Abstract
The PGPR elicit plant immunity referred to as induced systemic tolerance (IST) to cope up with abiotic stresses. The common modes of PGPR include fixing N2, increasing the availability of nutrients in the rhizosphere, positively influencing root growth, promoting beneficial plant–microbe symbioses and succumb diseases. The present review deals case study of salt-tolerant rhizobacteria with respect to its functional plant growth promotional activities. Genomic DNA was isolated from bacterial strain AK-1, and gene-specific primers were used to amplify the 16S ribosomal DNA, ACC deaminase gene (acdS gene), IAA gene (ipdC gene), P-solubilizing gene (gcd and gad gene), ectoine production gene (EctC gene) and glycine betaine gene (betA gene). Gene amplification using specific gene primers showed sharp bands of the specific genes near to desired amplicon size. Bacterial-inoculated plants were exhibited superior tolerance against salt stress, as shown by their higher plant biomass, water content, chlorophyll content and lower osmotic stress injury as compared to non-inoculated plants during salt stress. Increased proline accumulation and antioxidant activity in bacterial-inoculated plants also contributed to salt tolerance. This study was conducted to assess the PGPR that are associated with the rhizosphere of soybean grown in semiarid areas of Rajasthan. We also sought to identify and characterize representative PGPR with respect to growth-promoting attributes and studied their salinity tolerance.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
Abiotic stress is a very serious environmental factor worldwide that restricts the growth and productivity of plants worldwide. Once a seed germinates, plants being immobile are destined to stay where they are. Thus, they experience heavy selection pressure in their environments. Plants have developed many traits that help them to evolve and succeed across the globe under different environmental regimes. Following changes occurred in plants during stress condition like modification of cell wall, changes in cell cycle and cell division, production of osmolytes, etc. Many stress-responsive genes are also expressed, which includes the synthesis of osmoprotectants, detoxifying enzymes and transporters, as well as genes that encode regulatory proteins such as transcription factors, protein kinases and phosphatases during stress condition. Different plant species show different response with respect to their environment. Harsh environmental condition, which is harmful for one plant species (sensitive plant), might not be stressful for another plant (tolerant plant). Agriculture lands are frequently facing abiotic challenges like salinity, drought and high- and low-temperature conditions which ultimately affect plant growth and reduce crop yields, productivity and quality. Among these stresses, soil salinity contributes a major proportion in destruction of cultivated land area and reduction of crop productivity (Choudhary et al. 2016).
A saline soil is generally defined as one in which the electrical conductivity (EC) of the saturation extract (ECe) in the root zone exceeds 4 dSm−1 (approximately 40 mMNaCl). The yield of most plants is reduced at this salinity level. It has been estimated that the salinized areas are increasing at a rate of 10% annually which affected 20% of total cultivated and 33% of irrigated agricultural lands worldwide (Shrivastava and Kumar 2015). According to the Food and Agricultural Organization (FAO), if corrective measures are not taken, the arable land would be 50% salinized by the year 2050. Various number of salts, e.g. sodium chloride (NaCl), sodium sulphate (Na2SO4), sodium nitrate (NaNO3), magnesium sulphate (MgSO4), magnesium chloride (MgCl2), potassium sulphate (K2SO4), calcium carbonate (CaCO3), etc., could be dissolved in saline soil, although NaCl causes most of the salt problems for higher plants in nature. The dominant sources of soil salinity are weathering of parental rocks and rainfall. Weathering of rocks release various types of soluble salts, mainly chloride salts, while rainfall contains seawater salts, mainly sodium chloride. Rain containing 10 mg/kg of sodium chloride would affect the land by deposition of 10 kg/ha of salt during each 100 mm of rainfall per year. The other cause of accumulation is the intrusion of seawater on land which deposits a huge amount of salts in soils of coastal lands. Furthermore, human activities also contribute in soil salinity like poor-quality irrigation water, insufficient drainage, land clearing and the replacement of perennial vegetation with annual crops. An increase in the salts limit inhibits plant growth by an osmotic and ion stress. The former osmotic stress immediately comes over plant in accordance with a rise in salt levels outside the roots, which leads to inhibition of water uptake, cell expansion and lateral bud development (Shafique et al. 2014). The ionic stress develops when toxic level of Na+ accumulates in plants particularly in leaves over threshold level leading to leaf mortality with chlorosis and necrosis, a decreased essential cellular metabolic activities including photosynthesis and reduced enzyme activities. The harmful effects of salinity are not only on agricultural production but also on low economic returns due to high cost of cultivation (Munns and Tester 2008).
Increasing human population and reduction in land available for cultivation are two threats for agricultural sustainability. Today, it is big challenge to increase the efficiency and sustainability of global agriculture system. Because this system is regularly marked by scarcity of water resources, environmental pollution and increased salinization of soil and water. These challenges create continued poverty and food insecurity by which people become chronically malnourished.
2.2 Soybean Numero Uno Crop
Soybean (Glycine max L. Merrill) belongs to Fabaceae family, the subfamily Faboideae and the genus Glycine. The characteristic of this family member plants is that they have a mutualistic relationship with specific bacteria, in the case of soybean, Bradyrhizobium japonicum, a rhizobial species. During this interaction, the rhizobia provide nitrogen source to the plant by fixing atmospheric nitrogen. In turn, rhizobia receive sugars and minerals from the soybean plants to survive in the soil environment. It is an annual herbaceous bushy plant that can vary in height (0.2–2.0 m.). It grows well in warm and moist climate. A temperature of 26.5–30 °C appears to be optimum for most of the varieties. A well-drained and fertile loam soil with a pH between 6.0 and 7.5 are most suitable for cultivation. Its pods, leaves and stems are typically covered with brown or grey hairs. The leaves are trifoliate with three to four leaflets per leaf. Flowers are self-fertile white, pink or purple in colour and turn into pods. Each pod is 4–7 cm long, covered with hair and contains two to four seeds with 6–10 mm in diameter. The seed coat is hard, water resistant and protects the seed from damage and drying. Soybean seeds are an important source of oil and protein. Together, oil and protein content covered almost 60% of dry weight of soybean (protein at 40% and oil at 20%) and the remaining includes 35% carbohydrate and 5% ash. Its protein is used as a major source of dietary protein which contains all the essential amino acids particularly glycine, tryptophan and lysine, vitamins (A and D) and minerals used in place of cow’s milk. The oil produced from soybean is highly digestible, contains no cholesterol and used mainly in cooking, margarine and salad dressings. Soybean is used in non-fermented foods (soy milk, tofu and tofu skin) and fermented foods (soy sauce, fermented bean paste, natto and tempeh). On the other hand, the extreme efforts on alternative sources of energy stimulated soy oil-based lubricant and fuel products that replace non-renewable petroleum products. Based on these attributes, soybean is the most promising component of the climate-smart agriculture concept (FAO 2013). It finds a cheaper source of high-quality proteins and has potential to reduce malnutrition, a dominant problem in poor sections of society in the country.
According to USDA report (2015), soybean contributed about 60% of the total 536 million metric tons of oilseeds produced globally by major oil crops (sunflower, copra, peanut, cotton, palm and rapeseed).The USA and China were dominated countries in world soybean production through 1950–1970, growing more than 75 and 25% of the world soybean crop, respectively. In the early 1970s, a worldwide shortage of feed protein led to the initiation of soybean production in several other countries, most notably Argentina, Brazil and India. Comparing the world soybean production during 1965 and now, there has been a significant increase in 11.1 times. However, the USA and China shares of the world’s soybean production had shrunk to 36% and 4%, respectively. In the present time, the top five countries, the USA (34%), Brazil (30%), Argentina (19%), China (4%) and India (3%), produce 90% of the world’s soybeans (Fig. 2.1). It is exclusively grown in Northwest and Central part of India during kharif season (June to September). USDA (2015) estimated 9.0 million metric tons soybean production in all over India during 2014/2015 that was lowest in the last 5 years, while the area was estimated at 11.65 million hectares, increased by 0.7 million hectares from last year, and historically the second largest. These data indicate the poor levels of productivity of soybean crop in India as compared to other countries. The major soybean-producing states are Madhya Pradesh (60%), Maharashtra (30%) and Rajasthan (5%) (Fig. 2.2).Rajasthan consists of three climatic zones, namely, arid zone, semiarid temperate zone and semiarid tropical zone. Soybean is mainly grown in semiarid tropical zone of Rajasthan, regularly affected by high temperature, soil salinity, low pH and metal toxicity which cause a dramatic reduction in crop yield annually. The Rajasthan’s soybean production is gradually decreasing since the last 5 years, and the lowest growing area and production were estimated 6.8 lakh Hectares and 5.6 lakh MT, respectively, in 2014/2015 (SOPA report 2014/2015) (Fig. 2.3).
Several abiotic factors are responsible for deprived production of soybean in India. Most of the areas under soybean cultivation are rainfed; hence, drought is a major constraint in soybean production. The Northwest areas of India are frequently facing an erratic behaviour of monsoon, which affects planting. A short intense drought can cause significant damage to plant and harm crop productivity. There is cross talk between drought and salt stress as both of these stresses create osmotic imbalance interference with nutrient availability and photosynthetic capacity and dehydration of the cell, which affects cell division, cell enlargement and differentiation (Nakashima et al. 2014). Soybeans consume large amounts of potassium (K) resulting low availability of K in soils. Potassium deficiency tends to have weak stems, which become more susceptible to pathogen attack.
Soybean is classified as moderately salt-sensitive crop, severely affected by decreasing symbiotic interaction which results in reduction of nodule formation and the amount of nitrogen fixed (Prudent et al. 2015). During salinity stress, malate concentration decreased that led to reduced bacteroid respiration and antioxidant content. High level of salts initiates water stress which leads to decrease in leghaemoglobin content, and accumulation of ureides in nodule decreases nitrogen fixation. Salinity is also reported to hasten the seed filling rate and decrease grain filling duration, which affect to seed protein and oil contents. Furthermore, the presence of large amount of salt alters the pH of soil, which leads to reduction in mobility of nutrient elements, such as P, K, Fe, Zn and Ca.
2.2.1 Selection and Characterization of PGPR Strains
A potent PGPR strain is selected from several root-colonizing bacteria by screening on the basis of their ability to produce PGP activity, inhibit the growth of various phytopathogens and a positive interaction with the host plant (Bhattacharyya and Jha 2012). Pure cultures of PGPR strains are applied on seeds in in vitro glasshouse trials. Seeds are treated with pure and fresh bacterial suspension and then planted in soil for test. During the experiment, those PGPRs that found significant enhances in plant growth and alleviates negative symptoms of stresses are selected for further field trials (Compant et al. 2005).Primary characterizations of new isolates are done based on biochemical characteristics as in Bergey’s Manual of Determinative Bacteriology (Holt et al. 1994). After that, for molecular characterization, DNA- and RNA-based homology testing, ribosomal protein profiling through MALDI and fatty acid profiling through GC-MS analysis are considered as the most reliable tools for identification of PGPR strains (Bhattacharyya and Jha 2012). It is reported that 16S ribosomal RNA is a component of the 30S small subunit of prokaryotic ribosome. Through evolution this region of the gene remained conserved and hence widely used to define molecular phylogeny and taxonomy of bacteria since the last decade (Sun et al. 2008). PGPRs are becoming a frequent practice for enhancement of legume plants growth and its nutritional value. Most of the studies focused on co-inoculations of PGPR and rhizobium for the improvement of nitrogen-fixing capacity under stress conditions (Egamberdieva et al. 2015). Some recent reports studied on PGPR effects on soybean are presented in Table 2.1.
Many resident microflora of stress environment perform all functions of life for survival of their own and associated biological entities. Some genera, like Bacillus, Paenibacillus and Pseudomonas, are actively being used to alleviate abiotic stresses (reviewed by Choudhary et al. 2016). In saline environment, halophilic bacteria and their metabolites have exhibited many potential which are suitable for vast agricultural, industrial and environmental applications. The successful restoration of plant growth under salinity condition after inoculation with halophilic bacteria provides the basis for a suitable alternative to improve crop growth and yield in saline soils. In this regard, the present study focused on application of salt-tolerant bacteria to improve soybean plant growth and reduce negative effect of salt stress. Salt-tolerant bacterial isolates were recovered from soybean rhizospheric soil grown in semiarid region of Rajasthan, India. The mechanisms of bacterial-mediated IST in soybean plant were studied at biochemical and molecular level.
2.2.2 PGPR Characteristics: Case Studies
Study performed with reference strain AK (MTCC number 12058).
2.2.2.1 Qualitative and Quantitative Estimation of Phosphate Solubilization Under Salt
Inorganic phosphate solubilization in plate assay revealed that as the strains were able to solubilize phosphate in selected salt stress treatments 0–500 mMNaCl (Fig. 2.4a, b). All the recovered strains were able to solubilize Pi and form halo zone around the colonies. The strains AK-1 and VS-1 showed maximum solubilization index in salt stress as well as non-salt stress (Fig. 2.5).
The strain AK-1 showed solubilization index in the range of 2.55–1.77, and VS-1 showed in the range of 2.43–1.69. All the selected bacterial strains were checked for inorganic phosphate solubilization in different salt concentrations quantitatively also. All bacterial strains were able to solubilize inorganic phosphate in controlled condition. The tendency to solubilize Pi decreased as the concentrations of NaCl increased from 0 to 500 mM (Fig. 2.6a, 2.6b, 2.6c, 2.6d, and 2.6e). The two bacterial strains AK-1 and VS-1 were able to solubilize maximum Pi in all the salt concentrations. The strain AK-1 produced maximum soluble P of 4.43 μg/mL in 0 mMNaCl and 3.4 μg/mL in 500 mMNaCl, and the strain VS-1 produced maximum soluble P of 4.1 μg/mL NaCl and 3.4 μg/mL in 0 mM and 500 mMNaCl, respectively.
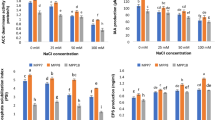
2.2.2.2 ACC Deaminase Activity in Different Salt Concentrations
Two bacterial strains AK-1 and VS-1 were tested for ACC utilization by bacterial strains as nitrogen source (Fig. 2.7a, 2.7b, and 2.7c). The strains were able to metabolize ACC and fulfil their nitrogen requirement. TTC acted as electron donor and respiratory indicator and formed pink-coloured insoluble compound with the growth of bacterial strains. As the bacterial strains were less stressed in less NaCl concentrations, so more coloured compound was formed in 0 mM than other NaCl concentrations (0–500 mM).
2.2.2.3 Proline Determination in Different Salt Concentrations
Compatible solute proline’s concentration was determined in bacterial strain AK-1 which revealed that the bacterial strain was able to form proline as cellular osmolyte in salt stress (Fig. 2.8a). Cellular osmolyte tends to decrease with the increasing salt concentration from 200 to 500 mM due to decreased bacterial growth in salt stress. Maximum 300 μg/g weight proline accumulation was found in 200 mMNaCl (Fig. 2.8b). The control (0 mM) NaCl containing AK-1 showed minimum proline content of 160 μg/g weight proline.
2.2.2.4 Exopolysaccharide Production
The beneficial role of exopolysaccharide-producing bacteria is known to remove various toxic heavy metals. Bacterial EPSs bind to cations including Na+; when the population density of EPS-producing bacteria is increased in the root zone, it would decrease Na+ available for the plant uptake and thus helps in alleviation of salinity stress (Khodair et al. 2008). Bacteria produced 0.65 g fresh weight of exopolysaccharide in absence of NaCl, but when the NaCl concentration increased to 300 mM, fresh weight increased to 0.82 g, likewise dry weight also increased to from 0.32 g in 0 mMNaCl to 0.43 g in 300 mMNaCl (Table 2.2). This increase in EPSs can be attributed to survival of bacteria in salinity stress which can contrarily reduce available Na+ for plant uptake in soil. EPSs are also known to bind soil particles closely and avoid forming drought stress.
2.2.2.5 Molecular Characterization of Genes in Bacteria
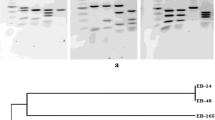
Genomic DNA was isolated from bacterial strain AK-1, and gene-specific primers were used to amplify the 16S ribosomal DNA, ACC deaminase gene (acdS gene), IAA gene (ipdC gene), P-solubilizing gene (gcd and gad gene), ectoine production gene (EctC gene) and glycine betaine gene (betAgene). Gene amplification using specific gene primers showed sharp bands of the specific genes near to desired amplicon size (Fig. 2.9a–g).
2.2.3 Sterilization and Viability and Seed Sensitivity
Seed sterilization was standardized by absence bacterial growth in nutrient both in minimum HgCl2 concentration and treatment time. The seeds were found sterilized when treated with 0.1% HgCl2 for 1 min (Fig. 2.10a). Absence of bacterial growth and seed germination on filter paper assay further standardized the seed sterilization protocol for plant experiments. Triphenyltetrazolium chloride is a redox indicator which was used as cellular respiration indicator to check the seed viability. TTC on reduction formed insoluble reddish-coloured formazan (Fig. 2.10b). The seeds selected for the study were found viable and showed reddish-coloured formazan.
Soybean seeds’ salt susceptibility was performed on the six varieties, viz. PK 1050, JS 9560, NR 7, JS 7105, PK 1025 and JS 9305. Out of six varieties, JS 9560 was found most susceptible for 200 mMNaCl and least salt tolerant (Fig. 2.10c). Hence, soybean seed variety JS 9560 was selected for the plant experiments.
2.2.4 Standardization for Bacterial Inoculums
Soybean seed variety JS 9560 (salt sensitive) was inoculated with 1 × 108 CFU/mL bacterial densities for all the 11 bacterial strains in filter paper assay as shown in Fig. 2.11. All the 11 bacterial strains augmented root length and lateral roots up to some or greater extent (Table 2.3). The two bacterial strain AK-1 and VS-1 increased the root length and lateral root utmost nearly 17.5 cm and 28.8 by AK-1, 16.1 cm and 27.6 by VS-1 with 100% germination by both the bacterial inoculants. Hence, bacterial strain AK-1 was selected as bacterial inoculant for further studies on plants. Some of the experiments were performed on VS-1 also.
Study performed with reference strain AU (MTCC number 12057).
2.2.4.1 Bacterial Growth Under Stress Conditions
The results of the effect of salt and drought concentrations on rhizobacterial growth are presented in Table 2.4. The bacterial isolates differed in their ability to tolerate salinity in form of NaCl. All isolates could tolerate salinity up to 2% NaCl. A decrease in isolate number was observed with increase in NaCl concentration. A number of 19 isolates could tolerate 4% NaCl, 10 isolates could tolerate 6% NaCl, 8 isolates could tolerate 8% NaCl, 4 isolates could tolerate 10% NaCl, and 2 isolates were able to tolerate 12% NaCl concentration.
All isolates were screened for their ability to tolerate PEG wherein 3 isolates were able to tolerate maximum 14% PEG whereas 7, 13 and 20 isolates could tolerate 12%, 10% and 8% PEG, respectively. All isolates could tolerate drought up to 6% PEG.
2.2.5 PGP Traits
A total number of 14 bacterial isolates which were showed growth on 8%, 10% and 12% NaCl were selected for PGP traits characterization (Fig. 2.12). Two bacterial strains AT7 and AM were not able to produce any PGP properties. A number of three bacterial strains were able to produce only single one ACC deaminase activity. A total number of six bacterial strains were able to produce more than one PGP activity, and three bacterial strains (AU, AU7 and AT11) were producing all PGP properties tested (Table 2.5).
2.2.5.1 IAA Production on Different Salt Concentration
A total number of nine bacterial strains were able to produce IAA in the presence of 100 μg/ml L-tryptophan; however, four bacterial strains (AT, AT4, AM3 and AU) were able to produce IAA without L-tryptophan also. IAA production was found inversely proportional to salt concentrations, when the concentration of salt increased from 2% to 12% IAA production decreased. Only three bacterial isolates AU, AT4 and AM3 were able to retain sufficient IAA producing activity (>10 μg/mL) at 10% NaCl concentration, and other bacterial strains lost their activity after 8% NaCl concentration. During stress condition, the highest IAA production was observed in AU isolate (13.6 μg/mL) followed by AM3 (12.1 μg/mL) and AT4 (11.4 μg/mL) at 6% NaCl (Fig. 2.13).
2.2.5.2 Phosphate Solubilization on Different Salt Concentration
When the isolates were subjected to qualitative test for P solubilization using Pikovskaya agar plates, seven isolates were able to form clear halo around the colonies indicating positive results. The strain AU, AT and AT11 were showed maximum solubilization index in salt stress as well as non-salt stress (Fig. 2.14a). The ranges of solubilization index were 2.83–1.86, 2.36–1.12 and 2.54–1.77 in AU, AT and AT11 isolates, respectively (Fig. 2.14b). Other isolates showed the development of hazy zones at higher salt concentration.
2.2.5.3 Siderophore Production on Different Salt Concentration
Qualitative estimation revealed that siderophore production was higher at 0% NaCl in a total number of five bacterial isolates as compared to other salt concentration. The index size was recorded in ranged from 4.6 to 1.2. Siderophore production decreased with increased salt concentration. The highest index during salt stress was measured in AU (2.6) followed by AU7 (2) at 8% as compared to other isolates (Fig. 2.15a and 2.15b).
2.2.5.4 ACC Deaminase Activity on Different Salt Concentration
The 14 strains were tested for ACC deaminase activity, and 6 strains were found to be positive. A number of three bacterial strains lost their activity at 6% NaCl; however, AU, AU7 and AT11 strains were able to retain their activity up to 8% NaCl. The highest activity was observed in cell-free extract of AU isolates at 0% NaCl (73 nmol α-ketobutyrate mg protein−1 h−1) and 8% NaCl (29 nmol α-ketobutyrate mg protein−1 h−1) (Fig. 2.16).
2.2.5.5 EPS Production on Different Salt Stress
A total number of seven bacteria were found to produce EPS production. The production was increased under salt stress. Bacterial isolates AT, AT11 and AT12 were showed highest production at 6%; AU7 and AU3 were showed at 8%; and AU was produced at 10% NaCl. The highest production was found in AT11 (1.3 g mL−100) followed by AU (1.17 g mL−100) (Fig. 2.17).
2.2.5.6 Phylogeny Analysis of PGP Gene Properties
Gene amplification using specific gene primers showed sharp bands for acds (410 bp), IaaM(330 bp), g6pd (630 bp), sid (360 bp), tre (510 bp), nr (630 bp) and p5cs (250 bp) (Fig. 2.19). All genes were sequenced and submitted in NCBI; GenBank accession numbers are listed in Table 2.6. As expected, all PGP genes of AU bacterial strain tested showed similar to the P. fluorescence intrageneric cluster. Regarding the phylogeny based on the IaaM and nrgene sequences, the AU bacterial strain forms an independent cluster, which includes P. simiae WCS417 (CP007637), whereas in acds, g6pd, sid, tre and p5cs genes phylogeny, AU bacterial strain was included with P. fluorescens PICF7 (CP005975) in an independent cluster. All other species from the genus Pseudomonas are found outside this cluster (Fig. 2.20a, 2.20b, and 2.20c).
It is consensus that salinity is a major limiting factor for crop productivity and a major cause of the reduction of cultivable lands in semiarid areas of the world. A wide range of strategies have been adopted to develop salt-tolerant crops but little success to date as few genetic traits for salt tolerance are identified (Schubert et al. 2009). Several studies have demonstrated that local adaptation of plants to habitat-imposed stresses is driven by their closely associated microbes (reviewed by Choudhary 2012). Soybean is a major oilseed crop in India, and it covers almost 4% of world’s soybean production. It is one of the salt-sensitive (glycophytes) agricultural crops. Salinity detrimentally affects growth and development of soybean by severely reducing the metabolic processes such as CO2 assimilation, oil and protein synthesis (Ghassemi-Golezani and Taifeh-Noori 2011). Salinity-related water stress has negative effect on the nodulation ability of soybean plants. It is now recognized that different types of signals (nitrogenous, oxidative, redox, etc.) in legume’s rhizospheric environment play a crucial role in establishing interaction between microbes and legumes (Karmakar et al. 2015). Therefore, soybean is a useful model for investigating the ecology of PGPR and their influence on plant growth under saline soils. This study was conducted to assess the PGPR that are associated with the rhizosphere of soybean grown in semiarid areas of Rajasthan. We also sought to identify and characterize representative PGPR with respect to growth-promoting attributes and studied their salinity tolerance. Rhizosphere environment is hot spot for various types of soil microorganisms due to rich nutrient availability. The bacteria present in stress affected rhizospheric environments have been isolated and identified and their beneficial effects on plant growth studied by many researchers (Jain et al. 2014; Vaishnav et al. 2015; Kumari et al. 2015).
Lane 1- DNA marker (100 bp), Lane 2- g6pd, Lane 3- p5cr, Lane 4- nr, Lane 5- amy, Lane 6- IaaM, Lane 7- sid, Lane 8- acds
The evolutionary distances were computed using the Kimura 2-parameter method and are in the units of the number of base substitutions per site. Values shown next to the branches are the percentage of replicate trees with associated taxa clustered together in the bootstrap test (1000 replicates). Evolutionary analyses were conducted in MEGA 6.
The evolutionary distances were computed using the Kimura 2-parameter method and are in the units of the number of base substitutions per site. Values shown next to the branches are the percentage of replicate trees with associated taxa clustered together in the bootstrap test (1000 replicates). Evolutionary analyses were conducted in MEGA 6.
The evolutionary distances were computed using the Kimura 2-parameter method and are in the units of the number of base substitutions per site. Values shown next to the branches are the percentage of replicate trees with associated taxa clustered together in the bootstrap test (1000 replicates). Evolutionary analyses were conducted in MEGA 6.
In all tested bacteria, the growth was reduced upon higher NaCl and PEG concentration. More than 65% of bacterial strains were severely affected by 8% NaCl and 10% PEG. Only a few isolates such as AT4, AT12, AM3, AM5 and AU showed a significant growth with 10% NaCl and 12% PEG. These results are in agreement with several studies, which showed that the number of viable colonies of rhizospheric strains declined with increasing concentration of salt, while isolated strains from saline soil were able to survive at higher salt concentration in media (Naz et al. 2009; Omar et al. 2009). A total number of 14 bacterial isolates which were showed significant growth on higher salt stress were screened in vitro for PGP activities. A number of three bacterial isolates were found to show all PGP properties tested, whereas six bacterial isolates were able to produce more than one PGP properties.
A total number of eight bacterial isolates were able to produce IAA in the presence of tryptophan; however, out of them four isolates were also capable to produce IAA in the absence of tryptophan. Various reports have been published on IAA production by PGPR strains either with or without the tryptophan supplement in culture media (Farina et al. 2012; Mia and Shamsuddin 2013). In our experiment the level of IAA produced in culture media was found higher with tryptophan. Strain AU was found to have higher ability to convert tryptophan to IAA than other isolates, while in the absence of tryptophan, its ability was similar to AM3 isolate. The production of IAA by PGPR is usually correlated with direct effects on plant growth (Cassán et al. 2014). It is responsible for division, extension and differentiation of plant cells and tissues. It also affects photosynthesis, pigment formation, biosynthesis of various metabolites and resistance to biotic and abiotic stress factors (Bashan and de-Bashan 2010). It has been proved that IAA participates in biofilm formation leading to quorum sensing (Hu et al. 2010). Inoculation of IAA producing Carnobacterium sp. was reported to increase soybean seedling growth against Fusarium wilt (Jain et al. 2013). The IAA producing wild and mutant type of Pseudomonas and Bacillus strains were found to enhance tolerance against salinity and drought stress in soybean and mung bean plants, respectively (Kumari et al. 2016). Only seven isolates were able to solubilize Ca3(PO4)2 present in the Pikovskaya medium in salt stress as well as non-salt stress. The capacity to solubilize Pi is a good characteristic for the selection of bacteria capable of increasing P content in the rhizosphere, and their application to increase plant protection against adverse abiotic factors has now been an upcoming strategy (reviewed by Vassilev et al. 2012). The solubilization of calcium phosphate observed in this study may be due to the production of organic acids by the bacteria which decreases pH in the culture medium (Marra et al. 2012). Similarly phosphate-solubilizing bacteria have been isolated from sunflower (Ambrosini et al. 2012), canola (Farina et al. 2012) and Turkish tea (Çakmakçı et al. 2010). Phosphate-solubilizing bacteria (PSB) isolated from canola plants were tested for growth-promoting effects and found to promote plant growth (Farina et al. 2012). Likewise novel PSB, ‘Pantoea cypripedii PS1’ along with Enterobacter aerogenes PS16 and Rhizobium ciceri enhanced the growth of chickpea (Cicer arietinumL.) (Singh et al. 2014). Besides, Burkholderia was found as a dominant rhizospheric bacterial genus associated with sunflower plants and showed a stimulatory effect on plant growth through siderophore and solubilized phosphate (Ambrosini et al. 2012).
Another important trait of rhizospheric bacteria is production of siderophores that may directly or indirectly influence the plant growth. Directly, plant roots uptake iron from siderophore, and indirectly, siderophores are making iron unavailable to the phytopathogens and protecting plant health (Rajkumar et al. 2010). In the present study, five isolates were found positive on CAS medium and produced siderophore up to 8% NaCl. Likewise, a total of 108 bacteria isolated from canola plants displayed the ability to produce siderophores in which two bacterial genus Klebsiella and Pseudomonas were able to promote canola plant growth (Farina et al. 2012). Siderophore-producing Paenibacillus sp. isolates were recovered from wheat rhizosphere and chosen for in vivo experiments in a greenhouse and found to be very efficient in wheat plant growth promotion (Beneduzi et al. 2008).
The ACC-D-containing PGPRs have been reported for improving the salinity tolerance in plants by many researchers (Siddikee et al. 2011; Nautiyal et al. 2013; Kumari et al. 2015; Singh et al. 2015). A number of six bacterial isolates were exhibited ACC-D activity in which only three isolates AU, AU7 and AT11 were able to show activity up to 8% NaCl. The inoculation of Klebsiella sp. SBP-8 containing ACC-deaminase on wheat (Triticum aestivum) under salt condition improved growth of the plant and protects from salt stressors through more than one mechanism including an effect on plant biomass, chlorophyll content and on K+/Na+ ratio (Singh et al. 2015). ACC deaminase containing Pseudomonas sp. mediated saline tolerance in soybean (Glycine max) plants (Kumari et al. 2015) and drought stress in drying soil in mung bean (Vigna radiata) (Kumari et al. 2016).
Introduction of EPS-producing microorganisms in the drought and saline environment can alleviate stress in the crop plants. In this study, salt-tolerant EPS-producing rhizobacteria from soybean rhizosphere were screened. Only seven bacterial isolates produced mucoid growth. These isolates showed efficient EPS production up to 8% NaCl. The EPS production was higher in the presence of NaCl stress than non-stress condition, and it increased by increasing stress level in the most of the isolates, indicating that EPS production in bacteria occurs as a response to the stress and protects cells against desiccation (Qurashi and Sabri 2011). EPS-producing bacteria in the root zone were reported to enhance soil aggregation and water holding capacity and decrease the content of Na+ available for plant uptake and thus help in alleviating salt and drought stress in plants (Choudhary et al. 2015). An EPS-producing bacterial strain Pseudomonas putida GAP-P45 was recovered from alfisol of sunflower rhizosphere and found to increase the survival, plant biomass and root adhering soil/root tissue ratio of sunflower seedlings under drought stress (Vardharajula et al. 2009). Upadhyay et al. (2011) isolated 11 bacterial strains from wheat grown on salt-affected soils, which showed tolerance up to 80 g L−1NaCl and also exhibited an EPS-producing potential. The isolated bacterial strains were employed as inocula in wheat plants and found to increase biomass compared to the uninoculated plants during salt stress.
Pseudomonads are predominant bacteria in the rhizosphere with versatile functions. They are known to produce plant hormones, siderophores, antibiotics, enzymes like proteases and glucanases and solubilize minerals which have made them the most promising group of PGPR involved in the biocontrol of plant diseases and abiotic stress tolerance (reviewed by Choudhary 2012). They were capable of rapid growth and utilize various substrates as nutrients therefore show efficient colonization with a wide variety of crops including cereals, pulses, oilseeds and vegetables (Santoyo et al. 2012). In addition, PCR amplification confirmed the presence of PGP genes, namely, acdS, nr, amy, IaaM, sid, g6pd and p5csin bacterial isolate AU. Presence of ACC-D has been confirmed by amplification and sequence analysis of acdS, a structural gene encoding ACC-D. Several pair of primers has been designed by various researchers to detect the presence of acdS gene in bacteria (Hontzeas et al. 2004; Cheng et al. 2008; Duan et al. 2009; Jha et al. 2012). The acdS gene is commonly found in Actinobacteria, Deinococcus-Thermus, three classes of Proteobacteria (α, β and γ), various fungi belonging to Ascomycota and Basidiomycota and in some Stramenopiles (Singh et al. 2015).
In the study performed by our group, an inoculation of PGPB Pseudomonas spp. to soybean and guar in the 200 mMNaCl treatment alleviated the stress (Kumari et al. 2016, 2016; Vaishnav et al. 2015). All the treatment plants with bacterial inoculation showed better plant growth characteristic with respect to their control. Salt-treated plants showed least growth due to salinity-induced harmful physiological changes in plant system, while the plants inoculated with bacterial strains showed good growth characteristics. On comparing the results of Pseudomonas spp. inoculated soybean and guar plants, it was found that the strain AU was able to promote the growth of stress-tolerant guar plants along with soybean plants. Being stress tolerant guar showed more percentage seed germination than soybean plants, but the effect of PGPB can be clearly seen on the plants. Plant growth-promoting activities of bacteria make them efficient for alleviating biotic as well as abiotic stress (Choudhary et al. 2015).
References
Algar E, Gutierrez-Mañero FJ, Garcia-Villaraco A, García-Seco D, Lucas JA, Ramos-Solano B (2014) The role of isoflavone metabolism in plant protection depends on the rhizobacterial MAMP that triggers systemic resistance against Xanthomonas axonopodis pv. glycines in Glycine max (L.) Merr. cv. Osumi. Plant Physiol Biochem 82:9–16
Ambrosini A, Beneduzi A, Stefanski T, Pinheiro FG, Vargas LK, Passaglia LM (2012) Screening of plant growth promoting Rhizobacteria isolated from sunflower (Helianthus annuus L.). Plant Soil 356(1–2):245–264
Armendariz AL, Talano MA, Wevar Oller AL, Medina MI, Agostini E (2015) Effect of arsenic on tolerance mechanisms of two plant growthpromoting bacteria used as biological inoculants. J Environ Sci 33:203–210
Bashan Y, De-Bashan LE (2010) How the plant growth-promoting bacterium Azospirillum promotes plant growth: a critical assessment. Adv Agron 108:77–136
Beneduzi A, Peres D, da Costa PB, Zanettini MHB, Passaglia LMP (2008) Genetic and phenotypic diversity of plant-growth-promoting bacilli isolated from wheat fields in southern Brazil. Res Microbiol 159:244–250
Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol 28(4):1327–1350
Çakmakçı R, Dönmez MF, Ertürk Y, Erat M, Haznedar A, Sekban R (2010) Diversity and metabolic potential of culturable bacteria from the rhizosphere of Turkish tea grown in acidic soils. Plant Soil 332:299–318
Cassán F, Vanderleyden J, Spaepen S (2014) Physiological and agronomical aspects of phytohormone production by model plant-growth-promoting rhizobacteria (PGPR) belonging to the genus Azospirillum. J Plant Growth Regul 33:440–459
Cheng Z, Duncker BP, McConkey BJ, Glick BR (2008) Transcriptional regulation of ACC deaminase gene expression in Pseudomonas putida UW4. Can J Microbiol 54:128–136
Choudhary DK (2012) Microbial rescue to plant under habitat-imposed abiotic and biotic stresses. Appl Microbiol Biotechnol 96(5):1137–1155
Choudhary DK, Kasotia A, Jain S, Vaishnav A, Kumari S, Sharma KP, Varma A (2015) Bacterial-mediated tolerance and resistance to plants under abiotic and biotic stresses. J Plant Growth Regul 1:25
Choudhary DK, Kasotia A, Jain S, Vaishnav A, Kumari S, Sharma KP, Varma A (2016) Bacterial-mediated tolerance and resistance to plants under abiotic and biotic stresses. J Plant Growth Regul 35:276–300
Compant S, Duffy B, Jerzy N, Clement C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71(9):4951–4959
Duan J, Müller KM, Charles TC, Vesely S, Glick BR (2009) 1- Aminocyclopropane-1- carboxylate (ACC) deaminase genes in Rhizobia from southern Saskatchewan. Microb Ecol 57:423–436
Egamberdieva D, Shurigin V, Gopalakrishnan S, Sharma R (2015) Microbial strategies for the improvement of legume production in hostile environments. In: Azooz MM, Ahmad P (eds) Legumes under environmental stress: yield, improvement and adaptations, 1st edn. Wiley, Hoboken
FAO (2013) Climate-smart agriculture sourcebook
Farina R, Beneduzi A, Ambrosini A, de Campos SB, Lisboa SS, Wendisch V, Vargas LK, Passaglia LMP (2012) Diversity of plant growth-promoting rhizobacteria communities associated with the stages of canola growth. App Soil Ecol 55:44–52
Fernández-Bidondo L, Silvani V, Colombo R, Pérgola M, Bompadre J, Godeas A (2011) Pre-symbiotic and symbiotic interactions between Glomus intraradices and two Paenobacillus species isolated from AM propagules. In vitro and in vivo assays with soybean (AG043RG) as plant host. Soil Biol Biochem 43:1866–1872
Ghassemi-Golezani K, Taifeh-Noori M (2011) Soybean performance under salinity stress. INTECH Open Access Publisher, Rijeka
Holt JG, Krieg NR, Sneath PH, Staley JT, Williams ST (1994) International edition: Bergey’s manual of determinative bacteriology. Springer Science+Business Media, New York
Hontzeas N, Zoidakis J, Glick BR, Abu-Omar MM (2004) Expression and characterization of the 1-aminocyclopropane-1-carboxylic acid deaminase from the rhizobacterium P. putida UW4: a key enzyme in bacterial plant growth promotion. Biochem Biophys Acta 1703:11–19
Hu M, Zhang C, Mu Y, Shen Q, Feng Y (2010) Indole affects biofilm formation in bacteria. Ind J Microbiol 50(4):362–368
Jain S, Vaishnav A, Kasotia A, Kumari S, Gaur RK, Choudhary DK (2013) Bacteria-induced systemic resistance and growth promotion in Glycine max L. Merrill upon challenge inoculation with Fusarium oxysporum. Proc Natl Acad Sci India Biol Sci 83:561–567
Jain S, Vaishnav A, Kasotia A, Kumari S, Gaur RK, Choudhary DK (2014) Bacteria-induced systemic resistance and growth promotion in Glycine max L. Merrill upon challenge inoculation with Fusarium oxysporum. Proc Natl Acad Sci India Biol Sci 83:561–567
Jha B, Gontia I, Hartmann A (2012) The roots of the halophyte Salicornia brachiata are a source of new halotolerant diazotrophic bacteria with plant growth-promoting potential. Plant Soil 356:265–277
Juge C, Prévost D, Bertrand A, Bipfubusa M, Chalifour FP (2012) Growth and biochemical responses of soybean to double and triple microbial associations with Bradyrhizobium, Azospirillum and arbuscular mycorrhizae. Appl Soil Ecol 61:147–157
Karmakar K, Rana A, Rajwar A, Sahgal M, Johri BN (2015) Legume-rhizobia symbiosis under stress. In: Arora NK (ed) Plant microbes Symbiosis: applied facets, vol 241. Springer, India. https://doi.org/10.1007/978-81-322-2068-8_12
Khodair TA, Galal GF, El-Tayeb TS (2008) Effect of inoculating wheat seedlings with exopolysaccharide-producing bacteria in saline soil. J Appl Sci Res 4:2065–2070
Kumari S, Vaishnav A, Jain S, Varma A, Choudhary DK (2015) Bacterial-mediated induction of systemic tolerance to salinity with expression of stress alleviating enzymes in soybean (Glycine max L. Merrill). J Plant Growth Regul 34:558–573
Kumari S, Vaishnav A, Jain S, Varma A, Choudhary DK (2016) Induced drought tolerance through wild and mutant bacterial strain Pseudomonas simiae in mung bean (Vigna radiata L.). World J Microbiol Biotechnol 32(1): 1–0
Marks BB, Megías M, Nogueira MA, Hungria M (2013) Biotechnological potential of rhizobial metabolites to enhance the performance of Bradyrhizobium spp. and Azospirillum brasilense inoculants with soybean and maize. AMB Express 3:21
Marra LM, Soares CRFS, de Oliveira SM, Ferreira PAA, Soares BL, de Fráguas CR, de Lima JM, de Souza Moreira FM (2012) Biological nitrogen fixation and phosphate solubilization by bacteria isolated from tropical soils. Plant Soil 357:289–307
Masciarelli O, Llanes A, Luna V (2014) A new PGPR co-inoculated with Bradyrhizobium japonicum enhances soybean nodulation. Microbiol Res 169:609–615
Mia MB, Shamsuddin ZH (2013) Rhizobium as a crop enhancer and biofertilizer for increased cereal production. Afr J Biotechnol 9:6001–6009
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K (2014) The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front Plant Sci. https://doi.org/10.3389/fpls.2014.00170
Nautiyal CS, Srivastava S, Chauhan PS, Seem K, Mishra A, Sopory SK (2013) Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol Biochem 66:1–9
Naz I, Bano A, Hassan T-U (2009) Isolation of phytohormones producing plant growth promoting rhizobacteria from weeds growing in Khewra salt range, Pakistan and their implication in providing salt tolerance to Glycine max L. Afr J Biotechnol 8(21):5762–5766
Omar MNA, Osman MEH, Kasim WA, El-Daim A (2009) Improvement of salt tolerance mechanisms of barely cultivated under salt stress using Azospirillum brasilense. In: Ashraf M, Ozturk M, Athar HR (eds) Salinity and water stress, vol 44. Springer, Netherlands, pp 133–147
Prudent M, Vernoud V, Girodet S, Salon C (2015) How nitrogen fixation is modulated in response to different water availability levels and during recovery: a structural and functional study at the whole plant level. Plant Soil 399:1–12
Qurashi AW, Sabri AN (2011) Osmoadaptation and plant growth promotion by salt tolerant bacteria under salt stress. Afr J Microbiol Res 5:3546–3554
Rajkumar M, Ae N, Prasad MN, Freitas H (2010) Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol 28(3):142–149
Ramesh A, Sharma SK, Yadav N, Joshi OP (2014a) Phosphorus mobilization from native soil p-pool upon inoculation with phytate-mineralizing and phosphate solubilizing Bacillus aryabhattai isolates for improved p-acquisition and growth of soybean and wheat crops in microcosm conditions. Agric Res 3:118–127
Ramesh A, Sharma SK, Sharma MP, Yadav N, Joshi OP (2014b) Inoculation of zinc solubilizing Bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in Vertisols of Central India. Appl Soil Ecol 73:87–96
Salavati A, Bushehri AAS, Taleei A, Hiraga S, Komatsu S (2012) A comparative proteomic analysis of the early response to compatible symbiotic bacteria in the roots of a supernodulating soybean variety. J Proteome 75:819–832
Santoyo G, Orozco-Mosqueda MDC, Govindappa M (2012) Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: a review. Biocontrol Sci Technol 22:855–872
Schubert S, Neubert A, Schierholt A, Su¨mer A, Zörb C (2009) Development of salt-resistant maize hybrids: the combination of physiological strategies using conventional breeding methods. Plant Sci 177:196–202
Shafique A, Rehman S, Khan A, Kazi AG (2014) Improvement of legume crop production under environmental stresses through biotechnological intervention. In: Ahmad P (ed) Emerging Technologies and Management of Crop Stress Tolerance, vol 2. https://doi.org/10.1016/B978-0-12-800875-1.00001-6
Shrivastava P, Kumar R (2015) Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22:123–131
Siddikee MA, Glick BR, Chauhan PS, Yim W, Sa T (2011) Enhancement of growth and salt tolerance of red pepper seedlings (Capsicum annuum L.) by regulating stress ethylene synthesis with halotolerant bacteria containing 1-aminocyclopropane-1-carboxylic acid deaminase activity. Plant Physiol Biochem 49:427–434
Simonetti E, Viso NP, Montecchia M, Zilli C, Balestrasse K, Carmona M (2015) Evaluation of native bacteria and manganese phosphite for alternative control of charcoal root rot of soybean. Microbiol Res 180:40–48
Singh O, Gupta M, Mittal V, Kiran S, Nayyar H, Gulati A, Tewari R (2014) Novel phosphate solubilizing bacteria ‘Pantoea cypripedii PS1’along with Enterobacter aerogenes PS16 and Rhizobium ciceri enhance the growth of chickpea (Cicer arietinum L.). Plant Growth Regul 73:79–89
Singh RP, Jha P, Jha PN (2015) The plant-growth-promoting bacterium Klebsiella sp. SBP-8 confers induced systemic tolerance in wheat (Triticum aestivum) under salt stress. J Plant Physiol 184:57–67
Stefan M, Dunca S, Olteanu Z, Oprica L, Ungureanu E, Hritcu L, Mihasan M, Cojocaru D (2011) Soybean (Glycine max [L] Merr.) inoculation with Bacillus pumilus RS3 promotes plant growth and increases seed protein yield: relevance for environmentally-friendly agricultural applications. Carpathian J Earth Environ Sci 5:131–138
Sun L, Qiu FB, Zhang XX, Dai X, Dong XZ, Song W (2008) Endophytic bacterial diversity in rice (Oryza sativa L.) roots estimated by 16S rDNA sequence analysis. Microb Ecol 55:415–424
Upadhyay SK, Singh JS, Singh DP (2011) Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedosphere 21(2):214–222
USDA (2015) World agriculture production
Vaishnav A, Kumari S, Jain S, Varma A, Choudhary DK (2015) Putative bacterial volatile-mediated growth in soybean (Glycine max L. Merrill) and expression of induced proteins under salt stress. J Appl Microbiol 119:539–551
Vardharajula S, Ali SKZ, Grover M, Reddy G, Bandi V (2009) Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol Fertil Soils 46:17–26
Vassilev N, Eichler-Löbermann B, Vassileva M (2012) Stress-tolerant P-solubilizing microorganisms. Appl Microbiol Biotechnol 95:851–859
Wang C-J, Yang W, Wang C, Gu C, Niu D-D, Liu H-X, Wang Y-P, Guo J-H (2012) Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLoS One 12:1–10
Acknowledgments
The research was supported by SERB-Grant no. SR/FT/LS-129/2012 to DKC. Some of the research has partially been supported by DBT grant no. BT/PR1231/AGR/21/340/2011 to DKC.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Vaishnav, A., Kasotia, A., Choudhary, D.K. (2018). Role of Functional Bacterial Phylum Proteobacteria in Glycine max Growth Promotion Under Abiotic Stress: A Glimpse on Case Study. In: Choudhary, D., Kumar, M., Prasad, R., Kumar, V. (eds) In Silico Approach for Sustainable Agriculture. Springer, Singapore. https://doi.org/10.1007/978-981-13-0347-0_2
Download citation
DOI: https://doi.org/10.1007/978-981-13-0347-0_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-0346-3
Online ISBN: 978-981-13-0347-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)