Abstract
Antarctica has the greatest diversity of lakes types on the planet including freshwater, brackish, saline and hypersaline systems, epishelf lakes, ice shelf lakes and lakes and cryoconite holes on glacier surfaces. Beneath the continental ice sheet, there are hundreds of subglacial lakes. These systems are dominated by microbial food webs, with few or no metazoans. They are subject to continuous cold, low annual levels of photosynthetically active radiation and little or no allochthonous nutrient inputs from their catchments. Subglacial lakes function in darkness. Heterotrophic bacteria are a conspicuous and important component of the simple truncated food webs present. Bacterial abundance and production vary between freshwater and saline lakes, the latter being more productive. The bacterioplankton functions throughout the year, even in the darkness of winter when primary production is curtailed. In more extreme glacial habitats, biomass is even lower with low rates of production during the annual melt season. Inter-annual variation appears to be a characteristic of bacterial production in lakes. The factors that control production appear to be phosphorus limitation and grazing by heterotrophic and mixotrophic flagellate protozoa. The evidence suggests high rates of viral infection in bacteria and consequent viral lysis, resulting in significant carbon recycling, which undoubtedly supports bacterial growth in winter. The biodiversity of lacustrine Antarctic heterotrophic bacteria is still relatively poorly researched. However, most of the main phyla are represented and some patterns are beginning to emerge. One of the major problems is that data for heterotrophic bacteria are confined to a few regions served by well-resourced research stations, such as the McMurdo Dry Valleys, the Vestfold Hills and Signy Island. A more holistic multidisciplinary approach is needed to provide a detailed understanding of the functioning, biodiversity and evolution of these communities. This is particularly important as Antarctic lakes are regarded as sentinels of climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
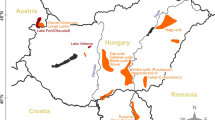
Despite being almost entirely covered by an ice sheet up to 4 km thick, Antarctica carries the widest diversity of lake and pond types on the planet. The ice-free areas that constitute around 2 % of the surface area contain freshwater lakes and ponds as well as brackish, saline and hypersaline lakes. Some hypersaline lakes are equivalent to ten times the salinity of seawater (Laybourn-Parry and Wadham 2014). Their waters are so loaded with salts that they never develop ice covers and in winter the water temperature can plummet to −17 °C (Ferris and Burton 1988). Epishelf lakes occur between the land and an ice shelf and are very unusual systems. There are two structural types, either a body of freshwater sits on seawater as is the case in Beaver Lake (Amery Oasis), or a freshwater lake is connected to the sea by a conduit under an ice shelf or a glacier, as is the case for the Bunger Hills epishelf lakes (Gibson and Andersen 2002; Laybourn-Parry et al. 2006). Because of the connection to the sea, epishelf lakes are tidal and are characterized by rafted freshwater ice on their shorelines. Logistic constraints have limited most data to regions where there are permanent stations that conduct microbial ecological research. Consequently, most data relate to the Maritime Antarctic (Signy Island), the McMurdo Dry Valleys (MDVs) and the coastal oases of the Vestfold and Larsemann Hills, with smaller data sets for the Syowa Oasis and Schirmacher Oasis (Fig. 1).
It is only in the last few decades that researchers have considered lake ice covers as potential habitats for microbial life (Felip et al. 1995; Alfreider et al. 1996). The perennial ice covers of some Dry Valley lakes develop liquid-water inclusions around particles of aeolian origin. These dark-coloured particles are heated by the Sun and melt the surrounding ice. It has been calculated that such liquid-water inclusions occur for up to 150 days in the annual cycle and that as much as 40 % of the lake ice cover volume can be liquid water (Priscu et al. 1998). The sediment particles act as a source of both organic and inorganic nutrients for communities of micro-organisms that include Cyanobacteria, heterotrophic bacteria and chemolithotrophic bacteria.
Both freshwater and saline lakes and ponds occur on the surface of ice shelves. These systems are relatively shallow and freeze to their bases in winter (Hawes et al. 2008). The surface of glaciers and the ice sheet support a range of aquatic habitats during the summer melt phase (Hodson et al. 2008). Cryoconite holes that are effectively mini lakes are common. These are shallow, small-diameter structures containing a sediment layer (the cryoconite), overlain by water and in Antarctica usually with a layer of surface ice cover. Supraglacial lakes or cryolakes are less well researched. They can be ephemeral, forming and then draining through a hole or Moulin to the glacier base. Others like those on the Canada Glacier in the MDVs are thought to be 2000–4000 years old. They are shallow systems with a water depth of less than 2 m overlying a sediment layer and are permanently ice-covered. In winter, they freeze to their bases (Bagshaw et al. 2010). They usually form near the base of an ice cliff from the coalescence of cryoconite holes. They grow progressively larger as they progress down the glacier until they reach the glacier snout where they disintegrate (Laybourn-Parry et al. 2010).
Below the continental ice sheet is a vast array of subglacial lakes, a total of 402 quoted in the most recent review (Siegert et al. 2016). Their presence has been determined by airborne radio-echo sounding since the 1970s (Oswald et al. 1973), with latest additions to the inventory derived from laser altimetry (Smith et al. 2009). The entry into Lake Whillans in 2013 has provided the first detailed information on its microbial communities (Christner et al. 2014).
All of these aquatic environments are the domain of mainly extremophile organisms that are confronted by continuous low temperatures close to freezing, and in saline water bodies below freezing. The ice cover adds to light attenuation in the underlying water columns. Moreover, the polar regions experience the lowest annual levels of photosynthetically active radiation, so the scope for photosynthesis is limited. This in turn limits exudation of photosynthate from autotrophs so that concentrations of dissolved organic carbon (DOC), essential for supporting heterotrophic bacterial growth, are low (Table 1). Unlike lakes at lower latitudes and many Arctic lakes, Antarctic lacustrine systems receive virtually no allochthonous carbon inputs from their catchments. Thus, DOC is entirely derived from autochthonous sources. The exceptions are water bodies close to penguin rookeries or seal wallows. As one would expect these various water bodies have low concentrations of inorganic phosphorus and nitrogen that are essential for supporting both bacterial and autotroph production (Laybourn-Parry and Wadham 2014).
Antarctic lacustrine habitats are characterized by truncated food webs. There are few zooplankton and no fish. The communities are dominated by micro-organisms, including microalgae, autotrophic and heterotrophic protozoans, Bacteria, Archaea and viruses. Based on concentrations of chlorophyll a, Antarctic lakes and ponds mostly fall within the ultra-oligotrophic to oligotrophic section of the trophic continuum (Table 1). The exceptions are those lakes that receive allochthonous carbon and nutrient inputs: for example, Heywood Lake (Signy Island), where a chlorophyll a concentration of 297.6 µg L−1 was recorded (Butler 1999) and Rookery Lake (Vestfold Hills) where a maximum value of 35 µg L−1 occurred (Laybourn-Parry et al. 2002). While high Arctic lakes closely resemble their Antarctic counterparts in having a largely microbial plankton, lower-latitude Arctic lakes have well developed zooplankton and fish communities (Vincent et al. 2008). Despite the harsh conditions, year-long studies of Antarctic lakes indicate that biological processes, including bacterial production, continue throughout the winter (Heath 1988; Bayliss et al. 1997; Butler 1999; Butler et al. 2000; Laybourn-Parry et al. 2004).
Environmental adaptations unique to psychrophilic micro-organisms have been thoroughly described from a range of cold habitats worldwide where psychrophilic micro-organisms thrive (Dolhi et al. 2013). Psychrophilic strains of bacteria are present and have been isolated from Antarctic lakes, such as Gillisia limnaea strain DSM 15749 (Van Trappen et al. 2004) and Carnobacterium iners sp. nov. (Snauwaert et al. 2013), including specialists, for example, cold-active halophilic bacteria from the ice-sealed Lake Vida (Mondino et al. 2009). Specific adaptations to the cold, such as amino acid substitutions that increase structural flexibility and protein function at low temperature, are present (Tang et al. 2013). However, Antarctic bacteria seem to have the ability to grow at a wide range of temperatures. While they function at low temperatures continuously, some of them have the ability to grow at 25 °C (Hosoi-Tanabe et al. 2010). A detailed study of six strains of psychrotolerant bacteria isolated from a depth of 50–55 m in Lake Vanda grew at temperatures close to zero to −2 °C below zero, but all had their optimum growth between 18 and 24 °C (Vander Schaaf et al. 2015). These data suggest the presence of universal genera, indicating that the bacteria in Antarctic lakes are not specific to this environment. Due to the presence of cosmopolitan strains, and the flushing of terrestrial bacteria through Antarctic lake systems, there is yet to be a consensus on whether Antarctic lake is predominantly psychrophiles or psychrotolerant mesophiles. This further highlights the need for detailed physiological investigation, rather than reliance on sequence-based evidence alone. For example, mesophiles would not be expected to grow at lake in situ temperatures most of the year except in summer. Also, mesophiles could be indicative of decomposition and remain preserved in the sediments.
Bacterial abundance and productivity
The concentrations of bacterioplankton in various Antarctic water bodies are listed in Table 2. What is immediately apparent is that saline lakes in the Vestfold Hills support higher concentrations of bacteria than freshwater lakes in same locality. The exception is Highway Lake, a brackish lake with a salinity close to freshwater (4 ‰). The freshwater lakes of the maritime Antarctic (Signy Island) support much higher abundances of bacteria than continental Antarctic lakes. Here, the climate is less severe and the presence of soil and moss beds provides some allochthonous carbon inputs. Heywood Lake receives large carbon and nutrient inputs from an adjacent fur seal wallow. The presence of fur seals has increased since the 1950s and 1960s leading to the progressive eutrophication of Heywood Lake (Smith 1988; Hodgson et al. 1998). Among the saline lakes listed, Rookery Lake is close to an Adelie penguin rookery and its elevated bacterial concentrations reflect allochthonous inputs of nutrients and carbon. When placed in a global context, these bacterial abundances fall within the range recorded for lower-latitude freshwater oligotrophic lakes. Across a range of temperate oligotrophic lakes, abundance ranged between 1.0 and 80 × 108 L−1 (Pick and Caron 1987; Vaqué and Pace 1992; Laybourn-Parry et al. 1994; Hofer and Sommaruga 2001; Lymer et al. 2008). Abundances found in Arctic freshwater lakes are also similar (Hobbie and Laybourn-Parry 2008). Comparable data for lower-latitude saline lakes are limited. Meromictic Mahoney Lake (British Columbia) supported bacterial concentrations of 40–85 × 108 L−1, though chlorophyll a concentrations were relatively low, usually around 1 µg L−1. However, DOC was high between 20 and 80 mg L−1, derived from allochthonous sources and upward diffusion from the lower monimolimnion waters (Overmann et al. 1996). Thus, the upper range reported for meromictic Antarctic lakes (Lakes Fryxell, Bonney and Ace Lake) are of the same order of magnitude.
Continuous low temperatures, limited DOC and nutrient limitation are likely to curtail the magnitude of bacterial growth. Rates of bacterial production are low (Table 2) but similar to values reported for Arctic lakes, for example Toolik Lake and a lake in Franz Joseph Land where rates ranged from 1.6 to 22.4 and 1.2 to 3.9 µg C L−1 day−1, respectively (O’Brien et al. 1997; Panzenböck et al. 2000). A review of bacterial production in predominately temperate freshwaters indicates an average rate of 26.4 ± 33.1 µg C L−1day−1 with a median of 11.5 µg C L−1day−1 (Cole et al. 1988). These values cover a wide range of trophic status. Reported rates for Antarctic lakes other than those in the maritime Antarctic are significantly lower (Table 2). However, they correspond to the lower rates in ranges reported for temperate oligotrophic lakes, for example a Swedish lake (3.84 µg C L−1 day−1) (Riemann and Bell 1990), Lake Michigan (1.5–90 µg C L−1 day−1) (Scavia et al. 1986), two humic Scandinavian lakes (3.6–22.8 µg C L−1 day−1) (Sundh and Bell 1992) and Loch Ness (0–46.2 µg C L−1 day−1 over a year) (Laybourn-Parry and Walton 1998). Bacterial growth is particularly low in ultra-oligotrophic Beaver Lake, the largest epishelf lake in Antarctica with an area of 800 km2 (Table 2). In this lake, concentrations of DOC limit bacteria growth at times during summer (Laybourn-Parry et al. 2006).
While saline lakes are common worldwide, there are few data on their bacterial productivity. During a 10-month study of Mahoney Lake (British Columbia), bacterial production in the mixolimnion varied between 0 and 11.6 µg C L−1 day−1 (Overmann et al. 1996). These rates being of the same order of magnitude as occur in Antarctic meromictic saline lakes (Table 2). Most studies of saline meromictic lakes have focused on upper the mixolimnion water, while the lower anoxic monimolimnion water is largely unexplored by comparison. Methanotrophic bacteria have been isolated from Ace Lake and Burton Lake in the Vestfold Hills; however, overall in Ace Lake they represent only <0.1–1 % of total bacteria (Bowman et al. 1997). Two species of methanogenic Archaea have been isolated from Ace Lake, Methanogenium frigidum and Methanococcoides burtonii (Franzmann and Rohde 1991; Franzmann et al. 1997). A single phylotype of Crenarchaeota was located close to the oxycline in Lake Fryxell (Karr et al. 2006). The biomass of methanogenic Archaea based on the identification and quantification phospholipid-derived ether lipids (PLEL) has been determined in Ace Lake and equates to cell concentrations of <1.0 × 108 to 7.4 × 108 cell L−1 between 17 and 23 m. Much higher concentrations were seen in the upper sediment layer of the lake with a maximum of 17.7 × 109 cells g−1 dry weight (Mancuso et al. 1990).
Sulphate-reducing bacteria are common in the sulphate-rich monimolimnia of meromictic lakes. The abundances of Desulfobacter and Desulfovibrio in Ace Lake increased from 0.5 × 108 and 4.8 × 108 cells L−1 and 0.1 and 0.9 × 108 cells L−1 between 10 and 23 m, respectively (Mancuso et al. 1990). However, sulphate concentrations decreased with depth in the water column and below 18 m at <1 mmol L−1 would have become limiting for sulphate reduction. These authors suggest that the increased numbers of sulphate-reducing bacteria with depth may have been due to the settling of cells from higher levels in the water column, and that they would probably have been inactive in the deep waters. Analysis of environmental DNA and isolates from Lake Fryxell revealed a diverse group of sulphate-reducing bacteria (see section on biodiversity below). There was clear localization of some groups in the water column in relation to chemical and physical conditions (Karr et al. 2005). Sulphur-oxidizing bacteria, specifically three strains of Thiobacillus thioparus, were isolated from 9, 10 and 11 m in Lake Fryxell. Most probable number culture analysis showed a peak in concentration at 9.5 m of 2 × 105 cells L−1 within a narrow zone where sulphide and oxygen coexist in the water column. However, sulphur-oxidizing bacteria were still detectable at 13 m well into the anoxic waters (Sattley and Madigan 2006).
Supraglacial aquatic habitats are more extreme than lakes and ponds in rock basins. Temperatures in cryoconite holes usually hover around freezing. They are nutrient-limited environments, and this is reflected in very low rates of bacterial growth in both the sediment and the overlying water (Table 2). Typically higher rates are sustained in the sediment. There are few data for supraglacial lakes and ponds or cryolakes, as they are poorly researched, though interest in their biology and biogeochemistry is growing (Table 2). Concentrations of bacteria in their water columns are similar to those seen in cryoconite holes.
Prior to the penetration of subglacial Lake Whillans, our knowledge of the bacteriology of subglacial lakes was based on accretion ice derived from cores drilled above Lake Vostok. This ice is refrozen lake water that has attached to the base of the overlying glacial ice (Priscu et al. 1999). Highest cell numbers in the accretion ice reached 380 ± 53 cells mL−1 (Christner et al. 2006). Lake Whillans is a comparatively small (60 km2) shallow, downstream lake, located some tens of kilometres from the grounding line of the Whillans Ice Stream (Fricker et al. 2007). It is a dynamic system that fills and drains on decadal timescales. In contrast, Lake Vostok is an ancient stable subglacial lake around 250 km long and 50 km wide. The concentration of cells in the waters of Lake Whillans is within the range seen in surface ultra-oligotrophic freshwater systems and is characterized by considerable morphological diversity. However, the levels of production achieved by these cells are extremely low (Table 2). DOC averaged 221 ± 55 µmols L−1, two times greater than estimates for Lake Vostok (Christner et al. 2014). Subglacial lakes are dark environments, so autotrophic production is bacterial via chemoautotrophy. The most abundant phylotypes were closely related to chemoautotrophic species that use nitrogen, iron and sulphur as energy sources (Christner et al. 2014).
Factors controlling production
Heterotrophic bacteria require a carbon source and nitrogen and phosphorus to sustain growth. As indicated above in continental Antarctic lakes, with few exceptions, the DOC pool is largely autochthonous derived from autotrophic production. In meromictic lakes, there is an upward of diffusion of nutrients and dissolved organic carbon derived from the underlying monimolimnion resulting from geochemical processes in the water, the sediments and microbial mats. The supply of DOC and its chemical makeup and the availability of inorganic phosphorus are regarded as prime factors that drive bacterial production in lakes (Toolan et al. 1991; Vrede et al. 1999; Vrede 2005; Jansson et al. 2006; Simek et al. 2006; Dorado-Garcia et al. 2014). However, temperature also has an effect (Vrede 2005; Hall et al. 2009) and the situation is complicated by factors that impact on bacterial mortality and the taxonomic makeup of the bacterial community (Weinbauer and Höfle 1998; Jacquet et al. 2013). Various studies quoted above indicate that there are differences in response to DOC and inorganic phosphorus loadings in relation to trophic status and in some instances season. Thus, unravelling the factors that control bacterioplankton productivity in time and space is a complex problem.
A correlation analysis of bacterial production versus primary production, inorganic phosphorus, inorganic nitrogen and DOC in a range of Antarctic freshwater and saline lakes produced no significant correlations for freshwater lakes (Table 3). However, on an individual lake basis there was a significant correlation between bacterial production and DOC in Crooked Lake (P < 0.001, n = 26), but not in the other lakes. In saline lakes, there was a correlation between bacterial production and primary production and between bacterial production and DOC (Table 3). These correlations are based on data collected over summers or entire years. In the MDV lakes summer bacterial abundances correlated positively and significantly with chlorophyll a, but not with inorganic phosphorus (Lisle and Priscu 2004). A detailed statistical analysis of long-term summer data from Lake Hoare between 1993 and 2004 failed to show any correlation between bacterial production and DOC (Herbei et al. 2010). A detailed discussion of the DOC pool and bacterial carbon demand is given below (under carbon cycling), which provides some insight into the difficulties of unravelling the factors that control bacterial production.
Studies aimed at unravelling the complexities of carbon and phosphorus dynamics on bacterial growth of necessity involve mesocosms or enclosures of some type, and filtration to remove phytoplankton and bacterial grazers. The responses of the bacterial community to additions of organic carbon and/or inorganic phosphorus relative to controls are relatively easy to assay in such experiments. Most of these studies relate to temperate latitudes. For example, in temperate lakes phosphorus limitation was evident in summer and carbon limitation in autumn and generally the addition of phosphorus-stimulated bacterial production, more so in oligotrophic lakes compared with eutrophic lakes (Vrede 2005). Sub-Arctic lakes have low concentrations of inorganic nutrients, and in these systems bacterial production can be limited by inorganic phosphorus availability when nutrient use efficiency is at its maximum, and by organic carbon when growth efficiency is at its maximum. Bacterial production is limited by both phosphorus and carbon when nutrient use efficiency and growth efficiency have suboptimal values (Jansson et al. 2006). There are few manipulation studies in Antarctic lakes. In both the East and West lobes of Lake Bonney (MDVs), bacterial production in the upper mixolimnion waters was limited by phosphorus availability rather than carbon. In the monimolimnion, phosphorus additions had no impact on growth (Ward et al. 2003). These results are contrary to those derived from the analysis of field-collected data for Dry Valley lakes mentioned above (Lisle and Priscu 2004).
Protozoan grazers, including heterotrophic and mixotrophic nanoflagellates and ciliates, consume bacterial production and can have a significant impact. For example, during the transition from summer to winter mixotrophic nanoflagellates grazed more than 100 % of bacterial production day−1 in Lake Bonney, thereby reducing biomass (Thurman et al. 2012). Viral lysis of infected bacterial cells is another source of mortality impacting on bacterial production. Virus abundances are high in both Antarctic freshwater and saline lakes (Madan et al. 2005; Säwström et al. 2007a). Typically, a high proportion of the bacterial community is infected. In freshwater Antarctic lakes up to 34 % compared with 2.2 % at lower latitudes, however, the burst size is low with a mean of 4 compared with 26 for lakes elsewhere (Säwström et al. 2007b). These high infection rates imply a high bacterial mortality. The balance between viral- and predator-induced mortalities is complex and likely to vary in time and space. At lower latitudes, the balance between grazing and viral-induced mortality varied with depth in the water column of a eutrophic lake in September. Viral lysis removed an average of 7.7–27.8 % of bacterial production in the epilimnion and 38.4–97.3 % in the hypolimnion, while protozoan predation accounted for 81.8–108 % of bacterial production in the epilimnion and 5.0–8.9 % in the hypolimnion (Weinbauer and Höfle 1998). Temporal changes in the abundances of predators and viruses also impact on the taxonomic makeup of the bacterial community and play an important role in shaping community composition (Jacquet et al. 2013). Viruses are species-specific, and bacterial predators are likely to practise feeding selectivity. Ciliated protozoans exhibit selective feeding on bacteria, choosing a preferred food species even when its concentration is low in a bacterial mixture (Thurman et al. 2010). It is highly likely that flagellates are also selective feeders.
Carbon cycling
As previously indicated, continental Antarctic lakes differ from their lower-latitude counterparts in receiving very little allochthonous input of carbon or nitrogen and phosphorus. Thus, the DOC pool is largely autochthonous produced in situ by phototrophic organisms, and in some environments by chemoautotrophs, which in turn supports bacterial growth (Matsumoto 1989; McKnight et al. 1991). Estimates of summer bacterial carbon demand in meromictic Lakes Fryxell and Bonney indicated that demand was greater than the estimated pool of DOC (Takacs et al. 2001). Major presumed sources of DOC were exudation from phytoplankton, input from streams and upward diffusion from the nutrient-rich monimolimnion over the chemocline. However, a major potential source of DOC is recycling as a result of viral lysis of bacterial cells. In freshwater Crooked Lake and Lake Druzhby (Vestfold Hills), it was estimated that viral lysis contributed >60 % of the DOC pool in winter and <20 % in summer when phytoplankton production was at its maximum (Säwström et al. 2007a). High viral infection rates seen in these lakes are a major factor in viral-mediated carbon cycling. Around 26 % of the bacterial community in Lake Bonney is lysogenic (Lisle and Priscu 2004). This fraction of bacterial cells represents a pool of 0.86 µg C L−1 that could be released as DOC, equal to around 23 % of bacterioplankton demand (Säwström et al. 2008). Clearly, the lysis of these bacteria would not be simultaneous, but would occur in pulses and might be important in the winter months when exudation from phototrophs is minimal. Annual changes in concentrations of DOC indicate that high values occur in winter (Fig. 2b) supporting some of the highest biomass in the annual cycle (Fig. 2a).
a Bacterial biomass over an annual cycle in Lake Druzhby and Crooked Lake and b Concentrations of dissolved organic carbon (DOC) in Lake Druzhby and Crooked Lake. Data from Laybourn-Parry et al. (2004). Filled circles Crooked Lake, open circles Lake Druzhby
In lower-latitude lakes, so-called sloppy feeding by zooplankton represents another contribution to the DOC pool. Zooplankton are very sparse in Antarctic continental lakes. For example, in the freshwater and slightly brackish lakes in the Vestfold Hills the single cladoceran zooplankter Daphniopsis studeri occurs in concentrations of <1.0 L−1 (Säwström et al. 2009). In the Dry Valley lakes, the only obvious zooplankton are rotifers that occur in concentrations up to 19 L−1 (Thurman et al. 2012), although very large water samples have revealed evidence of extremely scarce copepods (Hansson et al. 2011). Thus, in Antarctic lakes zooplankton probably make no significant contribution to the DOC pool.
The decomposition of organic matter in the anoxic water and sediments of meromictic Antarctic lakes is an area on which there are limited data. The upward diffusion of DOC from monimolimnion is one component of the DOC pool that drives bacterial production in the mixolimnion. The important microbial processes that mediate decomposition and the specific organisms involved have received some attention (e.g. Franzmann et al. 1988, 1991a, b; Mancuso et al. 1990; Takacs et al. 2001; Karr et al. 2005; Lauro et al. 2011). Rates of processes such as sulphate reduction and methanogenesis in Antarctic lakes are slow when compared with other aquatic environments (Franzmann et al. 1988, 1991a, b).
Annual variations
Investigations covering an annual cycle have shown that the bacterioplankton functions in winter sustained by a supply of DOC (Fig. 2a, b). Similar patterns have been noted in saline lakes (Madan et al. 2005; Laybourn-Parry et al. 2007). The DOC pool sustaining bacterial growth is derived from viral lysis and autotrophic production, which continues for most of the year under low levels of solar irradiation (Heath 1988; Bayliss et al. 1997; Henshaw and Laybourn-Parry 2002). During the winter months, the bacterioplankton are subject to predation from heterotrophic flagellates and mixotrophic phototrophic flagellates. The latter are a feature of many of Antarctic lakes where mixotrophy is an important survival strategy (Laybourn-Parry et al. 2005).
Where long-term studies have been undertaken, there are evident inter-annual variations (Figs. 3, 4). Relatively high rates of bacterial production are evident in the winter months in Crooked Lake, though the magnitude of production varies (Fig. 3). For example in 2003/2004, it was low in comparison with 1992/1993 and 1999/2000. Data for the months of March and April are lacking because vehicular access to the lake is only possible once the sea ice has formed. During the summer months, helicopters provide access. Routine determinations were taken twice each summer in Lake Fryxell (MDVs) over a long period and show similar inter-annual variations, particularly in the late 1990s. During the late nineties, rates of production were around 4–10 times higher. There is no clear explanation for these variations although factors that control nutrient and DOC concentrations and autotrophic production are undoubtedly involved. The light climate for primary production is determined by ice thickness, and in coastal lakes by the ice-free period, as well as annual climatic variations. Ace Lake is probably the most studied lake in Antarctica. There have been a number of attempts to produce models of carbon cycling within the system, for example using a molecular approach including metaproteome data, though based on only two sampling dates in late December (Lauro et al. 2011), and another based on a detailed review of long-term data (Rankin et al. 1999). The long-term data sets show clear inter-annual variations in physical and chemical conditions, which undoubtedly account for variations in bacteria abundance, and in bacterial and primary production (Laybourn-Parry and Bell 2014).
The biodiversity of Antarctic lake and glacial surface communities
Antarctica is an isolated continent, although there is evidence that propagules can reach the continent on air masses (Marshall 1996). This geographic isolation, linked to a large variety of highly specific selection pressures and a wide range of heterogeneous environments with strong environmental gradients, particularly obvious in sediments and in meromictic lakes, have shaped the bacterial diversity we see today.
Polar lacustrine environments present a surprisingly diverse range of prokaryotes, despite the relatively harsh nature of the environment. This diversity is mainly composed of specialist aquatic taxa (including freshwater, brackish and marine species), transient terrestrial taxa washed into and through during periods of snow and ice melt, aerial derived taxa which are blown in and can become established for different time periods and a series of specialist taxa normally associated with other ecological components, such as penguin guano and epiphytic moss and lichen-associated species. They encompass the full range of bacterial phyla known to date, although this appears to be far more heterogeneous than previously thought, perhaps owing to high divergence within the relatively restricted lineages that have successfully colonized Antarctic aquatic environments throughout its evolutionary history.
The development of molecular techniques, initially based on the analysis of the 16S rRNA gene sequences, has revolutionized the study of prokaryote diversity. In the early years, the 16S rRNA sequence databases were rather limited in the sequence information they contained, with a bias towards medically important prokaryotes. Environmental samples were significantly under-represented, if covered at all. The 16S rRNA gene sequencing data were then enhanced by multiple sequence targets using multilocus sequence typing, although this continued to be based on what was available in sequence databases. With the advent of high-throughput sequencing and environmental metagenomics, it is now possible to sequence a large portion of the diversity found in environmental samples; consequently, patterns are starting to emerge through bioinformatic techniques alone, without underpinning knowledge on the sequence or genome.
The lack of background knowledge on the diversity identified through informatics tools has, however, led to a credibility gap, which will be reduced in the coming years, as more complementary culture and physiological studies are published to inform the sequence databases. This credibility gap results from the interpretation of sequence data through homology alone or where a thorough understanding of what that sequence data mean in terms of physiological or ecological function is lacking. This is particularly pronounced where short high-throughput sequences of relatively low similarity to sequences in the established databases are used and is a result of the rush to sequence without understanding the fundamental microbial ecology underpinning that sequence information. Much of what we know today about Antarctic lacustrine prokaryotes comes from the analysis of whole genomes such as that of Exiguobacterium antarcticum B7, isolated from a biofilm from Ginger Lake, King George Island, Antarctica (Carneiro et al. 2012) and through comparative genomics. A number of key species have been cultured and their full genomes sequenced. These have then been compared to non-polar strains, and gene expression profiles have been compared in order to determine whether Polar strains respond differently to environmental challenges. For example, Pseudoalteromonas haloplanktis (Lanoil et al. 1996), the archaeons Methanococcoides burtonii and Methanogenium frigidum (Saunders et al. 2003) as described in more detail later in the review.
Our current understanding of Antarctic lacustrine prokaryote diversity is largely limited by the geographical coverage of the samples, due to the logistic constraints, limited sampling in some cases and an understanding of what the DNA sequences actually mean in terms of cellular and environmental function. Furthermore, the number of entries in sequence databases is considerably reduced for remote locations that are not served by nearby research stations, and many entries are not supported by process related data and physical and chemical ecological data.
Biodiversity and community structure
Antarctic lacustrine systems have been considered ideal for the study of prokaryote diversity and evolution as they form discrete units and have a wide and variable suite of selection pressures (Laybourn-Parry and Pearce 2007). From the growing data base on the biodiversity of polar aquatic systems, patterns are starting to emerge, such as the largely cold-adapted genera Psychrobacter and Exiguobacterium (Rodrigues et al. 2009) and Polynucleobacter (Hahn et al. 2015). We are, however, still far from a comprehensive understanding of the total level of microbial diversity in many environments. What is clear is that each system studied appears to have its own distinct composition of prokaryotes, although it is still possible to identify wide-scale patterns and taxa which appear to be well adapted to this type of environment.
Bacterial biomass tends to concentrate at the sediment–water interface, within the surface layers of sediment, and in meromictic lakes on the chemocline/oxycline and in the lower anoxic waters. Where water columns have been studied in detail, it is clear that community structure varies with depth (Izaguirre et al. 2003; Pearce et al. 2003, 2005; Pearce 2005; Foong et al. 2010; Villaescusa et al. 2010). This is particularly marked in meromictic lakes where there are strong physical and chemical gradients (Bowman et al. 1997; Karr et al. 2005; Glatz et al. 2006).
Biogeographical patterns are starting to emerge, for example the predominance of Flavobacterium, Pseudomonas and Polaromonas within freshwater prokaryotic communities in shallow lakes in the northern Victoria Land, East Antarctica (Michaud et al. 2012). A number of studies have focused on transects across and within geographical locations. Romina Schiaffino et al. (2011)analysed the latitudinal variation of bacterioplankton in 45 freshwater environments (lakes, shallow lakes and ponds) across a transect of more than 2100 km stretching from the Argentinean Patagonia (45°S) to the Maritime Antarctic (63°S). Of 76 operational taxonomic units identified in the lakes studied, 45 were common to Patagonian and Antarctic water bodies, 28 were present only in Patagonian lakes and three were restricted to the Antarctic lakes. More importantly, significant differences were found in bacterioplankton community composition between Patagonia and Antarctica. Statistical analysis showed that phosphate, light and latitude had significant effects on total bacterioplankton abundance. In the Vestfold Hills, Logares et al. (2012) studied the biogeography of bacterial communities in a range of freshwater lakes and saline lakes of differing salinity that had undergone different evolution. Their results indicated that bacterioplankton community composition was strongly correlated with salinity and weakly correlated with geographical distance between lakes. A few abundant taxa were shared between some lakes and coastal marine communities; nevertheless, lakes contained a large number of taxa not detected in the adjacent sea. Abundant and rare taxa within saline communities presented similar biogeography, suggesting that these groups have comparable environmental sensitivity. Habitat specialists and generalists were detected among abundant and rare taxa, with specialists being relatively more abundant at the extremes of the salinity gradient. An investigation of the occurrence and diversity of the family Legionellaceae in lakes on the Antarctic Peninsula supports the concept of biogeographical patterns of bacterial assemblages and suggest that both spatial and environmental factors control bacterioplankton community structure (Carvalho et al. 2008).
Variations between lakes types and cryoconite holes across Antarctica
Subglacial lakes
Analysis of accretion ice from Lake Vostok has revealed phylotypes belonging to the Alpha-proteobacteria, Beta-proteobacteria and Gamma-proteobacteria, the Firmicutes, the Actinobacteria and Bacteroidetes lineages (Priscu et al. 1999; Christner et al. 2001, 2006; Bulat et al. 2004). Among the Beta-proteobacteria, clone sequences were most closely related to aerobic methylotrophic species in the genera Methylobacillus and Methylophilus. These bacteria are able to use C-1 compounds as a substrate (e.g. methanol, formate, carbon monoxide) and carbon assimilation is via a ribose monophosphate pathway, suggesting the potential for methylotrophy (Christner et al. 2006). Lake Whillans was sampled for the first time in 2012 and is dominated by Beta-proteobacteria, Gamma-proteobacteria, Delta-proteobacteria, Bacteroidetes and Actinobacteria, as well as Chloroflexi and Thaumarchaeota. As stated under the section on Bacterial Abundance and Productivity, the most common phylotypes were related to chemoautotrophic species utilizing nitrogen, iron and sulphur as energy sources (Christner et al. 2014).
Surface lakes
Reported occurrence of common heterotrophic bacterial phyla is shown in Table 4. Caution should be exercised in interpreting the data, as in continental Antarctica saline lakes have been more intensively studied for bacterial diversity than freshwater lakes. Lakes Fryxell and Bonney in MDVs and Ace Lake in the Vestfold Hills are particularly well investigated. There is clear evidence of some degree of endemism based on what we know to date (e.g. Franzmann et al. 1997; McCammon et al. 1998; McCammon and Bowman 2000).
The lakes of the McMurdo Dry Valleys (MDVs) are old and have a complex evolutionary history. For example, a lake has existed in the Lake Bonney basin for about 300,000 years. The Dry Valley lakes are the remains of large proglacial lakes, and a number have undergone drying down and refilling phases in their history, as a result of climatic change (Hendy 2000; Green and Lyons 2009). In contrast, the saline lakes of coastal oases like the Vestfold Hills are in most cases young, postdating the last glacial maximum, and of marine origin, having been formed by a combination of isostatic uplift and changes in sea level (Zwart et al. 1998). The difference in the evolution of the lakes is to some extent reflected in the diversity picture we have to date with distinct sequences in some lakes such as Lake Bonney (Glatz et al. 2006). Meromictic lakes have strong physical and chemical vertical gradients, typified by an upper oxygenated layer, a transition zone (the chemocline) and lower often warmer, anoxic waters. Most data pertain to the lakes of the Taylor Valley, Lake Vanda in the Wright Valley (MDVs) and the Vestfold Hills. Lake Vanda differs from the MDV lakes in being dominated by Ca and Cl ions, whereas Lakes Bonney and Fryxell are dominated by Na and Cl ions (Green and Lyons 2009).
The saline lakes of the Vestfold Hills range from unstratified brackish systems, through stratified meromictic lakes to hypersaline systems that are thermally stratified in summer and mixed in winter, for example Deep Lake (Laybourn-Parry and Wadham 2014). The extremely hypersaline lakes do not form ice covers. Thus, these systems present a wide range of physical and chemical conditions. They are marine-derived, and their present day prokaryote and eukaryote biota have probably largely evolved from marine ancestors. However, most, if not all, are likely to still be present in the marine ecosystem, although no one has yet looked systematically.
The MDVs have few freshwater lakes. Lake Hoare and Lake Miers are the only two, and of these Lake Hoare is the only one where the biota have been studied in detail. There are also many ponds, both freshwater and saline, but these have not been subject to bacteriological analysis. In contrast, the Vestfold Hills has many freshwater lakes and ponds, but there are little taxonomic bacterial data, the focus has been on carbon cycling processes. The Larsemann Hills lies to the south of the Vestfold Hills and has many mainly freshwater and slightly brackish lakes on which there are few data. The microbial biodiversity of lakes in the Schirmacher Oasis has only been investigated in recent years, and thus data are limited (Huang et al. 2010, 2014; Peeters et al. 2012) (Table 4). However, based on the data it appears that these lakes have greater diversity than one of Antarctica’s largest perennially ice-covered surface lakes, Lake Untersee that lies 90 km SW of the Schirmacher Oasis. There were no common genera between the lakes of the Schirmacher Oasis and Lake Untersee (Huang et al. 2010).
The maritime Antarctic includes the Antarctic Peninsula and nearby groups of islands and has exclusively freshwater lakes and ponds. Here, the climate is less severe than in continental Antarctica and the catchments may have vegetation in the form of mosses. The lakes of this region are small and relatively shallow compared to many of the lakes in the MDVs, the Vestfold Hills and other ice-free coastal areas. Some of the Maritime Antarctic lakes have been subject to detailed bacterial diversity analysis (Table 4).
There have been few ambitious large-scale biodiversity studies. Van Trappen et al. (2002) studied the diversity of 746 heterotrophic bacteria isolated from microbial mats from ten freshwater and saline Antarctic lakes, including lakes from the MDVs (Lakes Hoare and Fryxell), the Vestfold Hills (Lakes Ace, Lake Druzhby, Grace Lake, Highway Lake, Pendant Lake, Organic Lake and Watts Lake) and the Larsemann Hills (Lake Reid), under heterotrophic growth conditions. These strains were divided into 41 clusters, containing 2–77 strains, 31 strains formed single branches. Several groups consisted of strains from different lakes from the same region, or from different regions. The 16S rRNA genes from 40 strains representing 35 different fatty acid groups were sequenced. The strains belonged to the Alpha-, Beta- and Gamma-Proteobacteria, the high (Actinobacteria) and low (Firmicutes) percent G+C Gram-positives, and to the Cytophaga–Flavobacterium–Bacteroides branch. For strains representing 16 clusters, validly named nearest phylogenetic neighbours showed pairwise sequence similarities below 97 %. This indicated that the clusters they represent belong to taxa that have not been sequenced, or are as yet unnamed new taxa, related to Alteromonas, Bacillus, Clavibacter, Cyclobacterium, Flavobacterium, Marinobacter, Mesorhizobium, Microbacterium, Pseudomonas, Saligentibacter, Sphingomonas and Sulfitobacter.
Peeters et al. (2011) used cultivation techniques to study the heterotrophic bacterial diversity in two microbial mat samples originating from the littoral zone of two continental Antarctic lakes (Forlidas Pond and Lundström Lake) in the Dufek Massif (within the Pensacola Mountains group of the Transantarctic Mountains) and Shackleton Range, respectively. Nearly 800 isolates were picked after incubation on several growth media at different temperatures. They were grouped using a whole-genome fingerprinting technique, repetitive element palindromic PCR and partial 16S rRNA gene sequencing. Phylogenetic analysis of the complete 16S rRNA gene sequences of 82 representatives showed that the isolates belonged to four major phylogenetic groups: Actinobacteria, Bacteroidetes, Proteobacteria and Firmicutes. A relatively large difference between the samples was apparent. Forlidas Pond has a perennial ice cover underlain by hypersaline brine, with summer thaw forming a less saline littoral moat. This was reflected in the bacterial diversity with a dominance of isolates belonging to Firmicutes, whereas isolates from the freshwater Lundström Lake revealed a dominance of Actinobacteria. A total of 42 different genera were recovered, including first records from Antarctica for Albidiferax, Bosea, Curvibacter, Luteimonas, Ornithinibacillus, Pseudoxanthomonas, Sphingopyxis and Spirosoma. Additionally, a considerable number of potential new species and new genera were recovered distributed over different phylogenetic groups. Comparison with public databases showed that overall 72 % of the phylotypes are cosmopolitan, whereas 23 % are currently only known from Antarctica. However, for the Bacteroidetes, the majority of the phylotypes recovered are at present known only from Antarctica and many of these represented previously unknown species.
In extreme saline lakes such as Deep Lake (Vestfold Hills) that has a salinity ten times that of seawater, the only viable prokaryotes recovered from water samples were halophytic Archaea (Halobacterium spp.) (McMeekin and Franzmann 1988). Organic Lake is another hypersaline Vestfold Hills lake (6.5 times seawater) that also exhibits low species diversity. It contains a bacterium (probably Halomonas) that produces dimethyl sulphide (Franzmann et al. 1987). Lake Vida is an unusual extreme lake in the MDVs. It is 3.5 km long and 1 km wide. It has a depth of 19 m and has an ice cover around 15 m maximum depth that overlies a concentrated brine layer around 5 m in depth (Doran et al. 2003). The brine contains 32 unique sequences distributed across a number of bacterial phyla: the Proteobacteria, Lentisphaerae, Firmicutes, Spirochaeta, Bacteroidetes, Verrucomicrobia, Candidatus Saccharibacteria (Albertsen et al. 2013) and Actinobacteria. A number of these have not been previously observed in high salinity systems (Murray et al. 2012).
Cryoconite holes
Relatively more data are available on bacterial community diversity for glaciers in the Arctic and Alpine regions. In Antarctica, the main focus has been on cryoconites holes on glaciers in the Taylor Valley (MDVs). Based on fluorescence in situ hybridization (FISH), there was a clear difference between the Canada Glacier and the Hughes Glacier. Alpha-Proteobacteria were low in abundance on the Canada Glacier (1.6 %), while they contributed 11.2 % of the community on the Hughes Glacier. The Beta-proteobacteria contributed a higher proportion of the community on both glaciers. The Cytophaga-Flavobacteria probe hybridized 87 % of total bacteria in the sediments of the Canada Glacier and 27.6 % on the Hughes Glacier (Foreman et al. 2007). Similar differences have also been noted among eukaryotic phyla in cryoconites holes on glaciers in the Taylor valley (Porazinska et al. 2004). Phylogenetic analysis of bacteria isolated from cryoconite holes on the Canada Glacier revealed members of a range of bacterial lineages, the Actinobacteria, Acidobacteria, Cytophagales, Gemmatimonas, Planctomycetes, Alpha-, Beta- and Gamma-Proteobacteria, Verrucomicrobia and the photosynthetic Cyanobacteria (Christner et al. 2003). A number of the isolates were species previously isolated from microbial mats in neighbouring Lake Fryxell, which the Canada Glacier feeds (Brambilla et al. 2001).
Marked differences in relative abundance of phyla were also observed between a small glacier on Signy Island (South Orkney Islands, Maritime Antarctic) and the Sørsdal Glacier that abuts the Vestfold Hills in continental Antarctica. The Alpha-, Beta-, Delta- and Gamma-Proteobacteria phylum represented around half of the abundance in Signy Island and 25 % on the Sørsdal Glacier. Other phyla present on both glaciers were Bacteroidetes, Actinobacteria, Acidobacteria and Firmicutes. On the Signy Island glacier Verrucomicrobia were present, and on the Sørsdal Glacier Gemmatimonadetes were detected. Interestingly, this phylum is fairly uncommon except in soils and was not found on any of the Arctic glaciers from the Greenland, Norway and Svalbard investigated in the study (Cameron et al. 2011).
Common patterns
As shown in the previous section, no system is entirely unique, and some discernible patterns can be identified, including patterns of abundance and diversity. Frequently encountered classifications include Actinobacteria, Proteobacteria (especially the Alpha-, Beta- and Gamma-proteobacteria), Chloroflexi, Bacteroidetes, Flavobacteria, Cytophaga, Bacilli and Janthinobacteria. Much of the genetic information we currently have relates to uncultivated prokaryotes. We know virtually nothing about their physiology and ecology. Most pre-metagenomic era investigations were restricted to a few tens to a few hundred sequences, most of which remain uncultured. However, there appears to be a close link between bacterial community composition and environmental heterogeneity in maritime Antarctic lakes, especially regarding trophic status (Pearce 2005; Schiaffino et al. 2009). Patterns are evident when a polyphasic approach is used. For example, Michaud et al. (2012) applied both PCR-dependent and PCR-independent molecular techniques to analyse the prokaryotic community in surface waters of small, shallow freshwater lakes in northern Victoria Land. The in situ abundance of different bacterial groups was determined by fluorescence in situ hybridization, whereas bacterial diversity was investigated by 16S rRNA gene sequencing of bacterial clones and isolates. The different approaches allowed the identification of the significant microbial components of the lake bacterioplankton communities, indicating a predominance of Flavobacterium, Pseudomonas and Polaromonas (up to about 56 % of total sequences). Interestingly, they found that the closest blast matches to their sequences were predominantly from polar lakes and ponds, in addition to streams and glaciers, suggesting a bipolar distribution of freshwater bacteria, reinforcing the idea that physicochemical and trophic status may affect the structure and composition of the bacterioplankton assemblages in Antarctic lakes.
Future directions
Future studies are likely to rely on environmental metagenomics, given the increasing cost-effectiveness and coverage of high-throughout sequencing techniques. Environmental metagenomics focuses on sequencing the entire environmental genome (rather than just specific genes such as the 16S rRNA gene) and has already been applied in an Antarctic Freshwater Lake Metagenome, registered with the JGI Genome Portal. The main advantage of metagenomics is that it provides information about the functional potential of the community. Yau et al. (2013) used shotgun metagenomics to study the unusual sulphur chemistry in Organic Lake, which they performed on size-fractionated samples collected along a depth profile. Ng et al. (2010) adopted a metaproteogenomic analysis of a dominant green sulphur bacterium from Ace Lake, Antarctica. Green sulphur bacteria dominate the bacteriochlorophylls of the lower chemocline in Ace Lake. The dominant species, designated C-Ace, possess chlorosomes with extremely efficient light-capturing capabilities enabling phototrophy and growth at very low light intensities. This, coupled with their ability to grow at low temperatures, is probably responsible for their dominance.
Much of the literature pertaining to bacterial diversity lacks information on the chemistry and physical conditions of the environment and data on processes such as bacterial production. Moreover, much sampling is short term and may not involve vertical sampling of the water column in lakes. There is a real need for more holistic, integrated, longer-term investigations so that we can understand what drives bacterial evolution and functioning in Antarctic lakes and on glaciers. It is evident that most of our data come from parts of the Maritime Antarctic, the MDVs and the Vestfold Hills. There are little data from other coastal lake regions such as the Syowa Oasis, the Bunger Hills and the Schirmacher Oasis (see Fig. 1). A more trans-Antarctic database is needed. An exemplar of the integrated approach is seen in the US National Foundation-funded Long Term Ecosystem Research Program in the MDVs.
There are some aquatic environments for which we have few or no bacteriological data: for example, epishelf lakes that occur in the polar regions (though mainly in Antarctica), cryolakes on glaciers and ponds and small lakes on ice shelves. Research in Antarctica is costly and logistically challenging, and this has limited the acquisition of data. However, it is now widely accepted that Antarctic and Arctic lakes respond quickly to climatic variation and are sensitive barometers of climate change (Quayle et al. 2002; Antoniades et al. 2007). Heterotrophic bacteria are an important component of the microbially dominated food webs of Antarctic lacustrine systems. An expansion of our knowledge of their biodiversity and functional dynamics is timely.
References
Albertsen M, Hugenholtz P, Skarshewski A, Nielsen KL, Tyson GW, Nielsen PH (2013) Genome sequences of rare, uncultured bacteria obtained by differential coverage binning of multiple metagenomes. Nat Biotechnol 31:533–538
Alfreider A, Pernhaler J, Amann R, Sattler B, Glockner F, Wille A, Psenner R (1996) Community analysis of the bacterial assemblages in the winter cover and pelagic layers of a high mountain lake by in situ hybridization. Appl Environ Microbiol 62:2138–2144
Anesio AM, Sattler B, Foreman C, Hodson AJ, Tranter M, Psenner R (2011) Carbon fluxes through bacterial communities on glacier surfaces. Ann Glaciol 51(56):32–40
Antoniades D, Crawley C, Douglas MSV, Pienitz R, Andersen D, Foran PT, Hawes I, Pollard W, Vincent WF (2007) Abrupt environmental change in Canada’s northernmost lake inferred from diatom and fossil pigment stratigraphy. Geophys Res Lett 34:L18708. doi:10.1029/2007Gl030947
Bagshaw EA, Tranter M, Wadham JL, Fountain AG, Basagic H (2010) Dynamic behaviour of supraglacial lakes on cold polar glaciers: Canada Glacier, McMurdo Dry Valleys, Antarctica. J Glaciol 56:366–368
Bayliss P, Ellis-Evans JC, Laybourn-Parry J (1997) Temporal patterns of primary production in a large ultra-oligotrophic Antarctic freshwater lake. Polar Biol 18:363–370
Bell EM, Laybourn-Parry J (1999) Annual plankton dynamics in an Antarctic saline lake. Freshw Biol 41:507–519
Bowman JP, McCammon SA, Skerratt JH (1997) Methylosphaera hansonii gen. nov., sp. nov., a psychrophilic, group I methanotroph from Antarctic marine-salinity, meromictic lakes. Microbiology 143:1451–1459
Bowman JP, Rea SM, McCammon SA, McMeekin TA (2000) Diversity and community structure within anoxic sediment from marine meromicic lakes and a coastal marine basin, Vestfold Hills, Eastern Antarctica. Environ Microbiol 2:227–237
Bowman JP, Nichols CM, Gibson JAE (2003) Algoriphagus ratkowskyi gen.nov., sp. nov., Brumimicrobium glaciale gen. nov., sp. nov., Cryomorpha ignava gen. nov., sp. nov. and Crocinitomix catalasitica gen. nov., sp. nov, novel Flavobacteria isolated from various polar habitats. Int J Syst Evol Microbiol 53:1343
Brambilla E, Hippe H, Hagelstein A, Tindall BJ, Stackebrandt E (2001) 16S rDNA diversity of cultured and uncultured prokaryotes of a mat sample from Lake Fryxell, McMurdo Dry Valleys, Antarctica. Extremophiles 5:23–33
Bulat SA, Alekhina IA, Blot M, Petit J-R, de Angelis M, Wagenbach D, Lipenkov VY, Vasilyena LP, Wloch DM, Raynaud D, Lukin VV (2004) DNA signature of thermophilic bacteria from the aged accretion ice of Lake Vostok, Antarctica; implications for searching for life in extreme icy environments. Int J Astrobiol 3:1–12
Butler HG (1999) Seasonal dynamics of the planktonic microbial community in a maritime Antarctic lake undergoing eutrophication. J Plankton Res 21:2393–2419
Butler HG, Edworthy MG, Ellis-Evans JC (2000) Temporal plankton dynamics in an oligotrophic maritime Antarctic lake. Freshw Biol 43:215–230
Cameron KA, Hodson AJ, Osborn AM (2011) Structure and diversity of bacterial, eukaryotic and archaeal communities in glacial cryoconite holes from the Arctic and the Antarctic. FEMS Microbiol Ecol 82:254–267
Carneiro AR, Ramos RT, Dall’Agnol H, Pinto AC, de Castro Soares S, Santos AR, Guimarães LC, Almeida SS, Baraúna RA, das Graças DA, Franco LC, Ali A, Hassan SS, Nunes CI, Barbosa MS, Fiaux KK, Aburjaile FF, Barbosa EG, Bakhtiar SM, Vilela D, Nóbrega F, dos Santos AL, Carepo MS, Azevedo V, Schneider MP, Pellizari VH, Silva A (2012) Genome sequence of Exiguobacterium antarcticum B7, isolated from a biofilm in Ginger Lake, King George Island, Antarctica. J Bacteriol 194:6689–6690
Carvalho FR, Nastasi FR, Gamba RC, Foronda AS, Pellizari VH (2008) Occurrence and diversity of Legionellaceae in polar lakes of the Antarctic peninsula. Curr Microbiol 57:294–300
Christner BC, Mosley-Thompson E, Thompson LG, Reeve JN (2001) Isolation of bacteria and 16S rDNAs from Lake Vostok accretion ice. Environ Microbiol 3:570–577
Christner BC, Kvitko BH, Reeve JN (2003) Molecular identification of bacteria and Eukarya inhabiting an Antarctic cryoconite hole. Extremophiles 7:177–183
Christner BC, Royston-Bishop G, Foreman CM, Arnold BR, Tranter M, Welch KA, Lyons WB, Tsapin AI, Studinger M, Priscu JC (2006) Limnological conditions in subglacial Lake Vostok. Limnol Oceanogr 51:2485–2501
Christner BC, Priscu JC, Achberger AM, Barbante C, Carter SP et al (2014) Subglacial Lake Whillans: a microbial ecosystem beneath the West Antarctic ice sheet. Nature 512:310–313
Clocksin KM, Deborah O, Madigan J, Madigan MT (2007) Cold-active chemoorganotrophic bacteria from permanently ice-covered Lake Hoare, Mcmurdo Dry Valleys, Antarctica. Appl Environ Microbiol 73:3077–3083
Cole JJ, Findlay S, Pace ML (1988) Bacterial production in fresh and saltwater ecosystems: a cross-system overview. Mar Ecol Prog Ser 43:1–10
Collins MD, Lawson PA, Labrenz M, Tindall BJ, Weiss N, Hirsch P (2002) Nesterenkonia lacusekhoensis sp. nov., isolated from hypersaline Ekho Lake, East Antarctica, and emended description of the genus Nesterenkonia. Int J Syst Evol Microbiol 4:1145–1150
Dolhi JM, Maxwell DP, Morgan-Kiss RM (2013) The Antarctic Chlamydomonas raudensis: an emerging model for cold adaptation of photosynthesis. Extremophiles 17:711–722
Dorado-Garcia I, Medina-Sánchez JM, Herrera G, Cabrerizo MJ, Carrillo P (2014) Quantification of carbon and phosphorus co-limitation in bacterioplankton: new insights on an old topic. PLoS Biol 9(6):e999288
Doran PT, Fritsen CH, McKay CP, Priscu JC, Adams EE (2003) Formation and character of an ancient 19-m ice cover and underlying trapped brine in an ‘ice-sealed’ east Antarctic lake. Proc Natl Acad Sci U S A 100:26–31
Ellis-Evans JC, Laybourn-Parry J, Bayliss P, Perriss SJ (1998) Physical, chemical and microbial community characteristics of lakes of the Larsemann Hills, continental Antarctica. Arch Hydrobiol 141:29–230
Felip M, Sattler B, Psenner R, Catalan J (1995) Highly active microbial communities in the ice and snow cover of high mountain lakes. Appl Environ Microbiol 61:2394–2401
Ferris JM, Burton HR (1988) The annual cycle of heat content and mechanical stability of hypersaline Deep Lake, Vestfold Hills, Antarctica. Hydrobiologia 165:115–128
Foong CP, Wong Vui Ling CM, González M (2010) Metagenomic analyses of the dominant bacterial community in the Fildes Peninsula, King George Island (South Shetland Islands). Polar Sci 4:263–273
Foreman CM, Sattler B, Mikucki JA, Porazinska DL, Priscu JC (2007) Metabolic activity and diversity of cryoconites in the Taylor Valley, Antarctica. J Geophys Res 112:32
Franzmann PD, Rohde M (1991) An obligately anaerobic, coiled bacterium from Ace Lake, Antarctica. J Gen Microbiol 137:2191–2196
Franzmann PD, Deprez PP, Buron HR, van den Hoff J (1987) Limnology of Organic Lake, Antarctica, a meromictic lake that contains high concentrations of dimethyl sulfide. Aust J Mar Freshw Res 38:409–417
Franzmann PD, Skyring GW, Burton HR, Deprez PP (1988) Sulfate reduction rates and some aspects of the limnology of four lakes and a fjord in the Vestfold Hills, Antarctica. Hydrobiologia 165:25–33
Franzmann PD, Höpfl P, Weiss N, Tindall BJ (1991a) Psychrotrophic, lactic acid-producing bacteria from anoxic waters in Ace Lake, Antarctica; Carnobacterium funditum sp. nov. and Carnobacterium alterfunditum sp. nov. Arch Microbiol 156:255–262
Franzmann PD, Roberts NJ, Mancuso CA, Burton HR, McMeekin TA (1991b) Methane production in meromictic Ace lake, Antarctica. Hydrobiologia 210:191–201
Franzmann PD, Liu Y, Balkwill DL, Aldrich HC, Conway E et al (1997) Methanogenium frigidum sp. Nov., a psychrophilic, H2-using methanogen from Ace Lake. Int J Syst Bacteriol 47:1068–1072
Fricker HA, Scambos T, Bindschadler R, Padman L (2007) An active subglacial water system in West Antarctica mapped from space. Science 315:1544–1548
Fukui F, Torii T, Okabe S (1985) Vertical distribution of nutrients and DOC in lake waters near Syowa Station, Antarctica. Antarct Rec 86:28–35
Gibson JAE, Andersen DT (2002) Physical structure of the epishelf lakes of the southern Bunger Hills, East Antarctica. Antarct Sci 14:253–261
Gilbert J, Davies P, Laybourn-Parry J (2005) A hyperactive calcium dependent antifreeze protein in an Antarctic bacterium. FEMS Microbiol Lett 245:67–72
Glatz RE, Lepp PW, Ward BB, Francis CA (2006) Planktonic microbial community composition across steep physical/chemical gradients in permanently ice-covered Lake Bonney, Antarctica. Geobiology 4:53–67
Green WJ, Lyons WB (2009) The saline lakes of the McMurdo Dry Valleys, Antarctica. Aquat Geochem 15:321–348
Hahn MW, Koll U, Jezberová J (2015) Global phylogeography of pelagic Polynucleobacter bacteria: restricted geographic distribution of subgroups, isolation by distance and influence of climate. Environ Microbiol 17:829–840
Hall EK, Dzialowski AR, Stoxen SM, Cotner JB (2009) The effect of temperature on the coupling between phosphorus and growth in lacustrine bacterioplankton communities. Limnol Oceanogr 54:880–889
Hansson L-A, Hylander H, Dartnall HJG, Lidström S, Svensson J-E (2011) High zooplankton diversity in the extreme environments of the McMurdo Dry Valley lakes, Antarctica. Antarct Sci 24:131–138
Hawes I, Howard-Williams C, Fountain AG (2008) Ice-based freshwater ecosystems. In: Vincent WF, Laybourn-Parry J (eds) Polar lakes and rivers, limnology of Arctic and Antarctic ecosystems. Oxford University Press, Oxford, pp 103–118
Heath CW (1988) Annual primary productivity of an Antarctic continental lake: phytoplankton and benthic algal mat production strategies. Hydrobiologia 165:77–87
Hendy CH (2000) Late quaternary lakes in the McMurdo sound region of Antarctica. Geogr Ann A 82:411–432
Henshaw T, Laybourn-Parry J (2002) The annual patterns of photosynthesis in two large freshwater, ultra-oligotrophic Antarctic lakes. Polar Biol 25:744–752
Herbei R, Lyons WB, Laybourn-Parry J, Gardner C, Priscu JC, McKnight DM (2010) Physiochemical properties influencing biomass abundance and primary production in Lake Hoare, Antarctica. Ecol Model 221:1184–1193
Hobbie JE, Laybourn-Parry J (2008) Heterotrophic microbial processes in polar lakes. In: Vincent WF, Laybourn-Parry J (eds) Polar lakes and rivers, limnology of Arctic and Antarctic ecosystems. Oxford University Press, Oxford, pp 197–212
Hodgson DA, Johnston NM, Caulkett AP, Jones VJ (1998) Palaeolimnology of Antarctic fur seal Arctocephalus gazelle populations and implications for Antarctic management. Biol Conserv 83:145–154
Hodson AJ, Anesio AM, Tranter M, Fountain AG, Osborn M, Priscu JC, Laybourn-Parry J, Sattler B (2008) Glacial ecosystems. Ecol Monogr 78:41–67
Hodson AJ, Paterson H, Westwood K, Cameron K, Laybourn-Parry J (2013) A blue ice ecosystem on the margins of the East Antarctic ice sheet. J Glaciol. doi:10.3189/2013JoG12J052
Hofer JS, Sommaruga R (2001) Seasonal dynamics of viruses in an alpine lake: importance of filamentous forms. Aquat Microb Ecol 26:1–11
Hosoi-Tanabe S, Zhang H, Zhu D, Nagata S, Ban S, Imura S (2010) Comprehensive analysis of an antarctic bacterial community with the adaptability of growth at higher temperatures than those in Antarctica. Biocontrol Sci 15:57–62
Huang JP, Hoover RB, Swain A, Murdock C, Bej AK (2010) Comparison of the microbial diversity and abundance between the freshwater land-locked lakes of the Schirmacher Oasis and the perennially ice-covered Lake Untersee in East Antarctica. NASA Technical Report: ntrs.nasa.gov/archive/nasa/casi,ntrs.gov/20100040620
Huang JP, Swain AK, Anderson DT, Bej AK (2014) Bacterial diversity within five unexplored freshwater lakes interconnected by surface channels in East Antarctica dronning maud land (Schirmacher Oasis) using amplicon pyrosequencing. Polar Biol 37:359–366
Izaguirre I, Allende L, Marinone MC (2003) Comparative study of the planktonic communities of three lakes of contrasting trophic status at Hope Bay (Antarctic Peninsula). J Plankton Res 25:1079–1097
Jacquet S, Domaizon I, Chardon C, Personnic S (2013) Are small grazers and/or viruses a structuring factor of free-living bacterial community in Lake Geneva? Adv Microbiol 3:233–248
James SR, Burton HR, McMeekin TA, Mancus CA (1994) Seasonal abundance of Halomonas meridian, Halomonas subglaciescola, Flavobacerium gondwanense and Flavobacterium salegens in four Antarctic lakes. Antarct Sci 6:325–332
James MR, Pridmore RD, Cummings VJ (1995) Planktonic communities of melt ponds on the McMurdo Ice Shelf, Antarctica. Polar Biol 15:555–567
Jansson M, Bergström A-K, Lymer D, Vrede K, Karlsson J (2006) Bacterioplankton and nutrient use efficiencies under variable organic carbon and inorganic phosphorus ratios. Microb Ecol 52:358–364
Karr EA, Sattley WM, Rice MR, Jung DO, Madigan MT, Achenbach LA (2005) Diversity and distribution of sulfate-reducing bacteria in permanently frozen Lake Fryxell, McMurdo Dry Valleys, Antarctica. Appl Environ Microbiol 71:6353–6359
Karr EA, Ng JM, Belchik SM, Sattley WM, Madigan MT, Achenbach LA (2006) Biodiversity of methanogenic and other Archaea in the permanently frozen Lake Fryxell, Antarctica. Appl Environ Microbiol 72:1663–1666
Kurosawa N, Sato S, Kawarabayasi Y, Imura S, Naganuma T (2010) Archaeal and bacterial community structures in the anoxic sediment of Antarctic meromictic lake Nurume-Ike. Polar Sci 4:421–429
Labrenz M, Collins MD, Lawson PA, Tindall BJ, Braker G, Hirsch P (1998) Antarctobacter heliothermus gen. nov., sp. nov., a budding bacterium from hypersaline and heliothermal Ekho Lake. Int J Syst Bacteriol 48:1363–1372
Lanoil BD, Ciuffetti LM, Giovannoni SJ (1996) The marine bacterium Pseudoalteromonas haloplanktis has a complex genome structure composed of two separate genetic units. Genome Res 6(12):1160–1169
Lauro FM, DeMaere MZ, Yau S, Brown MV, Ng C, Wilkins D, Raftery MJ, Gibson JAE, Andrews-Pfannkoch C, Lewis M, Hoffman JM, Thomas T, Cavicchioli R (2011) An interactive study of a meromictic lake ecosystem in Antarctica. ISME J 5:879–895
Lawson PA, Collins MD, Schumann P, Tindall BJ, Hirsch P, Labrenz M (2000) New LL-diaminopimelic acid-containing actinomycetes from hypersaline, heliothermal and meromictic Antarctic Ekho Lake: Nocardioides aquaticus sp. nov. and Friedmanniella [correction of Friedmannielly] lacustris sp. nov. Syst Appl Microbiol 23:219–229
Laybourn-Parry J, Bayliss B (1996) Seasonal dynamics of the planktonic community of Lake Druzhby, Princess Elizabeth Land, Eastern Antarctica. Freshw Biol 35:57–67
Laybourn-Parry J, Walton M (1998) Seasonal heterotrophic flagellate and bacterial plankton dynamics in a large oligotrophic lake—Loch Ness. Freshw Biol 39:1–8
Laybourn-Parry J, Pearce DA (2007) The biodiversity and ecology of Antarctic lakes—models for evolution. Philos Trans R Soc Lond B 362:2273–2289
Laybourn-Parry J, Bell EM (2014) Ace Lake: three decades of research on a meromictic, Antarctic lake. Polar Biol 37:1685–1699
Laybourn-Parry J, Wadham JL (2014) Antarctic lakes. Oxford University Press, Oxford
Laybourn-Parry J, Walton M, Young J, Jones RI, Shine A (1994) Protozooplankton and bacterioplankton in a large oligotrophic lake—Loch Ness, Scotland. J Plankton Res 16:1655–1670
Laybourn-Parry J, Bayliss P, Ellis-Evans JC (1995) The dynamics of heterotrophic nanoflagellates and bacterioplankton in a large ultra-oligotrophic Antarctic lake. J Plankton Res 17:1835–1850
Laybourn-Parry J, Quayle W, Henshaw T (2002) The biology and evolution of Antarctic saline lakes in relation to salinity and trophy. Polar Biol 25:542–552
Laybourn-Parry J, Henshaw T, Jones DJ, Quayle W (2004) Bacterioplankton production in freshwater Antarctic lakes. Freshw Biol 49:735–744
Laybourn-Parry J, Marshall WA, Marchant HJ (2005) Flagellate nutritional versatility as a key to survival in two contrasting Antarctic saline lakes. Freshw Biol 50:830–838
Laybourn-Parry J, Madan NJ, Marshall WA, Marchant HJ, Wright SW (2006) Carbon dynamics in an ultra-oligotrophic epishelf lake (Beaver Lake, Antarctica) in summer. Freshw Biol 51:1116–1130
Laybourn-Parry J, Marshall WJ, Madan NJ (2007) Viral dynamics and patterns of lysogeny in saline Antarctic lakes. Polar Biol 30:351–358
Laybourn-Parry J, Tranter M, Hodson AJ (2010) The ecology of snow and ice environments. Oxford University Press, Oxford
Lisle JT, Priscu JC (2004) The occurrence of lysogenic bacteria and microbial aggregates in the lakes of the McMurdo Dry Valleys, Antarctica. Microb Ecol 47:427–439
Logares R, Lindström ES, Langenheder S, Logue JB, Paterson H, Laybourn-Parry J, Rengefors K, Tranvik L, Bertilsson S (2012) Biogeography of bacterial communities exposed to progressive long-term environmental change. ISME J 7:937–948
Lymer DJB, Logue CPD, Brussard A-C, Baudoux K, Vrede K, Lindström ES (2008) Temporal variation in freshwater viral and bacterial community composition. Freshw Biol 53:1163–1175
Madan NJ, Marshall WA, Laybourn-Parry J (2005) Virus and microbial loop dynamics over an annual cycle in three contrasting Antarctic lakes. Freshw Biol 50:1291–1300
Mancuso CA, Franzmann PD, Burton HR, Nichols PD (1990) Microbial community structure and biomass estimates of a methanogenic Antarctic lake ecosystem as determined by phospholipid analysis. Microb Ecol 19:73–95
Marshall WA (1996) Biological particles over Antarctica. Nature 383:680
Marshall WA, Laybourn-Parry J (2002) The balance between photosynthesis and grazing in Antarctic mixotrophic cryptophytes during summer. Freshw Biol 47:2060–2070
Matsumoto GI (1989) Biogeochemical study of organic-substances in Antarctic lakes. Hydrobiologia 172:265–289
McCammon SA, Bowman JP (2000) Taxonomy of Antarctic Flavobacterium species: description of Flavobacterium gillisiae sp. nov., Flavobacterium tegetincola sp. nov. and Flavobacterium xanthum sp.nov. review and reclassification of (Flavobacterium) salegans as Salegentibacter salegens gen. nov., comb. nov. Int J Syst Evol Microbiol 50:1055–1063
McCammon SA, Innes BH, Bowman JP, Franzmann PD, Dobson SJ, Holloway PE, Skerratt JH, Nicols PD, Rankin JL (1998) Flavobacterium hibernum sp. nov. a lacose utilizing bacterium from a freshwater Antarctic lake. Int J Syst Evol Microbiol 48:1405–1412
McKnight DM, Aiken GR, Smith RL (1991) Aquatic fulvic-acids in microbially based ecosystems—results from two desert lakes in Antarctica. Limnol Oceanogr 36:998–1006
McMeekin TA, Franzmann PD (1988) Effect of temperature on the growth rates of halotolerant and halophilic bacteria isolated from Antarctic saline lakes. Polar Biol 8:281–285
Michaud L, Caruso C, Mangano S, Interdonato F, Bruni V, Lo Giudice A (2012) Predominance of Flavobacterium, Pseudomonas, and Polaromonas within the prokaryotic community of freshwater shallow lakes in the northern Victoria Land, East Antarctica. FEMS Microbiol Ecol 82:391–404
Mondino LJ, Asao M, Madigan MT (2009) Cold-active halophilic bacteria from halophytic bacteria from the ice-sealed Lake Vida, Antarctica. Arch Microbiol 191:785–790
Murray AE, Kenig F, Fritsen CH, McKay CP, Cawley KM, Edwards R, Kuhn E, McKnight DM, Ostrom NE, Peng V, Ponce A, Priscu JC, Samarkin V, Townsend AT, Wagh P, Young SA, Yungg PT, Doran PT (2012) Microbial life at −13 °C in the brine of an ice-sealed Antarctic lake. PNAS 109:20626–20631
Naganuma T, Hua PN, Okamoto T, Ban S, Imura S, Kanda H (2005) Depth distribution of euryhaline bacteria in Suribati Ike, a meromictic lke in East Antarctica. Polar Biol 12:964–970
Ng C, Demaere MZ, Williams TJ, Lauro FM, Raftery M, Gibson JAE, Andrews-Pfannkoch C, Lewis M, Hoffman JM, Thomas T, Cavicchioli R (2010) Metaproteogenomic analysis of a dominant green sulfur bacterium from Ace Lake, Antarctica. ISME J 4:1002–1019
O’Brien WJ, Bahr M, Hershey AE, Hobbie JE, Kipphut GW, Kling GW, Kling H, McDonald M, Miller MC, Rublee P, Vestal JR (1997) The limnology of Toolik Lake. In: Milner AM, Oswood MW (eds) Freshwaters of Alaska. Springer, New York, pp 61–106
Oswald GKA, Robin GQ (1973) Lakes beneath the Antarctic ice sheet. Nature 245:251–254
Overmann J, Beatty JT, Hall KJ (1996) Purple sulfur bacterial control the growth of aerobic heterotrophic bacterioplankton in a meromictic salt lake. Appl Environ Microbiol 62:3251–3258
Panzenböck M, Möbes-Hansen B, Albert R, Herndl GJ (2000) Dynamics of phyto- and bacterioplankton in a high Arctic lake on Franz Joseph Land archipelago. Aquat Microb Ecol 21:265–273
Pearce DA (2003) Bacterioplankton community structure in a maritime Antarctic oligotrophic lake during a period of holomixis, as determined by denaturing gradient gel electrophoresis (DGGE) and fluorescence in situ hybridisation (FISH). Microb Ecol 46(1):92–105
Pearce DA (2005) The structure and stability of the bacterioplankton community in Antarctic freshwater lakes, subject to extremely rapid environmental change. FEMS Microbiol Ecol 53:61–72
Pearce DA, van der Gast C, Lawley B, Ellis-Evans JC (2003) Bacterioplankton community diversity in a maritime Antarctic lake, as determined by culture dependent and culture independent techniques. FEMS Microbiol Ecol 45:59–70
Pearce DA, van der Gast CJ, Woodward K, Newsham KK (2005) Significant changes in the bacterioplankton community structure of a maritime Antarctic freshwater lake following nutrient enrichment. Microbiology 151:3237–3248
Peeters K, Hodgson DA, Convey P, Willems A (2011) Culturable diversity of heterotrophic bacteria in Forlidas Pond (Pensacola Mountains) and Lundström Lake (Shackleton Range), Antarctica. Microb Ecol 62:399–413
Peeters K, Verleyen E, Hodgson DA, Convey P, Ertz D, Vyverman W, Willems A (2012) Heterotrophic bacterial diversity in aquatic microbial mat communities from Antarctica. Polar Biol 35:543–554
Pick FR, Caron DA (1987) Picoplankton and nanoplankton biomass in Lake Ontario: relative contribution of phototrophic and heterotrophic communities. Can J Fish Aquat Sci 44:2164–2172
Porazinska DL, Fountain AG, Nylen TH, Tranter M, Virginia RA, Wall DH (2004) The biodiversity and biogeochemistry of cryoconite holes from McMurdo Dry Valley glaciers, Antarctica. Arct Antarct Alp Res 36:84–91
Priscu JC, Priscu LR, Vincent WF, Howard-Williams C (1987) Photosynthate distribution by microplankton in permanently ice-covered Antarctic desert lakes. Limnol Oceanogr 32:260–270
Priscu JC, Fritsen CH, Adams EE, Giovannoni SJ, Paerl HW, McKay CP, Doran PT, Gordon DA, Lanoil BD, Pinckney JL (1998) Perennial Antarctic lake ice: an oasis for life in a polar desert. Science 280:2095–2098
Priscu JC, Adams EE, Lyons WB, Voytek MA, Mogk DW, Brown RL, McKay CP, Takacs CD, Welch KA, Wolf CF, Kirshtein CD, Avci R (1999) Geomicrobiology of subglacial ice above Lake Vostok, Antarctica. Science 286:2141–2144
Quayle WC, Peck LS, Peat H, Ellis-Evans JC, Harrigan PR (2002) Extreme responses to climate change in Antarctic lakes. Science 295:645
Rankin LM, Gibson JAE, Franzmann PD, Burton HR (1999) The chemical stratification and microbial communities of Ace Lake, Antarctica: a review of the characteristics of a marine-derived meromictic lake. Polarforschung 66:33–52
Riemann B, Bell RT (1990) Advances in estimating bacterial biomass and growth in aquatic systems. Arch Hydrobiol 118:385–402
Roberts EC, Laybourn-Parry J (1999) Mixotrophic cryptophytes and their predators in the Dry Valley lakes of Antarctica. Freshw Biol 41:737–746
Roberts EC, Priscu JC, Laybourn-Parry J (2004) Microplankton dynamics in a perennially ice-covered Antarctic lake—Lake Hoare. Freshw Biol 49:853–869
Rodrigues DF, de Jesus E, Ayala-del-Río HL, Pellizari VH, Gilichinsky D, Sepulveda-Torres L, Tiedje JM (2009) Biogeography of two cold-adapted genera: Psychrobacter and Exiguobacterium. ISME J 3:658–665
Romina Schiaffino M, Unrein F, Gasol JM, Massana R, Balagué V, Izaguirre I (2011) Bacterial community structure in a latitudinal gradient of lakes: the roles of spatial versus environmental factors. Freshw Biol 56:1973–1991
Sattley WM, Madigan MT (2006) Isolation, characterization, and ecology of cold-active, chemolithotrophic, sulfur-oxidizing bacteria from perennially ice-covered Lake Fryxell, Antarctica. Appl Environ Microbiol 72:5562–5568
Saunders NF, Thomas T, Curmi PM, Mattick JS, Kuczek E, Slade R, Davis J, Franzmann PD, Boone D, Rusterholtz K, Feldman R, Gates C, Bench S, Sowers K, Kadner K, Aerts A, Dehal P, Detter C, Glavina T, Lucas S, Richardson P, Larimer F, Hauser L, Cavicchioli R (2003) Mechanisms of thermal adaptation revealed from the genomes of the Antarctic Archaea Methanogenium frigidum and Methanococcoides burtonii. Genome Res 13:1580–1588
Säwström C, Anesio AM, Granéli W, Laybourn-Parry J (2007a) Seasonal viral loop dynamics in two large ultra-oligotrophic Antarctic freshwater lakes. Microb Ecol 53:1–11
Säwström C, Granéli W, Laybourn-Parry J, Anesio AM (2007b) High viral infection rates in Antarctic and Arctic bacterioplankton. Environ Microbiol 9:250–255
Säwström C, Laybourn-Parry J, Anesio AM, Priscu JC, Lisle J (2008) Bacteriophage in polar inland waters. Extremophiles 12:167–175
Säwström C, Karlson J, Laybourn-Parry J, Granéli W (2009) Zooplankton feeding on algae and bacteria under ice in Lake Druzhby, East Antarctica. Polar Biol 32:1195–1202
Scavia D, Laird GA, Fahnenstiel GL (1986) Production of planktonic bacteria in Lake Michigan. Limnol Oceanogr 31:612–626
Schiaffino MR, Unrein F, Gasol JM, Farias ME, Estevez C, Balagué V, Izaguirre I (2009) Comparative analysis of bacterioplankton assemblages from maritime Antarctic freshwater lakes with contrasting trophic status. Polar Biol 32:923–936
Siegert MJ, Ross N, Le Brocq AM (2016) Recent advances in understanding Antarctic subglacial lakes and hydrology. Philos Trans R Soc A 374:20140306. doi:10.1098/rsta.2014.0306
Simek K, Horñák K, Jezbera J, Nedoma J, Vrba J, Straskrábová V, Macek M, Dolan JR, Hahn WH (2006) Maximum growth rates and possible life strategies of different bacterioplankton groups in relation to phosphorus availability in a freshwater reservoir. Environ Microbiol 8:1613–1624
Smith RIL (1988) Destruction of Antarctic terrestrial ecosystems by a rapidly increasing fur seal population. Biol Conserv 45:55–72
Smith JA, Hodgson DA, Bentle MJ, Verleyen E, Leng MJ, Roberts SJ (2006) Limnology of two Antarctic epishelf lakes and their potential to record periods of ice shelf loss. J Paleolimnol 35:373–394
Smith BE, Fricker HA, Joughin IR, Tulaczyk S (2009) An inventory of active subglacial lakes in Antarctica detected by Icesat (2003–2008). J Glaciol 55:573–595
Snauwaert I, Hoste B, De Bruyne K, Peeters K, De Vuyst L, Willems A, Vandamme P (2013) Carnobacterium iners sp. nov., a psychrophilic, lactic acid-producing bacterium from the littoral zone of an Antarctic pond. Int J Syst Evol Microbiol 63:1370–1375
Stingl U, Foo W, Vergin KL, Lanoil B, Giovannoni SJ (2008) Dilution-top-extinction culturing of psychrotolerant planktonic bacteria from permanently ice-covered lakes in the McMurdo Dry Valleys, Antarctica. Microb Ecol 55:395–405
Sundh I, Bell RT (1992) Extracellular dissolved organic carbon released from phytoplankton as a source of carbon for heterotrophic bacteria in lakes of different humic content. Hydrobiologia 229:93–106
Takacs CD, Priscu JC (1998) Bacterioplankton dynamics in the McMurdo Dry Valley lakes, Antarctica: production and biomass over four seasons. Microb Ecol 36:239–250
Takacs CD, Priscu JC, McKnight DM (2001) Bacterial dissolved organic carbon demand in McMurdo Dry valley lakes, Antarctica. Limnol Oceanogr 46:1189–1194
Tang C, Madigan MT, Lanoil B (2013) Bacterial and archaeal diversity in sediments of west Lake Bonney, McMurdo Dry Valleys, Antarctica. Appl Environ Microbiol 79:1034–1038
Telling J, Anesio AM, Tranter M, Fountain AG, Nylen T, Hawkings J, Singh VB, Kaur P, Wadham GL (2014) Spring thaw ionic pulses boost nutrient availability and microbial growth in entombed Antarctic Dry Valley cryoconite holes. Front Microbiol. doi:10.3389/fmicb.2014.00694
Thurman J, Parry J, Hill PJ, Laybourn-Parry J (2010) The filer-feeding ciliates Colpidium striatum and Tetrahymena pyriformis display selective feeding behaviours in the presence of mixed, equally sized, bacterial prey. Protist 161:577–588
Thurman J, Parry J, Hill PJ, Priscu JC, Vick TJ, Chiuchiolo A, Laybourn-Parry J (2012) Microbial dynamics and flagellate grazing rates during transition to winter in Lakes Hoare and Bonney, Antarctica. FEMS Microbiol Ecol 82:449–458
Tindall BJ, Brambilla E, Steffan M, Neumann R, Pukall R, Kroppenstedt RM, Stackebrandt E (2000) Cultivatable microbial biodiversity: gnawing at the Gordian knot. Environ Microbiol 2:310–318
Tominaga H, Fukui F (1981) Saline lakes at Syowa Oasis, Antarctica. Hydrobiologia 82:375–389
Toolan T, Wehr JD, Findlay S (1991) Inorganic phosphorus stimulation of bacterioplankton production in a meso-eutrophic lake. Appl Environ Microbiol 57:2074–2078
Van Trappen S, Mergaert J, Van Eygen S, Dawyndt P, Cnockaert MC, Swings J (2002) Diversity of 746 heterotrophic bacteria isolated from microbial mats from ten Antarctic lakes. Syst Appl Microbiol 25:603–610
Van Trappen S, Vandecandelaere I, Mergaert J, Swings J (2004) Flavobacterium degerlachei sp. nov., Flavobacterium frigoris sp. nov. and Flavobacterium micromati sp. nov., novel psychrophilic bacteria isolated from microbial mats in Antarctic lakes. Int J Syst Evol Microbiol 54:85–92
Vander Schaaf NA, Cunningham AMG, Cluff BP, Kraemer CK, Reeves CL, Riester CJ, Slater LK, Madigan MT, Sattley WM (2015) Cold-adapted, heterotrophic bacteria from the highly oligotrophic waters of Lake Vanda, Antarctica. Microorganisms 3:391–406
Vaqué D, Pace ML (1992) Grazing on bacteria by flagellates and cladocerans in lakes of contrasting food web structure. J Plankton Res 14:307–321
Villaescusa JA, Casamayor EO, Rochera C, Velázquez D, Chicote Á, Quesada A, Camacho A (2010) A close link between bacterial community composition and environmental heterogeneity in maritime Antarctic lakes. Int Microbiol 13:67–77
Vincent WF (1981) Production strategies in Antarctic inland waters: phytoplankton ecophysiology in a permanently ice-covered lake. Ecology 62:1215–1224
Vincent WF, Hobbie JE, Laybourn-Parry J (2008) Introduction to the limnology of high-latitude lake and river ecosystems. In: Vincent WF, Laybourn-Parry J (eds) Polar lakes and rivers, limnology of Arctic and Antarcyic aquatic ecosystems. Oxford University Press, Oxford, pp 1–18
Vrede K (2005) Nutrient and temperature limitation of bacterioplankton growth in temperate lakes. Microb Ecol 49:245–256
Vrede K, Vrede T, Isaksson A, Karlsson A (1999) Effects of nutrient (phosphorus, nitrogen and carbon) and zooplankton on bacterioplankton and phytoplankton—a seasonal study. Limnol Oceanogr 44:1616–1624
Ward BB, Granger J, Maldonado MT, Wells ML (2003) What limits bacterial production in the suboxic region of permanently ice-covered Lake Bonney, Antarctica? Aquat Microb Ecol 31:33–47
Webster-Brown J, Gall M, Gibson J, Wood S, Hawes I (2010) The biogeochemistry of meltwater habitats in the Darwin Glacier region (80°S). Victoria Land, Antarctica. Antarct Sci 22:646–661
Weinbauer MG, Höfle MG (1998) Significance of viral lysis and flagellate grazing as factors controlling bacterioplankton production in a eutrophic lake. Appl Environ Microbiol 64:431–438
Xiao X, Yin X, Lin L, Sun L, You Z, Wang P, Wang F (2005) Chitinase genes in lake sediments of Ardley Island, Antarctica. Appl Environ Microbiol 71:7904–7909
Yau S, Lauro FM, Williams TJ, DeMaere MZ, Brown MV, Rich J, Gibson JAE, Cavicchioli R (2013) Metagenomic insights into strategies of carbon conservation and unusual sulfur biogeochemistry in a hypersaline Antarctic lake. ISME J 7:1944–1961
Zwart D, Bird M, Stone J, Lambeck K (1998) Holocene sea-level change and ice-sheet history in the Vestfold Hills, East Antarctica. Earth Planet Sci Lett 155:131–145
Acknowledgments
The work of the authors quoted here was funded by the Natural Environment Research Council (UK) the Levehulme Trust (UK), the National Science Foundation (USA) and the Australian Antarctic Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laybourn-Parry, J., Pearce, D. Heterotrophic bacteria in Antarctic lacustrine and glacial environments. Polar Biol 39, 2207–2225 (2016). https://doi.org/10.1007/s00300-016-2011-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-016-2011-1