Abstract
Background
Dural arteriovenous fistulas (DAVF) are commonly encountered lesions that can be treated both transvenously, transarterially or using a combined approach.
Objective
Transvenous coil embolization of DAVF is a recognized treatment option but can be challenging. In this context this article presents clinical experience using the Kaneka ED10 ExtraSoft coils in combination with the Marathon microcatheter to treat high grade DAVF. The physical properties of these coils and the microcatheter were also determined.
Material and Methods
All patients with high grade DAVF treated with the Marathon and the Kaneka ED COIL ∞10 ExtraSoft coils were retrospectively identified. The clinical presentation, location, grade of the lesion, clinical and radiological follow-up data were recorded. Bench side studies were performed to determine the physical properties of the Marathon catheter in comparison to the SL10 and Headway Duo as well the maximum width of the Kaneka pusher wire in comparison to Hypersoft, Target and Axium Prime coils.
Results
A total of 8 patients with 9 DAVF with 3 Cognard 3 and 6 Cognard 4 lesions were identified. All the DAVF’s were occluded either at the end of the procedure or on follow-up imaging. On bench side tests the Marathon microcatheter had the most flexible distal tip and distal shaft in comparison to the SL10 and Headway Duo. The proximal shaft of the Marathon was stiffer than the SL10. The Kaneka ED COIL ∞10 ExtraSoft had the smallest distal width and were the only coils tested that could be deployed through a Marathon microcatheter.
Conclusion
The combination of the Marathon microcatheter and Kaneka ED COIL ∞10 ExtraSoft is useful for the treatment of high grade DAVF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dural arteriovenous fistula (DAVF) is a frequently encountered entity that is believed to be an acquired disease in adults. Although the exact pathogenesis of DAVF is unknown there is an association between these lesions and venous thrombosis [1] as well as evidence to suggest that angiogenesis is involved [2,3,4,5,6]. There two accepted classification systems, Borden and Merland-Cognard, both of which focus on the venous drainage pathways and in particular the presence of cortical venous reflux (CVR; [7, 8]). Low-grade lesions, those without CVR (Borden I, Cognard I and IIa), are less likely to present with hemorrhage; however, they can evolve over time into higher grade lesions and consequently an increase in the risk of hemorrhage. Lesions with CVR (Borden II and III, Cognard IIb, IIa+b, III, IV and V) are associated with an aggressive clinical presentation, which is defined as either intracranial hemorrhage or non-hemorrhagic neurological deficit [9]. In the work of Cognard et al. [7] low grade DAVF’s did not present with hemorrhage; however, the hemorrhagic risk associated with less favorable venous configurations progressively increased; 11% in type IIb and IIa+b, 40% in type III lesions, and 65.5% in type IV lesions.
Treatment for high grade DAVF’s is often recommended given the high risk of intracranial hemorrhage associated with these lesions. The treatment of low-grade lesions may also be recommended depending on the symptoms, e. g. pulsatile tinnitus causing intractable insomnia. The aim of treatment is to completely obliterate the fistulous connection since partial treatment can result in the recruitment of new arterial supply and/or a change in the venous drainage that will alter the hemorrhagic risk profile of the lesion or the symptoms [10]. Several different treatment options exist including surgery, endovascular surgery, and radiotherapy.
From an endovascular perspective the treatment of these lesions can be transarterial, transvenous or a combined approach. The aim of the treatment is to occlude the fistulous point. A variety of embolic agents have been used to treat these lesion including n‑butyl cyanoacrylate (n-BCA; Cordis, Miami Lakes, FL, USA), ethylene vinyl alcohol copolymer (Onyx; Medtronic, Dublin, Ireland), occlusion of the fistulous sinus segment with platinum coils, and even venous sinus stenting [11, 12].
Although the individual anatomy must be taken into account when trying to determine the optimal treatment strategy, a venous approach can offer the advantage of reducing the possibility of inadvertent occlusion of an important artery. Similarly, if access to the fistulous point is difficult due to small arterial feeders, a venous approach may allow access to the fistulous point [13]. Venous access to the fistula can be difficult and although liquid embolic agents have been used transvenously [14] they are not ideal given the direction of flow. Liquid embolic agents can be used with lower profile microcatheters, e. g. the Apollo and Marathon microcatheters (Medtronic, Dublin, Ireland). These microcatheters are often more navigable than larger microcatheters, such as the Echelon 10 (Medtronic), that are designed for coil embolization.
This article describes experiences of using the Kaneka ED COIL ∞10 ExtraSoft (Kaneka, Kanagawa, Japan) and the Marathon microcatheter to embolize high-grade DAVF. Additionally, bench side tests are presented demonstrating the beneficial features of the Marathon microcatheter and Kaneka ED COIL ∞10 ExtraSoft.
Material and Methods
Clinical Study
Patient Population
A retrospective search of our prospectively maintained database was performed to find all patients with Cognard 3 and 4 dural arteriovenous fistulas that were treated using coil embolization via a transvenous route. The database was searched to identify all patients between January 2017 and January 2018. For each patient the demographic data, clinical presentation, location of the DAVF, therapeutic intervention including the number of coils deployed, immediate angiographic result, and radiological follow-up information were recorded. Due to the retrospective nature of this work ethics approval was not required.
Endovascular Treatment
All treatments were performed with the patient under general anesthesia. All procedures were performed via a venous transfemoral or transjugular route using an 8 Fr access system and intermediate catheter, typically a Navien (Medtronic) as standard. The transvenous approach was chosen in all cases because of potential access issues from a transarterial approach. The Marathon microcatheter was used to access the fistulous point in all cases. Using a 4 Fr sheath in the right common femoral artery a 4 Fr angiography catheter was placed into the external carotid artery on the ipsilateral side of the DAVF to monitor shunting across the DAVF during the procedure and at the end of the procedure. All procedures were performed under heparin anticoagulation with a 5000 IU bolus dose at the start of the procedure and subsequent 1000 IU bolus doses every hour to maintain the activated clotting time between 2–2.5 times the baseline.
Procedural Assessment and Follow-Up
Neurological examinations were performed to evaluate for potential ischemic or hemorrhagic complications in the post-operative period (<24 h post-procedure) and at each subsequent follow-up. Follow-up angiography was performed at 6–12 months post-operatively as standard. Standard Towne’s and lateral angiographic projections were performed. Additionally, angiographic runs, including high frame rate and magnified runs were performed if persistent shunting was suspected.
Bench-side Study
Microcatheters
Bench-side studies were performed on three commonly used microcatheters, the SL10 (Stryker, Kalamazoo, MI, USA; n = 1), the Headway Duo (Microvention, Aliso Viejo, CA, USA; n = 1), and the Marathon microcatheters (n = 1). In order to determine the stiffness of the microcatheter shaft a 3-point bending test was performed. After warming the catheters to body temperature in a water bath the catheter was fixed between two clamps set 6 mm apart. The force required to displace the section of the microcatheter between the clamps by 1 mm was recorded (Fig. 1). This was repeated along the microcatheter from 20–400 mm from the distal tip of each catheter. The test was repeated up to 1600 mm from the distal tip for the SL10 and Marathon catheters to determine which catheter had the stiffer proximal shaft.
In order to determine the flexibility of the distal tip each microcatheter was clamped 7 mm from the distal end of the microcatheter after warming to body temperature in a water bath. The force to displace the end of the microcatheter by 3 mm was calculated for each of the microcatheters (Fig. 2).
Coils
Bench-side studies were performed on 4 different types of endovascular coils, Target (3.5 × 60 mm; Stryker), Axium Prime (3.5 × 60 mm; Medtronic), Hypersoft (3 × 60 mm; Microvention) and the ED COIL ∞10 ExtraSoft (3.5 × 30mm; Kaneka). High-resolution photography using a DFK 33UP5000 camera was performed after imaging of the coils at ×80 magnification using a SMZ1270i high resolution stereoscopic microscope (Nikon, Tokyo, Japan). The maximum diameter of the coil pusher wire was measured using the NIS-Elements (D) documentation software (Nikon).
Results
Clinical Results
The results are summarized in Table 1. A total of 8 patients (7 male) were identified with an average age of 62.9 ± 15.1 years. A single patient had bilateral DAVFs. Of the lesions three were located in the ethmoidal region, two of the lesions were tentorial, one lesion involved the cavernous sinus and one patient had bilateral sphenoidal fistulas. All the lesions were classified as Borden 3 with 3 lesions classified as Cognard 3 and the remaining 6 lesions classified as Cognard 4. Of the lesions four were found incidentally. The remaining lesions were symptomatic and a single lesion presented with hemorrhage.
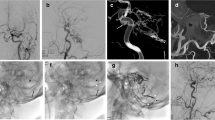
An average of 17.4 ± 10.2 coils were placed at the fistulous point. Of the lesions six showed complete occlusion at the end of the procedure with the remaining three lesions showing persistent although markedly reduced shunting. In these three cases the significantly reduced flow was believed to be sufficient to induce thrombosis of the vein and obliteration of the DAVF. There were no intra-operative complications (Figs. 3 and 4).
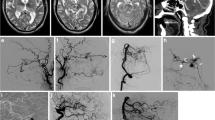
Patient 1 had an ethmoidal dural arteriovenous fistula (DAVF) classified as Borden 3 and Cognard 4 (a). An approach via the superior sagittal sinus was considered; however, it was felt an approach via the basal vein of Rosenthal and inferior frontal vein would be simpler (b; black arrow identified the distal microcatheter tip marker en route to the fistulous point). After coiling there was complete occlusion of the shunt and this was confirmed on angiography at the 3‑month follow-up (c)
Follow-up angiography was available in 6 patients (mean 8.3 months, range 1–17 months post-operatively) and in all patients the dural arteriovenous fistula (DAVF) was occluded. All the DAVFs were occluded either at the end of the procedure or on follow-up imaging.
Bench-side Results
Microcatheter
The distal shaft of the Marathon microcatheter was more flexible than the distal shaft of the Headway Duo and the SL10 (Fig. 5). The Marathon microcatheter has a stiffer proximal shaft than the SL10 (Fig. 6) with nearly three times the force required to deflect the proximal shaft of the Marathon in comparison to the SL10.
The Marathon microcatheter had the most flexible distal tip with 0.02 N of force required to deflect the catheter tip by 3 mm. The SL10 had a less flexible tip than the Headway Duo and required 0.0275 N to deflect the microcatheter tip to the same degree (Fig. 7).
Coils
The coil with the widest distal end/distal pusher wire was the Target coil (0.337 mm) followed by the Axium Prime (0.343 mm) and the Hypersoft (0.342 mm), with the Kaneka ED COIL ∞10 ExtraSoft having the smallest diameter (0.291 mm; Fig. 8). The only coil able to pass through the distal opening of the Marathon microcatheter, according to these measurements, is the Kaneka ED COIL ∞10 ExtraSoft coil.
Discussion
In recent times a transarterial approach for the treatment of DAVF has gained widespread acceptance. This is, at least in part, due to the introduction of newer embolic agents, such as Onyx and PHIL (precipitating hydrophobic injectable liquid; Microvention [15, 16]). Although this approach is often successful, alternative approaches may often be required particularly if the arterial feeders are small, very tortuous or when the arterial branches involved supply cranial nerves, the globe, or there is concern for extracranial-intracranial anastomoses [10]. In these scenarios a transvenous approach may be more appropriate; however, access to the actual fistulous point can prove challenging. Naturally, a flexible microcatheter is required in order to access the fistulous point and for these reasons microcatheters such as the Magic 1.2 Fr and 1.5 Fr (Balt Extrusion, Montmercy, France) are appropriate when using glue. The dimethyl sulfoxide (DMSO) compatible microcatheters, such as the Sonic 1.2 Fr (Balt Extrusion) and Apollo (Medtronic) are appropriate when using Onyx. Coil occlusion of DAVF is a recognized and accepted treatment modality and by its nature is more controllable when compared to the use of liquid embolic agents. The main problem, in particular for higher grade lesions, such as Cognard 3 or 4 lesions, is gaining access to the fistulous point with the microcatheter. Accessing the fistulous point via tortuous vessels will require flexible microcatheters and this often means microcatheters of small diameter. This represents a potential problem since the inner lumen of many of these catheters will not accommodate coils. For example, the Excelsior SL10 has an outer diameter of 0.6 mm and an inner luminal diameter of 0.42 mm whereas the outer diameter of the Marathon is only 0.51 mm with an inner diameter of 0.33 mm. Similarly, the Marathon has a smaller inner and outer diameter than the Headway Duo. The inner lumen of the DMSO compatible detachable tip Apollo microcatheter is 0.33 mm and the Sonic 1.5 Fr is 0.3 mm.
As was shown in the bench-side examinations, the Marathon microcatheter would be unable to accommodate most coils with the widest point of Target, Axium Prime and Hypersoft coils all being greater than 0.33 mm and only the ED COIL ∞10 ExtraSoft coils able to fit into the microcatheter (maximum width 0.291 mm). This could be advantageous for the treatment of small aneurysms and other authors have documented the use of the Marathon microcatheter to coil aneurysms as well for the treatment of DAVFs and AVMs [17,18,19,20]. Chau et al. [17] recently published a case of a basilar perforated aneurysm treated with stents and coiling using the Kaneka ED COIL ∞10 ExtraSoft coils and they noted no catheter kickback from the placement of these coils; however, kickback was seen when they attempted to place a Target Nano coil. Microcatheter kickback is thought to be, at least in part, caused by the size and stiffness of the terminal joint between the coil and the distal pusher wire [21]. It is suspected that the smaller diameter of the Kaneka ED COIL ∞10 ExtraSoft is at least partly responsible for decreased kickback reported by Chau et al. The stability of the microcatheter is important when treating DAVF as it allows accurate placement of coils at the fistulous point but also helps to minimize repeated catheter movements and hence overall procedure time and radiation exposure. The ability to pass coils through the microcatheter also appears dependent on the vessel tortuosity with evidence to suggest that coils with smaller pusher wire diameters can be used in more tortuous vessels [18]. This has been our experience with successful embolization achieved in the vast majority of cases despite prominent proximal tortuosity. Similarly, in order to achieve greater microcatheter stability a tri-axial technique would often be used. The longer length of the Marathon microcatheter, 165 cm compared to 150 cm for coiling catheters, such as the Echelon, is also of benefit particularly when navigating very tortuous venous structures.
In the bench-side testing the Marathon microcatheter required the least force to cause microcatheter tip deflection meaning that in comparison to the Headway Duo and SL10 catheters it has the softest distal tip. This is not particularly surprising given that the catheter was designed principally to be used for accessing distal structures with liquid embolic agents; however, since this catheter was not designed for coiling it does not have a proximal catheter marker as standard coiling catheters do. This poses a problem with respect to using the Marathon as a coiling catheter; however, the Kaneka coils offer an advantage in this respect. The coils are detached by electrolytically melting the polyvinyl alcohol (PVA) that connects the coil and delivery wire. The current generator box can detect changes in the electrical resistance of a circuit formed by connecting electrodes to the proximal end of the delivery wire and the return electrode or a needle inserted into the patient’s skin. When the PVA and distal end of the delivery wire is exposed to blood there is a rapid decrease in the resistance of the circuit. This decrease in the electrical conductance causes a change in the sound signal and in the color of the light on the control handset, which alerts the operator to the fact that the coil has been fully pushed out from the microcatheter and can be detached. In addition to this alarm the coil also has a proximal radio-opaque marker that allows the user to determine the coil position in the standard fashion. It has been found that the combination of these features, the flexibility and softness of the Marathon microcatheter, the softness and small size of the Kaneka ED COIL ∞10 ExtraSoft, and the alert signal provided by the detachment handset, is ideal for treating DAVF and may also be very suited to treating distal aneurysms or even using the pressure cooker technique [22, 23] for arteriovenous malformations.
This study suffers from the inherent limitations of a retrospective study with small numbers as well as selection bias towards patients, in whom the transvenous approach was considered feasible. Furthermore, the Marathon microcatheters were only compared to those that are frequently used in the department and to coils that are commonly used in the department.
Conclusion
The combination of the Marathon microcatheter and Kaneka ED COIL ∞10 ExtraSoft coils can be used to treat high grade DAVF via a transvenous approach.
References
Kutluk K, Schumacher M, Mironov A. The role of sinus thrombosis in occipital dural arteriovenous malformations−development and spontaneous closure. Neurochirurgia (Stuttg). 1991;34:144–7.
Lawton MT, Jacobowitz R, Spetzler RF. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg. 1997;87:267–74.
Zhu Y, Lawton MT, Du R, Shwe Y, Chen Y, Shen F, Young WL, Yang GY. Expression of hypoxia-inducible factor-1 and vascular endothelial growth factor in response to venous hypertension. Neurosurgery. 2006;59:687–96. discussion 687–696.
Klisch J, Kubalek R, Scheufler KM, Zirrgiebel U, Drevs J, Schumacher M. Plasma vascular endothelial growth factor and serum soluble angiopoietin receptor sTIE-2 in patients with dural arteriovenous fistulas: a pilot study. Neuroradiology. 2005;47:10–7.
Li Q, Zhang Q, Huang QH, Fang YB, Zhang ZL, Xu Y, Liu JM. A pivotal role of the vascular endothelial growth factor signaling pathway in the formation of venous hypertension-induced dural arteriovenous fistulas. Mol Med Rep. 2014;9:1551–8.
Bhogal P, Yeo LL, Henkes H, Krings T, Söderman M. The role of angiogenesis in dural arteriovenous fistulae: the story so far. Interv Neuroradiol. 2018;24:450-4.
Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A, Chiras J, Merland JJ. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671–80.
Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995;82:166-79. Erratum in: J Neurosurg. 1995;82:705-6.
Davies MA, TerBrugge K, Willinsky R, Coyne T, Saleh J, Wallace MC. The validity of classification for the clinical presentation of intracranial dural arteriovenous fistulas. J Neurosurg. 1996;85:830–7.
VanLandingham M, Fox B, Hoit D, Elijovich L, Arthur AS. Endovascular treatment of intracranial dural arteriovenous fistulas. Neurosurgery. 2014;74 Suppl 1:S42-9.
Liebig T, Henkes H, Brew S, Miloslavski E, Kirsch M, Kühne D. Reconstructive treatment of dural arteriovenous fistulas of the transverse and sigmoid sinus: transvenous angioplasty and stent deployment. Neuroradiology. 2005;47:543–51.
Kirsch M, Liebig T, Kühne D, Henkes H. Endovascular management of dural arteriovenous fistulas of the transverse and sigmoid sinus in 150 patients. Neuroradiology. 2009;51:477–83.
Lekkhong E, Pongpech S, ter Brugge K, Jiarakongmun P, Willinsky R, Geibprasert S, Krings T. Transvenous embolization of intracranial dural arteriovenous shunts through occluded venous segments: experience in 51 Patients. AJNR Am J Neuroradiol. 2011;32:1738–44.
Albuquerque FC, Ducruet AF, Crowley RW, Bristol RE, Ahmed A, McDougall CG. Transvenous to arterial Onyx embolization. J Neurointerv Surg. 2014;6:281–5.
Lamin S, Chew HS, Chavda S, Thomas A, Piano M, Quilici L, Pero G, Holtmannspolter M, Cronqvist ME, Casasco A, Guimaraens L, Paul L, Gil Garcia A, Aleu A, Chapot R. Embolization of intracranial dural arteriovenous fistulas using PHIL liquid embolic agent in 26 patients: a multicenter study. AJNR Am J Neuroradiol. 2017;38:127–31.
Leyon JJ, Chavda S, Thomas A, Lamin S. Preliminary experience with the liquid embolic material agent PHIL (Precipitating Hydrophobic Injectable Liquid) in treating cranial and spinal dural arteriovenous fistulas: technical note. J Neurointerv Surg. 2016;8:596–602.
Chau Y, Sachet M, Sédat J. Super-selective coil embolization of a basilar perforator artery aneurysm previously treated by the stent-in-stent technique, using an extremely soft bare coil delivered through a one-marker microcatheter. Interv Neuroradiol. 2017;23:492–6.
Beckett JS, Duckwiler GR, Tateshima S, Szeder V, Jahan R, Gonzalez N, Vinuela F. Coil embolization through the marathon microcatheter: advantages and pitfalls. Interv Neuroradiol. 2017;23:28–33.
Horie N, Hayashi K, Morikawa M, Izumo T, Nagata I. A novel method for super-selective coil embolization using an extremely soft bare coil through a liquid embolic delivery microcatheter. Neurol Med Chir (Tokyo). 2015;55:605–9.
Harada K, Morioka J. Initial experience with an extremely soft bare platinum coil, ED coil-10 Extra Soft, for endovascular treatment of cerebral aneurysms. J Neurointerv Surg. 2013;5:577–81.
Miyachi S, Izumi T, Matsubara N, Naito T, Haraguchi KI, Wakabayashi T. The mechanism of catheter kickback in the final stage of coil embolization for aneurysms: the straightening phenomenon. Interv Neuroradiol. 2010;16:353–60.
Chapot R, Stracke P, Velasco A, Nordmeyer H, Heddier M, Stauder M, Schooss P, Mosimann PJ. The pressure cooker technique for the treatment of brain AVMs. J Neuroradiol. 2014;41:87–91.
Zhang G, Zhu S, Wu P, Xu S, Shi H. The transvenous pressure cooker technique: A treatment for brain arteriovenous malformations. Interv Neuroradiol. 2017;23:194–9.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
P. Bhogal and M. Aguilar Pérez serve as proctors and consultants for phenox. H. Henkes is a co-founder and shareholder of phenox. M. AlMatter, V. Hellstern, H. Bäzner and O. Ganslandt declare that they have no competing interests.
Ethical standards
For this type of retrospective study formal approval is not required.
Additional information
Author Contribution
P. Bhogal, M. AlMatter, V. Hellstern data collection, manuscript preparation; H. Bäzner, O. Ganslandt review, editing, manuscript preparation; H. Henkes study design and concept, review; M. Aguilar Pérez guarantor
Rights and permissions
About this article
Cite this article
Bhogal, P., AlMatter, M., Hellstern, V. et al. High-Grade Dural Arteriovenous Fistulas. Clin Neuroradiol 29, 653–660 (2019). https://doi.org/10.1007/s00062-018-0724-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-018-0724-y