Abstract
Soil salinity is one of the main yield-limiting factors in various crops. Under different environmental stresses, many rhizobacteria have demonstrated encouraging role in enhancing plant growth and tolerate stress conditions. In this study, three potential 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase- and exopolysaccharides (EPS)-secreting bacterial strains including Bacillus megaterium, B. tequilensis, and Pseudomonas putida have been assessed for their growth-promoting characteristics. These bacterial strains positively affected the physiology, biochemistry, and antioxidant enzymatic activities of wheat plant, under salinity stress. Results of this study depicted that the inoculation of PGPR positively invigorates growth attributes like relative water content and photosynthetic pigments of wheat seedling under saline conditions. Moreover, plants inoculated with PGPR also showed decreased concentration of malondialdehyde (MDA) and hydrogen peroxide (H2O2). Inoculation of PGPR reduced electrolytic leakage and enhanced enzymatic activity for the scavenging of reactive oxygen species (ROS). These PGPR also increased the production of proline and total soluble sugar. Expression analysis of selected genes by qPCR revealed higher expression of Salt Overly Sensitive (SOS1 and SOS4) genes and predicted their potential role in stress tolerance. These genes can be further overexpressed in wheat plant to tolerate salinity stress. On the basis of these findings, it can be concluded that the priming of seeds with aforementioned PGPR can decrease the adverse effects of salinity on wheat plant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Numerous environmental stresses such as water deficiency, flooding, salinity, and extreme temperature conditions can affect the growth and development of plants and ultimately reduce their yield (Bano and Fatima 2009; Jha et al. 2011). Among these abiotic stresses, soil salinity is one of the most prominent abiotic stresses that restrict crop productivity (Munns and Gilliham 2015). Pakistan has mainly calcareous soils and salinity affects the overall yield of a variety of crops (Shrivastava and Kumar 2015). It has been reported that 6% of the cultivated land in Pakistan is highly saline (Ilangumaran and Smith 2017). More than 20% of cultivated areas, across the globe, have been reported to be under the influence of salt stress and this percentage is increasing, rapidly (Ruan et al. 2010; Lakhdar et al. 2009).
In dry and semi-dry areas of the world, soil salinity adversely disturbs the production of crops. It restricts the growth and production of crops by the generation of reactive oxygen species (ROS), sodium and chloride toxicity, and osmotic and nutrient imbalances (Mishra et al. 2013). Elevated level of ROS under various stresses can threat plant cells by oxidizing proteins, damaging DNA and RNA, doing lipid peroxidation, activating apoptosis, and inhibiting enzymes (Nxele et al. 2017; Ghassemi-Golezani and Farhangi-Abriz 2019). Under salinity stress, plants imitate various alterations in their homeostasis, mostly, because of the decrease in accessibility of water, inadequate absorption, and distribution of ions, which ultimately causes nutritive inequity (Hessini et al. 2019). Plants synthesize various compatible solutes like proline and total soluble sugars to maintain an osmotic balance (Hmidi et al. 2018).
Many studies have described the use of different plant growth-promoting rhizobacteria (PGPR) to tolerate salinity stress. PGPR progress the development of plants by colonizing their roots (Lugtenberg and Kamilova 2009). They can decrease the severity of plant damage under various environmental stresses by the changing various biochemical, molecular, and physiological processes of the host plant (Meena et al. 2015). ACC deaminase-producing halotolerant microorganisms can be used for seed priming and bio-augmentation to create stress resistance and enhance the production of crops (Li et al. 2017). Moreover, root-colonizing bacteria along with extracellular polysaccharide matrix (EPS) defend plants against salt stress by decreasing the uptake of sodium ions and increasing the water-holding capacity. Exopolysaccharides-secreting PGPR provide protection against various abiotic and biotic stresses (Kumar et al. 2015).
Various mechanisms have been adapted by PGPR to cope with various stresses. Active salt-tolerant ACC deaminase-producing microbes are used to improve stress tolerance and plant yield by the bio-augmentation of seeds (Ilangumaran and Smith 2017). The ACC deaminase is released as root exudate and is converted into α-ketobutyrate and ammonia, producing ethylene, which has a significant impact on plant growth and function under stress (Zhang et al. 2018). Furthermore, exopolysaccharides maintain higher moisture content and plant growth under stress conditions. Exopolysaccharides provide safety in dehydration, microbial accumulation, plant–microbe interaction, surface attachment, and bioremediation (Naseem et al. 2018). PGPR also promotes plant growth and reduces salt stress by producing the phytohormones and increasing the intake of nutrients. The drastic effects of synthetic fertilizers on crop productivity have also been reduced by PGPR (Kumar et al. 2015). The IAA raises the discharge of plant root exudates that expeditiously fill in as an energy provider for PGPR and improve their growth and colonization (Etesami et al. 2015). Besides providing phosphorus to the plants, phosphate-solubilizing bacteria also improve the growth of plant by promoting nitrogen fixation, increasing accessibility of trace elements, and producing phytohormones (Kumar et al. 2013). Higher accumulation of proline and total soluble sugar is observed in plants under stress condition. Proline is hydroxyl radical scavenger that also adjusts the osmotic pressure in the cells to tolerate stress (Gururani et al. 2013). Increased total soluble sugar content is another important resistance approach of plants in salt toxicity (Upadhyay et al. 2012). Chlorophyll content is considered as a stability indicator under different stresses. Carotenoids are non-enzymatic scavengers of ROS and they are present in substantial amounts in plants (Ashraf 2009). Salinity stress causes the intense oxidative damage to the plants by forming ROS. Oxidative stress resistance is empathetically related to activities of ROS-scavenging enzymes like SOD, POD, and CAT (Miller et al. 2008).
During the interaction of plants with various mutualistic and symbiotic microorganisms, plant undergoes various cellular and molecular changes. These changes might be associated with various interactions between plants and microorganisms. PGPR also induce various changes that affect the development and nutrition of plants (Bashan et al. 2004). With considerable advancements in our knowledge, halotolerant rhizobacteria are being studied to identify genes that are responsible for salinity stress tolerance. Ionic stress at cellular level causes negative impacts on crop yield and production. In plants, numerous genes that are upregulated under stress conditions have been reported to be involved in various metabolic pathways and they regulate the mechanism of transcription, signal transduction, and ion transport (Deinlein et al. 2014). Salt overlay sensitive (SOS) pathways and various ion transporters help in the alleviation of ionic stress in plants (Zhu 2000). SOS1 is an important plasma membrane sodium and hydrogen ions antiporter that helps the plants to cope with salinity stress. Pyridoxal-5-phosphate is an important cofactor for various enzymes. Its synthesis is regulated by pyridoxal kinase that is encoded by SOS4 gene. Moreover, SOS4 gene is also related with the production of IAA (Shi et al. 2002; Mahajan et al. 2008).
The aim of the current study was to screen out the ACC deaminase- and EPS-producing bacterial strains. These bacterial isolates were also screened for their plant growth-promoting activity and their salt stress tolerance potential was tested in wheat. To understand the probable intricate mechanisms, adapted by PGPR to ameliorate salinity tolerance in wheat plants, the expressions of SOS1 and SOS4 genes were also analyzed.
Materials and Methods
Collection of Rhizobacterial Strains
Three plant growth-promoting rhizobacterial strains (Bacillus megaterium MPP7, Bacillus tequilensis MPP8, and Pseudomonas putida MPP18) were obtained from Molecular Plant Pathology Lab, Quaid-i-Azam University, Islamabad, Pakistan. These strains were formerly isolated from the rhizosphere of Justicia adhatoda, Chenopodium murale, and Cenchrus ciliaris, growing in Khewra salt mine, Pakistan (32°37′44.1″ N and 73°00′47.1″ E). Previous studies have characterized B. megaterium (Dahmani et al. 2020), B. tequilensis (Kang et al. 2019), and P. putida (Kumar et al. 2016) as PGPR.
Bioassays of Isolated Bacterial Strains
ACC Deaminase Activity
Activity of 1-aminocyclopropane-1-carboxylic acid deaminase (ACCD) of all three bacterial strains was assessed by quantifying α-ketobutyrate. The bacterial strains were cultured in DF salt minimal medium supplemented with 0, 25, 50, and 100 mM concentration of NaCl and 3 mM ACC. Culture broth were positioned in a shaker incubator at 30 ± 2 °C and 150 rpm for 1 day. The ACCD activity was assessed by following the procedure of Penrose and Glick (2003). Optical density values were recorded at 540 nm. A standard curve of α-ketobutyrate concentration was plotted against absorbance value of each tested sample and finally the α-ketobutyrate was quantified in µM/mg protein/h.
Exopolysaccharide Production
To quantify the production of exopolysaccharide, the bacterial strains were cultured in ATCC no. 14 broth. This culture media was prepared by adding KH2PO4 (0.2 g), K2HPO4 (0.8 g), MgSO4·7H2O (0.2 g), CaSO4·2H2O (0.1 g), FeCl3 (10 mg), Na2MoO4·2H2O (10 mg), yeast extract (0.5 g), sucrose (20 g), and agar (15 g) in distilled water (1000 ml). Before autoclave, pH of media was adjusted to 7.2. Bacterial strains were cultured and placed in shaker incubator for 48 h at 30–35 °C and 200 rpm. The bacterial suspensions were centrifuged at 9000 rpm for 10 min after adding 1 mM EDTA to produce pellet. Supernatant containing exopolysaccharides and chilled acetone were mixed in 1:3 ratio and again centrifuged at 15,000 rpm for 3 min to precipitate exopolysaccharides mass, as pellet. It was then washed with sterilized water and allowed to dry. The purified EPS was quantified gravimetrically using an analytical balance (Zainab et al. 2020).
Synthesis of Indole Acetic Acid
IAA synthesis potential of selected three bacterial isolates was assessed by following the protocol of Gordon and Weber (1951). For this purpose, LB broth was prepared and supplemented with varying NaCl concentrations (i.e., 0, 25, 50, and 100 mM) and l-tryptophan (100 mg/l). The culture was incubated in shaker incubator at 35 °C ± 2 °C and 150 rpm for 1 day and each culture (5 ml) was centrifuged at 15,000 rpm for 2 min. Supernatant and Salkowski's reagent were mixed in 1:2 ratio and placed in dark for 30 min. IAA production was checked by Salkowski’s reagent. Color development was considered as an indicator of IAA production. The OD of solution (supernatant mixed with Salkowski’s reagent) was measured at 535 nm. To calculate IAA production, standard curve was drawn with 10–100 μg/ml IAA.
Phosphate Solubilization
For the screening of phosphate-solubilizing bacteria, plate assay method was used. Bacteria were allowed to grow on Pikoyskaya media, supplemented with various salt concentrations (i.e., 0, 25, 50, and 100 mM) and the Petri plates were positioned in an incubator at 27–28 °C ± 2 °C for 7–8 days (Pikovskaya 1948; Fischer et al. 2007). After clear zone appearance around the bacterial strains, phosphate solubilization index was calculated by the following formula:
Salt Tolerance Assay of Selected Bacterial Strains, In Vitro
To see the salt tolerance, selected bacterial strains were separately inoculated into the LB broth, amended with 0–100 mM concentration of NaCl (Barra et al. 2016) in 100 ml flasks. The inoculated flasks were positioned in shaker incubator at 35 °C ± 2 °C for 1 day at 250 rpm. The growth of bacteria under saline conditions was observed for next seven days by measuring the OD of growth media at 600 nm.
Salt Tolerance Assay of Selected Bacterial Strains, In Vivo
For this analysis, a pot experiment was conducted (in three replicates) in a complete randomized design (CRD). Seeds of wheat variety Morocco were obtained from National Agriculture Research Center (NARC), Islamabad, Pakistan. Bacterial suspensions were prepared in LB broth by placing inoculated media in incubator shaker at 35 °C ± 2 °C and 120 rpm. After 2 days, broth cultures were centrifuged at 3000 rpm for 10 min and pellets were re-suspended in distilled water to get an optical density (OD) of 1, at 600 nm. Bio-priming was performed by soaking certified wheat seeds (Morocco variety) in the selected bacterial suspensions (Naseem and Bano 2014). A mixture of sand, clay, and peat moss (1:1:1 ratio) was sieved through 2 mm mesh to remove soil micro-organisms and gravel. Following the standard protocols of McLean, the pH and EC of the experimental soil were measured. In each treatment, salt stress was induced with different concentrations of NaCl (0 mM, 25 mM, 50 mM, and 100 mM). Each pot was filled with 200 g of autoclaved soil and seeds were sown in sets of the following treatments: soil containing non-primed (normal) seeds (C); soil containing seeds primed with B. megaterium (B1), soil containing seeds primed with B. tequilensis (B2), and soil containing seeds primed with P. putida (B3).
Germination Percentage
Seeds were said to be germinated when the 2 mm radical emerged from the seed coat. The germination percentage was recorded by the following formula of Manmathan and Lapitan (2013):
Biochemical and Physiological Parameters of Plants
After 21 days of sowing, following biochemical and physiological parameters were studied.
Growth Attributes
Lengths of freshly harvested shoots and roots of wheat plants were measured with the help of measuring tape. Fresh weights of the plants were measured using electrical balance after uprooting the whole plants. The dried weights of the plants were measured after drying them in an oven at 70 °C for 72 h.
Osmoprotectants
Proline contents of wheat leaves were estimated by the procedure of Bates et al. (1973). The absorbance of obtained mixture was measured at 520 nm, and the amount of proline was determined in μg/g FW. The total soluble sugar contents were assessed by following the protocol of Hahm et al. (2017). The optical density (OD) was measured at a wavelength of 620 nm, and based on the constructed standard curve ranging from 0 to 10 mg of dextrose sugar, the total soluble sugar contents (μg/g FW) were measured.
Photosynthetic Pigments
Fresh leaves (0.1 g) were grinded in 80% acetone and kept in dark for 24 h. For the determination of carotenoid and chlorophyll contents, absorbance of the extracts was measured at 645 nm (for chlorophyll a), 663 nm (for chlorophyll b), and 480 nm (for carotenoids) (Stockburger and Mitchell 1999; Saeidi and Zabihi-e-Mahmoodabad 2009).
Relative Water Content, Relative Electrolytic Leakage, and Salt Tolerance Index
Relative water contents (RWC) of fresh leaves were assessed by measuring the turgid weight of fresh leaves, followed by its drying in hot air oven, till constant dry weight was obtained. RWC were calculated by following the formula of Whetherley (1950). The relative electrolytic leakage (REL) was measured by the standard protocol of Lutts et al. (1996) and salt tolerance index (STI) was calculated by the following formula:
Antioxidant Enzymes Assay
The assessment of Superoxide dismutase (SOD) was performed by the method of Beauchamp and Fridovich (1971). Peroxidase (POD) activity was determined by the method of Vetter et al. (1958), with little modifications (Gorin and Heidema 1976). Catalase activity (CAT) was analyzed by calculating the value of hydrogen peroxide disappearance (Luck 1974).
Oxidative Burst
Malondialdehyde (MDA) activity was measured by the protocol of Yasin et al. (2018) and absorbance was recorded at 532 and 600 nm. The production of H2O2 was assessed by mixing 0.2 g of leaf sample in 5 ml of 0.1% chilled trichloroacetic acid (Loreto and Velikova 2001). The absorbance was measured at a wavelength of 390 nm.
Expression Analysis of Stress-Related Genes
The total RNA of control, salinity-stressed, and PGPR (B. tequilensis)-inoculated wheat plants was extracted by CTAB method (Yu et al. 2017). Quantitative real-time PCR was used for the expression analysis of selected salt stress-related genes (SOS1 and SOS4) using forward and reverse primers (Table 1). Actin was used as a housekeeping gene. Three replicates were taken from each treatment. Protocol of Ho-Kim et al. (2008) was used for the preparation of PCR mixture. Standard thermal cycling conditions were 95 °C for 1 min, followed by 40 cycles of denaturation at 95 °C for 15 s, 57 °C for 15 s, and 72 °C for 45 s.
Statistical Analysis
The experiments were performed in triplicates and their mean and standard error were calculated using Excel 2016. The data were subjected to one-way ANOVA, followed by Tukey’s least significant difference (LSD) method using Statistix version 8.1. Furthermore, principal component analysis (PCA) was performed by XLSTAT 2016 to compare different experimental treatments.
Results
Characterization of Plant Beneficial Traits
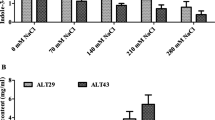
All tested bacterial strains were able to produce ACC deaminase, EPS, and IAA and were also able to solubilize inorganic phosphate, even under salt stress condition (Fig. 1). The production of ACCD by the selected bacterial strains was ranging from 0.52 to 1.83 μM/mg protein/h. Among all tested strains, B. tequilensis was found to be more proficient and showed highest ACCD activity at 0 mM (1.83 μM/mg protein/h) and 25 mM salt concentration (1.70 μM/mg protein/h). In comparison, at 100 mM NaCl concentration, the strain synthesized 0.95 μM/mg protein/h. The range of IAA production by all three selected PGPR was 89.44–79.4 μM/ml. Moreover, B. tequilensis synthesized maximum amount of IAA in tryptophan-supplemented media, under salt stress. The maximum PSI of B. tequilensis MPP8 was observed at 0 mM salt concentration (5.49) followed by 25 mM concentration (5.1) and 100 mM concentration (3.95). Under varying salt concentrations, strain B. tequilensis MPP8 was found to be more efficient in accumulating EPS than B. megaterium and P. putida. Maximum increase in EPS accumulation (1.33 mg/ml) was observed at 100 mM concentration by B. tequilensis.
Analysis of plant growth-promoting properties of B. megaterium (MPP7), B. tequilensis (MPP8), and P. putida (MPP18). Under different salt stress conditions, ACC deaminase activity (a), IAA production (b), phosphate solubilization index (c), and EPS production (d) were observed. Values are described as means and bars denote standard errors. Dissimilar alphabets demonstrate significantly different values (p < 0.05) from each other, as calculated by Tukey’s least significant difference (LSD) test
Salt Tolerance Assay of Selected Bacterial Strains, In Vitro
The growth rate of B. megaterium (MPP7), B. tequilensis (MPP8), and P. putida (MPP18) was successfully observed under different NaCl concentrations (0 mM, 25 mM, 50 mM, and 100 mM) for seven days (Fig. 2). All the selected bacterial strains exhibited variable potential of salt tolerance. Till 5th day of incubation, both species of Bacillus revealed highest growth rate and it declined, thereafter. P. putida showed highest growth till 4th day of incubation, under different concentrations of NaCl.
Growth curve of B. megaterium (MPP7), B. tequilensis (MPP8), and P. putida (MPP18) under different salinity stress levels (0 mM, 25 mM, 50 mM, and 100 mM). Bars denote standard errors. Dissimilar alphabets demonstrate significantly different values (p < 0.05) from each other, as calculated by Tukey’s least significant difference (LSD) test
Germination Percentage
Bacterial strains were successfully inoculated with seed priming and germinated in experimental soil. The texture of the soil was loamy with 6.3 pH and 0.005 ds/m EC. These characteristics declared this soil to be ideal for plant growth. In the bacterial inoculated treatments, the germination rate of seeds was observed to be more than the control. Increasing concentration of salt negatively affected the seed germination. The minimum germination rate was observed in control treatment at 100 mM concentration of salt. Increase in germination rate was observed in bacterial inoculated treatments. The germination rate was in the subsequent order B2 > B1 > B3 > C and the maximum germination percentage was observed in B2 treatment, even at 100 mM salt concentration (Fig. 3).
Influence of various treatments on seed germination. Four different treatments including soil containing non-primed (normal) seeds (C), soil containing seeds primed with B. megaterium (B1), soil containing seeds primed with B. tequilensis (B2), and soil containing seeds primed with P. putida (B3) were used under various concentrations (0–100 mM) of NaCl. Bars denote standard errors. Dissimilar alphabets demonstrate significantly different values (p < 0.05) from each other, as calculated by Tukey’s least significant difference (LSD) test
Biochemical and Physiological Parameters of Plants
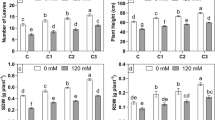
Different biochemical and physiological parameters helped us to understand the response of plants under various treatments. Significant elevations of proline and sugar contents were observed with increasing concentrations of NaCl in all bacterial inoculated treatments (B1, B2, and B3). Among these, B2 treatment resulted in maximum increase of both proline and sugar contents (Fig. 4a).
Effects of different treatments on various biochemical and physiological parameters of PGPR-inoculated wheat seedlings under various levels of salinity stress (0 mM, 25 mM, 50 mM, and 100 mM). Four treatments including soil containing non-primed (normal) seeds (C), soil containing seeds primed with B. megaterium (B1), soil containing seeds primed with B. tequilensis (B2), and soil containing seeds primed with P. putida (B3) were used. Bars denote standard errors. Dissimilar alphabets demonstrate significantly different values (p < 0.05) from each other, as calculated by Tukey’s least significant difference (LSD) test. RWC Relative water content, REL Relative electrolyte leakage
Salinity stress directly affected the growth of wheat seedlings. Among all treatments, the minimum root length and shoot length were observed in control (C treatment) at 100 mM NaCl concentration. The maximum root length and shoot length were observed in B2 treatment, under different concentrations of salt (Fig. 4b). It was obvious from the growth analyses that the inoculation of bacterial strains significantly increased the fresh and dry weight of plants in both stressed and non-stressed conditions (Fig. 4c).
The application of bacterial strains triggered the production of photosynthetic pigments and these were decreased under salinity stress condition. Similar effects of salt stress on carotenoid content were also observed (Fig. 4d).
RWC of wheat plants was reduced significantly with the increasing concentration of NaCl. By the application of PGPR, noticeable increase in RWC under salt stress condition was observed. The maximum RWC was observed in treatment B2, even under higher salt stress conditions (Fig. 4e). Salinity stress significantly increased electrolytic leakage. Priming of seeds with bacterial strains resulted in significant reduction of electrolytic leakage in all treatments (Fig. 4e). The salt tolerance index of wheat plants was significantly reduced under salinity conditions. However, PGPR inoculation increased the salt tolerance index and the maximum salt tolerance was observed in B. tequilensis-treated plants (Fig. 4f).
Antioxidant Enzymes Activity
The production of antioxidant enzymes was significantly increased in PGPR-inoculated plants, under salt stress conditions (Fig. 5). Among all bacterial strains, the highest enzymatic activities were exhibited by B. tequilensis at 100 mM salt stress.
Antioxidant enzymatic activities in PGPR-inoculated wheat seedlings under various levels of salinity stress (0 mM, 25 mM, 50 mM, and 100 mM). Four treatments including soil containing non-primed (normal) seeds (C), soil containing seeds primed with B. megaterium (B1), soil containing seeds primed with B. tequilensis (B2), and soil containing seeds primed with P. putida (B3) were used. Bars denote standard errors. Dissimilar alphabets demonstrate significantly different values (p < 0.05) from each other, as calculated by Tukey’s least significant difference (LSD) test
Oxidative Burst
In the present study, under 100 mM salt stress condition, increased production of MDA and H2O2 was observed in wheat seedlings. Though the inoculation of all PGPR decreased their production, B. tequilensis (B2) was more useful in reducing the levels of MDA and H2O2 (Fig. 6).
Changes in malondialdehyde and hydrogen peroxide production in PGPR-inoculated wheat seedlings under various levels of salinity stress (0 mM, 25 mM, 50 mM, and 100 mM). Four treatments including soil containing non-primed (normal) seeds (C), soil containing seeds primed with B. megaterium (B1), soil containing seeds primed with B. tequilensis (B2), and soil containing seeds primed with P. putida (B3) were used. Bars denote standard errors. Dissimilar alphabets demonstrate significantly different values (p < 0.05) from each other, as calculated by Tukey’s least significant difference (LSD) test
Expression Analysis of Stress-Related Genes
For better understanding of plant defense mechanism, the expression levels of two stress-related genes viz. SOS1 and SOS4 were observed in wheat plants under salinity and PGPR treatments. In this trial, only the most efficient strain (B. tequilensis MPP8) was used. Application of PGPR significantly affected the relative expression of SOS1 and SOS4 genes in the leaves of wheat seedlings (Fig. 7). A higher expression of these genes was observed after salt stress and bacterial inoculation. As compared to control, the application of B. tequilensis enhanced the expression of SOS1 and SOS4 protein genes and depicted their potential role in salinity tolerance.
Relative expression of SOS1 and SOS4 genes in the leaves of wheat plants. Relative expression was observed in plants grown without PGPR and salt stress (C), plants grown under salt stress (NaCl), plants inoculated with halotolerant PGPR (B. tequilensis) and plants grown under salinity stress and inoculated with B. tequilensis (100 mM NaCl + B. tequilensis). Bars denote standard errors. Dissimilar alphabets demonstrate significantly different values (p < 0.05) from each other, as calculated by Tukey’s least significant difference (LSD) test
Principal Component Analysis (PCA)
Principal component analysis (PCA) confirmed the role of PGPR in the growth of wheat plant under different stress conditions (Fig. 8). The biplot among the charted statistics (F1 and F2) showed 83.66% differences (F1 presented about 61.58% and F2 presented about 22.08% differences). Blue color dots are indicating correlation among the different experimental treatments and red dots are representing the correlation among different variables. Positively correlated variables were found to be located near to each other in the same quadrant. Biplot revealed that plant growth attributes, photosynthetic pigments, RWC, and STI are positively correlated with each other and negatively correlated with all other parameters.
Discussion
Plant growth-promoting rhizobacteria help to promote the growth of plants under various biotic and abiotic stresses (Dimkpa et al. 2009). This study has validated the efficiency of rhizobacteria in inducing salinity tolerance by promoting various plant growth-promoting traits.
The selected bacterial strains efficiently produced ACC deaminase. It has been reported earlier that ACC deaminase-producing Bacillus strains efficiently improved the growth of wheat plants under salinity stress (Din et al. 2019). The findings of the current analysis showed an increased production of EPS under salinity stress and suggested the protective role of halotolerant bacterial strains (Li et al. 2017). The auxin-synthesizing bacteria have been previously reported to enhance root growth and uptake of nutrient, which help plants to cope with the salinity stress (Yasin et al. 2018). Various species of Bacillus, Azotobacter, and Pseudomonas have been stated to synthesize auxin (Cassán et al. 2014; Verma et al. 2018). The current study showed that all the tested strains have the ability to produce IAA, indicating their potential use as PGPR. All the selected bacterial strains were able to solubilize phosphate which is an important plant growth-promoting trait. Our results depicted that among all the studied bacterial strains, B. tequilensis produced the highest levels of ACCD, EPS, and IAA. B. tequilensis also exhibited efficient phosphate-solubilizing ability and proved it to be the best PGPR.
Seed priming with PGPR increased the germination percentage and improved various growth attributes of wheat plant. In the current research, both sugar and proline contents were increased in bacterial inoculated wheat seedlings grown under saline conditions. Consequently, the PGPR inoculants enhanced the growth of plant under various levels of salinity stress by improving metabolic resistance mechanisms (Ilangumaran and Smith 2017).
In the current study, salinity stress significantly reduced chlorophyll content of plants, while bacterial inoculation significantly increased chlorophyll a and chlorophyll b contents. Reduction in chlorophyll a and b content is an indication of photo-oxidation (Rahdari et al. 2012). Scientists have previously stated improved production of photosynthetic pigments in bacterial inoculated plants, under salt stress conditions (Sapre et al. 2018). In this study, carotenoid contents were noticeably increased due to bacterial application under stressed and non-stressed conditions. High carotenoid content attributes to genotype tolerance since they are responsible for breakdown of singlet oxygen (Efeoğlu et al. 2009).
Salinity results in osmotic stress by reducing the RLWC (Fahad et al. 2015). Our results showed improved RLWC in plants, treated with PGPR, while RLWC were decreased in diseased plants. In previous studies, substantial decrease in RLWC has been repeated under the stress conditions (Dekov et al. 2000; Nayyar and Gupta 2006). Our findings also revealed relative electrolyte leakage under stress conditions, which might have enhanced POD and CAT activity. Among all treatments, bacterial inoculation diminished the adversity of stress by decreasing electrolytic leakage. Previously, Bacillus sp. has also been reported to decrease electrolytic leakage and imparting membrane stability (Vardharajula et al. 2011). The outcomes of the current analysis revealed that STI of wheat seedlings was significantly reduced in salt stress condition. However, PGPR inoculants showed elevated STI value, correspondingly. Previous studies have also reported increased STI value in Capsicum annum (Yasin et al. 2018).
After the inoculation of PGPR under salinity stress, all three inoculated bacteria showed considerable increase in both root and shoot length and fresh and dry weights of root and shoot. Application of PGPR has been reported to increase root shoot length and overall plant vigor (Farooq and Bano 2013). Karlidag et al. (2013) also reported improved growth attributes due to bacterial inoculation, under salt stress.
In the current study, antioxidant enzymes activities (SOD, POD, and CAT) in wheat plants inoculated with PGPR were significantly increased, when compared with their respective un-inoculated control plants. Our results are supported by the results of Hmaeid et al. (2019), who also reported that the activities of ROS-scavenging enzymes were enhanced in PGPR-inoculated Sulla carnosa, under the influence of salinity stress.
Under the influence of excess salt, plants start producing excessive level of MDA. The results of this study depicted that MDA content was high in plants under the salinity stress. Bacterial inoculation alleviated the stress tolerance potential in wheat plants by decreasing the level of MDA. Our results are similar to the findings of Singh and Jha (2017), who also noted that decrease in malondialdehyde production in S. maltophilia SBP-9 inoculated wheat plants, under salinity stress. Hydrogen peroxide level was increased in the plants under salt stress but bacterial inoculated plants showed low production of hydrogen peroxide. PGPR has been well documented to lower the levels of lipid peroxidation, superoxide anions, and hydrogen peroxide and stimulate defense mechanisms (Gupta et al. 2017).
The SOS protein family is clearly shown to be able to mediate salt tolerance directly and indirectly (Ramezani et al. 2013). The findings of the current research showed high expression of SOS genes in the plants inoculated with halotolerant B. tequilensis, under salinity stress and described their potential role in salinity tolerance. It has previously been reported that the rice SOS genes play a significant role in the adaptive mechanism to salinity tolerance (El Mahi et al. 2019).
Conclusion
This is the first comprehensive study in which three halotolerant ACCD- and EPS-producing PGPRs including Bacillus megaterium, B. tequilensis, and Pseudomonas putida have been studied on wheat seedlings, simultaneously. These bacterial inoculants showed ability to solubilize phosphate and produce indole-3-acetic acid to maintain plant growth. Bacterial inoculation increased salinity stress tolerance in wheat by increasing expression levels of ROS-scavenging enzymes. Higher expression of SOS1 and SOS4 genes also predicted their potential role in stress tolerance. These genes can be further overexpressed in wheat plant to tolerate salinity stress. All these results make this study very reliable and novel and provide an environmental solution to salinity stress.
References
Ashraf M (2009) Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol Adv 27(1):84–93
Bano A, Fatima M (2009) Salt tolerance in Zea mays (L). following inoculation with rhizobium and pseudomonas. Biol Fert Soils 45:405–413
Barra PJ, Inostroza NG, Acuña JJ, Mora ML, Crowley DE, Jorquera MA (2016) Formulation of bacterial consortia from avocado (Persea americana Mill.) and their effect on growth, biomass and superoxide dismutase activity of wheat seedlings under salt stress. App Soil Ecol 102:80–91
Bashan Y, Holguin G, De-Bashan LE (2004) Azospirillum-plant relationships: physiological, molecular, agricultural, and environmental advances (1997–2003). Can J Microbiol 50(8):521–577
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(1):276–287
Cassán F, Vanderleyden J, Spaepen S (2014) Physiological and agronomical aspects of phytohormone production by model plant-growth-promoting rhizobacteria (PGPR) belonging to the genus Azospirillum. J Plant Growth Regul 33(2):440–459
Dahmani MA, Desrut A, Moumen B, Verdon J, Mermouri L, Kacem M, Coutos-Thévenot P, Kaid-Harche M, Bergès T, Vriet C (2020) Unearthing the plant growth-promoting traits of Bacillus megaterium RmBm31, an endophytic bacterium isolated from root nodules of Retama monosperma. Front Plant Sci 11:124
Deinlein U, Stephan AB, Horie T, Luo W, Xu G, Schroeder JI (2014) Plant salt-tolerance mechanisms. Trends Plant Sci 19(6):371–379
Dekov I, Tsonev T, Yordanov I (2000) Effects of water stress and high-temperature stress on the structure and activity of photosynthetic apparatus of Zea mays and Helianthus annuus. Photosynthetica 38(3):361–366
Dimkpa C, Weinand T, Asch F (2009) Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ 32(12):1682–1694
Din BU, Sarfraz S, Xia Y, Kamran MA, Javed MT, Sultan T, Chaudhary HJ (2019) Mechanistic elucidation of germination potential and growth of wheat inoculated with exopolysaccharide and ACC-deaminase producing bacillus strains under induced salinity stress. Ecotoxicol Environ Saf 183:109466
Efeoğlu B, Ekmekci Y, Cicek N (2009) Physiological responses of three maize cultivars to drought stress and recovery. S Afr J Bot 75(1):34–42
El Mahi H, Pérez-Hormaeche J, De Luca A, Villalta I, Espartero J, Gámez-Arjona F, Quintero FJ (2019) A critical role of sodium flux via the plasma membrane Na+/H+ exchanger SOS1 in the salt tolerance of rice. Plant Physiol 180(2):1046–1065
Etesami H, Alikhani HA, Hosseini HM (2015) Indole-3-acetic acid (IAA) production trait, a useful screening to select endophytic and rhizosphere competent bacteria for rice growth promoting agents. MethodsX 2:72–78
Fahad S, Hussai S, Matloob A, Khan FA, Khaliq A, Saud S, Faiq M (2015) Phytohormones and plant responses to salinity stress: a review. Plant Growth Regul 75(2):391–404
Farooq U, Bano A (2013) Screening of indigenous bacteria from rhizosphere of maize (Zea mays L.) for their plant growth promotion ability and antagonism against fungal and bacterial pathogens. J Anim Plant Sci 23:1642–1652
Fischer SE, Fischer SI, Magris S, Mori GB (2007) Isolation and characterization of bacteria from the rhizosphere of wheat. World J Microbiol Biotechnol 23(7):895–903
Ghassemi-Golezani K, Farhangi-Abriz S (2019) Biochar alleviates fluoride toxicity and oxidative stress in safflower (Carthamus Tinctorius L.) seedlings. Chemosphere 223:406–415
Gordon SA, Weber RP (1951) Colorimetric estimation of indoleacetic acid. Plant Physiol 26(1):192
Gorin N, Heidema FT (1976) Peroxidase activity in golden delicious apples as a possible parameter of ripening and senescence. J Agric Food Chem 24(1):200–201
Gupta R, Singh A, Ajayakumar PV, Pandey R (2017) Microbial interference mitigates Meloidogyne incognita mediated oxidative stress and augments bacoside content in Bacopa monnieri L. Microbiol Res 199:67–78
Gururani MA, Upadhyaya CP, Baskar V, Venkatesh J, Nookaraju A, Park SW (2013) Plant growth-promoting rhizobacteria enhance abiotic stress tolerance in Solanum tuberosum through inducing changes in the expression of ROS-scavenging enzymes and improved photosynthetic performance. J Plant Growth Regul 32(2):245–258
Hahm MS, Son JS, Hwang YJ, Kwon DK, Ghim SY (2017) Alleviation of salt stress in pepper (Capsicum annum L.) plants by plant growth-promoting rhizobacteria. J Microbiol Biotechnol 27(10):1790–1797
Hessini K, Issaoui K, Ferchichi S, Saif T, Abdelly C, Siddique KH, Cruz C (2019) Interactive effects of salinity and nitrogen forms on plant growth, photosynthesis and osmotic adjustment in maize. Plant Physiol Biochem 139:171–178
Hmaeid N, Wali M, Mahmoud OMB, Pueyo JJ, Ghnaya T, Abdelly C (2019) Efficient rhizobacteria promote growth and alleviate NaCl-induced stress in the plant species Sulla carnosa. Appl Soil Ecol 133:104–113
Hmidi D, Abdelly C, Ashraf M, Messedi D (2018) Effect of salinity on osmotic adjustment, proline accumulation and possible role of ornithine-δ-aminotransferase in proline biosynthesis in Cakile maritima. Physiol Mol Biol Plants 24:1017–1033
Ho-Kim Y, Yang I, Bae YS, Park SR (2008) Performance evaluation of thermal cyclers for PCR in a rapid cycling condition. Biotechniques 44(4):495–505
Ilangumaran G, Smith DL (2017) Plant growth promoting rhizobacteria in amelioration of salinity stress: a systems biology perspective. Front Plant Sci 8:1768
Jha Y, Subramanian RB, Patel S (2011) Combination of endophytic and rhizospheric plant growth promoting rhizobacteria in Oryza sativa shows higher accumulation of osmoprotectant against saline stress. Acta Physiol Plant 33:797–802
Kang SM, Khan AL, Waqas M, Asaf S, Lee KE, Park YG, Kim AY, Khan MA, You YH, Lee IJ (2019) Integrated phytohormone production by the plant growth-promoting rhizobacterium Bacillus tequilensis SSB07 induced thermotolerance in soybean. J Plant Interact 14(1):416–423
Karlidag H, Yildirim E, Turan M, Pehluvan M, Donmez F (2013) Plant growth-promoting rhizobacteria mitigate deleterious effects of salt stress on strawberry plants (Fragaria×ananassa). Hort Sci 48(5):563–567
Kumar V, Singh P, Jorquera MA, Sangwan P, Kumar P, Verma AK, Agrawal S (2013) Isolation of phytase-producing bacteria from Himalayan soils and their effect on growth and phosphorus uptake of Indian mustard (Brassica juncea). World J Microbiol Biotechnol 29(8):1361–1369
Kumar A, Bahadur I, Maurya BR, Raghuwanshi R, Meena VS, Singh DK, Dixit J (2015) Does a plant growth-promoting rhizobacteria enhance agricultural sustainability. J Pure App Microbiol 9(1):715–724
Kumar M, Mishra S, Dixit V, Kumar M, Agarwal L, Chauhan PS, Nautiyal CS (2016) Synergistic effect of Pseudomonas putida and Bacillus amyloliquefaciens ameliorates drought stress in chickpea (Cicer arietinum L.). Plant Signal Behav 11(1):e1071004
Lakhdar A, Rabhi M, Ghnaya T, Montemurro F, Jedidi N, Abdelly C (2009) Effectiveness of compost use in salt affected soil. J Hazard Mater 171:29–37
Li H, Lei P, Pang X, Li S, Xu H, Xu Z, Feng X (2017) Enhanced tolerance to salt stress in canola (Brassica napus L.) seedlings inoculated with the halotolerant Enterobacter cloacae HSNJ4. Appl Soil Ecol 119:26–34
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127(4):1781–1787
Luck H (1974) Estimation of catalase, methods in enzymatic analysis. Academic Press, New York
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Ann Rev Microbiol 63:541–556
Lutts S, Kinet JM, Bouharmont J (1996) NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78(3):389–398
Mahajan S, Pandey GK, Tuteja N (2008) Calcium-and salt-stress signaling in plants: shedding light on SOS pathway. Arch Biochem Biophys 471(2):146–158
Manmathan H, Lapitan NL (2013) Measuring germination percentage in wheat. Bio-protocol 3(16):e866
Meena RK, Singh RK, Singh N, Meena SK, Meena VS (2015) Isolation of low temperature surviving plant growth—promoting rhizobacteria (PGPR) from pea (Pisum sativum L.) and documentation of their plant growth promoting traits. Biocatal Agric Biotechnol 4:806–811
Miller G, Shulaev V, Mitter R (2008) Reactive oxygen signaling and abiotic stress. Physiol Plant 133:481–489
Mishra P, Bhoomika K, Dubey RS (2013) Differential responses of antioxidative defense system to prolonged salinity stress in salt-tolerant and salt-sensitive indica rice (Oryza sativa L.) seedlings. Protoplasma 250:3–19
Munns R, Gilliham M (2015) Salinity tolerance of crops what is the cost? New Phytol 208:668–673
Naseem H, Bano A (2014) Role of plant growth promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J Plant Interact 9:689–701
Naseem H, Ahsan M, Shahid MA, Khan N (2018) Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. J Basic Microbiol 58(12):1009–1022
Nayyar H, Gupta D (2006) Differential sensitivity of C3 and C4 plants to water deficit stress: association with oxidative stress and antioxidants. Environ Exp Bot 58(1–3):106–113
Nxele X, Klein A, Ndimba BK (2017) Drought and salinity stress alter ROS accumulation, water retention, and osmolyte content in sorghum plants. S Afr J Bot 108:261–266
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant 118(1):10–15
Pikovskaya RI (1948) Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 17:362–370
Rahdari P, Tavakoli S, Hosseini SM (2012) Studying of salinity stress effect on germination, proline, sugar, protein, lipid and chlorophyll content in purslane (Portulaca oleracea L.) leaves. J Stress Physiol Biochem 8(1):182–193
Ramezani A, Niazi A, Abolimoghadam AA, Babgohari MZ, Deihimi T, Ebrahimi M, Akhtardanesh H, Ebrahimie E (2013) Quantitative expression analysis of TaSOS1 and TaSOS4 genes in cultivated and wild wheat plants under salt stress. Mol Biotechnol 53(2):189–197
Ruan CJ, da Silva JAT, Mopper S, Qin P, Lutts S (2010) Halophyte improvement for a salinized world. Crit Rev Plant Sci 29:329–359
Saeidi M, Zabihi-e-Mahmoodabad R (2009) Evaluation of drought stress on relative water content and chlorophyll content of sesame (Sesamum indicum L.) genotypes at early flowering stage. Res J Environ Sci 3:345–350
Sapre S, Gontia-Mishra I, Tiwari S (2018) Klebsiella sp. confers enhanced tolerance to salinity and plant growth promotion in oat seedlings (Avena sativa). Microbiol Res 206:25–32
Shi H, Quintero FJ, Pardo JM, Zhu JK (2002) The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 14(2):465–477
Shrivastava P, Kumar R (2015) Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22(2):123–131
Singh RP, Jha PN (2017) The PGPR Stenotrophomonas maltophilia SBP-9 augments resistance against biotic and abiotic stress in wheat plants. Front Microbiol 8:19–45
Stockburger KZ, Mitchell AK (1999) Photosynthetic pigments: a bibliography, vol 383. Pacific Forestry Centre, Victoria
Upadhyay SK, Singh JS, Saxena AK, Singh DP (2012) Impact of PGPR inoculation on growth and antioxidant status of wheat under saline conditions. Plant Biol 14(4):605–611
Vardharajula S, Zulfikar AS, Grover M, Reddy G, Bandi V (2011) Drought-tolerant plant growth promoting Bacillus spp.: effect on growth, osmolytes, and antioxidant status of maize under drought stress. J Plant Interact 6(1):1–14
Verma JP, Jaiswal DK, Krishna R, Prakash S, Yadav J, Singh V (2018) Characterization and screening of thermophilic Bacillus strains for developing plant growth promoting consortium from hot spring of Leh and Ladakh region of India. Front Microbiol 9:1293
Vetter JL, Steinberg MP, Nelson AI (1958) Enzyme assay, quantitative determination of peroxidase in sweet corn. J Agric Food Chem 6(1):39–41
Whetherley PE (1950) Studies in the water relations of cotton plants. I. The field measurement of water deficit in leaves. New Phytol 49:81–87
Yasin NA, Akram W, Khan WU, Ahmad SR, Ahmad A, Ali A (2018) Halotolerant plant-growth promoting rhizobacteria modulate gene expression and osmolyte production to improve salinity tolerance and growth in Capsicum annum L. Environ Sci Pollut Res 25(23):23236–23250
Yu G, Hatta A, Periyannan S, Lagudah E, Wulff BB (2017) Isolation of wheat genomic DNA for gene mapping and cloning. In: Periyannan Sambasivam (ed) Wheat rust diseases. Humana Press, New York, pp 207–213
Zainab N, Din BU, Javed MT, Afridi MS, Mukhtar T, Kamran MA, Chaudhary HJ (2020) Deciphering metal toxicity responses of flax (Linum usitatissimum L.) with exopolysaccharide and ACC-deaminase producing bacteria in industrially contaminated soils. Plant Physiol Biochem 152:90–99
Zhang S, Fan C, Wang Y, Xia Y, Xiao W, Cui X (2018) Salt-tolerant and plant-growth-promoting bacteria isolated from high-yield paddy soil. Can J Microbiol 64(12):968–978
Zhu JK (2000) Genetic analysis of plant salt tolerance using Arabidopsis. Plant Physiol 124(3):941–948
Acknowledgements
We are grateful to National Agricultural Research Centre (NARC), Islamabad, Pakistan, for providing wheat seeds.
Funding
This study was financially supported by university research fund (URF 2019-20), Quaid-i-Azam University Islamabad.
Author information
Authors and Affiliations
Contributions
UH executed the experiments and wrote the manuscript; MFHM designed the experiments, supervised the work, read and approved the final manuscript; FL and MK did initial characterization of bacterial strains; MA, AK, and HJC did data analysis; KT and SSB did statistical analysis; MA revised manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Axel Mithofer.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Haroon, U., Khizar, M., Liaquat, F. et al. Halotolerant Plant Growth-Promoting Rhizobacteria Induce Salinity Tolerance in Wheat by Enhancing the Expression of SOS Genes. J Plant Growth Regul 41, 2435–2448 (2022). https://doi.org/10.1007/s00344-021-10457-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-021-10457-5