Abstract
Euryhaline halophiles grow over a wide range of salinity, from <3% NaCl (seawater equivalent) to >15% NaCl and even saturation level (about 30% NaCl). Several species of euryhaline halophiles occur worldwide, especially in marine environments and also in aquatic and terrestrial habitats of the Antarctic ice-free areas. A biogeographic view of Antarctic halophiles is that their migration among lakes on land is more difficult than in marine setting. Ponds and lakes on land may thus serve as “islands” which facilitate the selection and separation of unique species. We isolated euryhaline halophiles from the saline lake, Suribati Ike, near Syowa Station and placed them into seven groups, each demonstrating a clear depth-related distribution. Six of the seven groups probably represent new species of the genera Halomonas and Marinobacter. This result suggests that Antarctic saline lakes exhibit high selectivity of unique euryhaline halophiles and possibly of other microbial groups.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Antarctic climate is characterized by extreme cold and dryness. Under these conditions, seawater entrapped in coastal ice-free zones was “lyophilized” to become concentrated and yield saline to hypersaline water bodies. Organisms inhabiting the Antarctic saline lakes include euryhaline halophiles such as Halomonas species (James et al. 1990; Okamoto et al. 2004), but these species in turn have not been reported from lakes in similar geographical settings such as less brine Lake Fryxell (Brambilla et al. 2001; Karr et al. 2003). Euryhaline halophiles grow in a broad range of salinity, from less than 3% NaCl to more than 15% NaCl and even saturation (Ventosa et al. 1998). Given the tolerance of euryhaline halophiles to low and high temperature, dryness and various salinities (Oren 2002), it is reasonable to consider that they are active participants in biological and biogeochemical processes in Antarctic saline lakes. These lakes may have similar physical and chemical features but be separated geographically, and there has been phenotypic variation in phylogenetically coherent strains. An example is the strains closely related to Halomonas variabilis isolated from a temporal saline pond near Terra Nova Bay Station and Suribati Ike near Syowa Station. The strains showed 100% similarity in 16S rDNA sequences at a distance of 3,000 km over a vast ice sheet, but differed in physiological features (Okamoto et al. 2004). We applied this approach to vertical distribution of halomonads and other euryhaline halophiles, previously characterized for meromictic lakes formed in Antarctic terrains (Labrenz and Hirsch 2001).
Antarctic meromictic lakes are characterized by an upper layer of cold aerobic freshwater and lower layer of warm (heliothermal) anaerobic brine (Green et al. 1998; Voytek et al. 1998). They likely represent a source of novel halophiles of phylogenetic and biotechnological interest (Franzmann and Dobson 1993; McMeekin et al. 1993; Gilbert et al. 2004). Although the range of temperature and salinity they cover is within the known growth and survival ranges of euryhaline halophiles (Ventosa et al. 1998), the influence of oxygen availability on the distribution of euryhaline halophiles has been little considered.
Here, we report the vertical distribution of euryhaline halophilic strains isolated from a typical meromictic lake, Suribati Ike, East Antarctica. Particular attention is given to the aerobic and anaerobic nature of the isolated strains.
Materials and methods
Study site and sample collection
Lake Suribati Ike is located at approximately 69°29′S and 39°41′E, Skarvsnes, Soya Coast, East Antarctica. Although the lake is 400 m distant from the shoreline, its marine origin is suggested by its surface altitude of −33 m and its salt composition (Hirabayashi and Ossaka 1977). It is thought to be a relic of seawater land-locked by postglacial isostatic uplift several thousand years ago, as schematized for the older saline lake Vestfold Hills (Laybourn-Parry et al. 2002). Suribati Ike is 1,070×780 m across, and is 0.4 km2 with a maximum depth of 31.2 m (Imura et al. 2003). It shows a typical meromictic stratification of upper (>8 m) and lower (<8 m) layers of cold, less saline and warm brine waters, respectively (Fig. 1). Measurement of dissolved oxygen concentration (DO, mll−1) with a Portable Water Quality Checker (Model WQC-22A, DKK-TOA Corp., Tokyo, Japan) showed that the lower layer was anaerobic with a sulfidic odor and blackish sulfide particles due to high organic accumulation (Fukui et al. 1985), while DO in the upper layer was over-saturated due to highly active photosynthetic oxygen release as suggested by a chlorophyll maximum and high abundance of flagellated algae (Ban et al. 2001). Conductivity measurement was tried only unsuccessfully due to the damage of the probe by high concentration of sulfide. Therefore, salinity was calculated as “equivalent salinity” from the measured density of water samples. For example, a density of 1.150 corresponds to an equivalent salinity of 15% NaCl.
Profiles of water temperature (°C), equivalent salinity (%; calculated from density), and dissolved oxygen concentration (mll−1) in the meromictic Antarctic lake, Suribati Ike. The shaded depth zone (about 10 m deep) indicates the chemolimnion between the aerobic upper layer and the anaerobic lower layer
Water samples were collected from the surface to a depth of 24 m at an interval of 2 m at a southeastern point (bottom, ca 25 m deep) in the central basin using autoclaved Heyroth glass bottles. The stoppers of the bottles were pulled-open to collect water at targeted depths, and then closed by releasing the pull wire. Some bottles were used at more than one depth, but washed with water of the same depth to minimize “carry-over” from the previous sampling depth. Water samples were transferred to autoclaved plastic bottles to the brim with no headspace left, and were kept refrigerated for live preservation of euryhaline halophiles for isolation 3 months later. Considering the possible changes in microbial abundance and species composition during preservation, quantitative and qualitative aspects of these species were not focused on in this study.
Isolation and cultivation of euryhaline halophiles
Portions of refrigerated water samples were inoculated in 1/20 LBG liquid medium containing 0.05% bactopeptone (BD Diagnostic Systems, MD, USA), 0.025% yeast extract (BD Diagnostic Systems), 0.01% dextrose and 11.5% NaCl in artificial seawater (Marine Art SF, Senju Pharmaceutical Co. Ltd, Osaka, Japan). This medium, with a final NaCl concentration of 15%, facilitates isolation of euryhaline halophiles (Ventosa et al. 1984; Okamoto et al. 2004). After 2 weeks of enrichment in the dark at room temperature, liquid cultures were spread on 1/20 LBG medium solidified with 1.5% agar. Single colonies were repeatedly picked and re-spread on fresh agar plates. Colony-forming strains of halophiles were thus isolated and maintained on agar plates containing identical ingredients. These halophilic isolates were then tested for colony formation at 4°C and at 3 and 20% NaCl on otherwise identical 1/20 LBG agar. Anaerobic growth was examined by cultivation on agar plates in a glove box under an 80% N2:10% CO2:10% H2 atmosphere.
Preparation of bacterial DNA and amplification of 16S rDNA
Genomic DNA samples were done by direct extraction from 5- to 15-day-old colonies of the purified isolates (Okamoto et al. 2004). Colonies of each isolate were suspended in 100μl of sterilized distilled water, vortex-stirred for 10 min and sonicated for 10 min. The suspensions were heat-shocked to denature DNA at 95°C for 10 min, ice-cooled, and briefly centrifuged at 3,000 g for 10 s to remove cell fragments. The supernatants were used as DNA template solutions for amplification of 16S rDNA.
Partial 16S rDNA sequences were amplified by PCR with the common primers Eubac 27F and Eubac 1492R (Borosius et al. 1978; DeLong 1992) using Ampli Taq DNA polymerase (Applied Biosystems, Foster City, CA, USA) on a Peltier Thermal Cycler (PTC-100, MJ Research, Inc., Waltham, MA, USA). PCR conditions were set as 25 cycles of denaturation at 94°C for 20 s, annealing at 55°C for 40 s, and elongation at 72°C for 40 s.
Sequencing and phylogenetic analysis of 16S rDNA
The PCR products were purified with the QIA Quick PCR Purification Kit (QIAGEN Inc., Valencia, CA, USA) for use as sequencing templates. The 16S rDNA amplicons were fully sequenced with the primer set reported by Okamoto et al. (2004), using the ABI Prism Big Dye Terminator Cycle Sequencing Ready Reaction Kit on an ABI 3100 DNA sequencer (Applied Biosystems, Foster City, CA, USA).
The 16S rDNA sequences were examined for similarity using FASTA (Pearson and Lipman 1988) and BLAST (Karlin and Altschul 1990; Tatusova and Madden 1999) at the DNA Data Bank of Japan (DDBJ, http://www.ddbj.nig.ac.jp) and National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov/bl2seq). They were then used to construct phylogenetic trees by the neighbor-joining (NJ), maximum parsimony (MP) and minimum evolutionary (ME) algorithms using MEGA ver 2.1 (Saitou and Nei 1987; Kumar et al. 2001; Nei and Kumar 2001). The 16S rDNA sequences determined in this study were deposited in the DDBJ under the accession numbers AB085653 and AB166905-AB166926.
Results
Isolation and phylogenetic affiliation of euryhaline halophiles
A total of 23 strains (SYO-J42, J51–J55, J57–J58 and J60–J74) were isolated and shown to grow at both 3 and 20% NaCl and at both 4°C and room temperature of 20°C (Table 1). All strains showed aerobic growth, while some Marinobacter strains (J51, J60, J62, J63, and J68) also showed anaerobic growth in the N2–CO2–H2 (80:10:10) atmosphere. The facultatively anaerobic strains were capable of denitrification as shown by positive results from the bubble formation test (data not shown).
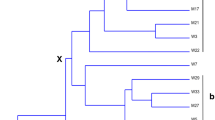
The isolated strains were clustered into five groups and affiliated with the genera Halomonas, Idiomarina, and Marinobacter based on 16S rDNA sequences (Table 1, Fig. 2). The previously reported strain J42 (Okamoto et al. 2004) and strain J52 that was lost during sub-culture were identical in 16S rDNA sequence, and most closely related to H. glaciei from Adelie Land, Antarctica (Reddy et al. 2003). Strains J53, J54, J65, and J71 were identical to each other and related to an alkalohalophile Halomonas sp., ML-185, from the sediment of the hypersaline Mono Lake; however, the Antarctic strains showed different growth properties (Table 1). Strain J57 was affiliated with the euryhaline halophilic and piezophilic bacterium Idiomarina loihiensis from a Hawaiian submarine volcano (Loihi Seamount; Hou et al. 2004). Strains J51 and J55 were related to Marinobacter sp. CK47 from Kerguelen Gulf seawater. The other 14 strains formed a phylogenetically coherent group related to a metal cyanide-degrading Marinobacter sp. from gold mine tailing dams. Strains J58 and J60 were identical in 16S rDNA sequences but differentiated in growth properties, particularly anaerobic growth.
Phylogenetic tree based on 16S rDNA sequences of the euryhaline halophilic strains isolated from the meromictic Antarctic lake, Suribati Ike. The tree was constructed by the ME method, and rooted with Carnimonas nigrifaciens (Y13299; Garriga et al. 1998) and Zymobacter palmae (D14555; Okamoto et al. 1993). Values at nodes indicate bootstrap values for 1,000 replicates. Values less than 50 are not reported. The scale bar represents the average number of nucleotide substitutions per site
Depth distribution of the isolated strains
The halophilic strain groups showed different patterns of depth-related distribution. Strains J42 and J52, related to Halomonas glaciei, as well as strain J57, related to I. loihiensis, were isolated from the upper aerobic layer of lake Suribati Ike. Strains J51 and J55, related to Marinobacter sp., were also isolated from the upper aerobic layer. J55 showed a capacity for anaerobic growth and could thus have been found even in the lower anaerobic layer. The Halomonas-related group of strains J53, J54, J65, and J71 showed a distribution over the upper aerobic and lower anaerobic layers, while strains J65 and J71 from the anaerobic depths showed no anaerobic growth under the conditions studied.
The largest group of isolates was the second group of the Marinobacter cluster that contained strains J58–J74 (Table 2). All strains of this group were isolated from the lower anaerobic layer (Table 2), and four (J60, J62, J63, and J68) were capable of anaerobic growth under the study conditions (Table 1). The distribution of this group contrasted with that of the aerobic groups of J42, J52, and J57, which were found only in the upper aerobic layer, and thus habitat segregation for these groups were suggested.
Discussion
Twenty-three strains of euryhaline halophiles were isolated from the meromictic Suribati Ike, and affiliated with the common halophilic genera Halomonas, Idiomarina, and Marinobacter (Table 1). Halomonads are readily isolated from coastal Antarctic seawater and sea ice (e.g., Bowman et al. 1997), with the cosmopolitan H. variabilis being one of the most easily grown species. The most readily isolated species are not necessarily the most common, as shown by the comparison of isolates and 16S rDNA clones in Antarctic pack ice (Brinkmeyer et al. 2003), although strains related to H. variabilis have been isolated from various extreme habitats with phenotypic variations (Okamoto et al. 2004). Isolation of such phenotypically diverse halophiles despite phylogenetic coherence agrees with the dominance of Halomonas species in the meromictic Ekho Lake in Vestford Hills (James et al. 1994), but contrasts with undetectable abundance of halomonads within anoxic sediments and microbial mats of the Vestfold Hills meromictic lakes (Bowman et al. 2000a, 2000b; Van Trappen et al. 2002) and in the amictic, less brine Lake Fryxell (Laybourn-Parry et al. 1997; Brambilla et al. 2001; Karr et al. 2003). The chemolimnion of Suribati Ike may favor the habitation of euryhaline halophiles.
The Halomonas and Marinomonas strains of Suribati Ike formed phylogeneticially coherent groups diverged from their nearest non-Antarctic relatives long before the development of stable ice-sheet (Franzmann 1996). They may have developed over the long period diverse physiological features that benefit the populations in thriving in the various niches of the lake. Serogroups of Halomonas species comprised 5–39% of total planktonic bacteria in the saline lakes of the Vestfold Hills (James et al. 1994). Dissolved organic carbon (DOC) concentrations were correlated with total counts but not with Halomonas immunofluorescence counts, suggesting that Halomonas abundance is unaffected by DOC concentration. If this applies to the eutrophic lake Suribati Ike, whose DOC concentration ranges from 20 mgl−1 at the surface to 125 mgl−1 in the bottom waters (Fukui et al. 1985), depth-related distribution of Halomonas phylotypes would be ascribed to non-DOC factors such as DO concentration, or in other words aerobic/anaerobic conditions. The scarcity of Halomonas in the Suribati Ike anaerobic layer agrees with the absence of Halomonas phylotypes in highly reducing lake sediments (Bowman et al. 2000a, 2000b).
A key feature of Suribati Ike is its meromictic structure of upper aerobic and lower anaerobic layers, which is primarily the result of meromictic stratification of ice-melt freshwater and evaporated relic seawater. The chemocline (ca 10 m deep) was outlined by the maximum DO concentration due to photosynthesis by flagellated algae (Ban et al. 2001). A marked abundance of dinoflagellates has also been recognized at corresponding salinities of the saline lakes in Vestford Hills (Perris and Laybourn-Parry 1997). The Halomonas (J65 and J71) and Marinomonas (J58, J61, J64, J66, J67, J69, J70, J72, J73, and J74) strains from the anaerobic depths showed no anaerobic growth under the study conditions. However, considering the various energy-generating processes available to bacteria, the capacity of these strains for anaerobic growth is not ruled out. For example, the microbial preference for organic ingredients may affect the intensity of fermentation, and anaerobic respiration may require specific concentrations and combinations of electron donors (organic ingredients) and acceptors (sulfate, nitrate, etc). Comprehensive tests for the presence of such anaerobic processes are now underway.
We consider the Halomonas and Marinomonas strains of Suribati Ike as meeting the concept “similar genotypes but distinct phenotypes” (Table 1, Fig. 2). A key phenotype is the capacity for anaerobiosis, which is associated with the water depth of the lake. It remains unclear whether the depth-related distribution is the result of dispersion of facultatively anaerobic halophiles into Suribati Ike or physiological divergence of halophiles (Rauch and Bar-Yam 2004). Because re-colonization of the Antarctic saline lakes is only a recent event (<10,000 years), dispersion should be more realistic than divergence (Franzmann 1996), as also presumed for wide-distributed freshwater bacteria (Zwart et al. 1998). Comparative studies with other isolated saline lakes may lead to the answer.
J57 was closely related to I. loihiensis, which was originally isolated from a hydrothermal vent of a Hawaiian submarine volcano (Donachie et al. 2003), with a 99.7% similarity of 16S rDNA sequence. Phylogenetic similarity of Antarctic and hydrothermal vent halophiles was also reported for H. variables (Okamoto et al. 2001). Another Antarctic strain closely related to I. loihiensis, along with Halomnas/Marinobacter strains, was isolated from the isohaline Triple Lake in the Vestfold Hills and found to produce antifreeze proteins (Gilbert et al. 2004). Role of antifreezing proteins in euryhaline halophiles is not fully elucidated, and study of these microorganisms in Antarctic lakes may increase the molecular and biochemical diversity of such proteins.
References
Ban S, Imura S, Kudo I (2001) Biological characteristic in Lake Suribachi. Abstracts for XXIV Symposium on Polar Biology, National Institute of Polar Research, Tokyo, Japan, 74
Borosius J, Palmer ML, Kennedy PJ, Noller HF (1978) Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci USA 75:4801–4805
Bowman JP, McCammon SA, Brown MV, Nichols DS, McMeekin TA (1997) Diversity and association of psychrophilic bacteria in Antarctic Sea Ice. Appl Environ Microbiol 63:3068–3078
Bowman JP, McCammon SA, Rea SM, McMeekin TA (2000a) The microbial composition of three limnologically disparate hypersaline Antarctic lakes. FEMS Microbiol Lett 183:81–88
Bowman JP, Rea SM, McCammon SA, McMeekin TA (2000b) Diversity and community structure within anoxic sediment from marine salinity meromictic lakes and a coastal meromictic marine basin, Vestfold Hills, Eastern Antarctica. Environ Microbiol 2:227–237
Brambilla E, Hippe H, Hagelstein A, Tindall BJ, Stackebrandt E (2001) 16S rDNA diversity of cultured and uncultured prokaryotes of a mat sample from Lake Fryxell, McMurdo Dry Valleys, Antarctica. Extremophiles 5:23–33
Brinkmeyer R, Knittel K, Jürgens J, Weyland H, Amann R, Helmke E (2003) Diversity and Structure of Bacterial Communities in Arctic versus Antarctic Pack Ice. Appl Environ Microbiol 69:6610–6619
DeLong EF (1992) Archaea in coastal marine environments. Proc Natl Acad Sci USA 89:5685–5689
Donachie SP, Hou S, Gregory TS, Malahoff A, Alam M (2003) Idiomarina loihiensis sp. nov., a halophilic gamma-Proteobacterium from the Loihi submarine volcano, Hawaii. Int J Syst Evol Microbiol 53:1873–1879
Franzmann PD (1996) Examination of Antarctic prokaryotic diversity through molecular comparisons. Biodivers Conserv 5:1295–1305
Franzmann PD, Dobson SJ (1993) The phylogeny of bacteria from a modern Antarctic refuge. Antarct Sci 5:267–270
Fukui F, Torii T, Okabe S (1985) Vertical distribution of nutrients and DOC in lake waters near Syowa Station, Antarctica (in Japanese with English abstract). Antarct Rec 58:93–107
Garriga M, Ehrmann M, Arnau M, Hugas M, Vogel RF (1998) Carnimonas nigrificans gen. nov., sp. nov., a bacterial causative agent for black spot formation on cured meat products. Int J Syst Bacteriol 48:677–686
Gilbert JA, Hill PJ, Dodd CER, Laybourn-Parry J (2004) Demonstration of antifreeze protein activity in Antarctic lake bacteria. Microbiology 150:171–180
Green WJ, Canfield DE, Nixon P (1998) Cobalt cycling and fate in Lake Vand. In: Priscu JC (ed) Ecosystem dynamics in a polar desert. American Geophysical Union, Washington DC, pp 205–215
Hirabayashi J, Ossaka J (1977) Seasonal variation in chemical composition and the origin of the saline lakes around Syowa Station, Antarctica. Antarct Rec 86:28–35
Hou S, Saw JH, Lee KS, Freitas TA, Belisle C, Kawarabayasi Y, Donachie SP, Pikina A, Galperin MY, Koonin EV, Makarova KS, Omelchenko MV, Sorokin A, Wolf YI, Li QX, Keum YS, Campbell S, Denery J, Aizawa S-I, Shibata S, Malahoff A, Alam M (2004) Genome sequence of the deep-sea gamma-proteobacterium Idiomarina loihiensis reveals amino acid fermentation as a source of carbon and energy. Proc Natl Acad Sci USA 101:18036–18041
Imura S, Bando T, Seto K, Ohtani S, Kudoh S, Kanda H (2003) Distribution of aquatic mosses in the Soya Coast region, East Antarctica. Polar Biosci 16:1–10
James SR, Dobson SJ, Franzmann PD, McMeekin TA (1990) Halomonas meridiana, a new species of extremely halotolerant bacteria isolated from Antarctic saline lakes. Syst Appl Microbiol 13:270–278
James SR, Burton HR, McMeekin TA, Mancuso CA (1994) Seasonal abundance of Halomonas meridiana, Halomonas suglaciescola, Flavobacterium gondowanense and Flavobacterium salegens in four Antarctic lakes. Antarct Sci 6:325–332
Karlin S, Altschul SF (1990) Methods for assessing the statistical significance of molecular sequence features using general scoring schemes. Proc Natl Acad Sci USA 87:2264–2268
Karr EA, Sattley WM, Jung DO, Madigan MT, Achenbach LA (2003) Remarkable diversity of phototrophic purple bacteria in a permanentaly frozen Antarctic Lake. Appl Environ Microbiol 69:4910–4914
Kumar S, Tamura K, Jakobsen IB, Nei M (2001) MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244–1245
Labrenz M, Hirsch P (2001) Physiological diversity and adaptations of aerobic heterotrophic bacteria from different depths of hypersaline, heliothermal and meromictic Ekho Lake (East Antarctica). Polar Biol 24:320–327
Laybourn-Parry J, James MR, McNight D, Priscu J, Spaulding S, Shiel R (1997) The microbial plankton of Lake Fryxell, Southern Victoria Land, Antarctica. Polar Biol 17:54–61
Laybourn-Parry J, Quayle W, Henshaw T (2002) The biology and evolution of Antarctic saline lakes in relation to salinity and trophy. Polar Biol 25:542–552
McMeekin TA, Nichols PD, Nichols DS, Juhasz A, Franzmann PD (1993) Biology and biotechnological potential of halotolerant bacteria from Antarctic saline lakes. Experientia 49:1042–1046
Nei M, Kumar S (2001) Molecular evolution and phylogenetics. Oxford University Press, Oxford, UK
Okamoto T, Taguchi H, Nakamura K, Ikenaga H, Kuraishi H, Yamasato K (1993) Zymobacter palmae gen. nov., sp. nov., a new ethanol-fermenting peritrichous bacterium isolated from palm sap. Arch Microbiol 160:333–337
Okamoto T, Fujioka K, Naganuma T (2001) Phylogenetic similarity of aerobic gram-negative halophilic bacteria from a deep-sea hydrothermal mound and Antarctic habitats. Polar Biosci 14:1–9
Okamoto T, Maruyama A, Imura S, Takeyama H, Naganuma T (2004) Comparative phylogenetic analyses of Halomonas variabilis and related organisms based on 16S rRNA, gyrB and ectBC gene sequences. Syst Appl Microbiol 27:323–333
Oren A (2002) Halophilic microorganisms and their environments. Kluwer Academic, The Netherlands
Pearson WR, Lipman DJ (1988) Improved tools for biological sequence comparison. Proc Natl Acad Sci USA 85:2444–2448
Perris SJ, Laybourn-Parry J (1997) Microbial communities in saline lakes of the Vestfold Hills (eastern Antarctica). Polar Biol 18:135–144
Rauch EM, Bar-Yam Y (2004) Theory predicts the uneven distribution of genetic diversity within species. Nature 431:449–452
Reddy GS, Raghavan PU, Sarita NB, Prakash JS, Nagesh N, Delille D, Shivaji S (2003) Halomonas glaciei sp. nov. isolated from fast ice of Adelie Land, Antarctica. Extremophiles 7:55–61
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Tatusova TA, Madden TL (1999) Blast 2 sequences—a new tool for comparing protein and nucleotide sequences. FEMS Microbiol Lett 174:247–250
Van Trappen S, Mergaert J, Van Eygen S, Dawyndt P, Cnockaert MC, Swings J (2002) Diversity of 746 heterotrophic bacteria isolated from microbial mats from ten Antarctic lakes. Syst Appl Microbiol 25:603–610
Ventosa A, Rodriguez-Valera F, Poindexter JS, Reznikoff WS (1984) Selection of moderately halophilic bacteria by gradual salinity increases. Can J Microbiol 30:1279–1282
Ventosa A, Nieto JJ, Oren A (1998) Biology of moderately halophilic aerobic bacteria. Microbiol Mol Biol Rev 62:504–544
Voytek MA, Ward BB, Priscu JC (1998) The abundance of ammonium-oxidizing bacteria in Lake Bonney, Antarctica determined by immunofluorescence, PCR and in situ hybridization. In: Priscu JC (ed) Ecosystem dynamics in a polar desert. American Geophysical Union, Washington DC, pp 217–228
Zwart G, Hiorns WD, Methe BA, van Agterveld MP, Huismans R, Nold SC, Zehr JP, Laanbroek HJ (1998) Nearly identical 16S rDNA sequences from lakes in North America and Europe indicate the existence of clades of globally distributed freshwater bacteria. Syst Appl Microbiol 21:546–556
Acknowledgments
This study was partly supported by the NIPR collaborative research program No. 99 (2002–2004) and the Grants-in-Aid for Scientific Research (14340268) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naganuma, T., Hua, P.N., Okamoto, T. et al. Depth distribution of euryhaline halophilic bacteria in Suribati Ike, a meromictic lake in East Antarctica. Polar Biol 28, 964–970 (2005). https://doi.org/10.1007/s00300-005-0026-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-005-0026-0