Abstract
Background
Onyx has emerged in recent years for the endovascular treatment of brain arteriovenous malformations (AVMs). However, the role of Onyx embolization is still under discussion. We report our initial experiences in the treatment of brain AVMs with Onyx embolization.
Methods
Between January 2004 and December 2007, 86 patients with brain AVMs were embolized with Onyx. Clinical presentation included intracerebral hemorrhage in 32 patients, seizures in 25 patients, headaches in 20 patients, neurologic deficits in 3 patients, and in 6 patients the AVM was an incidental finding. According to the Spetzler–Martin scale, three AVMs were grade I, 13 were grade II, 45 were grade III, 19 were grade IV, and 6 were grade V. Seventy-four AVMs were located in eloquent regions.
Results
Initial complete obliteration after final embolization was achieved in 16 patients (18.6%), with an average of 80.5% (range, 30–100%) volume reduction. Partial embolization was followed by surgery in 18 patients, whereas 17 AVMs were cured. In 48 patients treated by embolization and radiosurgery, four patients were lost to follow-up. Three-year follow-up angiography was performed on 30 patients and showed complete obliteration after radiosurgery in 23 patients. The remaining 14 patients are awaiting 3-year postradiosurgery results. Embolization-related permanent morbidity was 3.5%, whereas mortality was 1.2%.
Conclusions
Although Onyx allows moderate obliteration rates, combined management, such as adjunctive embolization with microsurgery or radiosurgery, may be effective for selected large AVMs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Modern treatment of brain arteriovenous malformations (AVMs) includes the following modalities alone or in combination: endovascular embolization, microsurgical resection, and stereotactic radiosurgery [5, 24, 32, 33]. Microsurgical resection is preferred for the treatment of AVMs in accessible areas [7, 27]. Radiosurgery is recommended for AVMs in anatomically difficult locations [27, 33]. Endovascular embolization is mostly commonly used to reduce the size of the AVM nidus and occlude deep feeding vessels that are difficult to access, to enhance the safety of surgery, or to make the AVM amenable to radiosurgical ablation [4, 6, 9, 11, 12, 39, 40]. Occasionally, small AVMs with a limited number of feeders that can easily be catheterized can be embolized alone for total obliteration or other obvious target for embolization such as pseudoaneurysms or large fistulae [33]. Although controversial, some investigators have advocated palliative embolization for large AVMs.
A variety of embolic agents has been used for endovascular embolization of brain AVMs, including particles, liquid polymers, and coils. Although having the advantage of variety of particle sizes, the particulate agents such as polyvinyl alcohol and microspheres are associated with increased frequency of recanalization and potential for intraprocedural hemorrhage [34, 37]. Coils are commonly used for the occlusion of arteriovenous fistulae within the AVM nidus. Until recently, the most widely used liquid embolics are N-butyl cyanoacrylate (n-BCA). Because of its flow characteristics, it allows easy injection via small, flow-guided catheters atraumatically. Its ability of rapidly solidifying liquid tissue adhesive allows permanent embolization with durable occlusion of the pedicle. However, there are still several disadvantages of n-BCA. Because of its rapid polymerization, the catheter must be quickly withdrawn after each injection of n-BCA and before any reflux has occurred. Furthermore, it also somewhat limits the amount of glue that can be injected through the microcatheter. Multiple catheterizations of the malformation are necessary to achieve a high rate of occlusion [3, 13, 20, 22].
Recently, a new liquid embolic agent Onyx consisting of ethylene–vinyl alcohol dissolved in DMSO became available for the endovascular treatment of brain AVMs [1]. The physical characteristics of Onyx reduce the possibility of the catheter gluing to the injected polymer [26]. Due to lava-like flow patterns, it allows for more prolonged and controlled injections. Thus, it is possible to occlude larger portions of the nidus per injection. In this study, we report our clinical experience with Onyx embolization in the treatment of brain AVMs.
Materials and methods
Patient population
Between January 2004 and December 2007, 86 patients with AVMs were treated by embolization with Onyx at our institution. The mean patient age was 30.3 years (range, 8–55 years). There were 51 men and 35 women. Presenting symptoms are outlined in Table 1, including intracranial hemorrhage in 32 patients (37.2%, 32/86), seizures in 25 patients (29.1%, 25/86), headaches in 20 patients (23.2%, 20/86), neurologic deficit in 3 patients (3.5%, 3/86), and in 6 patients (7.0%, 6/86) the AVM was an incidental finding. Of 86 patients with AVM, five (5.8%, 5/86) were located in the cerebellum, ten (11.6%, 10/86) were located in the deep brain (six in the corpus callosum and four in the basal ganglia), and one (1.2%, 1/86) was a brain stem lesion. The locations of the remaining AVMs were frontal (15.1%, 13/86), temporal (19.8%, 17/86), parietal (11.6%, 10/86), occipital (9.3%, 8/86), fronto-parietal (9.3%, 8/86), fronto-temporal (7.0%, 6/86), temporo-parietal (3.5%, 3/86), temporo-occipital (2.3%, 2/86), parieto-occipital (1.2%, 1/86), and fronto-temporo-parietal (2.3%, 2/86; Table 2).
According to the Spetzler–Martin grading scale [35], 3 AVMs (3.5%, 3/86) were grade I, 13 (15.1%, 13/86) were grade II, 45 (52.3%, 45/86) were grade III, 19 (22.1%, 19/86) were grade IV, and 6 (7.0%, 6/86) were grade V (Table 3). A total of 74 (86%) of the AVMs were located in eloquent regions. The average AVM volume before embolization was 18.5 cm3 (range, 1.4–59.0 cm3), and the AVMs were divided into three subgroups: <10 cm3 (15), 10–20 cm3 (48), and >20 cm3 (23 patients). The sizes of the AVMs were determined (excluding the feeding arteries and draining veins) by correlating the measurements from the corresponding digital subtraction angiography (DSA) and MR imaging. AVM volume was calculated retrospectively by the formula that Pasquelin et al. [29] described (V = width × height × length × 0.52). Reduction in AVM volume was calculated as follows: [pre-embolization V − post-embolization V]/pre-embolization V × 100.
Treatment strategy
All patients were evaluated by a joint meeting of neurosurgeons with expertise in endovascular embolization or microsurgery resection, and neuroradiologists, based on magnetic resonance imaging (MRI) and DSA. In our hospital, small AVMs (<3 cm) located in non-eloquent areas with superficial venous drainage are usually treated by surgery alone, unless with angioarchitecture suitable for curative embolization (few feeding pedicles) or other targets for embolization such as pseudoaneurysms or large fistulae. For AVMs larger than 3 cm, endovascular embolization is usually performed to decrease the size of the nidus, to enhance the safety of surgery, or to make the remaining AVM amenable for radiosurgery (the largest diameter of the remaining nidus is <3 cm). Radiosurgery alone is preferentially used for centrally located AVMs with a nidus size <3 cm.
Embolization procedure
All procedures were performed with the patients under general anesthesia at a biplane angiography unit, and informed consent was obtained from all patients. Systolic blood pressure during the procedure was controlled between 100 and 110 mmHg. A 6-F sheath was placed in the femoral artery after Seldinger’s puncture and catheterization. After a 6-F guiding catheter (Envoy, Cordis, Miami Lakes, FL, USA) was inserted in one of the main feeding pedicles (internal carotid artery or dominant vertebral artery), a flow-directed microcatheter compatible with DMSO (Marathon or Ultraflow, ev3) was navigated into the nidus of AVMs with the aid of blood flow and a 0.008- or a 0.010-in. guidewire (Mirage or Silverspeed, ev3). Once the microcatheter tip was placed at an intra- or perinidal position, superselective angiography was performed to confirm a stable distal tip position of the microcatheter, clear angioarchitecture of the nidus, and visible drainage vein. After the tip of the microcatheter is in place, we always perform a global angiography to assess microcatheter position within the nidus and estimate an acceptable length of reflux. Then, we proceeded with the injection of Onyx as follows: (1) The microcatheter was flushed with 5 mL of normal saline; (2) 0.25 mL of DMSO was injected into the microcatheter to fill the dead space; (3) Onyx was aspirated into a 1-mL syringe and 0.25 mL of this amount was injected slowly at a rate of 0.1 mL/min to fill the microtheter and replace the DMSO in the dead space; and (4) slow injection of the Onyx was then continued under subtracted fluoroscopy (blank road mapping). Injection as slow as 0.10–0.15 mL/min was recommended to achieve full penetration of Onyx into the nidus. The injection was stopped if the reflux was larger than determined earlier, drainage veins were filled, or leptomeningeal collaterals were embolized. Injection procedure was then resumed after a waiting period of 30 s to 2 min to allow for solidification and ensure continuous penetration of Onyx into the nidus.
“Push-and-plug” technique is the key to improve penetration of Onyx into the nidus. After multiple rounds of injection, reflux, and waiting, a reflux as long as 1.0–1.5 cm could be formed at the tip of the microcatheter. Finally, a plug could be created around the tip, completely blocking the blood flow and resulting in a distinctive change in pressure gradients within the nidus, until when Onyx could penetrate into the nidus continuously to embolize the AVMs subtotally or totally. The microcatheter would be withdrawn after the embolization or if the reflux exceeded 1.5–2.0 cm. When the catheter was withdrawn, the microcatheter would be pulled straight first, then applied with a gradually increased force and either snapped out of the Onyx cast or slowly pulled out. Staged sessions of embolization were planned for partially occluded AVMs.
Post-embolization care
After embolization, CT scan was performed to exclude hemorrhagic complications. Subsequently, the patient was monitored for 24 h at the intensive care unit. If a large volume of the nidus has been occluded, we always attempt to maintain a low systolic blood pressure (<100–110 mmHg) after the procedure. Steroids were given intravenously (10 mg of dexamethasone) after the procedure. Anti-epileptic medicine was administered intravenously after the intervention to prevent seizure. In patients with catheter trapping in the arterial feeder, low-molecular-weight heparin was subcutaneously injected at 12 h (750–1,000 U/h) for 48 h, followed by oral aspirin for 3 months at a dose of 100 mg/day.
Follow-up
At our institution, angiographic examinations were scheduled in patients with complete AVM obliteration by embolization after 6 months and in patients with surgical removal of the AVM after embolization at discharge. After completion of staged embolization, radiosurgery was soon performed with a Leksell Gamma Knife at our own institution. Patients who have been treated with radiosurgery were followed with yearly MRI. Control cerebral angiography was usually performed at 3 years after radiosurgery. If after 3 years the AVM was still not obliterated, repeated radiosurgery was considered. Clinical outcome was measured before and after embolization procedure and graded according to the modified Rankin scale (mRS, 0–6): 0, no symptoms; 1, minor symptoms; 2, some restrictions in lifestyle; 3, significant restriction; 4, partially dependent; 5, fully dependent; and 6, death. Long-term outcomes were also recorded.
Results
A total of 86 patients were treated by endovascular embolization alone or in combination with microsurgical resection and radiosurgery (Table 4). A total of 123 endovascular procedures were performed with a mean of 1.43 (range, 1–3) per patient: 53 patients were embolized once, 29 patients were embolized twice, and 4 patients were embolized thrice. The mean number of endovascular sessions in SM grades III to IV was higher than those in SM grades I and II. An average of 5.8 ml (range, 1–13.5 ml) Onyx was used per patient. In 82 patients, Onyx was used alone; in three patients, Onyx and n-BCA was used; and in one patient, a combination of Onyx and coils was used.
Anatomic results
Initial complete obliteration of the AVM at the end of all embolization procedures was achieved by embolization alone in 16 patients (18.6%, 16/86), in which two AVMs were grade I, seven were grade II, four were grade III, and three were grade IV (Table 4). An average of 80.5% (range, 30%–100%) volume reduction was achieved after embolization (<50%, 6; 50–69%, 10; 70–79%, 10; 80–89%, 21; 90–99%, 23; 100%, 16). After a mean follow-up of 6.5 months of the 16 initially completely occluded AVMs, two angiographic recurrences were evident. These two patients underwent radiosurgery, and follow-up showed complete obliteration.
Among the 86 patients, one patient died of embolization-related complication. Three patients with partially embolized AVMs refused further embolization or radiosurgery. Of the remaining 66 patients, 18 patients had additional surgery (including the two patients who received hematoma evacuation and removal of the malformation following acute hemorrhagic complications from embolization). Seventeen AVMs were cured by embolization and surgery, which was confirmed with angiography before discharge. Only one patient’s angiography showed a minor residual AVM, and this patient received additional radiosurgery.
Forty-eight patients were treated with Gamma Knife radiosurgery after a sufficient reduction of the AVM size (<3 cm) had been achieved. Four patients were lost to follow-up because of address change. Control cerebral angiography was performed on 30 patients at the time this article was written and showed complete obliteration after radiosurgery in 23 patients (76.7%, 23/30). The remaining 14 patients are awaiting 3-year post-radiosurgery results. Currently, 56 of 86 AVMs (65%) have been obliterated completely.
Complications
Embolization-related complications occurred in 12 patients (14.0%, 12/86) and are summarized in Table 5. Periprocedurally, the microcatheter was inadvertently glued to the vessel in three cases and was left in place without clinical sequelae. In one patient, the arterial perforation occurred when catheter manipulation was sealed with glue. The patient tolerated the perforation without a neurologic deficit. Severe intracerebral hemorrhage occurred in another patient, which was caused by obvious shifting of the nidus during microcatheter retrieval. Despite undergoing a craniotomy for hematoma evacuation and removal of the malformation, the patient was left with disabling hemiparesis. At follow-up, the patient’s condition was improved to a non-disabling residual hemiparesis. Post-interventionally, acute intracranial hemorrhage occurred in two patients between 1 and 4 h after embolization. In one patient, an external ventricular drainage was inserted, but the patient died of a brainstem hemorrhage. The other one was obviously caused by premature occlusion of the nidal draining vein. After being treated with emergency craniotomy and removal of the hematoma, the patient revived with a permanent disabling deficit. The remaining patients with one intraventricular hemorrhage and two subarachnoid hemorrhages presented with non-neurologic deficits. Two patients suffered ischemic complications after embolization, exhibiting new neurologic deficits. One patient with parietal AVM suffered from lower limb paresis due to a small infraction, and the paresis resolved at discharge. Another patient with occipital AVM experienced permanent visual field deficit due to glue reflux proximal to the tip of the microcatheter. Overall, the permanent morbidity rate related to embolization was 3.5% (3/86), and mortality was 1.2% (1/86) per patient.
Of 18 patients who had adjunctive embolization and surgery, 13 patients showed no neurologic deficits postoperatively; however, two patients experienced non-disabling neurologic deficits and three patients experienced disabling neurologic deficits, including the two patients who received hematoma evacuation and removal of the malformation following acute hemorrhagic complications from embolization.
Of 48 patient who had adjunctive embolization and radiosurgery, only one patient experience non-disabling neurologic deficit. No patient experienced hemorrhage after Gamma Knife radiosurgery. There was no death.
Clinical outcomes
After last embolization treatment, clinical status of 78 patients (90.7%, 78/86) remained unchanged or improved; three patients (3.5%, 3/86) experienced minor symptoms without neurologic deficit, one patient (1.2%, 1/86) had a non-disabling (mRS, 1–2) deficit, three patients (3.5%, 3/86) had disabling deficits (mRS, ≥3), and one patient (1.2%, 1/86) died. In the 18 patients treated with adjunctive embolization and surgery, postoperative clinical status worsened for three patients compared with results from final embolization, where one patient had a disabling neurologic deficit, whereas two patients experienced non-disabling neurologic deficits. In the 48 patients treated with adjunctive embolization and radiosurgery, only one patient experience non-disabling neurologic deficit. Because four patients (4.7%) were lost to long-term follow-up after radiosurgery, long-term outcomes were recorded in 82 patients (95.3%). On long-term follow-up (42.8 ± 27.2 months), in those who had new neurologic deficits after treatment, three patients had non-disabling neurological deficit and two patients had disabling neurologic deficits.
Discussion
The main purpose of the treatment of brain AVMs is complete obliteration of the nidus to prevent first or recurrent intracranial hemorrhage. Currently, microsurgical resection, endovascular embolization, or stereotactic radiosurgery are used either alone or in combination to achieve that goal. In recent years, treatment of brain AVMs in many centers has changed their goal of embolization from adjunctive therapy before surgery or radiosurgery to primary curative embolization [17, 25, 30, 31, 38]. However, the role of endovascular embolization still remains controversial. Although previous literature shows relatively high cure rates with embolization alone, it also provides high rates of complications. Here, we report our initial experiences in the treatment of brain AVMs with Onyx embolization, which was different from previous studies. Although Onyx allows moderate obliteration rates in our series, combined management, such as adjunctive embolization with microsurgery or radiosurgery, is effective for selected large AVMs.
Onyx is a new embolic agent consisting of EVOH dissolved in DMSO and mixed with micronized tantaluma powder to obtain adequate radiopacity. When Onyx comes into contact with blood or any aqueous solution, copolymer precipitates and solidifies itself into a spongy cast from the outside, like lava of a volcano, as initiated by rapid diffusion of DMSO. Before the cast is completely solidified, its liquid center still flows continuously. Due to lava-like flow patterns, it allows for more prolonged and controlled injections. Thus, it is possible to occlude larger portions of the nidus per injection. It also allows simultaneous angiographic control through the guiding catheter and assessment of the status of the embolization. Furthermore, it allows working with reflux and blocking flow in the feeder pedicle with a microcatheter threaded inside, permitting continued penetration of the different portions of the nidus. With the knowledge of morphologic characteristics of AVMs that are suitable for embolization with Onyx, high occlusion rates and low complication rates are feasible in treating a small number of feeders.
Up to now, several studies have demonstrated clinical experience of embolization of intracranial AVMs with Onyx. Jahan et al. [14] first reported a single-center experience in treating 23 AVMs with Onyx. They embolized 129 feeding arteries in 33 sessions. Although there was no cure rate after embolization, the average obliteration rate of AVMs was 63%. In 17 (74%) of 23 patients, 50% or greater reduction in AVM volume was achieved. Pierot et al. [31] reported their preliminary results of a multicentric study in treating 50 AVMs with Onyx. In 15 patients for whom embolization is completed, two cases (13%) were complete occluded, whereas 14 cases (93%) had a percentage of occlusion of the nidus >60%. Leonardi et al. [19] reported only two completely obliterated cases among 34 cases with AVMs of Spetzler–Martin grade 3 or more. Pérez-Higueras et al. [30] reported ten cases (22%) of complete occlusion among 45 cases.
More recently, van Rooij et al. [36] reported a series of patients undergoing embolization with Onyx, observing a cure rate of 16% (seven patients), all of which were small AVMs (grades I and II). Weber et al. [38] reported a complete obliteration rate of 20% alone in a series of 94 cases. Two recurrences were present at 3 months’ follow-up angiographic examination, resulting in a complete obliteration rate of 18%. In another series of 94 patients, Mounayer et al. [25] reported angiographic cure in 26 patients; in this series, the course of endovascular treatment was completed in 53 patients by using a combination of Onyx and n-BCA. Panagiotopoulos et al. [28] reported a complete initial occlusion of 20 patients (24.4%) in 82 cases, whereas the complete obliteration rate was 19.5% (16/82). Katsaridis et al. [17] reported substantially higher cure rates in a consecutive series of 101 patients. Among the 101 patients, there were 52 patients in whom the treatment was completed; 28 (53.9%) AVMs were totally occluded by endovascular procedures alone. However, in these series, higher complication rates occur. Moreover, follow-up angiography was absent in this issue.
In our series consisting of 86 patients, complete occlusion is 18.6% (16/86) and near-total occlusion (90–99%) was achieved in 26.7% (23/86) of patients (Fig. 1). Although the occlusion rate of the Panagiotopoulos and Katsaridis series was higher than ours, they did not report on the results of AVM treatment stratified by size, location, or SM grades. Moreover, when pursuing total occlusion rates of AVMs, higher complication rates also result. Most of our series, 74.4% (64/86), are large AVMs (>3 cm) and 86% (74/86) of AVMs were located in eloquent regions. Whereas small AVMs can be effectively treated either by surgery or radiosurgery, treatment of large AVMs represents a significant challenge. For large AVMs in accessible regions, we prefer preoperative embolization to decrease the blood supply to the malformation, thereby enhancing the safety of operation, whereas for large AVMs in eloquent regions, we commend staged embolization to reduce large AVMs to a manageable size (<3 cm) that is amenable to radiosurgical ablation (Figs. 2 and 3) as previous studies demonstrated that the size of the AVM after embolization and before radiosurgery is significantly associated with the outcome [6, 11, 12, 40]. At 3 years after radiosurgery, the obliteration rate is 76.7% (23/30). Moreover, no patient experienced hemorrhage after Gamma Knife radiosurgery. If after 3 years the AVM was still not obliterated, repeated radiosurgery was considered.
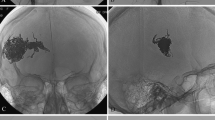
A 36-year-old patient with an SM grade III AVM in the left parietal lobe. a, d Left internal carotid angiogram in anteroposterior (AP) and lateral views showing arterial supply from multiple feeders arising mainly from the left anterior cerebral artery and middle cerebral artery with superficial venous drainage. b, e Left internal carotid angiogram in AP and lateral views demonstrating complete obliteration of the AVM with two injections of Onyx (total of 4.5 mL). c, f Plain radiography in later and AP views showing the Onyx cast in the malformation
A 28-year-old patient with an SM grade III AVM in the left parietal lobe. a Left internal carotid angiogram (AP view) showing arterial supply from feeders of the left middle cerebral artery with superficial venous drainage. b Substantial reduction in the volume of the AVM with Onyx injections of 9.0 mL. c Plain radiography in AP view. d Complete obliteration of the AVM 3 years after gamma knife radiosurgery
A 28-year-old patient with an SM grade IV AVM in the right temporo-parietal lobe. Right vertebral artery angiogram (a), right internal carotid angiogram (b), and left internal carotid angiogram (c) in lateral views showing AVM supplied by right posterior cerebral artery and bilateral anterior cerebral artery with deep venous drainage. d–f AVM shows 80% obliteration with two sessions of Onyx injections (total of 9.0 mL). g–i Complete obliteration of the AVM 3 years after Gamma Knife radiosurgery
There are also some disadvantages to the use of Onyx embolization of brain AVMs, such as catheter entrapment and angiotoxicity. The reflux of Onyx during the procedure acts as a double-edged sword. On the one hand, proper reflux helps the continuous penetration of Onyx into the nidus to achieve satisfactory embolization compared with n-BCA; on the other hand, improper reflux makes it difficult to withdraw the microcatheter, which may result in hemorrhagic complications or catheter entrapment. In our opinion, the tortuosity of the feeding artery is the main cause of catheter entrapment. The length of the Onyx reflux, as well as the injection time, is also a potential cause. Onyx is not a good embolic agent for obviously small tortuous feeding artery. If Onyx has to be used in such a case, the use of microcatheter with detachable tips may help reduce some of the risks associated with non-detachable microcatheters [23]. Anigotoxicity with vasospasm or angionecrosis has also been associated with Onyx embolization due to the action potential-reducing effects of DMSO [14]. Another potential disadvantage of Onyx is the amount of radiation exposure required for prolonged injections. However, a recent study seems to resolve this issue [21]. Using two catheters for the delivery of Onyx has allowed a more efficient embolization with shorter injection and X-ray exposure.
The permanent disabling morbidity and mortality rated in several recent large series using Onyx have ranged from 6.8% to 17.7% [17, 25, 28, 30, 31, 36, 38]. In our study, the permanent morbidity rate of embolization is 3.5% (3/86) and mortality was 1.2% (1/86) per patient. Our results are in the range of recent large series using predominantly or exclusively n-BCA [8, 10, 16, 18], which have ranged from 1.6% to 6.5%.
The major clinically significant complication is embolization-related hemorrhage. Periprocedural hemorrhage may be caused by mechanical vessel rupture during microcatheter placement into small and tortuous arteries, during microcatheter retrieval, or intranidal aneurysm rupture. Thus, gentle manipulation should be stressed and guidewire was recommended to enter the nidus. If such arterial rupture occurred during catheter manipulation, immediate injection of n-BCA might be helpful to occlude the bleeding. Acute post-embolization hemorrhage is thought to be caused by inappropriate occlusion of the nidal draining vein, increased pressure in feeder arteries, delayed thrombosis in the draining veins due to partial obliteration of the AVM, and hemodynamic changes attributable to normal perfusion pressure breakthrough (NPPBS) [2]. Regardless of better penetration feature of Onyx, we prefer staged embolization sessions in large AVMs in order to avoid the occurrence of NPPBS syndrome. Meanwhile, postoperative blood pressure control should also be taken to reduce post-procedural hemorrhage.
Ischemic procedure-related complications may be attributed to catheter-induced thrombotic emboli or embolization of normal brain arteries, or the venous system. Onyx refluxes during the procedure and occludes normal arterial branches. On the other hand, venous thrombosis may be caused by decreasing blood flow within the venous outlet of the AVMs. However, not all ischemic events result in neurologic deficits. Although some researchers mentioned the role of platelet glycoprotein IIb/IIIa antagonists, antiplatelet treatment was not required in our series.
Catheter gluing is unlikely to happen due to the cohesive features of Onyx. The difficulty in catheter removal depends on the tortuosity of feeding pedicle, the length of time of injection, and the distance of Onyx reflux. In our opinion, quick catheter withdrawal is easier for straight and large feeding pedicle. In those cases with tortuous feeding artery, slow constant traction should be maintained on the catheter on the basis of no shifting of the nidus. When trapped, it is safer to leave the catheter in place and cut off at the groin sheath. If properly treated, the patient suffers no clinical symptom with a microcatheter left in the body.
Initial experiences in recent studies have shown that Onyx is the better agent to treat AVMs, which allows long-duration injections and enhances therapeutic effect. However, the complication rate is still a major worrisome concern in contrast to n-BCA [15]. The use of the microcatheter with detachable tip for Onyx injections may reduce some of the complications associated with non-detachable microcatheters [23].
Conclusions
Our initial experience with Onyx embolization for the treatment of brain AVMs has been encouraging. Although Onyx allows moderate obliteration rates, combined management, such as adjunctive embolization with microsurgery or radiosurgery, may be effective for selected large AVMs.
References
Alexander MJ, Tolbert ME (2006) Targeting cerebral arteriovenous malformations for minimally invasive therapy. Neurosurgery 59:S178–S183
Chyatte D (1997) Normal pressure perfusion breakthrough after resection of arteriovenous malformation. J Stroke Cerebraovasc Dis 6:130–136
Debrun GM, Aletich V, Ausman JI, Charbel F, Dujovny M (1997) Embolization of nidus of brain arteriovenous malformations with n-butyl cyanoacrylate. Neurosurgery 40:112–121
Fiorella D, Albuquerque FC, Woo HH, McDougall CG, Rasmussen PA (2006) The role of neuroendovascular therapy for the treatment of brain arteriovenous malformations. Neurosurgery 59:S163–S177
Fleetwood IG, Steinberg GK (2002) Arteriovenous malformations. Lancet 359:863–873
Gobin YP, Laurent A, Merienne L, Schlienger M, Aymard A, Houdart E, Casasco A, Lefkopoulos D, George B, Merland JJ (1996) Treatment of brain arteriovenous malformations by embolization and radiosurgery. J Neurosurg 85:19–28
Hamilton MG, Spetzler RF (1994) The prospective application of a grading system for arteriovenous malformations. Neurosurgery 34:2–7
Hartmann A, Pile-Spellman J, Stapf C, Sciacca RR, Faulstich A, Mohr JP, Schumacher HC, Mast H (2002) Risk of endovascular treatment of brain arteriovenous malformations. Stroke 33:1816–1820
Hauck EF, Welch BG, White JA, Purdy PD, Pride LG, Samson D (2009) Preoperative embolization of cerebral arteriovenous malformations with Onyx. AJNR Am J Neuroradiol 30:492–495
Haw CS, terBrugge K, Willinsky R, Tomlinson G (2006) Complications of embolization of arteriovenous malformations of the brain. J Neurosurg 104:226–232
Henkers H, Nahser HC, Berg-Dammer E, Weber W, Lange W, Kühne D (1998) Endovascular therapy of brain AVMs prior to radiosurgery. Neurol Res 20:479–492
Izawa M, Chernov M, Hayashi M, Iseki H, Hori T, Takakura K (2009) Combined management of intracranial arteriovenous malformations with embolization and Gamma Knife radiosurgery: comparative evaluation of the long-term results. Surg Neurol 71:43–53
Jafar JJ, Davis AJ, Berenstein A, Choi IS, Kupersmith MJ (1993) The effect of embolization with N-butyl cyanoacrylate prior to surgical resection of cerebral arteriovenous malformations. J Neurosurg 78:60–69
Jahan R, Murayama Y, Gobin YP, Duckwiler GR, Vinters HV, Viñuela F (2001) Embolization of arteriovenous malformations with Onyx: clinicopathological experience in 23 patients. Neurosurgery 48:984–987
Jayaraman M, Cloft HJ (2009) Embolization of brain arteriovenous malformations for cure: because we could or because we should? AJNR Am J Neuroradiol 30:107–108
Jayaraman MV, Marcellus ML, Hamilton S, Do HM, Campbell D, Chang SD, Steinberg GK, Marks MP (2008) Neurologic complications of arteriovenous malformation embolization using liquid embolic agents. AJNR Am J Neuroradiol 29:242–246
Katsaridis V, Papagiannaki C, Aimar E (2008) Curative embolization of cerebral arteriovenous malformations (AVMs) with Onyx in 101 patients. Neuroradiology 50:589–597
Ledezma CJ, Hoh BL, Carter BS, Pryor JC, Putman CM, Ogilvy CS (2006) Complications of cerebral arteriovenous malformation embolization: multivariate analysis of predictive factors. Neurosurgery 58:602–611
Leonardi M, Simonettti L, Cenni P, Raffi L (2005) Brain AVM embolization with Onyx: analysis of treatment in 34 patients. Interv Neuroradiol 11:185–204
Loh Y, Duckwiler GR (2010) A prospective, multicenter, randomized trial of the Onyx liquid embolic system and N-butyl cyanoacrylate embolization of cerebral arteriovenous malformations. J Neurosurg 113:733–741
Lopes DK, Bagan B, Wells K (2010) Onyx embolization of arteriovenous malformation using 2 microcatheters. Neurosurgery 66:616–618
Lundqvist C, Wihkolm G, Svendsen P (1996) Embolization of cerebral arteriovenous malformations. Part II. Aspects of complications and late outcome. Neurosurgery 39:460–467
Maimon S, Strauss I, Frolov V, Margalit N, Ram Z (2010) Brain arteriovenous malformation treatment using a combination of Onyx and a new detachabale tip microcatheter, SONIC: short-term results. AJNR Am J Neuroradiol 31:947–954
Mattle HP, Schroth G, Seiler RW (2000) Dilemmas in the management of patients with arteriovenous malformations. J Neurol 247:917–928
Mounayer C, Hammami N, Piotin M, Spelle L, Benndorf G, Kessler I, Moret J (2007) Nidal embolization of brain arteriovenous malformations using Onyx in 94 patients. AJNR Am J Neuroradiol 28:518–523
Murayama Y, Vinuela F, Ulhoa A, Akiba Y, Duckwiler GR, Gobin YP, Vinters HV, Greff RJ (1998) Nonadhesive liquid embolic agent for cerebral arteriovenous malformations: preliminary histopathological studies in swine rete mirabile. Neurosurgery 43:1164–1175
Ogilvy CS, Stieg PE, Awad I, Brown RD Jr, Kondziolka D, Rosenwasser R, Young WL, Hademenos G, Special Writing Group of the Stroke Council, American Stroke Association (2001) AHA scientific statement: recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Stroke 32:1458–1471
Panagiotopoulos V, Gizewski E, Asgari S, Regel J, Forsting M, Wanke I (2009) Embolization of intracranial arteriovenous malformations with ethylene–vinyl alcohol copolymer (Onyx). AJNR Am J Neuroradiol 30:99–106
Pasqualin A, Barone G, Cioffi F, Rosta L, Scienza R, Da Pian R (1991) The relevance of anatomic and hemodynamic factors to a classification of cerebral arteriovenous malformations. Neurosurgery 28:370–379
Pérez-Higueras A, Rossi Lopez R, Quinones Taria D (2005) Endovascular treatment of cerebral AVM: our experience with Onyx. Interv Neuroradiol 11:141–157
Pierot L, Januel AC, Herbreteau D, Barreau X, Drouineau J, Berge J, Sourour N, Cognard C (2005) Endovascular treatment of brain arteriovenous malformations using Onyx: preliminary results of a prospective multicenter study. Interv Neuroradiol 11:159–164
Richling B, Killer M, Al-Schameri AR, Ritter L, Agic R, Krenn M (2006) Therapy of brain arteriovenous malformations: multimodality treatment from a balanced standpoint. Neurosurgery 59:S148–S157
Söderman M, Andersson T, Karlsson B, Wallace MC, Edner G (2003) Management of patients with brain arteriovenous malformations. Eur J Radiol 46:195–205
Sorimachi T, Koike T, Takeuchi S, Minakawa T, Abe H, Nishimaki K, Ito Y, Tanaka R (1999) Embolization of cerebral arteriovenous malformations achieved with polyvinyl alcohol particles: angiographic reappearance and complications. AJNR Am J Neuroradiol 20:1323–1328
Spetzler RF, Martin NA (1986) A proposed grading system for arteriovenous malformations. J Neurosurg 65:476–483
van Rooij WJ, Sluzewsld M, Beute GN (2007) Brain AVM embolization with Onyx. AJNR Am J Neuroradiol 28:172–177
Wallace RC, Flom RA, Khayata MH, Dean BL, McKenzie J, Rand JC, Obuchowski NA, Zepp RC, Zabramski JM, Spetzler RF (1995) The safety and effectiveness of brain arteriovenous malformation embolization using acrylic and particles: the experiences of a single institution. Neurosurgery 37:606–618
Weber W, Kis B, Siekmann R, Kuehne D (2007) Endovascular treatment of intracranial arteriovenous malformation with Onyx: technical aspects. AJNR Am J Neuroradiol 28:371–377
Weber W, Kis B, Siekmann R, Jans P, Laumer R, Kühne D (2007) Preoperative embolization of intracranial arteriovenous malformations with Onyx. Neurosurgery 61:244–254
Zabel-du Bois A, Milker-Zabel S, Huber P, Huber P, Schlegel W, Debus J (2007) Risk of hemorrhage and obliteration rates of LINAX-based radiosurgery for cerebral arteriovenous malformations treated after prior partial embolization. Int J Radiat Oncol Biol Phys 68:999–1003
Acknowledgment
We thank all the anonymous reviewers for their helpful suggestions on the quality improvement of our paper.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xu, F., Ni, W., Liao, Y. et al. Onyx embolization for the treatment of brain arteriovenous malformations. Acta Neurochir 153, 869–878 (2011). https://doi.org/10.1007/s00701-010-0848-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-010-0848-6