization of craniospinal lesions. Interv Neuroradiol 9:157–164
Ozanne A, Alvarez H, Krings T, Lasjaunias P (2007) Pediatric neurovascular malformations: vein of Galen arteriovenous malformations (VGAM), pial arteriovenous malformations (pial AVM), dural sinus malformations (DSM). J Neuroradiol 34:145–166
Ozturk MH, Unal H, Dinc H (2008) Embolization of an AVM with acrylic glue through a new microcatheter with detachable tip: an amazing experience. Neuroradiology 50:903–904
Panagiotopoulos V, Gizewski E, Asgari S, Regel J, Forsting M, Wanke I (2009) Embolization of intracranial arteriovenous malformations with ethylene–vinyl alcohol copolymer (Onyx). AJNR Am J Neuroradiol 30:99–106
Perez-Higueras A, Lopez RR, Tapia DQ (2005) Endovascular treatment of cerebral AVM: our experience with Onyx. Interv Neuroradiol 11:141–157
Pierot L, Januel AC, Herbreteau D, Barreau X, Drouineau J, Berge J, Sourour N, Cognard C (2005) Endovascular treatment of brain arteriovenous malformations using onyx: preliminary results of a prospective multicenter study. Interv Neuroradiol 11:159–164
Pierot L, Cognard C, Herbreteau D, Fransen H, van Rooij WJ, Boccardi E, Beltramello A, Sourour N, Kupcs K, Biondi A, Bonafe A, Reith W, Casasco A (2013) Endovascular treatment of brain arteriovenous malformations using a liquid embolic agent: results of a prospective, multicentre study (BRAVO). Eur Radiol 23:2838–2845
Rodesch G, Malherbe V, Alvarez H, Zerah M, Devictor D, Lasjaunias P (1995) Nongalenic cerebral arteriovenous malformations in neonates and infants. Review of 26 consecutive cases (1982–1992). Childs Nerv Syst 11:231–241
Saatci I, Geyik S, Yavuz K, Cekirge HS (2011) Endovascular treatment of brain arteriovenous malformations with prolonged intranidal Onyx injection technique: long-term results in 350 consecutive patients with completed endovascular treatment course. J Neurosurg 115:78–88
Sasaki T, Kurita H, Saito I, Kawamoto S, Nemoto S, Terahara A, Kirino T, Takakura K (1998) Arteriovenous malformations in the basal ganglia and thalamus: management and results in 101 cases. J Neurosurg 88:285–292
Song D, Leng B, Gu Y, Zhu W, Xu B, Chen X, Zhou L (2005) Clinical analysis of 50 cases of BAVM embolization with Onyx, a novel liquid embolic agent. Interv Neuroradiol 11:179–184
Stapf C, Mast H, Sciacca RR, Choi JH, Khaw AV, Connolly ES, Pile-Spellman J, Mohr JP (2006) Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology 66:1350–1355
Strauss I, Frolov V, Buchbut D, Gonen L, Maimon S (2013) Critical appraisal of endovascular treatment of brain arteriovenous malformation using Onyx in a series of 92 consecutive patients. Acta Neurochir (Wien) 155:611–617
Turjman F, Massoud TF, Vinuela F, Sayre JW, Guglielmi G, Duckwiler G (1995) Correlation of the angioarchitectural features of cerebral arteriovenous malformations with clinical presentation of hemorrhage. Neurosurgery 37:856–860, discussion 860–852
van Rooij WJ, Sluzewski M, Beute GN (2007) Brain AVM embolization with Onyx. AJNR Am J Neuroradiol 28:172–177, discussion 178
Velat GJ, Reavey-Cantwell JF, Sistrom C, Smullen D, Fautheree GL, Whiting J, Lewis SB, Mericle RA, Firment CS, Hoh BL (2008) Comparison of N-butyl cyanoacrylate and onyx for the embolization of intracranial arteriovenous malformations: analysis of fluoroscopy and procedure times. Neurosurgery 63:ONS73–ONS78, discussion ONS78–80
Weber W, Kis B, Siekmann R, Kuehne D (2007) Endovascular treatment of intracranial arteriovenous malformations with onyx: technical aspects. AJNR Am J Neuroradiol 28:371–377
Xu F, Ni W, Liao Y, Gu Y, Xu B, Leng B, Song D (2011) Onyx embolization for the treatment of brain arteriovenous malformations. Acta Neurochir (Wien) 153:869–878
Yamada S, Takagi Y, Nozaki K, Kikuta K, Hashimoto N (2007) Risk factors for subsequent hemorrhage in patients with cerebral arteriovenous malformations. J Neurosurg 107:965–972
Conflict of interest
There was no financial support given for this retrospective case series. None of the authors have any affiliation with industry materials used in this retrospective case series.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Altschul, D., Paramasivam, S., Ortega-Gutierrez, S. et al. Safety and efficacy using a detachable tip microcatheter in the embolization of pediatric arteriovenous malformations. Childs Nerv Syst 30, 1099–1107 (2014). https://doi.org/10.1007/s00381-014-2404-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-014-2404-9