Abstract
Rapid industrialization and intensive agriculture activities have led to a rise in heavy metal contamination all over the world. Chhattisgarh (India) being an industrial state, the soil and water are thickly contaminated with heavy metals, especially from arsenic (As). In the present study, we isolated 108 arsenic-resistant bacteria (both from soil and water) from different arsenic-contaminated industrial and mining sites of Chhattisgarh to explore the bacterial gene pool. Further, we screened 24 potential isolates out of 108 for their ability to tolerate a high level of arsenic. The sequencing of the 16S rRNA gene of bacterial isolates revealed that all these samples belong to different diverse genera including Bacillus, Enterobacter, Klebsiella, Pantoea, Acinetobacter, Cronobacter, Pseudomonas and Agrobacterium. The metal tolerance ability was determined by amplification of arsB (arsenite efflux gene) and arsC (arsenate reductase gene) from chromosomal DNA of isolated RnASA11, which was identified as Klebsiella pneumoniae through in silico analysis. The bacterial strains RpSWA2 and RnASA11 were found to tolerate 600 mM As (V) and 30 mM As (III) but the growth of strain RpSWA2 was slower than RnASA11. Furthermore, atomic absorption spectroscopy (AAS) of the sample obtained from bioremediation assay revealed that Klebsiella pneumoniae RnASA11 was able to reduce the arsenic concentration significantly in the presence of arsenate (44%) and arsenite (38.8%) as compared to control.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal contaminations in nature are a major concern for environmentalists. Arsenic (As) contamination is very severe in India (Indo-Gangetic plains) amongst all other heavy metals naturally found in South-east Asia. Arsenic occurs naturally in the soil through pedogenetic processes of weathering; however, the quantum of increase in its concentration in recent times can be attributed to several anthropogenic activities [1]. Arsenic is mainly the byproduct of mining and smelting industries. It is also an ingredient commonly found in several fertilizers and pesticides whose over application leads to arsenic contamination in soil and water [2]. Exposure to arsenic either causes adverse health issues like nausea, vomiting, abnormal heartbeat, profuse watery diarrhoea, and skin diseases, and in extreme cases, it may also lead to cancer [3, 4].
Arsenic also influences the growth, morphology, and biochemical activities of microorganisms and plants [5]. However, microbes, especially bacteria, are often found to be more resistant to arsenic as it employs a wide array of mechanisms like biotransformation, extrusion, bioaccumulation, and biosorption. Several systems found in bacteria to overcome arsenic toxicities like arsenate system (ars system), anaerobic arsenate respiration system (arr system), arsenite oxidation system (aio system), and arsenic methylation system (arsM system). A bacterium can have more than one arsenic resistance system operating within itself but amongst all the most common system found in the ars system. Genes regulating the ars operon consist of three (arsRBC) or five genes (arsRDABC) but may have several other genes like arsH, arsI, and arsM [6]. When arsenate, i.e. As (V) is taken up by the bacterium, the arsenate reductase gene (arsC) immediately converts it into arsenite, i.e. As (III). It is then extruded from the cell through an energy-dependent efflux pump (arsB gene), thus conferring resistance to the bacterium. Therefore, arsenic-resistant bacteria can be effectively used for the bioremediation process that ensures a more efficient clean-up of arsenic-polluted soil and water [7].
In India, Chhattisgarh is one of the seven worst-affected states from arsenic contamination [8]. In past, many researchers have reported arsenic contamination above permissible limits in different parts of Chhattisgarh [9,10,11]. However, very few research works have been carried out regarding the microbiological aspect and its possible implications for bioremediation purposes from this state [12]. Therefore, the present investigation was designed to evaluate the bioremediation property of indigenous arsenic-resistant bacterial populations for removing arsenic from bacterial isolates.
Materials and Methods
Collection of Soil and Water Samples
Surface soil samples (0–15 cm) were collected from different industrial and mining areas of Chhattisgarh. A total of 45 soil samples were collected in a sterile polythene bag from 23 villages/cities: eight districts of Chhattisgarh. Similarly, 26 water samples were collected from 20 villages/cities of Chhattisgarh. All the samples were stored in a refrigerator at 10 °C until further use. The list of all sample collection sites along with their coordinates is shown in Table S1 and Fig. S1.
Isolation and Screening of Arsenic (As)-Resistant Bacteria
The collected soil and water samples were serial diluted (up to 10–3 times) and 100 µL each was plated on minimal agar plates (dextrose, 1 g/L; dipotassium phosphate, 7 g/L; monopotassium phosphate, 2 g/L; sodium citrate, 0.5 g/L; magnesium sulphate, 0.1 g/L; ammonium sulphate, 1 g/L along with 1.8% agar) supplemented with 10 mM sodium arsenate (Na2HAsO4⋅7H2O). The isolates retrieved from sodium arsenate-supplemented media plates were also streaked on minimal media supplemented with 1 mM sodium arsenite (NaAsO2). All the chemicals and solutions were purchased from HiMedia Pvt. Ltd. (India). The plates were incubated at 37 °C until growth appears and individual colonies having distinct morphology were selected for further studies.
Determination of Minimum Inhibitory Concentration (MIC)
The minimum inhibitory concentration (MIC) of bacterial isolates was determined by taking 25 mL minimal broth supplemented with different concentrations of sodium arsenate (10, 50, 100, 200, 300, 400, 500, 600, and 700 mM) and sodium arsenite (1, 5, 10, 20, 30, and 40 mM). Overnight-grown (approx.16 h) log phase culture of each isolate (100 µL) was used as the initial inoculum. The flasks were then incubated at 37 °C, whilst being kept for continuous shaking at 100 rpm for 48 h. The growth of bacterial isolates was determined by measuring the optical density (O.D.) at 600 nm using UV-spectrophotometer (Systronics, India). Twenty-four best grown isolates were selected for further studies out of 108 isolates.
Antibiotic Susceptibility Test and Silver Nitrate Assay
The selected 24 isolates were checked for their co-tolerance towards heavy metals and antibiotics. The cultures were first grown in minimal broth supplemented with 1 mM Na3AsO4 and then incubated overnight at 37 °C. Further, bacterial cultures (100 µL) were evenly spread over nutrient agar plates having 1 mM Na3AsO4 along with HiPer antibiotic sensitivity discs (HiMedia Laboratories Pvt. Ltd., India) consisting of eight antibiotics. Simultaneously, the silver nitrate assay was also performed as per Simeonova et al. [13] for checking the ability of these isolates to convert the arsenic salts from one form to the other, i.e. either from As (III) to As (V) or from As (V) to As (III). The experiments were performed in duplicates.
Amplification of 16S rRNA, arsB, and arsC Gene
The overnight-grown culture (100 μL) with 0.6 OD was inoculated in minimal broth (with 1 mM Na3AsO4) followed by incubation at 37 °C. It was being kept for constant shaking at 100 rpm for 16 h. The genomic DNA of isolates was extracted using a genomic DNA isolation Kit (HiMedia Laboratories Pvt. Ltd., Mumbai, India). The isolated genomic DNA of all bacterial isolates were amplified using reported gene-specific primers, i.e. 16S rRNA [14], arsB [15], and arsC [16].
Bioremediation Assay
Bacterial isolate RnASA11 was selected based on MICs and two separate bioremediation assays were performed for each metal salts, i.e. sodium arsenate (100 ppm or 0.3 mM) and sodium arsenite (100 ppm or 0.77 mM). The experiment was conducted in the minimal broth along with negative control (without inoculum) and positive control (without arsenic salts). The flasks were continuously kept for shaking (at 100 rpm) for 7 days and its growth was periodically monitored every 24 h at 600 nm wavelength in a UV-spectrophotometer. Further, all the samples were centrifuged at 6000 rpm for 5 min at the end of the 7th day. The cell-free supernatant (diluted) was subjected to atomic absorption spectrometry (AAS) analysis (Elico Ltd., India).
DNA Sequencing Data
All the amplified products were sequenced by Sanger sequencing. The primers are enlisted in Table S2. Furthermore, the sequences were compared with the known sequences in the NCBI BLAST to find out their identities. The 16S rRNA gene sequences along with the BLAST sequences were aligned by ClustalW1.6 and a phylogenetic relationship was established amongst these isolates by constructing a phylogram through neighbour-joining method using MEGA6. The 16S rRNA gene was partially sequenced through the Sanger sequencing technique and the gene sequences were submitted to the NCBI GenBank database with accession numbers from MH793469 to MH793492. Both arsB and arsC CDS (coding sequences) sequences were also submitted to NCBI GenBank with accession number MH908969 and MH908972, respectively.
Statistical Analysis
All the observations were recorded and tabulated in a systematic manner. This bioremediation assay was conducted with four treatments, i.e. T1: control with arsenic salt; T2: without As + isolate RnASA11; T3: As (V) + isolate RnASA11; and T4: As (III) + isolate RnASA11. All treatments were replicated thrice. The bioremediation experiment was a complete randomized design (CRD). The obtained data were the mean of independent experiments with three replicates. Student’s t-test was used to analyse the data and p values < 0.05 were considered as statistically significant.
Results
Screening and Minimum Inhibitory Concentration (MIC) of Arsenic-Resistant Bacterial Isolates
A total of 108 arsenic-resistant bacterial isolates were screened, collected from contaminated soil and water samples of Chhattisgarh. All the isolates were maintained on minimal agar containing 10 mM sodium arsenate (Na2HAsO4⋅7H2O). The isolation of bacterial strains was based on their ability to grow in the presence of As (V) salt. The individual colonies with distinct morphology and culture characteristics were selected for further analysis. Out of 108 isolates, only 24 isolates were able to grow in the presence of 400 mM sodium arsenate in which only eight isolates grew on media containing 20 mM sodium arsenite. Further, only a single bacterium was able to grow in the presence of 30 mM sodium arsenite and 600 mM sodium arsenate. Thus, due to its higher tolerance ability towards arsenic salts, it was selected for further studies and identified as Klebsiella pneumoniae by 16S rRNA gene sequencing. It was recorded that Staphylococcus sp. strain tolerating 30 mM arsenite; however, it could resist only 250 mM sodium arsenate [17]. Similarly, Mujawar et al. [18] have reported that K. pneumoniae could grow at 21 mM arsenite.
Antibiotic Susceptibility Test and Silver Nitrate Assay
A qualitative silver nitrate (AgNO3) test was conducted to check the ability of bacterial isolate that can oxidize arsenite to arsenate or reduce arsenate to arsenite. It was found that all 24 isolates were arsenate reducers, i.e. they convert arsenate to arsenite by producing arsenate reductase enzyme. Further, all the 24 selected arsenic-resistant bacterial isolates were plated on the minimal agar plates having 1 mM Na3AsO4. This concentration of arsenic had been used by several workers for the screening of arsenic-resistant bacteria [19,20,21]. All the isolates showed a varied level of sensitivity towards eight antibiotics, i.e. streptomycin, tetracycline chloramphenicol, rifamycin, ampicillin, kanamycin, gentamycin, and nalidixic acid. The results of antibiotic sensitivity tests revealed that the majority of isolates showed resistance towards ampicillin and rifamycin. However, two bacterial strains KDWA1 and KDWA2 showed complete resistance against antibiotics streptomycin, ampicillin, and chloramphenicol. Bacterial strain RnASA11 showed less resistance against streptomycin, chloramphenicol, rifamycin, ampicillin, kanamycin, and gentamycin, whereas it was susceptible towards tetracycline and nalidixic acid. Shakoori et al. [22] reported that some arsenic-resistant bacterial species showed tolerance against a wide spectrum of antibiotics like erythromycin, kanamycin, nalidixic acid, and tetracycline.
The 16S rRNA-Based Characterization
All the selected 24 isolates were further characterized to understand their phylogenetic relationship through 16S rRNA gene sequencing. Results of gene sequencing revealed that both gram-positive and gram-negative arsenic-tolerant isolates were present, belonging to nine genera. The biochemical properties of all the selected isolates are summarized in Table 1. The 16S rRNA gene sequencing and phylogenetic analysis revealed that the isolates belonged to diverse groups of bacterial communities ranging from Firmicutes (13 species) to Proteobacteria (11 species). Results showed that amongst Proteobacteria, two isolates were from the Alphaproteobacteria group and rest belonged to the Gammaproteobacteria.
Amplification of Arsenic-Resistant Genes
PCR amplification of arsenic resistance genes (arsB and arsC) was performed to understand the genetic mechanism for arsenic resistance and arsenic transformation in the bacterial isolates. The PCR products were further sequenced to re-confirm the detection of genes. The PCR products yield approximately 750 bp and 350 bp amplicon of arsB and arsC gene, respectively. The strains RpSWA2 and RnASA11 showed the highest tolerance of metal salts; they were also positive for arsB and arsC genes (Figs. S3 and S4). It confirmed the presence of ars operon that was the main reason showing resistance by these isolates towards arsenic. The sequencing of these gene amplicons confirmed the presence of their respective coding regions (CDS) in the sequences (Fig. 1).
Phylogenetic trees based on 16S rRNA gene sequences of 24 arsenic-resistant bacteria strains constructed by neighbour-joining method using MEGA6 software. Bootstrap values, expressed as a percentage of 1000 replications, are given at branching points when ≥ 50%. Bar, 0.1 substitutions per nucleotide position. a Gram-positive strains, b Gram-negative strains
Bioremediation Assay for Arsenic
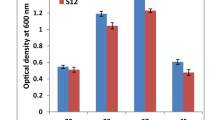
The two strains RpSWA2 and RnASA11 were able to tolerate the same concentration of the metal salts. However, the growth of RpSWA2 strain was comparatively slow as compared to RnASA11 (Fig. S2). Both of these strains were also positive for arsB and arsC gene amplifications. The final bioremediation assay was conducted with RnASA11. A growth curve was prepared by periodically taking the optical densities at 600 nm using UV-spectrophotometer for 10 days or till the ending of the death phase. In the absence of As (V) and As (III), the bacteria maintained its enhanced growth throughout the log phase till 3rd day and after that entered into the declining phase. However, in the presence of arsenic, a prolonged log phase was observed for 5 days with a brief stationary phase and entered into a declining phase from the 5th day onwards. The prolonged log phase in presence of As (V) and As (III) as compared to the shorter one in its absence exhibited the accelerated growth. It was achieved when the bacteria are grown under As (V) and As (III) stress conditions (Fig. 2). The results were supported by the previously published reports [23,24,25] and revealed that the log phase was prolonged in bacteria when it was exposed to arsenic. However, Butt and Rehman [23] reported that the growth pattern of K. pneumonia and K. variicola was significantly different as compared to the controls. The growth rate of bacterial isolates was a bit lower in presence of As (III) where the lag phase was slightly delayed in the presence of As (III) in both the bacterial isolates. The reduction in the number of arsenic concentration by these bacterial isolates was determined through AAS analysis. AAS is still a method of choice for the detection of a large number of elements, despite the variety of critical analytical techniques for the determination of trace elements. The RnASA11 strain showed a significant reduction in arsenic concentration (44.1%) when arsenate was used and average 38.8% reduction when arsenite was used as a source of arsenic in comparison to the control. This reduction was observed at 5% level of significance (Fig. 3). Thus, the arsenic-tolerant ability of bacterial isolate RnASA11 may be used effectively for the bioremediation of arsenic.
PCR amplification of arsB (750 bp) and arsC (350 bp) genes in arsenic-tolerant bacteria K. pneumoniae RnASA11 and CDS obtained from NCBI-CD search results for conserved domains within coding nucleotide sequences of arsB and arsC obtained from K. pneumoniae RnASA11. Agarose gel (1.2%) electrophoresis was separately conducted for both the gene amplicons along with a suitable DNA marker
Growth curve of K. pneumoniae RnASA11 in presence of arsenate (100 ppm) and arsenite (100 ppm) during 10 days long bioremediation assay. This bioremediation assay was conducted with four treatments, i.e. T1: control with arsenic salt; T2: without As + isolate RnASA11; T3: As (V) + isolate RnASA11; and T4: As (III) + isolate RnASA11. Each data represent the mean ± SE obtained from independent experiments with three replicates. Treatment T1 and T2 were compared with rest of the treatments
Discussion
Arsenic contamination is gradually gaining the status of a major form of pollution worldwide. However, arsenic pollution occurs due to both natural and anthropogenic sources. Arsenic being a heavy metal significantly determines the type of bacterial diversity in soil and water. Chhattisgarh is a popular state in India famous for its industrial and mining activities. In recent times, it is found to be contaminated with arsenic in both soil and water sources. The sequencing of the 16S rRNA gene revealed diverse groups of bacterial communities. Arsenic-tolerant isolates were identified to be of different genera such as Bacillus, Enterobacter, Klebsiella, Pantoea, Acinetobacter, Cronobacter, Pseudomonas, and Agrobacterium. The nucleotide sequence coding for the 16S rRNA gene were matched from the NCBI GenBank data. It revealed a similarity percentage of 91–99%. Results exhibited that maximum (13 in number) arsenic-resistant isolates (Fig. 1) belonged to the Bacillaceae family and they represented eight different species. The two isolates BSSA1 and RnAWA3 belonged to Priestia megateriums, which is a well-known plant growth promoter. It has reported previously for the alleviation of heavy metal toxicity in plants [26]. The isolates RgCSA1 and RpSSA7 were identified as P. aryabhattai and this species has recently reported for reducing arsenic phytotoxicity in plants by Ghosh et al. [27]. To the best of our knowledge, species like B. haynesii and B. nakamurai have not reported previously for arsenic resistance. Furthermore, the bacterial isolates, four (BGWA1, RgCWA5, RpUWA1, and RpSWA3) from genus Enterobacter and two (RgCWA1 and RnASA11) from genus Klebsiella belong to the family of Enterobacteriaceae, are well known for arsenic resistance [24]. Results also showed that some species of genus Enterobacter, i.e. E. tabaci, E. xiangfangensi, and E. bugandensis were resistant towards arsenic. Except for two isolates KDWA1 and KDWA2, which belong to genus Pantoea, the rests BHWA1, BHSA6, RgCSA2, KWA2, and RpSWA4 were from genus Acinetobacter, Cronobacter, Pseudomonas, Agrobacterium, and Cedecea, respectively (Fig. 4). All the above genera have previously reported for arsenic resistance [28].
The average arsenic concentrations detected after AAS analysis of samples collected from bioremediation assay. All the samples were further diluted for AAS analysis. This bioremediation assay was conducted with four treatments, i.e. T1: control with arsenic salt; T2: without As + isolate RnASA11; T3: As (V) + isolate RnASA11; and T4: As (III) + isolate RnASA11. Each data represent the mean ± SD obtained from independent experiments with three replicates. Student’s t test was used to analyse the data and *P < 0.05 compared with T2: without As + isolate RnASA11 (0 μM)
Antibiotics have been extensively used in agriculture and aquaculture. Bacteria are showing resistance against antibiotics due to their uncontrolled use in the environment. The presence of heavy metals often acts as a selective agent for antibiotic resistance in bacteria. It exerts a selection pressure in the environment, thereby resulting in the co-selection of metal and antibiotic resistance [29, 30]. Besides, numerous antibiotic resistance mechanisms in prokaryotes have seen to be influenced by metals. It has reported that a positive correlation exists in bacteria (i.e. between antibiotic resistance and heavy metal tolerance) and the mechanism for this co-selection is based on the cross-resistance and co-resistance phenomenon [31,32,33]. The results of this study are supported by previous works where a close association between arsenic tolerance and antibiotic resistance exist [34, 35]. Therefore, the findings of the present study help us in giving a better understanding of public health issues occurring in Chhattisgarh (India) related to the multi-resistance of antibiotics towards human and animal pathogens. Besides, it is also important to mention that bacteria previously exposed to the arsenic environment are showing resistance towards new antibiotics due to selection pressure. Therefore, to prevent bacteria from becoming a “superbug”, we must find ways of preventing arsenic contaminations in the environment.
The most widely considered mechanism for arsenic resistance in bacteria is the ars operon system which mainly consists of 3/5 genes arranged in a single transcriptional unit. In this study, we amplified the two most important genes in ars operon (arsC and arsB) to better understand the resistant mechanisms of the above bacterial genera isolated from different arsenic-contaminated areas. These genes are located in genomic or plasmid DNA [36, 37]. The arsC gene encodes a monomeric protein having 135 amino acids with three essential cysteine residues. Bacteria uptake arsenate through their phosphate channels when exposed to an arsenic environment. It occurs when the arsC gene comes into play and causes a reduction of cytoplasmic arsenate by converting it to arsenite. Arsenite is extruded from the cell with the help of an ATP-driven arsenite efflux pump formed by arsB gene with an association of arsA [15]. Sometimes, the presence of three copies of arsC gene enhances the levels of arsenate reduction in bacteria [19]. In this study, successful amplification of arsB and arsC genes confirmed the presence of arsenic detoxification mechanism for the reduction of As (V) in the isolates. Sequencing of arsB and arsC genes revealed that the functional protein of these genes belongs to anion permease family (arsB) and thioredoxin-like superfamily (arsC). However, the expression of both genes is regulated by a third gene, i.e. arsR [38]. The above discussion represents the genetic mechanisms of these bacterial isolates under an arsenic-rich environment. Further, it can be noted that the presence of ars operon is very essential for the survival of these bacterial isolates when exposed to arsenic stress.
It can be concluded from the study that the arsenic-contaminated soil and water of Chhattisgarh harbour diverse genera of bacteria. Additionally, it was found that repeated and persistent exposure of bacterial isolates to the arsenic environment caused resistance against this heavy metal, which is a point to ponder over for protecting the environment.
Conclusion
This study gave a picture of the bacterial diversity seen in the arsenic-contaminated soil and water of Chhattisgarh state. Finally, out of 24 isolates, one potential isolate RnASA11 was selected. The results obtained after PCR amplification and bioremediation assay indicated the presence of the arsenic resistance system in Klebsiella pneumoniae RnASA11. The presence of arsC gene reduced arsenate into arsenite, whilst arsB gene confirmed the efflux mechanism available in this isolate through which it extruded arsenite from the cell. This process depicted its arsenic resistance mechanism. Moreover, AAS analysis showed a reduction of arsenate (44.1%) and arsenite (38.8%), which were quite significant, and therefore, its arsenic resistance ability can be further used for bioremediation purpose, that this potential isolate could.
References
Dash B, Soni R, Goel R (2019) Rhizobacteria for reducing heavy metal stress in plant and soil. In: Sayyed RZ, Arora NK, Reddy MS (eds) Plant growth promoting rhizobacteria for sustainable stress management volume 1: rhizobacteria in abiotic stress management. Springer, Singapore, pp 179–203
Soni R, Dash B, Kumar P, Mishra UN, Goel R (2019) Microbes for bioremediation of heavy metals. In: Singh DP, Prabha R (eds) Microbial interventions in agriculture and environment volume: soil and crop health management. Springer, Singapore, pp 129–142
Ratnaike RN (2003) Acute and chronic arsenic toxicity. Postgrad Med J 79:391–396. https://doi.org/10.1136/pmj.79.933.391
Sher H, Perry Z, Arbel S, Reuveni H (2019) Prescription patterns of antidepressants: the effect of the black box warning among pediatric patients. Res Health Sci 4:1. https://doi.org/10.22158/rhs.v4n1p1
Roane TM, Pepper IL (1999) Microbial responses to environmentally toxic cadmium. Microb Ecol 38:358–364. https://doi.org/10.1007/s002489901001
Yang HC, Rosen BP (2016) New mechanisms of bacterial arsenic resistance. Biomed J 3(9):5–13. https://doi.org/10.1016/j.bj.2015.08.003
Moghannem S, Refaat B, Elsherbiny G, El-Sayed M, Elsehemy I, Kalaba M (2015) Characterization of heavy metal and antibiotic-resistant bacteria isolated from polluted localities in Egypt. Egypt Pharmaceut J 14:158. https://doi.org/10.4103/1687-4315.172856
Chakraborti D, Rahman MM, Das B, Chatterjee A et al (2017) Groundwater arsenic contamination and its health effects in India. Hydrogeol J 25:1165–1181. https://doi.org/10.1007/s10040-017-1556-6
Patel KS, Shrivas K, Brandt R, Jakubowski N, Corns W, Hoffmann P (2005) Arsenic contamination in water, soil, sediment and rice of central India. Environ Geochem Health 27:131–145. https://doi.org/10.1007/s10653-005-0120-9
Shrivastava A, Ghosh D, Dash A, Bose S (2015) Arsenic contamination in soil and sediment in India: sources, effects, and remediation. Curr Pollution Rep 1(2015):35–46. https://doi.org/10.1007/s40726-015-0004-2
Yadav A et al (2020) Assessment of arsenic and heavy metal pollution in Chhattisgarh. India J Hazard Toxic Radioact Waste. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000478
Kumar P, Gupta SB, Anurag SR (2019) Bioremediation of cadmium by mixed indigenous isolates Serratia liquefaciens BSWC3 and Klebsiella pneumoniae RpSWC3 isolated from industrial and mining affected water samples. Pollution 5:351–360. https://doi.org/10.22059/POLL.2018.268603.533
Simeonova D, Lievremont D, Lagarde F, Muller D, Groudeva V, Lett MC (2004) Microplate screening assay for detection of arsenite oxidizing and arsenate-reducing bacteria. FEMS Microbiol Lett 237:249–253
Lu JJ, Perng CL, Lee SY, Wan CC (2000) Use of PCR with universal primers and restriction endonuclease digestions for detection and identification of common bacterial pathogens in cerebrospinal fluid. J Clin Microbiol 38(6):2076–2080
Achour AR, Bauda P, Billard P (2007) Diversity of arsenite transporter genes from arsenic-resistant soil bacteria. Res Microbiol 158:128–137. https://doi.org/10.1016/j.resmic.2006.11.006
Saltikov CW, Olson BH (2002) Homology of Escherichia coli R773 arsA, arsB, and arsC genes in arsenic-resistant bacteria isolated from raw sewage and arsenic-enriched creek waters. Appl Environ Microbiol 68:280–288. https://doi.org/10.1128/aem.68.1.280-288.2002
Sher S, Zajif Hussain S, Rehman A (2020) Multiple resistance mechanisms in Staphylococcus sp. strain AS6 under arsenite stress and its potential use in amelioration of wastewater. J King Saud Univ Sci 32(7):3052–3058. https://doi.org/10.1016/j.jksus.2020.08.012
Mujawar SY, Shamim K, Vaigankar DC, Dubey SK (2019) Arsenite biotransformation and bioaccumulation by Klebsiella pneumoniae strain SSSW7 possessing arsenite oxidase (aioA) gene. Biometals 32:65–76. https://doi.org/10.1007/s10534-018-0158-7
Li X, Krumholz LR (2007) Regulation of arsenate resistance in Desulfovibrio desulfuricans G20 by an arsRBCC operon and an arsC gene. J Bacteriol 189:3705–3711. https://doi.org/10.1128/jb.01913-06
Banerjee S, Datta S, Chattyopadhyay D, Sarkar P (2011) Arsenic accumulating and transforming bacteria isolated from contaminated soil for potential use in bioremediation. J Environ Sci Health A Tox Hazard Subst Environ Eng 46:1736–1747. https://doi.org/10.1080/10934529.2011.623995
Naher U, Rahman F, Islam SMM, Sarkar MI, Biswas J (2016) Isolation of arsenic oxidizing-reducing bacteria and reclamation of As (III) in in vitro condition. Bangladesh Rice J 19:94. https://doi.org/10.3329/brj.v19i2.28170
Shakoori FR, Tabassum S, Rehman A, Shakoori AR (2010) Isolation and characterization of Cr6+ reducing bacteria and their potential use in bioremediation of chromium containing wastewater. Pakistan J Zool 42(6):651–658
Butt AS, Rehman A (2011) Isolation of arsenite-oxidizing bacteria from industrial effluents and their potential use in wastewater treatment. World J Microbiol Biotechnol 27:2435–2441. https://doi.org/10.1007/s11274-011-0716-4
Abbas SZ, Riaz M, Ramzan N, Zahid MT, Shakoori FR, Rafatullah M (2014) Isolation and characterization of arsenic resistant bacteria from wastewater. Braz J Microbiol 45:1309–1315. https://doi.org/10.1590/s1517-83822014000400022
Daware V, Gade WN (2015) Mechanism of arsenic tolerance in Klebsiella pneumoniae (HQ857583). Ind J Sci Res 6:457–469. https://doi.org/10.21608/eajbsg.2015.16483
Titah HS, Abdullah SRS, Idris M, Anuar N et al (2018) Arsenic resistance and biosorption by isolated rhizobacteria from the roots of Ludwigia octovalvis. Int J Microbiol 1–10:3101498. https://doi.org/10.1155/2018/3101498
Ghosh PK, Maiti TK, Pramanik K, Ghosh SK, Mitra S, De TK (2018) The role of arsenic resistant Bacillus aryabhattai MCC3374 in promotion of rice seedlings growth and alleviation of arsenic phytotoxicity. Chemosphere 211:407–419
Anderson CR, Cook GM (2004) Isolation and characterization of arsenate-reducing bacteria from arsenic-contaminated sites in New Zealand. Curr Microbiol 48:341–347. https://doi.org/10.1007/s00284-003-4205-3
Baker-Austin C, Wright MS, Stepanauskas R, McArthur JV (2006) Co-selection of antibiotic and metal resistance. Trends Microbiol 14:176–182. https://doi.org/10.1016/j.tim.2006.02.006
Jiang H, Yu T, Yang Y, Yu S, Wu J, Lin R, Li Y, Fang J, Zhu C (2020) Co-occurrence of antibiotic and heavy metal resistance and sequence type diversity of vibrio parahaemolyticus isolated from Penaeus vannamei at freshwater farms, seawater farms, and markets in Zhejiang province, China. Front Microbiol 11(1294):1–14. https://doi.org/10.3389/fmicb.2020.01294
Seiler C, Berendonk TU (2012) Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front Microbiol 3:399. https://doi.org/10.3389/fmicb.2012.00399
Pal C, Asiani K, Arya S, Rensing C, Stekel DJ, Larsson DGJ, Hobman JL (2017) Metal resistance and its association with antibiotic resistance. Adv Microb Physiol 70:261–313. https://doi.org/10.1016/bs.ampbs.2017.02.001
Zhang M, Wan K, Zeng J, Lin W, Ye C, Yu X (2020) Co-selection and stability of bacterial antibiotic resistance by arsenic pollution accidents in source water. Environ Int 135(105351):1–11. https://doi.org/10.1016/j.envint.2019.105351
Tewari S, Ramteke P, Tripathi M, Kumar S, Garg S (2013) Plasmid mediated transfer of antibiotic resistance and heavy metal tolerance in thermotolerant water borne coliforms. Afr J Microbiol Res 7:130–136. https://doi.org/10.5897/AJMR12.1563
Chen J, Rosen BP (2020) The arsenic methylation cycle: how microbial communities adapted methylarsenicals for use as weapons in the continuing war for dominance. Front Environ Sci 8(43):1–14. https://doi.org/10.3389/fenvs.2020.00043
Kaur S, Kamli MR, Ali A (2011) Role of arsenic and its resistance in nature. Can J Microbiol 57:769–774. https://doi.org/10.1139/w11-062
Dash B, Sahu N, Singh AK, Gupta SB, Soni R (2021) Arsenic efflux in Enterobacter cloacae RSN3 isolated from arsenic-rich soil. Folia Microbiol 66:189–196. https://doi.org/10.1007/s12223-020-00832-2
Chen J, Rosen BP (2014) Biosensors for inorganic and organic arsenicals. Biosensors 4:494–512. https://doi.org/10.3390/bios4040494
Acknowledgements
Author RS wants to acknowledge his university, i.e. Indira Gandhi Krishi Vishwavidyalaya, Raipur, India, for financial assistance. However, this research did not receive any specific grant from funding agencies in the public, commercial, or nonprofit sectors. We also acknowledge the Department of Soil Science and Agril. Chemistry, Indira Gandhi Krishi Vishwavidyalaya, Raipur, for providing instrumentation facilities during this research.
Author information
Authors and Affiliations
Contributions
The concept, design, data analyses, manuscript preparation, and revision were performed by RS and PK. The experiments, data curation, manuscript preparation, and validation were performed by RS, PK, BD, and SBG. The project supervision, final validation, fund management, and final manuscript revision were performed by RS, SBG, and TC. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflict of interest regarding the publication of this manuscript.
Ethical Approval
This study does not describe any experimental work related to human.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
284_2021_2602_MOESM1_ESM.jpg
Supplementary file1 Pictorial view of sample collection sites in Industrial and Mining affected area of Chhattisgarh (India). (JPG 109 kb)
284_2021_2602_MOESM2_ESM.jpg
Supplementary file2 Comparative screening of strain RpSWA2 and RnASA11. The experiment was conducted using seven treatments with three replications of each. Treatment without metal salt was treated as a positive control whereas absolute control did not contain any metal salts and bacterial culture. ( JPG 34 kb)
Rights and permissions
About this article
Cite this article
Kumar, P., Dash, B., Suyal, D.C. et al. Characterization of Arsenic-Resistant Klebsiella pneumoniae RnASA11 from Contaminated Soil and Water Samples and Its Bioremediation Potential. Curr Microbiol 78, 3258–3267 (2021). https://doi.org/10.1007/s00284-021-02602-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-021-02602-w