Abstract
Some rhizobacteria have demonstrated a noteworthy role in regulation of plant growth and biomass production under biotic and abiotic stresses. The present study was intended to explicate the ameliorative consequences of halotolerant plant growth-promoting rhizobacteria (HPGPR) on growth of capsicum plants subjected to salt stress. Salt stress was ascertained by supplementing 1 and 2 g NaCl kg−1 soil. The HPGPR positively invigorated growth attributes, chlorophyll, protein contents, and water use efficiency (WUE) of supplemented capsicum plants under salinity stress conditions. Bacillus fortis strain SSB21 caused highest significant increase in shoot length, root length, and fresh and dry biomass production of capsicum plants grown under saline conditions. This multi-trait bacterium also increased biosynthesis of proline and up-regulated the expression profiles of stress related genes including CAPIP2, CaKR1, CaOSM1, and CAChi2. On the other hand, B. fortis strain SSB21 inoculated plants exhibited reduced level of ethylene, lipid peroxidation, and reactive oxygen species (ROS). All these together contribute to activate physiological and biochemical processes involved in the mitigation of the salinity induced stress in capsicum plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The salinity is gradually affecting the global agricultural area every day and it is anticipated that at present soil salinity has affected over 800 million ha area. Therefore, it is indispensable to formulate appropriate stratagem to remediate lands for successful crop production. In spite of a number of modern management techniques, the salinity has been affecting millions of acres of agricultural area causing restricted crop choice and abridged yield. A minute quantity of ROS including hydrogen peroxide (H2O2), superoxide anion radical (O2−), and hydroxyl radical (OH−) are generated during normal physiological activities of plants. Plants genes encode antioxidant enzymes including superoxide dismutases, ascorbate peroxidases, catalases, and peroxidases. These enzymes in conjunction with some other metabolic and antioxidant systems are implicated in the regularization and scavenging of ROS and succeeding fortitude of the plants growing under trivial stress (Mittler 2002). Conversely, the elevated salt stress enhances O2− and H2O2 at very higher levels, detrimental to subjected plants causing oxidative damage (Yasin et al. 2017a). This elevated ROS level induce oxidative injury of membranes, lipids, proteins, DNA, and chlorophyll, leading to a progression of harmful metabolic activities and poor growth in affected plants (Khan et al. 2017a). Saline soils usually have a higher amount of sodium salts. The higher sodium contents in plant tissues impede phosphorylating and non-phosphorylating electron transport pathways in juxtaposition with reduced respiratory and photosynthetic activity (Jacoby et al. 2016). In general, it is a difficult task to raise profitable agronomic and horticultural crops in salt affected zones.
Luckily, the cross talk between plants and microbes stimulate multifaceted mechanisms which trigger stress tolerance in plants (Smith et al. 2017). The plant growth-promoting rhizobacteria (PGPR) elicit plant growth by means of direct or indirect mechanism. This has attracted researchers to develop biotechnological approaches involving the use of HPGPR as a bio-resource to alleviate salinity stress and improve growth of stressed plants. As a result, the application of PGPR in saline area agriculture has increased to accomplish the expedient effect of these microbes on stress mitigation, growth, and yield of inoculated crops (Ilangumaran and Smith 2017). The Bacillus megaterium BOFC15 improved stress tolerance in plants by manipulating abscisic acid-related stress response and root system architecture, which in return regulates stomatal conductance and plant water relation (Zhou et al. 2016). The Pseudomonas fluorescens isolate obtained from rhizospheric area of date palm was quite capable to mitigate salinity stress and improve root growth of Zea mays plants (Zerrouk et al. 2016). The halotolerant Serratia sp. Sl-12 supplemented Triticum aestivum plants exhibited enhanced salinity tolerance and shoot growth (Singh and Jha 2016). Likewise, T. aestivum seeds primed with Bacillus aquimaris reduced sodium contents, improved assimilation of total soluble sugars, and enhanced nutrient uptake and growth and biomass production in plants under saline soil (Upadhyay and Singh 2015). Enterobacter sp. EJ01-assisted salinized tomato plants exhibited reduced oxidative stress due to accelerated activity of ascorbate peroxidase (Kim et al. 2014), while the inoculation of Enterobacter sp. UPMR18 increased activity of ROS-scavenging enzymes and up-regulated the expression of ROS-pathway genes resulting improved salt tolerance and growth in Abelmoschus esculentus plants (Habib et al. 2016). The Pantoea dispersa (PSB3) inoculated chickpea plants showed reduced electrolyte leakage and Na+ contents. Conversely, inoculated plants demonstrated higher chlorophyll and relative leaf water and K+ contents under saline conditions (Panwar et al. 2016).
Growth-promoting bacteria can modulate expression of genes profile to alleviate plant stress. Bacillus amyloliquefaciens NBRISN13 manifested up-regulation of genes involved in ionic and osmotic stress tolerance of rice plants growing in saline conditions (Nautiyal et al. 2013). In another study, Bacillus subtilis GB03 inoculation decreased Na+ uptake in Puccinellia tenuiflora plants by modulating PtHKT2 genes in plants roots (Niu et al. 2016). Likewise, B. amyloliquefaciens SQR9 inoculation mitigated salt stress in maize plants by modulating expressions of genes encoding RuBisCo subunits, H+ pumping pyrophosphatase, and 9-cis-epoxycarotenoid dioxygenase (Chen et al. 2016). Similarly, higher proline synthesis and up-regulated expression of stress responsive genes improved salt stress amelioration in Dietzia natronolimnaea inoculated wheat plants (Bharti et al. 2016).
Capsicum (Capsicum annum Mill.) is an imperative medicinal and economical vegetable. Nevertheless, most of the capsicum-growing areas have become salt affected due to frequent use of pesticides and fertilizers mandatory for the cultivation of this crop (Hahm et al. 2017). In the current research, halotolerant rhizobacterial strains were isolated and their beneficial effect on growth of capsicum plants under high salt stress was elucidated. It was established that these native halotolerant rhizobacterial strains have enormous potential to be exploited for the successful capsicum production in saline areas.
Methodology
Isolation of rhizobacterial strains
Soil samples obtained from rhizospheric area of vigorous capsicum plants, growing in salt-affected areas, were used for isolation of halotolerant rhizobacteria. The soil sample (10 g) was thoroughly mixed with 90 ml deionized water and placed at 32 °C for 1 h. The soil suspension was serially diluted up to 107. An aliquot of this suspension was stretched on LB broth medium plates supplemented with NaCl (20%). The treated plates were kept at 30 °C for 24 h. The prominent bacterial colonies were isolated and re-streaked in fresh LB broth medium plates and incubated in the same way. This process was repeated three times to attain pure rhizobacterial colony. The pure rhizobacterial colony was once again inoculated on 20% NaCl contaminated LB broth media and kept at 32 °C for 3 days. The optical density (OD) of rhizobacterial culture was measured and those showing OD > 1 were regarded halotolerant. The screened halotolerant rhizobacteria were used for downstream experiments.
Evaluation of halotolerant rhizobacteria for mitigation of salt stress
A greenhouse experiment was conducted at Main Nursery, University of the Punjab, Lahore. The capsicum seeds were sterilized by immersing in 10% Clorox for 10 min followed by rinsing thrice with sterile deionized water. These seeds were air dried and five capsicum seeds were sown in each allocated pot (15 × 7 cm) containing autoclaved sandy loam soil. The physicochemical properties of soil have been demonstrated in Table 1. The greenhouse had natural light, 68–87% relative humidity, and 24–29 °C/18–23 °C (day/night temperature). The treated pots were uniformly watered at an interval of 24 h. Pots were fertilized every seventh day by applying half-strength Hoagland’s solution. The salt stress was induced through application of 1 and 2 g NaCl kg−1 soil in selected pots. One hundred milliliters of aqueous rhizobacterial cell suspension (108 cells ml−1) was used as bacterial inoculum (Ahmad et al. 2013). Distilled sterile water was provided to control plants as a replacement of rhizobacterial cell suspension. The plants were harvested after 50 days post-inoculation to evaluate the effect of different treatments.
Bioassays for existence of plant growth promoting attributes
The halotolerant rhizobacterial strains were assessed for the presence of growth-promoting attributes. The configuration of α-ketobutyrate (α-KB) produced through the enzymatic cleavage of 1-aminocyclopropane-1-carboxylate (ACC) was determined (Honma and Shimomura 1978) to evaluate the 1-aminocyclopropane-1-carboxylate deaminase (ACCD) activity of rhizobacterial cell extracts. The 12-h-old rhizobacterial culture (50 μl) was inoculated in 10 ml of nitrogen-free bromothymol blue malate (NFb) medium plates in the absence and presence of 0.05% l-tryptophan. The inoculated plates were placed on rotary shaker at 180 rpm for 2 days at 30 °C. Afterwards, the rhizobacterial culture was centrifuged for 5 min at 13,000 rpm. The aliquot of resulting supernatant (1 ml) was vortexed with Salkowski’s reagent (2 ml) and kept in the dark for 20 min at 25 °C. The development of pink color exhibited indole-3-acetic acid (IAA) production capability of bacterial isolate (Patten and Glick 2002).
The capability of rhizobacteria to synthesize siderophores was qualitatively assessed by growing it in chrome azurol S agar medium according to Schwyn and Neilands (1987). The rhizobacteria (10 μl) was spot inoculated on chrome azurol S agar plates. These plates were placed in shade for 5 days at 30 °C. The development of orange halos surrounding rhizobacterial colonies confirmed siderophore production. For determination of phosphate solubilization, a loopful of rhizobacterial isolate was inoculated on Pikovskaya’s agar (Pikovskaya 1948) medium plates supplemented with calcium phosphate as the inorganic form of phosphate. The plates were kept at 32 °C for 5 days. The development of clear halos surrounding rhizobacterial colony demonstrated the phosphate-solubilizing potential of microbes.
Molecular identification of screened halotolerant rhizobacteria
The HPGPR which exhibited maximum plant growth potential were screened and grown in LB broth medium and their DNA was extorted by following instructions of DNA extraction kit (Enzynomics, Korea). The rhizobacterial 16S rRNA genes were amplified by routine PCR using universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) according to Yu et al. (2014). The PCR was performed by using 2× nTaq ready mixes (Enzynomics, Korea) while Qiaquick PCR purification kit (Qiagen, Germany) was used for the purification of amplified products. The rhizobacterial gene sequence was forwarded to database of GenBank (NCBI) to attain accession number. The gene sequences were subjected to BLAST analysis to make the alignment speed expedient for the similarity of genomic sequences. The rhizobacterial gene and related sequences recovered from NCBI were aligned with the help of Clustal W program. The UPGMA method in association of genomic workbench was applied for the development of phylogenetic tree.
Evaluation of lipid peroxidation, proline, and H2O2 contents
The leaf sample (0.2 g) was vortexed in 0.1% trichloroacetic acid (10 ml) and centrifuged at 2000 rpm for 10 min. In 1 ml of resulting supernatant, 4 ml of 0.5% thiobarbituric acid in 20% trichloroacetic acid was mixed. This solution was placed over water bath at 95 °C for 0.5 h followed by quick cooling by ice bath prior to centrifugation at 2000 rpm for 10 min. The spectrophotometric absorbance of supernatant was observed at 532 and 600 nm. From absorbance values, the lipid peroxidation values in term of malondialdehyde (MDA) were estimated according to Heath and Packer (1968).
Proline contents were estimated by the modified method of Bates et al. (1973). Fresh leaf sample (50 mg) was homogenized with 40% methanol (2 ml). From this homogenate, 1 ml was vortexed with 1 ml of solution containing 25 mg ninhydrin, 6 M glacial acetic acid, and orthophosphoric acid in the ratio of 3:2 (v/v) and placed at 100 °C for 1 h. After cooling, 5 ml toluene was mixed and absorbance was determined at 528 nm and compared with standard curve.
For assessment of H2O2 contents, small pieces obtained from fresh leaf sample (0.2 g) were vortexed in 5 ml of 0.1% (w/v) ice-cold TCA (Loreto and Velikova 2001). The solution was centrifuged at 4 °C for 15 min at 12,000 rpm and supernatant (0.5 ml) was added to equal volume of 10 mM potassium phosphate buffer and 1 ml of 1 M potassium iodide at normal pH. The absorbance of solution was measured at 390 nm and compared with standard curve of H2O2.
Assessment of stomatal conductance, transpiration rate, photosynthetic rate, and transient water use efficiency
The portable photosynthesis system (LI-COR, Lincoln-Nebraska, USA) was used to determine stomatal conductance, transpiration rate (Tr), and photosynthesis rate (Pr) from capsicum leaves at 10 am. The transient leaf WUE was measured according to Liu et al. (2017) from the following equation:
Electrolyte leakage and leaf relative water content
The electrolyte leakage level from cell membranes is usually deemed to be a pivotal indicator of membrane injury. Leaf membrane damage caused by salt stress was estimated by measuring electrolyte leakage. For this purpose, leaf discs of uniform size were placed in clean glass tubes containing deionized water (25 ml). The glass tubes were shaken for 10 s and the initial electrical conductivity (EC0) of the mixture was calculated. The test tubes were then incubated for 12 h at 4 °C to evaluate EC1. Lastly, the glass tubes were autoclaved for 20 min at 121 °C to estimate EC2, and electrolyte leakage was measured according to Yang et al. (1996) from the following equation:
The pre-weighed full-sized fresh leaves were immersed in deionized water at 10 °C for 1 day. The leave samples were then wrapped in blotting paper for 2 min and their turgid weight was measured. Afterwards, leaves were kept in oven for 1 day at 72 °C and subsequently the dry weight was measured to estimate leaf relative water content (LRWC) as described by Smart and Bingham (1974) from the following equation:
where FW = fresh weight of leaf, DW = dry weight of leaf, and TW = turgid weight of leaf.
Quantification of photosynthetic pigments
The total chlorophyll (Chl), chlorophyll a (Chl a), and chlorophyll b (Chl b) contents from fresh capsicum leaves were measured according to Arnon (1949). The 0.5 g fresh leaf sample was immersed in acetone (80%) and placed on a rotary shaker. After complete bleaching of leaf sample, the solution was centrifuged for 10 min at 13,000 rpm. The spectrophtometric absorbance of Chl a from supernatant was measured at 663 nm and Chl b at 645 nm.
Salt tolerance index
The total weight of these harvested plants was termed as fresh weight. For determination of biomass production, the pre-weighted plant was oven dried for 2 days at 72 °C. The salt tolerance index (STI) was analyzed as described by Shetty et al. (1995) from the following equation:
where BPS = biomass of plant under salt stress, BPI = biomass of inoculated plant, and BPN = biomass of non-stressed/un-inoculated plants.
Analysis of changes in ethylene production
The ethylene produced by capsicum plants treated with two best bacterial performers, viz., Pseudomonas aeruginosa SSB13 and Bacillus fortis SSB21 was evaluated by keeping freshly removed root pieces into 1 ml of water placed in falcon tubes. These tubes were immediately covered with a gas-proof septum (Wu et al. 2011). The covered tubes were placed in dark at 30 °C for 4 h. From falcon tube, 1 ml gas was withdrawn with the help of Hamilton gastight syringe and injected into a gas chromatograph for estimation of ethylene on the basis of fresh weight of root samples.
Analysis of total soluble protein and expression of stress-related genes
Another identical greenhouse experiment was performed to denote changes in quantification of total soluble protein and expression levels of some stress-related genes in salinized capsicum plants under influence of rhizobacterial inducers. However, during this experiment, only best-performing strain “B. fortis SSB21” was used to evaluate changes in expression of stress-related genes. For protein estimation, 1 g leaf sample was extracted with 10 ml cold phosphate buffer saline at pH 7.4 (Sambrook and Russell 2001). The solution was centrifuged for 0.5 h at 14,000 rpm. The total soluble protein was estimated according to Bradford by using bovine serum albumin as standard. Leaf samples from inoculated plants were taken after 1 week. From leaf samples, RNA was extracted with the help of Tri reagent (Enzynomics, Korea) according to provided methodology. The qRT-PCR was performed with in Bio-Rad RT PCR system (Bio-Rad, USA). The PCR replications were performed by using SYBR Green-based one-step RT-PCR kit (Enzynomics, Korea) as described by Guo et al. (2012). Primers’ detail is provided in Table 2.
Determination of potassium and sodium ions
The oven-dried plant sample (100 mg) was digested with the help of nitric acid (2 ml). The Na+ and K+ ions were quantified by employing flame photometer Holiday and Preedy (1953).
Statistical analysis
Each experiment was performed and repeated in triplicate. The data obtained were statistically analyzed by ANOVA (analysis type according to the factors affecting the experiment) and differences among mean values were compared by DMRT at P < 0.05 using DSAASTAT software (Onofri Italy).
Results
Screening of halotolerant rhizobacteria
The salt-tolerant rhizobacterial strains were capable to grow in 20% NaCl-contaminated LB broth media. A total of nine rhizobacterial strains, exhibiting OD of > 1 at 600 nm, were regarded as halotolerant (Table 3).
Bioassay of halotolerant rhizobacteria for growth-promoting traits
The salt-tolerant rhizobacterial strains were analyzed for presence of plant growth-promoting attributes (Table 4). Four bacterial strains showed auxin synthesis capability by developing pink color in specific media (Table 4). The rhizobacterial strains SSB07, SSB13, SSB14, and SSB21 accomplished siderophore synthesis (Table 4). Five rhizobacterial strains were capable to solubilize phosphorus (Table 4). The rhizobacterial strains SSB03, SSB07, SSB19, and SSB26 exhibited ACCD activity (Table 4).
Evaluation of halotolerant rhizobacteria to mitigate salt stress
Among these halotolerant rhizobacterial strains, higher growth in capsicum plants was induced by strains SSB13 and SSB21 under salt stress (2 g NaCl kg−1 soil) conditions (Table 5). The shoot length was increased by 49.03 and 37.18% in case of SSB21 and SSB13 application, respectively, to the salinized (2 g NaCl kg−1 soil) capsicum plants as compared with respective control (Table 5). In the same way, root length was increased up to 87.3 and 69.8% in salt-stressed (2 g NaCl kg−1 soil) capsicum plants under the influence of SSB21 and SSB13, respectively, as compared to control plants grown under similar saline conditions. Correspondingly, for strains SSB21 and SSB13, capsicum plants showed higher fresh and dry weight and chlorophyll contents (Tables 5 and 8). The SSB21 significantly increased fresh and dry weight by 76.2 and 63.8%, respectively, as compared to salinized (2 g NaCl kg−1 soil) control plants (Table 6). In the same way, these two strains induced similar up-regulation in total chlorophyll contents of capsicum plants growing in salt-supplemented and control media (Table 8).
Identification of screened halotolerant growth-promoting rhizobacterial strains
The rhizobacterial strains SSB21 and SSB13 which demonstrated best stress alleviation results were identified as B. fortis SSB21 and P. aeruginosa SSB13. The genetic sequences of these microbes were submitted to NCBI under accession numbers MG563939 and MG563940, respectively. The B. fortis SSB21 strain showed 98% homology with B. fortis E5.Pb5 and 87% resemblance with already existing B. fortis RB12 (Fig. 1). Test strain P. aeruginosa SSB13 exhibited 100% similarity with P. aeruginosa SM9 (Fig. 1).
Determination of gas exchange parameters and water use efficiency
During the current study, the capsicum plants subjected to salinity stress demonstrated reduction in gas exchange attributes and water use efficiency as compared to the corresponding controls (Table 6). The photosynthetic rate, transpiration rate, and stomatal conductance decreased up to 27, 15, and 25%, respectively, in the capsicum plants under salt stress as compared to the control treatments. However, bacterial inoculations improved the gas exchange traits in capsicum plants grown under normal and saline conditions. The B. fortis SSB21-assisted plants raised under salt stress exhibited 25, 9, and 39% higher photosynthetic rate, transpiration rate, and stomatal conductance, respectively, compared to that of the analogous un-inoculated ones. On the other hand, the value of WUE also improved in bacterized plants. The B. fortis SSB21- and P. aeruginosa SSB13-inoculated plants grown under salt stress showed 15 and 7% higher values of WUE compared with the respective un-inoculated plants (Table 6).
Changes in ethylene level
Salinity stress induced capsicum plants for increased endogenous biosynthesis of ethylene. B. fortis SSB21 significantly lowered ethylene contents (32.9%) in capsicum plants subjected to salt stress in contrast to salinized control plants. Salinity stress alone increased production of ethylene by 57.9% as compared to control plants (Fig. 2). P. aeruginosa SSB13 inoculated C. annum plants treated with 2 g NaCl kg−1 soil exhibited 19.7% less ethylene contents as compared with salinized control plants.
Analysis of protein and expression of stress-related gene
The plants under stress synthesized higher protein contents as compared to un-inoculated control. The quantity of soluble protein was further enhanced by bacterial inoculation (Fig. 3). The bacterized plants under salt stress exhibited higher protein contents as compared to plants without salt stress. The B. fortis SSB21-inoculated capsicum plants raised under salt stress demonstrated 8% higher protein contents in contrast with salinized control plants. The B. fortis SSB21-inoculated plants at 7 days post-inoculation were evaluated to estimate the changes in expression level of different stress related genes including CAPIP2, CaKR1, CaOSM1, and CAChi2 (Fig. 4). The inoculated plants exhibited significant up-regulations in transcript levels of CAPIP2, CAChi2, and CaOSM1 by 2.3-, 1.6-, and 1.9-fold as compared to salinized control plants (Fig. 4). At this juncture, least increase (0.8-fold) in expression levels of CaKr1 was also recorded in salinized inoculated plants compared to un-inoculated control. The salt stress alone also slightly up-regulated expression of these stress-related genes as compared to plants receiving rhizobacterial inducers without salt stress (Fig. 4).
Changes in malondialdehyde, hydrogen peroxide, and proline
During the current study, salt stress enhanced the quantities of MDA and H2O2 in capsicum plants up to 29 and 35%, respectively, as compared to control. However, B. fortis SSB21 inoculation reduced the levels of MDA and H2O2 up to 39 and 45%, respectively, in C. annum plants grown under salt stress as compared to corresponding un-inoculated ones (Table 7). The value of proline contents were also significantly augmented in C. annum plants treated with salinity than that of control. Nevertheless, B. fortis SSB21- and P. aeruginosa SSB13-inoculated C. annum plants exhibited 23 and 11% higher proline contents, respectively, under salt stress compared with analogous un-inoculated plants.
Electrolyte leakage and leaf relative water content
The results of present study showed enhanced electrolyte leakage in C. annum plants grown in salt-stressed soils as compared to control. However, P. aeruginosa SSB13 and B. fortis SSB21 supplementation in salinized plants decreased electrolyte leakage up to 15 and 27%, respectively, as compared to un-inoculated plants subjected to salinity stress. The value of leaf relative water content was significantly declined in C. annum plants under salinity stress than that of control, whereas P. aeruginosa SSB13- and B. fortis SSB21-inoculated plants raised in saline conditions demonstrated 14 and 21% higher LRWC as compared to respective un-inoculated plants (Table 7).
Changes in chlorophyll a, chlorophyll b, and total chlorophyll contents
During the current research, C. annum plants grown in salt-contaminated soil exhibited lower values of chlorophyll a, chlorophyll b, and total chlorophyll contents as compared to control (Table 8). However, bacterial inoculation improved the levels of chlorophyll a, chlorophyll b, and total chlorophyll contents in salt-stressed C. annum plants as compared to salinity control treatment. The B. fortis SSB21 was proved most competent strain and it enhanced chlorophyll a, chlorophyll b, and total chlorophyll contents up to 25, 19, and 24%, respectively, in inoculated plants under salt stress as compared to un-inoculated plants treated with salinity control.
Salt tolerance index
The results of the present study demonstrated that salt tolerance index of C. annum plants was significantly lowered in saline soil. However, P. aeruginosa SSB13- and B. fortis SSB21-inoculated plants, when cultivated under salt-treated soil, indicated 73 and 78% higher salt tolerance index value, respectively, than that of un-inoculated plants under salt stress.
Determination of potassium and sodium ions
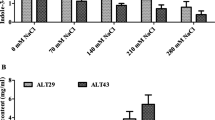
The sodium contents in root and shoot of treated plants were proportional to the applied salt concentration in the medium (Figs. 5 and 6). The roots exhibited higher Na+ as compared to shoot for all salt levels. The highest Na+ contents (8.23 mg g−1 DW) were observed in roots of un-inoculated plants growing in media contaminated with 2 g NaCl kg−1 soil. Bacterial inoculation reduced Na+ uptake by roots and subsequent translocation of this cation to shoot of treated plants. Salt stress reduced K+ contents in root and shoot tissues of subjected plants. A minute increase in K+ uptake was noticed in un-stressed inoculated plants. However, bacterial inoculation considerably enhanced K+ uptake and translocation in treated plants under salt stress In general, bacterial inoculation showed noteworthy decrease in Na+/K+ ratio (Figs. 5 and 6).
Discussion
The isolation and screening of halotolerant rhizobacterial strains from healthy capsicum plants growing in salt-affected soils was implied as the principal strategy in the present study. From isolated rhizobacterial strains, nine strains were capable to tolerate and grow in salt-amended media (Table 3). During the next phase of the study, capsicum plants growing in salt-amended soil under greenhouse conditions were inoculated with halotolerant rhizobacterial strains. These results demonstrated that different rhizobacterial strains exhibited inconsistent capability of plant growth promotion under salt stress. It is observed that SSB21 and SSB13 inoculation exhibited utmost growth promotion in plants under saline conditions. Therefore, these two strains were screened for downstream experimentation. The bioassays of SSB21 and SSB13 strains demonstrated their capability to exhibit ACCD activity, solubilize phosphate, synthesize IAA, and produce siderophore. These growth-promoting traits of rhizobacteria perhaps enhanced plant growth and biomass production in salinized plants under greenhouse conditions (Günes et al. 2014).
The auxin synthesis capability of bacteria may enable capsicum plants to alleviate salt stress by improving root growth and nutrient uptake. Similarly, the reduced ethylene level and enhanced root growth due to ACCD activity of rhizobacteria help in mitigation of salinity stress in assisted plants (Upadhyay and Singh 2015).
The chlorophyll degradation caused by chlorophyllase and other enzymes reduce production of photosynthetic pigments under stress (Szafrańska et al. 2017). Bacterial inoculation probably reduced chlorophyll degradation. Bacterized capsicum plants were capable to tolerate stress and exhibited production of higher photosynthetic pigments including Chl a, Chl b, and total chlorophyll, leading to improved photosynthetic activity and plant growth (Hahm et al. 2017). Other researchers have also reported increased production of photosynthetic pigments in bacterized plants under salt stress (Sapre et al. 2018).
Proline is an important amino acid which is implicated in the stabilization of cell membranes and other amino acids. It maintains cell homeostasis, acts as osmolyte, and scavenges free radicals and buffer redox potential under stress (Szabados and Savoure 2009). Proline provides energy required for physiological activities of plants indispensable for root growth and endurance of plants under stress (Hoque et al. 2007). Higher proline accumulation scavenges free radicals and alleviates drought and salt stress. The gene modulation under stress induces proline biosynthesis in subjected plants (Marco et al. 2015). The higher proline contents were observed in tomato plants due to up-regulation of stress-relevant genes under saline conditions (Gharsallah et al. 2016). The higher proline accumulation in plants sustains leaf turgidity and impedes chlorophyll biodegradation following improved photosynthesis in salinized plants (Pottosin et al. 2014). Our results for stress alleviation in B. fortis SSB21-inoculated capsicum plants due to increased proline synthesis are in accordance with Rojas-Tapias et al. (2012). Some other researchers have also documented the role of higher proline contents in alleviation of plant stress through reduction of electrolyte leakage by maintaining membrane stability, sustaining cellular osmotic balance or turgidity, and decreasing ROS overproduction and oxidative burst (Hayat et al. 2012). Osmotin and other analogous proteins enhance the formation of proline contents in plants (Holmström et al. 2000). Osmotin assists in compartmentation of solutes and ions (Koyama et al. 2001; Anil-Kumar et al. 2015). Osmotin is associated with tonoplast and facilitate compartmentation of sodium ions in plant cells (Yen and Duh 1994). The higher proline and total soluble protein contents observed during the current study advocate enhanced synthesis of osmotin-like proteins resulting in amelioration of salt stress in inoculated plants.
The MDA quantification helps to evaluate salt tolerance because plants subjected to salt stress show overproduction of MDA contents. The HPGPR-assisted capsicum plants showed salinity stress alleviation by reducing MDA contents. These findings are harmonious with Singh and Jha (2017), who observed that Stenotrophomonas maltophilia SBP-9 inoculation reduced MDA production in wheat plants and improved salinity tolerance. The capsicum plants under salt stress exhibited elevated electrolyte leakage. The higher electrolyte leakage under saline conditions enhances cell membrane injury in plants (Wu et al. 2017). The ROS synthesized by NADPH oxidase generate a superoxide anion radical (O−2) and a single oxygen species leading to H2O2 production. This H2O2 is consequently converted into hydroxyl radical ion. The overproduction of hydroxyl radical degrades plant cell membrane (Huang et al. 2014). The salt diffusion effect resulted due to the symbiotic relationship between salt-stressed capsicum plants and B. fortis SSB21 decreased cell membrane damage. Pantoea dispersa PSB3 showing ACCD activity and IAA production also reduced electrolyte leakage and Na+ uptake resulting in higher biomass production and improved salt stress mitigation in affected chick pea plants (Panwar et al. 2016).
Salt-persuaded stress injures stomatal surface and lowers stomatal conductivity (Kim et al. 2016). The stomatal closure under osmotic stress affects cells turgidity and reduces transpiration rate (Chedlia et al. 2007). From the current study, it was observed that salinity-induced osmotic stress perturbs gas exchange attributes including transpiration rate and stomatal conductivity. The stress alleviation encourages plant growth by increasing leaf area and stomatal conductance (Yasin et al. 2017b). The higher stomatal conductivity and increased transpiration rate in B. fortis SSB21-inoculated capsicum plants improved photosynthetic activity, growth, and biomass production.
Root is the first organ of a plant, influenced by salt stress. The water use efficiency (WUE) portrays a relationship between water and growth of a specific plant. Salinity causes osmotic stress in plants and reduces uptake and translocation of water to aerial parts of the subjected plants (Zhang et al. 2016). Plants growing under salt stress show reduced WUE, growth, and biomass production (Al-Omran et al. 2012). The improved root growth in bacteria-inoculated capsicum plants helped in the maintenance of stomatal conductivity and water balance. It was observed that Pseudomonas putida A20 and B. megaterium A12 ameliorated salinity stress in tomato by lowering ethylene level and improving WUE (Aslam et al. 2017). Furthermore, higher proline contents were recorded in HPGPR-inoculated capsicum plants. It is a well-documented fact that proline assists in plant metabolic activities and plays a pivotal role in the enhancement of WUE (Agami et al. 2016).
Salinity causes osmotic stress, and the reduced leaf relative water content is one of the most prominent symptoms of osmotic stress (Fahad et al. 2015). The osmolytes such as proline decrease hydric potential of the cell and thereby help it to evade losing water. Similarly, PGPR reduced ROS injury, enhanced plasma membrane integrity, and increased LRWC in salt-stressed plants (Cohen et al. 2015; Tiwari et al. 2016). The development of vigorous root system probably helped in uptake of water from wider rhizospheric area and enhanced LRWC in case of bacteria-assisted capsicum plants under salt stress.
B. fortis SSB21 promoted root growth and STI in supplemented plants. Mahmood et al. (2016) also reported that PGPR improve STI in plants by increasing root growth due to enhanced uptake of water and nutrients. Similar results for improved salt tolerance and plant–water relations were observed in Chenopodium quinoa seeds primed with halotolerant Bacillus sp. MN54 and Enterobacter sp. MN17 (Yang et al. 2016).
The IAA up-regulates ACC synthase genes, which in return enhances biosynthesis of ACC causing increased ethylene production. The higher level of ethylene synthesis during stress impedes auxin synthesis and limits normal plant growth. However, the surplus ACC is broken down by ACCD activity resulting in lower ethylene and improved plant growth in PGPR-inoculated plants under stress (Khan et al. 2017b). The ACCD activity converts ACC (precursor of ethylene) into ammonia and α-ketobutyrate and reduces stress inducible ethylene level and improve plant biomass production under stress (Ilangumaran and Smith 2017). The rhizobacteria exhibiting ACCD activity augment electron transport and photosynthetic activity while reducing xylem balancing pressure and stomatal resistance; on the whole, they improve the growth of inoculated plants under salt stress (Wang et al. 2016).
PGPR may dilute salt stress in inoculated plants by modulating the expression of stress-responsive genes (Barnawal et al. 2014). Therefore, during another greenhouse experiment of the current study, capsicum plants were co-cultivated with best-performing strain “B. fortis SSB21” to denote changes in expression of stress-related genes (CAPIP2, CaKR1, CaOSM1, and CAChi2). The CaChi2 gene in plants is believed to be associated with osmotic stress tolerance (Hong and Hwang 2006). Maurya et al. (2014) observed that up-regulation of CaKR1 and CaOSM1 induced salt stress tolerance in capsicum plants. The CAPIP2 gene encodes plasma membrane intrinsic protein involved in transportation of smaller neutral solutes and water (Jan et al. 2004). The H2O2-treated plants rapidly exhibited improved accumulation of CAPIP2 gene, demonstrating stress alleviation role of this gene (Dangl et al. 1996; Bestwick et al. 1997). The higher CAPIP2 expression has been observed in pepper plants under the influence of salinity stress (Lee et al. 2006). The up-regulated expression of CAPIP2 gene plays a significant role in alleviation of oxidative stress in pepper plants growing in saline conditions (Ishitani et al. 1998; Piao et al. 2001). Lee et al. (2006) have also revealed the role of CAPIP2 modulation in mitigation of biotic and abiotic stress.
The CaKR1 gene is an important member of ankyrin repeat zinc finger protein transcription factor family. This protein plays a crucial role in stress resistance (Walker-Simmons 1987). The over-expressed transgenic tomato plants with higher ankyrin repeat zinc finger protein exhibited enhanced antioxidant metabolic activities, reduced ROS production, and accelerated oxidative stress tolerance under saline conditions (Seong et al. 2007). The osmotin gene (CAOSM1) is associated with resistance against biotic and abiotic stresses (Choi et al. 2013). The CaOSM1gene in C. annuum is involved in suppression of biotic and salt stress (Hong et al. 2004; Choi and Hwang 2015). The enhanced accumulation of osmolytes including sugars and proline up-regulate CAOSM1 gene in various plants subjected to salt stress (Salzman et al. 1998; Hong et al. 2004). The putative up-regulated expression levels of these stress-related genes are directly or indirectly involved in mitigation of salinity stress tolerance in capsicum plants (Charu and Prasad 2011). The B. fortis SSB21 enhanced expression levels of the aforementioned stress-related genes resulting in alleviation of stress in inoculated salinized plants.
Additionally, the influence of B. fortis SSB21 vis-à-vis reduced ethylene biosynthesis and higher photosynthetic activity in supplemented capsicum plants are in agreement with the findings of Ali et al. (2014).
The increased uptake and translocation of toxic ions in plant leaves devastate chloroplast, reduce protein synthesis, and disrupt enzymatic and photosynthetic activity (Taiz and Zeiger 2002). Higher uptake of Na+ and reduced uptake of K+ ions by plants in response to increased sodium concentration of the media has been reported by many researchers (Martínez-Alcántara et al. 2015). The higher Na+ contents in salinized capsicum plants is possibly due to the competition of sodium ions with other cations, especially K+, causing disruption of ionic balance and enhancement of cytoplasmic membrane permeability. The bacterial exopolysaccharides bind Na+ ions within plant roots ensuing reduced upward translocation of these cations in assisted plants (Ashraf et al. 2004). The lower Na+/K+ ratio is an indication of efficacious salt stress alleviation in plants (Jeschke and Wolf 1988). During the current study, B. fortis SSB21 perhaps adjusted the cation uptake choice resulting in reduced Na+/K+ ratio in supplemented plants. The reduced Na+ contents and higher K+/Na+ ratio leading to stress alleviation was also observed by Samy et al. (2012) in PGPR-assisted egg plants growing under salt stress.
Conclusions
The current study revealed that plant growth-promoting attributes of halotolerant PGPR including IAA synthesis, ACCD activity, P solubilization, and siderophore production recuperated growth of assisted capsicum plants under saline conditions. The P. aeruginosa SSB13- and B. fortis SSB21-supplemented salinized capsicum plants exhibited higher chlorophyll contents, improved gas exchange parameters, augmented water use efficiency, and elevated leaf relative water contents. Bacterial inoculation also modulated proline contents and up-regulated the expression of stress-related genes such as CAPIP2, CaKR1, CaOSM1, and CAChi2. On the other hand, the reduced values of MDA, H2O2, ethylene, electrolyte leakage, and Na+/K+ ratio was recorded in PGPR-inoculated plants. B. fortis SSB21 proved comparatively better strain regarding salt stress alleviation and growth promotion. Altogether, the B. fortis SSB21 supplementation perked up the biochemical and physiogenetic system of capsicum plants to attenuate salinity stress. It is recommended to apply B. fortis SSB21 for stress alleviation and growth encouragement of capsicum plants growing in salt-affected soils. The further proteomics and metabolomics studies may elucidate the additional modules involved in the induction of salt stress tolerance and growth promotion in B. fortis SSB21-assisted capsicum plants. Additionally, the potential of P. aeruginosa SSB13 and B. fortis SSB21 formulation and consortium as a bio-fertilizer should be evaluated in plants subjected to different abiotic stresses under field conditions.
References
Agami RA, Medani RA, Abd El-Mola IA, Taha RS (2016) Exogenous application with plant growth promoting rhizobacteria (PGPR) or proline induces stress tolerance in basil plants (Ocimum basilicum L.) exposed to water stress. Int J Environ Agric Res 2(5):1–15
Ahmad M, Zahir ZA, Nazli F, Akram F, Arshad M, Khalid M (2013) Effectiveness of halo-tolerant, auxin producing Pseudomonas and rhizobium strains to improve osmotic stress tolerance in mung bean (Vigna radiata L.). Braz J Microbiol 44(4):1341–1348
Ali SKZ, Sandhya V, Rao LV (2014) Isolation and characterization of drought-tolerant ACC deaminase and exopolysaccharide-producing fluorescent Pseudomonas sp. Ann Microbiol 64:493–502
Al-Omran AM, Al-Harbi AR, Wahb-Allah MA, Nadeem M, Eleter A (2012) Management of irrigation water salinity in green house tomato production under calcareous sandy soil and drip irrigation. J Agric Sci Technol 14:939–950
Anil-Kumar S, Hima-Kumari P, Shravan-Kumar G, Mohanalatha C, Kavi-Kishor PB (2015) Osmotin: a plant sentinel and a possible agonist of mammalian adiponectin. Front Plant Sci 6:163. https://doi.org/10.3389/fpls.2015.00163
Arnon DI (1949) Copper enzymes in isolated chloroplasts polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15. https://doi.org/10.1104/pp.24.1.
Ashraf M, Hasnain S, Berge O, Mahmood T (2004) Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol Fertil Soils 40:157–162
Aslam H, Ahmad SR, Anjum T, Akram W (2017) Native halo-tolerant plant growth promoting bacterial strains can ameliorate salinity stress on tomato plants under field conditions. Int J Agric Biol 22(4):445–459. https://doi.org/10.17957/IJAB/15.0491
Barnawal D, Bharti N, Maji D, Chanotiya CS, Kalra A (2014) ACC deaminase-containing Arthrobacter protophormiae induces NaCl stress tolerance through reduced ACC oxidase activity and ethylene production resulting in improved nodulation and mycorrhization in Pisum sativum. J Plant Physiol 171:884–894. https://doi.org/10.1016/j.jplph.2014.03.007
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bestwick CS, Brown IR, Bennett MH, Mansfield JW (1997) Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell 9(2):209–221
Bharti N, Pandey SS, Barnawal D, Patel VK, Kalra A (2016) Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci Rep 6:34768. https://doi.org/10.1038/srep34768
Charu L, Prasad M (2011) Role of DREBs in regulation of abiotic stress responses in plants. J Exp Bot 62(14):4731–4748
Chedlia BA, Bechi BR, Boukhris M (2007) Effect of water deficit on olive trees cv. Chemlali under field conditions in arid region in Tunisia. Sci Hortic 113:267–277. https://doi.org/10.1016/j.scienta.2007.03.020
Chen L, Liu Y, Wu G, Veronican Njeri K, Shen Q, Zhang N, Zhang R (2016) Induced maize salt tolerance by rhizosphere inoculation of Bacillus amyloliquefaciens SQR9. Physiol Plant 158:34–44. https://doi.org/10.1111/ppl.12441
Choi HW, Hwang BK (2015) Molecular and cellular control of cell death and defense signaling in pepper. Planta 241(1):1–27
Choi DS, Hong JK, Hwang BK (2013) Pepper osmotin-like protein 1 (CaOSM1) is an essential component for defense response, cell death, and oxidative burst in plants. Planta 238(6):1113–1124
Cohen AC, Bottini R, Pontin M, Berli FJ, Moreno D, Boccanlandro H, Travaglia CN, Piccoli PN (2015) Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol Plant 153:79–90
Dangl JL, Dietrich RA, Richberg MH (1996) Death don’t have no mercy: cell death programs in plant–microbe interactions. Plant Cell 8(10):1793–1807
Fahad S, Hussain S, Matloob A, Khan FA, Khaliq A, Saud S, Hassan S, Shan D, Khan F, Ullah N, Faiq M, Khan MR, Tareen AK, Khan A, Ullah A, Ullah N, Huang J (2015) Phytohormones and plant responses to salinity stress: a review. Plant Growth Regul 75:391–404. https://doi.org/10.1007/s10725-014-0013-y
Gharsallah C, Fakhfakh H, Grubb D, Gorsane F (2016) Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in tomato cultivars. AoB Plants 8:55. https://doi.org/10.1093/aobpla/plw055
Günes A, Turan M, Güllüce M, Sahin F (2014) Nutritional content analysis of plant growth-promoting rhizobacteria species. Eur J Soil Biol 60:88–97. https://doi.org/10.1016/j.ejsobi.2013.10.010
Guo J, Chen L, Liu X, Gao Y, Zhang D, Yang L (2012) A multiplex degenerate PCR analytical approach targeting to eight genes for screening GMOs. Food Chem 132:1566–1573. https://doi.org/10.1016/j.foodchem.2011.11.096
Habib SH, Kausar H, Saud HM (2016) Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. Biomed Res Int 2016:6284547. https://doi.org/10.1155/2016/6284547
Hahm MS, Son JS, Hwang YJ, Kwon DK, Ghim SY (2017) Alleviation of salt stress in pepper (Capsicum annum L.) plants by plant growth-promoting rhizobacteria. J Microbiol Biotechnol 27(10):1790–1797. https://doi.org/10.4014/jmb.1609.09042
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments: a review. Plant Signal Behav 7(11):1456–1466
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast, kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Holiday ER, Preedy JR (1953) The precision of a direct-reading flame photometer for the determination of sodium and potassium in biological fluids. Biochem J 55(2):214–220
Holmström KO, Somersalo S, Mandal A, Palva TE, Welin B (2000) Improved tolerance to salinity and low temperature in transgenic tobacco producing glycine betaine. J Exp Bot 51(343):177–185
Hong JK, Hwang BK (2006) Promoter activation of pepper class II basic chitinase gene, CAChi2, and enhanced bacterial disease resistance and osmotic stress tolerance in the CAChi2 overexpressing Arabidopsis. Planta 223(3):433–448
Hong JK, Jung HW, Lee BK, Lee SC, Lee YK, Hwang BK (2004) An osmotin-like protein gene, CAOSM1, from pepper: differential expression and in situ localization of its mRNA during pathogen infection and abiotic stress. Physiol Mol Plant Pathol 64(6):301–310
Honma M, Shimomura T (1978) Metabolism of 1-aminocyclopropane-1carboxylicacid. Agric Biol Chem 42:1825–1831. https://doi.org/10.1271/bbb1961.42.1825
Hoque MA, Banu MNA, Okuma E, Amako K, Nakamura K, Shimoishi Y, Murata Y (2007) Exogenous proline and glycinebetaine increase NaCl-induced ascorbate-glutathione cycle enzyme activities, and proline improves salt tolerance more than glycinebetaine in tobacco bright Yellow-2 suspension-cultured cells. J Plant Physiol 164:1457–1468
Huang B, DaCosta M, Jiang Y (2014) Research advances in mechanisms of grass tolerance to abiotic stress from physiology to molecular biology. Crit Rev Plant Sci 33:141–189. https://doi.org/10.1080/07352689.2014.870411
Ilangumaran G, Smith DL (2017) Plant growth promoting rhizobacteria in amelioration of salinity stress: a systems biology perspective. Front Plant Sci 8:1768. https://doi.org/10.3389/fpls.2017.01768
Ishitani M, Xiong L, Lee H, Stevenson B, Zhu JK (1998) HOS1, a genetic locus involved in cold-responsive gene expression in Arabidopsis. Plant Cell 10(7):1151–1161
Jacoby RP, Che-Othman MH, Millar AH, Taylor NL (2016) Analysis of the sodium chloride-dependent respiratory kinetics of wheat mitochondria reveals differential effects on phosphorylating and non-phosphorylating electron transport pathways. Plant Cell Environ 39:823–833. https://doi.org/10.1111/pce.12653
Jan JY, Kim DG, Kim YO, Kim JS, Kang H (2004) An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Mol Biol 54(5):713–725
Jeschke WD, Wolf O (1988) External potassium supply is not required for root growth in saline conditions: experiments with Ricinus communis L. grown in a reciprocal split-root system. J Exp Bot 39:1149–1167. https://doi.org/10.1093/jxb/39.9.1149
Khan WU, Ahmad SR, Yasin NA, Ali A, Ahmad A, Akram W (2017a) Application of Bacillus megaterium MCR-8 improved phytoextraction and stress alleviation of nickel in Vinca rosea. Int J Phytorem 19(9):813–824. https://doi.org/10.1080/15226514.2017.1290580
Khan WU, Yasin NA, Ahmad SR, Ali A, Ahmad A, Akram W, Faisal M (2017b) Effect of Burkholderia cepacia CS8 on Cd-stress alleviation and phytoremediation by Catharanthus roseus. Int J Phytorem (Accepted)
Kim K, Jang YJ, Lee SM, Oh BT, Chae JC, Lee KJ (2014) Alleviation of salt stress by Enterobacter sp. EJ01 in tomato and Arabidopsis is accompanied by up-regulation of conserved salinity responsive factors in plants. Mol Cells 37:109–117. https://doi.org/10.14348/molcells.2014.2239
Kim J, Liu Y, Zhang X, Zhao B, Childs K (2016) Analysis of salt-induced physiological and proline changes in 46 switch grass (Panicum virgatum) lines indicates multiple responses modes. Plant Physiol Biochem 105:203–212. https://doi.org/10.1016/j.plaphy.2016.04.020
Koyama ML, Levesley A, Koebner RM, Flowers TJ, Yeo AR (2001) Quantitative trait loci for component physiological traits determining salt tolerance in rice. Plant Physiol 125(1):406–422
Lee SC, Kim SH, An SH, Yi SY, Hwang BK (2006) Identification and functional expression of the pepper pathogen-induced gene, CAPIP2, involved in disease resistance and drought and salt stress tolerance. Plant Mol Biol 62(1–2):151–164
Liu X, Chen X, Li R, Long F, Zhang L, Zhang Q, Li J (2017) Water-use efficiency of an old-growth forest in lower subtropical China. Sci Rep 7:42761. https://doi.org/10.1038/srep42761
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127:1781–1787
Mahmood S, Daur I, Al-Solaimani SG, Ahmad S et al (2016) Plant growth promoting rhizobacteria and silicon synergistically enhance salinity tolerance of mung bean. Front Plant Sci 7:876. https://doi.org/10.3389/fpls.2016.00876
Marco F, Bitrián M, Carrasco P, Rajam MV, Alcázar R, Antonio FT (2015) Genetic engineering strategies for abiotic stress tolerance in plants. Plant Biol Biotechnol 2:579–610
Martínez-Alcántara B, Martínez-Cuenca MR, Quiñones A, Iglesias DJ, Primo-Millo E, Forner-Giner MA (2015) Comparative expression of candidate genes involved in sodium transport and compartmentation in citrus. Environ Exp Bot 111:52–62
Maurya VK, Srinivasan R, Nalini E, Ramesh N, Gothandam KM (2014) Analysis of stress responsive genes in capsicum for salinity responses. Ann Res Rev Biol 6(1):66–78
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Nautiyal CS, Srivastava S, Chauhan PS, Seem K, Mishra A, Sopory SK (2013) Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol Biochem 66:1–9. https://doi.org/10.1016/j.plaphy.2013.01.020
Niu SQ, Li HR, Pare PW, Aziz M, Wang SM, Shi HZ et al (2016) Induced growth promotion and higher salt tolerance in the halophyte grass Puccinellia tenuiflora by beneficial rhizobacteria. Plant Soil 407:217–230. https://doi.org/10.1007/s11104-015-2767-z
Panwar M, Tewari R, Gulati A, Nayyar H (2016) Indigenous salt-tolerant rhizobacterium Pantoea dispersa (PSB3) reduces sodium uptake and mitigates the effects of salt stress on growth and yield of chickpea. Acta Physiol Plant 38:278. https://doi.org/10.1007/s11738-016-2284-6
Patten CL, Glick BR (2002) Regulation of indoleacetic acid production in Pseudomonas putida GR12-2 by ophan and the stationary-phase sigma factor RpoS. Can J Microbiol 48:635–642
Piao HL, Lim JH, Kim SJ, Cheong GW, Hwang I (2001) Constitutive over-expression of AtGSK1 induces NaCl stress responses in the absence of NaCl stress and results in enhanced NaCl tolerance in Arabidopsis. Plant J 27:305–314
Pikovskaya RI (1948) Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 17:362–370
Pottosin I, Velarde-Buendía AM, Bose J, Zepeda-Jazo I, Shabala S, Dobrovinskaya O (2014) Cross-talk between reactive oxygen species and polyamines in regulation of ion transport across the plasma membrane: implications for plant adaptive responses. J Exp Bot 65(5):1271–1283. https://doi.org/10.1093/jxb/ert423
Rojas-Tapias D, Moreno-Galvan A, Pardo-Diaz S, Obando M, Rivera D, Bonilla R (2012) Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl Soil Eco 61:264–272
Salzman RA, Tikhonova I, Bordelon BP, Hasegawa PM, Bressan RA (1998) Coordinate accumulation of antifungal proteins and hexoses constitutes a developmentally controlled defense response during fruit ripening in grape. Plant Physiol 117:465–472
Sambrook J, Russell D (2001) Molecular cloning: a laboratory manual. Editor. Cold Spring Harbor Lab. Press, Plainview
Samy AM, Abd-El-Azeem, Elwan MWM, Sung JK, Ok YS (2012) Alleviation of salt stress in eggplant (Solanum melongena L.) by plant-growth-promoting Rhizobacteria. Commun Soil Sci Plant Anal 43(9):1303–1315. https://doi.org/10.1080/00103624.2012.666305
Sapre S, Gontia-Mishra I, Tiwari S (2018) Klebsiella sp. confers enhanced tolerance to salinity and plant growth promotion in oat seedlings (Avena sativa). Microbiol Rese 206:25–32. https://doi.org/10.1016/j.micres.2017.09.009
Schwyn B, Neilands JB (1987) Universal chemical assay for detection and determination of siderophores. Anal Biochem 160:47–56
Seong ES, Cho HS, Choi D, Joung YH, Lim CK, Hur JH, Wang MH (2007) Tomato plants overexpressing CaKR1 enhanced tolerance to salt and oxidative stress. Biochem Biophys Res Commun 363(4):983–988
Shetty G, Hetrick D, Schwat P (1995) Effects of mycorrhizal fertilizers amendments on zinc tolerance of plants. Environ Pollut 88:308–314. https://doi.org/10.1016/0269-7491(95)93444-5
Singh RP, Jha PN (2016) Alleviation of salinity-induced damage on wheat plant by an ACC deaminase-producing halophilic bacterium Serratia sp SL-12 isolated from a salt lake. Symbiosis 69:101–111. https://doi.org/10.1007/s13199-016-0387-x
Singh RP, Jha PN (2017) The PGPR Stenotrophomonas maltophilia SBP-9 augments resistance against biotic and abiotic stress in wheat plants. Front Microbiol 8:1945. https://doi.org/10.3389/fmicb.2017.01945
Smart RE, Bingham GE (1974) Rapid estimates of relative water content. Plant Physiol 53(2):258–260
Smith DL, Gravel V, Yergeau E (2017) Editorial: signaling in the phytomicrobiome. Front Plant Sci 8:611. https://doi.org/10.3389/fpls.2017.00611
Szabados L, Savoure A (2009) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Szafrańska K, Reiter RJ, Posmyk MM (2017) Melatonin improves the photosynthetic apparatus in pea leaves stressed by paraquat via chlorophyll breakdown regulation and its accelerated de novo synthesis. Front Plant Sci. https://doi.org/10.3389/fpls.2017.00878
Taiz L, Zeiger E (2002) Plant physiology, 3rd edn. Publisher Sinauer, Sunderland
Tiwari S, Lata C, Chauhan PS, Nautiyal CS (2016) Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol Biochem 99:108–117
Upadhyay SK, Singh DP (2015) Effect of salt-tolerant plant growth-promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biol 17:288–293. https://doi.org/10.1111/plb.12173
Walker-Simmons MK (1987) ABA levels and sensitivity in developing wheat embryo's of sprouting resistant and susceptible cultivars. Plant Physiol 84:61–66
Wang QY, Dodd IC, Belimov AA, Jiang F (2016) Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase growth and photosynthesis of pea plants under salt stress by limiting Na+ accumulation. Funct Plant Biol 43:161–172. https://doi.org/10.1071/Fp15200
Wu JJ, Wang C, Zheng LQ, Wang L, Chen Y, Whelan J, Shou H (2011) Ethylene is involved in the regulation of iron homeostasis by regulating the expression of iron-acquisition-related genes in Oryza sativa. J Exp Bot 62:667–674
Wu W, Zhang Q, Ervin EH, Yang Z, Zhang X (2017) Physiological mechanism of enhancing salt stress tolerance of perennial ryegrass by 24-Epibrassinolide. Front Plant Sci 19(8):1017. https://doi.org/10.3389/fpls.2017.01017
Yang A, Miller CT, Turcoliver LD (1996) Simulation of correlated and uncorrelated packing of random size spheres. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics 53(2):1516–1524
Yang AZ, Akhtar SS, Iqbal S, Amjad M, Naveed M, Zahir ZA, Jacobsen SE (2016) Enhancing salt tolerance in quinoa by halo-tolerant bacterial inoculation. Funct Plant Biol 43:632–642. https://doi.org/10.1071/Fp15265
Yasin NA, Khan WU, Ahmad SR, Ali A, Ahmad A, Akram W (2017a) Imperative roles of halotolerant plant growth promoting rhizobacteria and kinetin in improving salt tolerance and growth of black gram (Phaseolus mungo). Environ Sci Pollut Res 5:4491–4505
Yasin NA, Zaheer MM, Khan WU, Ahmad SR, Ahmad A, Ali A, Akram W (2017b) The beneficial role of potassium in Cd-induced stress alleviation and growth improvement in Gladiolus grandiflora L. Int J Phytorem 20(3):274–283. https://doi.org/10.1080/15226514.2017.1374337
Yen GC, Duh PD (1994) Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J Agric Food Chem 42(3):629–632
Yu X, Li Y, Zhang C, Liu H, Liu J, Zheng W, Kang X, Leng X, Zhao K, Gu Y, Zhang X, Xiang Q, Chen Q (2014) Culturable heavy metal-resistant and plant growth promoting bacteria in V-Ti magnetite mine tailing soil from Panzhihua, China. PLoS One 9(9):e106618
Zerrouk IZ, Benchabane M, Khelifi L, Yokawa K, Ludwig-Muller J, Baluska F (2016) A Pseudomonas strain isolated from date-palm rhizospheres improves root growth and promotes root formation in maize exposed to salt and aluminum stress. J Plant Physiol 191:111–119. https://doi.org/10.1016/j.jplph.2015.12.009
Zhang P, Senge M, Dai Y (2016) Effects of salinity stress on growth, yield, fruit quality and water use efficiency of tomato under hydroponic system. Rev Agric Sci 4:46–55. https://doi.org/10.7831/ras.4.46
Zhou C, Ma Z, Zhu L, Xiao X, Xie Y, Zhu J, Wang J (2016) Rhizobacterial strain Bacillus megaterium BOFC15 induces cellular polyamine changes that improve plant growth and drought resistance. Int J Mol Sci 17:976. https://doi.org/10.3390/ijms17060976
Author information
Authors and Affiliations
Contributions
N.A.Y. and W.U.K. conceived, designed, and conducted the experiments. W.A. and Aq.A. helped in conducting experiments and analyzed the data and results. Am.A., N.A.Y., and W.U.K. wrote the manuscript. S.R.A. monitored the experimental work and critically commented on the manuscript. All authors read and approved the final manuscript.
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Yasin, N.A., Akram, W., Khan, W.U. et al. Halotolerant plant-growth promoting rhizobacteria modulate gene expression and osmolyte production to improve salinity tolerance and growth in Capsicum annum L.. Environ Sci Pollut Res 25, 23236–23250 (2018). https://doi.org/10.1007/s11356-018-2381-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2381-8