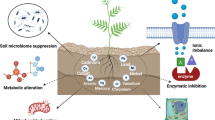
Abstract
The intensity of pollution expansion is increasing day by day of which heavy metal pollution has taken the center stage of discussion since the last few decades. Heavy metals have direct detrimental effect on our ecosystem in general and on the agroecosystem in particular, thereby proving to be hazardous for plants, animals, and microbes. One of the most common, low-cost, and eco-friendly strategies that can be employed to counter this problem effectively is through bioremediation. However among several types of bioremediation, microbial bioremediation with the use of rhizobacteria is best suited for alleviating heavy metal stresses in the agroecosystem.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
There exists a lot of misperception over the classification and definition of heavy metals. Still scientific groups have not reached into any consensus regarding this issue. In a report published in the International Union of Pure and Applied Chemistry (IUPAC), Duffus (2002) raised questions over the usage of the term “heavy metals” and its classifications. Therefore he suggested undertaking a much broader approach while classifying heavy metals based on the periodic table. In agreement with his views, Appenroth (2010) proposed for considering three groups of elements (transition elements, rare earth metals, and borderline elements) as heavy metals from the periodic table after thoroughly studying their chemical properties.
Keeping all these discussions aside, however, the most commonly followed definition of heavy metals is “These are the elements with an atomic weight between 63.5 and 200.6 followed by a specific gravity of more than 5.0” (Srivastava and Majumder 2008). In simple terms, we can say that they are heavier than water by five times or are having an atomic density >4 g/cm3 (Duruibe et al. 2007; Mahamood et al. 2012). They can also be defined as the block of all metals in Groups 3–16 that are present in period 4 and above, i.e., periods 5, 6, and 7 (Hawkes 1997). The term heavy metals in a broader sense are often used whenever there arises some implication for toxicity. As heavy metals are present in very minute quantity, i.e., 1 μg kg−1, these are often represented as trace elements (Tchounwou et al. 2012). Some of these trace metals are beneficial for plants (Zn, Mn, Fe, Cu, B, and Mo), while others are non-beneficial (Se and Co), and the rest (As, Hg, Pb, Cr, Cd, and Ni) are toxic (He et al. 2005).
10.1.1 Current Status of Heavy Metal Pollution
Pollution of heavy metals has been seen everywhere across the earth (lithosphere, atmosphere, and hydrosphere). It has been escalated to such an extent that it can be found even on the most extreme climatic conditions on earth starting from Mount Everest (Yeo and Langley-Turnbaugh 2010) to the deep ocean floor (Humbatov et al. 2015) and also underneath the topsoil layer (Wuana and Okieimen 2011; Su et al. 2014). Bioaccumulation of these metals can be seen on food items like milk (Tunegova et al. 2016), vegetables (Agrawal et al. 2007; Mishra and Tripathi 2008), fishes (Ebrahimpour et al. 2011; Abarshi et al. 2017), and livestock (Rajaganapathy et al. 2011; Okareh and Oladipo 2015). Rampant pollution had led to their worldwide distribution across every continent. Be it Asia (Rajindiran et al. 2015; Chen et al. 2015; Ghorbani et al. 2015) or Africa (Yabe et al. 2010), their presence can be felt everywhere. Rapid industrialization has also escalated their concentration in developed portions of the world like Europe (Panagos et al. 2013; Toth et al. 2016), Australia (Hart and Lake 1987), and South America (Smolders et al. 2003; Eichler et al. 2015). However, their presence in Antarctica seems to be quite surprising as it is so far uninhabited and unexplored as compared to the rest of the world (Evans et al. 2000; Santos et al. 2005). These things reflect the true situation of heavy metal pollution, thus a much needed eye-opener for us to save our ecosystem from further destruction.
Heavy metals are also found above permissible limits in our day-to-day utility commodities like food items (Mahaffey et al. 1975), soft drinks (Bingol et al. 2010; Godwill et al. 2015), and cosmetics (Borowska and Brzoska 2015). In some of the worst affected countries like India and Bangladesh, arsenic (As) is present above permissible limits in rice grains (Sinha and Bhattacharyya 2014; Meharg and Rahman 2003). Rice being the staple food in these countries leads to direct intake of arsenic. Not only in rice but also arsenic in cereals, pulses, vegetables, and forage crops has been reported by several researchers (Sharma et al. 2007; Santra et al. 2013). A regular dietary intake of these arsenic-contaminated food items (Signes et al. 2008) is a direct threat to one’s life. Therefore, different regulatory agencies like the World Health Organization (WHO), European Food Safety Authority (EFSA), and Agency for Toxic Substances and Disease Registry (ATSDR) have prescribed the maximum intake capacity of heavy metals as mentioned in Table 10.1.
Arsenic among all these heavy metals is ranked among the top ten hazardous chemicals by WHO. Besides this, it is also ranked number 1 by ATSDR (2017) on its substance priority list followed by lead and mercury. Lead till now is probably the most well-studied occupational toxin causing about 0.6% of all diseases worldwide (Gidlow 2004). More than 120 million people worldwide come under the threat lead toxicity with developing nations being the most affected (Venkatesh 2009).
The direct impact of heavy metal contamination is seen in soil and groundwater. The European Commission’s report on soil contamination and their impact on human health stated that heavy metals are the most frequently occurring contaminants on soil (35%) and groundwater (31%). Soils (around 33%) all over the world are facing serious heavy metal contamination problem (Roslan et al. 2016). Say for China, around 19.40% of Chinese farmland is facing heavy metal pollution (Zhang et al. 2015). Due to soil pollution, a loss of more than 10 billion US dollars is being incurred from over 10 million polluted sites out of which 50% contaminants happen to be heavy metals (He et al. 2015). Agricultural pesticides are one of the main sources of arsenic contamination in soil. A total of around 80–90% arsenic produced annually finds its way into soil through these chemicals (Nriagu and Pacyna 1988). Hutton and Symon (1986) reported that annually 1637 tons of lead and 111 tons of arsenic are being deposited into the arable soils of the United Kingdom through anthropogenic sources.
Atmospheric pollution of heavy metals after soil is the next biggest concern for researchers. About 30% of mercury per annum is released from anthropogenic sources into the atmosphere of which 50% comes from Asia alone (UNEP 2013). Excessive release of mercury into the air transports them to North America by wind, accounting for 5–36% of mercury deposition in the United States (Jaffe et al. 2005). Due to its long-range transport ability, even the Arctic region is also polluted from mercury contamination (Ilyin et al. 2004). Other than mercury, cadmium also contributes significantly to atmospheric heavy metal pollution. It has been reported that Spain and France equally contribute (i.e., 16%) for cadmium emission in Europe’s air (Dinis and Fiuza 2011). In recent times, Indian cities also show the presence of heavy metals in their atmosphere, often exceeding the maximum permissible limits (Chaudhari et al. 2012; Dey et al. 2014).
Groundwater heavy metal contamination is also an equally important global concern like soil and air heavy metal pollution. Among all heavy metals, arsenic contamination in groundwater is most noticed with South Asian countries like Bangladesh and India being worst affected (Ravenscroft et al. 2005; Pal et al. 2009). All over the world, nearly 130 million people come under the threat of arsenic contamination by drinking As-contaminated water, which is often above the prescribed limit (10 ppb) set by WHO (UNICEF 2008). Majority of these populations are inhabitants of two countries, i.e., Bangladesh (35–77 million) and India (12 million from the state of West Bengal alone), making them globally the worst hit countries (Smith et al. 2000; Ravenscroft et al. 2009). In India there are seven major states (West Bengal, Assam, Uttar Pradesh, Chhattisgarh, Bihar, Jharkhand, and Manipur) which find arsenic contamination in their groundwater (Chakraborti et al. 2017). The regulatory limits of heavy metals in drinking water prescribed by different agencies have been stated in Table 10.2.
10.1.2 Sources of Heavy Metal Pollution
Heavy metal contamination in the environment occurs through natural and anthropogenic means (Chen et al. 2009; INSA 2011). Heavy metals are nondegradable, due to which they are persistent in our environment and in the course of time get released into the soil, water, and air (Zaharescu et al. 2009; Aksu 2015; Van et al. 2016; Drira et al. 2017). During weathering and soil formation processes, they are released from rocks (metamorphic, sedimentary, and magmatic rocks) and minerals (oxides, hydroxides, and clay minerals) into the environment (Brad 2005). The fate of these metals on soil is governed by the type of parent materials and physiochemical properties of soil (Abdelilah et al. 2010; Roozbahani et al. 2015). Natural phenomena like volcanic eruptions, forest fires, and soil erosions play a major role in their distribution (Bielicka et al. 2005; Akpor et al. 2014). In aquatic systems, sediments are the chief storehouse of heavy metals, governing their overall distribution and transformation processes in water bodies (Wu et al. 2014). The level of heavy metals is often well regulated and rarely crosses their limits in natural environment.
However in contrast to natural sources, anthropogenic sources are more responsible for elevation of heavy metal concentration in natural environment (Xu et al. 2014). Human-driven activities like mining, intensive agricultural practices, and road constructions driven by urbanization and industrialization act as the perfect catalyst for their release into natural environment (Imperato et al. 2003; Liao et al. 2018). Intensive agricultural practices like excessive usage of pesticides and fertilizers coupled with sewage water for irrigation have led to the accumulation of heavy metals in cultivated soils (Sidhu 2016). Furthermore, runoff water passing through highways during rainfall contains heavy metals (Turer et al. 2001). Besides these, polyvinyl chloride (PVC) products, chargeable batteries, brake linings, tires, color pigments, furnace dusts, etc. are some other potential sources of heavy metals (Oves et al. 2016). Comprehensive descriptions for anthropogenic sources of heavy metals are listed in Table 10.3.
10.2 Effects of Heavy Metals on Life Forms
Due to their persistent nature, heavy metals accumulate in our body resulting in several health issues (Sharma et al. 2007; Garg et al. 2014). Heavy metals enter our body through food, air, and water. However the chief entry route of heavy metals into our body is through food (Darwish et al. 2015; Yadav et al. 2017). Regular intake of heavy metal-contaminated food can retard growth and weaken our immune system (Singh and Kalamdhad 2011). Alongside food, they can also make entry through the skin and air (Liang et al. 2017). Entry of these metals through food chain causes their bioaccumulation and paves the path for several cardiovascular, nervous, kidney, and bone diseases (Rani and Goel 2009; Ji-yun et al. 2016). Health issues occurring due to heavy metals are enlisted in Table 10.4.
The term heavy metal is often used in context of toxicity, but it should be noted that not all heavy metals (like Mn, Cu, Zn, Fe, etc.) are harmful (Flora et al. 2008). Some of these heavy metals are part of several metabolic pathways, while the rest are toxic to our body (Mahurpawar 2015; Al-Fartusie and Mohssan 2017). Chromium, for instance, has dual functions in our body. In its low concentration, it is used in a number of metabolic processes (like fat and protein metabolism), while excess exposure causes several respiratory diseases (Sathawara et al. 2004). In a similar way, copper is used for iron absorption and signaling. However when present in excess amount, it causes liver and kidney dysfunctions (Ashish et al. 2013). More often than not, we consider Zn (Zinc) as an essential element as it is part of numerous proteins and metalloenzymes. It is observed that excessive amount of Zn in our body may result in nausea and vomiting while its deficiency leads to neural disorders (Plum et al. 2010). Heavy metals are mutagenic and carcinogenic in nature (Silva et al. 2005; Fernandez-Luqueno et al. 2013). Heavy metal contamination causes a wide range of health issues related to developmental, gastrointestinal, dermal, respiratory, cardiovascular, immunological, and reproductive systems (Liu et al. 2013).
Heavy metals lead to oxidative stress, and to neutralize this effect, the cell produces antioxidants (catalase and superoxide dismutase) in its response. This balance is always maintained in our body, and any imbalances lead to altered gene expression, activation of signaling pathways, and production of cytokines (Salnikow et al. 2000; Leonard et al. 2004). Activation of metal-induced signaling pathways affects several signaling components (G-proteins, MAP kinases, tyrosine kinases, growth factor receptors, and nuclear transcription factors), thereby disrupting the normal functioning of the cell (Harris and Shi 2003; Flora et al. 2008). Researchers have also reported that heavy metal stresses induce apoptosis in cells (Wang and Shi 2001). Heavy metals cause cancer and are thus labeled as carcinogens (Galaris and Evangelou 2002). These metals damage DNA and cause mutation which leads to cancer (Durham and Snow 2006; Jadoon and Malik 2017), with lung and skin cancers being the most common among them (Harris and Shi 2003). However they also cause several other cancers like liver, kidney, bladder, prostate, lymphoma, leukemia, and breast (Pourahmad et al. 2003). The central nervous system (CNS) and hematopoietic system are also affected by the presence of these metals (Florea and Busselberg 2006). It has been reported that these metals are related to a wide range of neurological diseases like Wilson’s disease (Cu), Parkinson’s disease (Fe, Mn, and Cu), Alzheimer’s disease (Cd), Hallervorden-Spatz disease (Fe), multiple sclerosis, polycythemia, Minamata disease (Hg), muscular dystrophy, sideroblastic anemia, itai-itai disease (Cd), and blackfoot disease (As), among others (Montgomery 1995; Khan et al. 2013; Jaishankar et al. 2014; Draszawka-Bolzan 2014; Min and Min 2016). Metal toxins alter the functioning of neurotransmitters like catecholamines and bring about behavioral changes in humans (Shukla and Singhal 1984; Inoue 2013). Premature aging can occur due to heavy metal toxicity, thus paving the path for occurrence of numerous diseases (Mudgal et al. 2010).
Like humans, plants too uptake heavy metals, and their entry points are root and leaves. They get deposited in the cell wall, plasma membrane, or cytoplasm after traveling through xylem by means of apoplastic and symplastic pathways (Shahid et al. 2015; Clemens and Ma 2016). Uptake of heavy metal by plants is greatly influenced by the type of plant species and the defense mechanisms followed by them to overcome its toxicity (Alves et al. 2016). In agriculture, there are a lot of crop plants which show phytotoxicity to these metals (Forster 1954; Benzarti et al. 2008). The attributes that are hampered by heavy metal toxicity are seed germination, yield, nutrient uptake, and nitrogen fixation (Athar and Ahmad 2002; Guala et al. 2010; Sethy and Ghosh 2013). It has been observed that sometimes heavy metals besides competing with each other also try to compete with several other essential elements for their uptake, both at the cellular level and in the soil system (Krupa et al. 2002; Israr et al. 2011). For example, a certain concentration of As (arsenic) helps in the uptake of Mn, Cu, Fe, and P; however with its further increase in concentration, uptake of these metals decreases (Farnese et al. 2014).
There is significant reduction in the photosynthetic rate of plants due to heavy metal toxicity. This is due to the fact that these metals affect the enzymes of photosystem I and II which causes lower biomass production (Oves et al. 2016). Physiological and biochemical activities of plants like respiration, translocation, transcription, translation, mineral metabolisms, cell signaling, and cell cycle along with some developmental processes like flowering and embryogenesis are also affected (Ovecka and Takac 2014). Due to the presence of abiotic stresses (i.e., from heavy metals), lower root and shoot growth is observed in several crop plants which can be correlated with decrease in chlorophyll and protein content in these plants (Manios et al. 2002; John et al. 2009). Furthermore, heavy metal toxicity is dependent on plant growth stages (Cheng 2003; Peralta-Video et al. 2004).
Like humans, plants also produce reactive oxygen species (ROS) like H2O2, OH−, 1O2, and O2− and reactive nitrogen species (RNS) like nitric oxide and peroxynitrite ONOO− and free radicals in response to oxidative stress caused by heavy metals (Zengin and Munzuroglu 2005; Moller et al. 2007). Oxidative stress results in cellular toxicity and leads to oxidative degradation of biomolecules like carbohydrates, proteins, lipids, and nucleic acids (Aras et al. 2012). Arsenic toxicity displays a variety of symptoms in plant like leaf defoliation, chlorosis, necrosis, reduced fertility, stunted growth, and senescence and under severe condition may also cause death (Gulz et al. 2005; Abbas et al. 2018). Phosphate metabolism in plants gets affected by arsenic as arsenate mimics phosphate ion and can get substituted in its place (Kaur et al. 2011). Besides this, magnesium ion in chlorophyll molecule may also be substituted by other heavy metals (Zurek et al. 2014). Likewise, cadmium also interferes with several plant processes like photosynthesis, transpiration, mineral nutrition (N, K, Ca, Mg, P, and Fe), stomatal opening, and antioxidant metabolism (Benavides et al. 2005; Nazar et al. 2012). Nickel plays an essential role in nitrogen metabolism and seed germination. However, Ni toxicity results in chlorosis and yellowing of leaves which finally affect the normal functioning of plant (Selvaraj 2018). In some cases, it is interesting to see that two heavy metals have additive effects on their toxicity in plants. For instance, in barley plant, it has been observed that the combined effect of copper and cadmium resulted in lower root and shoot growth (Zaltauskaite and Sliumpaite 2013).
10.3 Heavy Metals and Microorganisms
Microorganisms also are subjected to heavy metal stress like any other life forms. Microbes (diatoms and microalgae) are often used for heavy metal pollution assessment and act as bioindicators (Sbihi et al. 2012; Djukic and Mandic 2018). Microbes are very sensitive to heavy metals and exhibit this sensitivity even at species and strain level (Giller et al. 1998). Microbes from different habitats and groups exhibit varied level of heavy metal tolerance (Sadler and Trudinger 1967). Generally, fungi are said to be more tolerant than bacteria to these metals (Rajapaksha et al. 2004).
Soil when exposed with heavy metals for a prolonged period of time resulted in decreased microbial biomass and reduced microbial diversity and activity with further change in their genetic composition (Chen et al. 2014; Kuzniar et al. 2018). These metals also considerably influence the bacterial community structure as revealed from metagenomic studies (Yao et al. 2017). These metals enhance microbial growth in its lower concentration while when present in excess quantity are harmful for the cell by affecting its membrane integrity, destroying its cellular organelles, and damaging its genetic materials (Sengor et al. 2009). Furthermore, an increase in lag time brings about reduction in growth of microbial cells (Gikas et al. 2009). Physiological activities like respiration and metabolism are affected due to heavy metals resulting in lower production of soil enzymes (Xie et al. 2016). Further, reproduction of several fungal species also gets influenced by the presence of these metals. Baldrian (2003) reported that the reproductive stages of saprophytic and mycorrhizal fungus were more affected as compared to their vegetative stages.
Microbes growing in the presence of heavy metals show certain morphological changes like transformation from one form to another. Certain bacteria change their shape from rod to spherical in copper’s presence (Sadler and Trudinger 1967). Similar findings have been reported in fungi where heavy metal induces certain morphological changes in fungal hyphae (Ali 2007). Soil-inhabiting fungus is also affected from these metals. Fungi play an important role in biodegradation process and biogeochemical cycles while influenced by the presence of heavy metals (Hartikainen et al. 2012; Khan et al. 2013). Nitrification, which is a crucial step in nitrogen cycle, is significantly inhibited by the presence of these metals (Park and Ely 2008; Hamsa et al. 2017). Microorganisms have the ability of uptaking heavy metals through certain metabolic or physiochemical pathways known as microbial biosorption. This metal uptake rate depends upon a wide array of factors like physiological state of cell, nature of growth medium, and type of microbes growing (Vijayadeep and Sastry 2014). By effectively utilizing this property, microbes can serve as a tool for alleviating heavy metal stress from the environment (Yamaji et al. 2016).
10.3.1 Bioremediation of Heavy Metal by Rhizobacteria
Soil pollutants can be extracted from the soil by employing several bioremediation techniques. Plant growth-promoting rhizobacteria (PGPR) are one of the better prospects for bioremediation of heavy metals in the rhizosphere. Rhizobacteria in combination with plants are more fruitful and provide better efficiency for bioremediation of heavy metals (Whiting et al. 2001). Upon exposure to heavy metal stress, rhizobacteria alter plant metabolism, due to which plants are able to withstand high concentrations of metals (Welbaum et al. 2004). The use of rhizobacteria in phytoremediation has therefore recently gained some momentum (de Souza et al. 1999). The symbiotic effectiveness of bacteria-plant system for the restoration of polluted soil from chromium and cadmium contamination was studied by Sobariu et al. (2017) where they utilized rhizospheric Azotobacter bacteria and Lepidium sativum plant for completing this task. They observed that the ability of heavy metal tolerance by plant improved under symbiotic condition. Furthermore, bacterial consortia native to heavy metal-contaminated soil, consisting of Bacillus mycoides and Micrococcus roseus, were found effective for phytoextraction and phytostabilization of Cd (Malekzadeh et al. 2012). Bioremediation of zinc was mediated by rhizobacteria (Bacillus megaterium and Pseudomonas aeruginosa) isolated from weed (Suaeda nudiflora) growing in chemically polluted site (Jha et al. 2017).
Important genera of cadmium-resistant rhizobacteria reported from some food crops (wheat, maize, barley, mustard, mung bean, black gram, and pumpkin) are Pseudomonas spp., Burkholderia sp., Flavobacterium sp., and Arthrobacter mysorens (Belimov and Dietz 2000; Ganesan 2008; Sinha and Mukherjee 2008; Kuffner et al. 2010; Xu et al. 2012; Saluja and Sharma 2014). Similarly, some arsenic-resistant gram-positive rhizobacteria are Bacillus megaterium, Bacillus pumilus, Bacillus cereus, Arthrobacter globiformis, and Staphylococcus lentus, while gram-negative rhizobacteria include Rhizobium radiobacter, Rhizobium rhizogenes, Enterobacter asburiae, Agrobacterium radiobacter, Sphingomonas paucimobilis, and Pantoea spp. (Wang et al. 2011; Titah et al. 2014; Lampis et al. 2015; Singh et al. 2015; Mesa et al. 2017). Rafique et al. (2015) reported some bacterial genera (Bacillus, Pseudomonas, and Cronobacter) capable of showing dual functions, i.e., simultaneously showing resistance for mercury, as well as capable of nitrogen fixation. Likewise, rhizobacteria capable of tolerating chromium are Pseudomonas, Ochrobactrum, Mesorhizobium, Bacillus, Paenibacillus, Cellulosimicrobium, and Rhodococcus (Faisal and Hasnain 2006; Trivedi et al. 2007; Chatterjee et al. 2009; Khan et al. 2012; Hemambika et al. 2013; Upadhyay et al. 2017).
10.3.2 Mechanisms of Heavy Metal Tolerance in Bacteria
Microbes are persistently able to survive in heavy metal-polluted environment by using a number of methods like biosorption, biomineralization, bioaccumulation, and biotransformation. Bioaccumulation is a process by which bacteria accumulate heavy metals in its cell which is influenced by various physical, chemical, and biological mechanisms operating inside its cell (Ayangbenro and Babalola 2017). Similarly, biosorption is defined as the passive uptake of metals by microbes (Malik 2004; Gadd 2009). Biomineralization is the process by which microbes form minerals. Likewise, biotransformation is another way of showing resistance toward heavy metals by microbes. It is the process of chemical alteration of chemicals such as nutrients, amino acids, toxins, and drugs by an organism. The two important factors involved in the biotransformation of heavy metals in soil are pH and carbon sources. Biotransformations of heavy metals are demonstrated in algae, fungi, and prokaryotes which convert these metals into metal sulfides. However, being insoluble in nature, these metal sulfides have reduced bioavailability (Scarano and Morelli 2003; Ayyasamy and Lee 2012). Further, microbial biofilms have the ability of accumulating or sequestering heavy metals by producing EPS (exopolysaccharides) which bind with these metals (Teitzel and Parsek 2003; Meliani and Bensoltane 2016).
Due to the presence of anionic structures, microbes have a net negative charge on their surface. This negative charge enables them to bind with metal cations. Furthermore, the polarized groups of the bacterial cell wall or the capsule enable them to bind with metal ions (El-Helow et al. 2000). Binding of these metal ions to the cell wall is governed by several attractive forces like van der Waals forces, electrostatic interactions, covalent binding, and alterations in redox potential. De et al. (2008) reported that Pseudomonas aeruginosa contains cysteine-rich transport proteins located in their cell membrane which enabled them to adsorb exceptionally high amount of mercury, i.e., up to 400 mg Hg g−1 dry cell mass. Microorganisms produce several organic and inorganic acids which help them in extracting metals from solid substrates.
10.4 Our Lead
Since the last 20 years, our group is pursuing a lot of studies related to bioremediation of heavy metals. In the case of microbial bioremediation of arsenic, we observed that the presence of a similar mechanism of resistance in the two bacterial strains isolated from two different sources may be due to horizontal gene transfer of the arsenic gene ars C from soil to water system and vice versa which is an alarming situation for global concern (Saluja et al. 2011). Gupta et al. (2002) developed heavy metal-resistant mutants of phosphate-solubilizing Pseudomonas sp. Similarly, Tripathi et al. (2004) characterize siderophore-producing lead- and cadmium-resistant Pseudomonas putida KNP9 strain. Gupta et al. (2005) did an in situ characterization of mercury-resistant growth-promoting fluorescent Pseudomonads. However, we also characterize some other cadmium-resistant strains (Rani and Goel 2009; Kumar et al. 2019). Rani et al. (2008) reported some rhizobacteria responsible for the decline of copper toxicity in pigeon pea and soil system. Besides this, our group has also reviewed several studies related to rhizobacterial detoxification of heavy metals for crop improvement and has compiled them for readers of scientific communities to comprehend its knowledge in a simpler way (Rani and Goel 2009; Goel et al. 2017; Saluja et al. 2011; Khan et al. 2011).
References
Abarshi MM, Dantala EO, Mada SB (2017) Bioaccumulation of heavy metals in some tissues of croaker fish from oil spilled rivers of Niger Delta region, Nigeria. Asian Pac J Trop Biomed 7(6):563–568
Abbas T, Muhammad R, Shafaqat A, Muhammad A, Abid M, Muhammad ZR, Muhammad I, Muhammad A, Muhammad Q (2018) Biochar application increased the growth and yield and reduced cadmium in drought-stressed wheat grown in an aged contaminated soil. Ecotoxicol Environ Saf 148:825–833
Abdelilah D, Zein OA, Mohamed EM (2010) Origins of trace elements in cultivated soils irrigated by sewage, Ourzirha Area (Meknes, Morocco). Agric Biol J N Am 1(6):1140–1147
Achparaki M, Thessalonikeos E (2012) Heavy metals toxicity. Aristotle Univ Med J 39(1):29–34
Agrawal S, Singh A, Sharma R, Agrawal M (2007) Bioaccumulation of heavy metal in leafy vegetables: a threat to human health (a review). Terrest Aquat Environ Toxicol 1:13–23
Akpor OB, Ohiobor GO, Olaolu TD (2014) Heavy metal pollutants in wastewater effluents: sources, effects, and remediation. Adv Biosci Bioeng 2(4):37–43
Aksu A (2015) Sources of metal pollution in the urban atmosphere (a case study: Tuzla, Istanbul). J Environ Health Sci Eng 13:79
Al-Fartusie FS, Mohssan SN (2017) Essential trace elements and their vital roles in human body. Indian J Adv Chem Sci 5(3):127–136
Ali EH (2007) Comparative study of the effect of stress by the heavy metals Cd+2, Pb+2, and Zn+2 on morphological characteristics of Saprolegnia delica Coker and Dictyuchus carpophorus Zopf. Pol J Microbiol 56(4):257–264
Alves LR, Reis AR, Gratao PL (2016) Heavy metals in agricultural soils: from plants to our daily life(a review). Científica 44(3):346-361
Appenroth KJ (2010) What are heavy metals in plant sciences? Acta Physiol Plant 32:615–619
Aras S, Aydin SS, Körpe DA, Dönmez Ç (2012) Comparative genotoxicity analysis of heavy metal contamination in higher plants. In: Begum G (ed) Ecotoxicology. Intech Open, Rijeka, pp 107–124
Arunakumara KKIU, Walpola BC, Yoon MH (2013) Current status of heavy metal contamination in Asia’s rice lands. Environ Sci Biotechnol 12(4):355–377
Ashish B, Neeti K, Haminashu K (2013) Copper toxicity: a comprehensive study. Res J Recent Sci 2:58–67
Ashraf MW (2011) Concentrations of cadmium and lead in different cigarette brands and human exposure to these metals via smoking. J Arts Sci Commer II(2):140–147
Athar R, Ahmad M (2002) Heavy metal toxicity: effect on plant growth and metal uptake by wheat, and on free-living acetobacter. Water Air Soil Pollut 138:165–180
ATSDR (1992) Toxicological profile for thallium. U.S. Department of Health and Human Services, Public Health Service, Atlanta
ATSDR (1999) Toxicological profile for mercury. U.S. Department of Health and Human Services, Public Health Service, Atlanta
ATSDR (2003) Toxicological profile for selenium. U.S. Department of Health and Human Services, Public Health Service, Atlanta
ATSDR (2004) Toxicological profile for copper. U.S. Department of Health and Human Services, Public Health Service, Atlanta
ATSDR (2005a) Toxicological profile for nickel. U.S. Department of Health and Human Services, Public Health Service, Atlanta
ATSDR (2005b) Toxicological profile for zinc. U.S. Department of Health and Human Services, Public Health Service, Atlanta
ATSDR (2007a) Toxicological profile for arsenic. U.S. Department of Health and Human Services, Public Health Service, Atlanta
ATSDR (2007b) Toxicological profile for lead. U.S. Department of Health and Human Services, Public Health Service, Atlanta
ATSDR (2012a) Toxicological profile for cadmium. U.S. Department of Health and Human Services, Public Health Service, Atlanta
ATSDR (2012b) Toxicological profile for chromium. U.S. Department of Health and Human Services, Public Health Service, Atlanta
ATSDR (2017) Minimal risk levels (MRLs) for hazardous substances list. Agency for Toxic Substances and Disease Registry, Atlanta
ATSDR (2018) Minimal risk levels (MRLs) for hazardous substances list. Agency for Toxic Substances and Disease Registry, Atlanta
Ayangbenro A, Babalola OO (2017) A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Res Public Health 14(1):1–16
Ayyasamy PM, Lee S (2012) Biotransformation of heavy metals from soil in synthetic medium enriched with glucose and Shewanella sp. HN-41 at various pH. Geomicrobiol J 29(9):843–851
Baldrian P (2003) Interactions of heavy metals with white-rot fungi. Enzym Microb Technol 32:78–91
Belimov AA, Dietz KJ (2000) Effect of associative bacteria on element composition of barley seedlings grown in solution culture at toxic cadmium concentrations. Microbiol Res 155:113–121
Bellows BC (2005) Arsenic in poultry litter: organic regulations. ATTRA, pp 1–12
Benavides MP, Gallego SM, Tomaro ML (2005) Cadmium toxicity in plants. Braz J Plant Physiol 17:21–34
Benzarti S, Mohri S, Ono Y (2008) Plant response to heavy metal toxicity: a comparative study between the hyperaccumulator Thlaspi caerulescens (Ecotype Ganges) and nonaccumulator plants: lettuce, radish, and alfalfa. Environ Toxicol 23(5):607–616
Bielicka A, Bojanowska I, Wiśniewski A (2005) Two faces of chromium-pollutant, and bioelement. Pol J Environ Stud 14:5–10
Bingol M, Yentür G, Er Demirhan B, Öktem AB (2010) Determination of some heavy metal levels in soft drinks from Turkey using ICP-OES method. Czech J Food Sci 28:213–216
BIS (2012) Annual report-2012-13, Bureau of Indian Standards
Borowska S, Brzóska MM (2015) Metals in cosmetics: implications for human health. J Appl Toxicol 35(6):551–572
Chakraborti D, Rahman MM, Das B, Chatterjee A, Das D, Nayak B, Pal A, Chowdhury UK, Ahmed S, Biswas BK, Sengupta MK, Hossain MA, Samanta G, Roy MM, Dutta RN, Saha KC, Mukherjee SC, Pati S, Kar PB, Mukherjee A, Kumar M (2017) Groundwater arsenic contamination and its health effects in India. Hydrogeol. J 25(4):1165–1181
Brad BH (2005) Sources and origins of heavy metals. In: Heavy metals in the environment: origin, interaction, and remediation. Elsevier Academic Press, Amsterdam, pp 1–27
Chatterjee S, Sau GB, Mukherjee SK (2009) Plant growth promotion by a hexavalent chromium reducing bacterial strain, Cellulosimicrobium cellulans KUCr3. World J Microbiol Biotechnol 10:1829–1836
Chaudhari PR, Gupta R, Gajghate DG, Wate SR (2012) Heavy metal pollution of ambient air in Nagpur City. Environ Monit Assess 184(4):2487–2496
Cheng S (2003) Heavy metals in plants and phytoremediation. Environ Sci Pollut Res Int 10(5):335–340
Chen T, Liu X, Li X, Zhao K, Zhang J, Xu J, Shi J, Dahlgren RA (2009) Heavy metal sources identification and sampling uncertainty analysis in a field-scale vegetable soil of Hangzhou. China Environ Pollut 157(3):1003–1010
Chen J, He F, Zhang X, Sun X, Zheng J, Zheng J (2014) Heavy metal pollution decreases microbial abundance, diversity, and activity within particle-size fractions of a paddy soil. FEMS Microbiol Ecol 87(1):164–181
Chen H, Teng Y, Lu S, Wang Y, Wang J (2015) Contamination features and health risk of soil heavy metals in China. Sci Total Environ 512-513:143-153
Chung JY, Yu SD, Hong YS (2014) Environmental source of arsenic exposure. J Prev Med Public Health 47(5):253–257
Clarkson TW (1992) Mercury: major issues in environmental health. Environ Health Perspect 100:31–38
Clemens S, Ma JF (2016) Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu Rev Plant Biol 67:489–512
Cvjetko P, Cvjetko I, Pavlica M (2010) Thallium toxicity in humans. Arh Hig Rada Toksikol 61:111–119
Darwish WS, Hussein MA, El-Desoky KI, Ikenaka Y, Nakayama S, Mizukawa H, Ishizuka M (2015) Incidence and public health risk assessment of toxic metal residues (cadmium and lead) in Egyptian cattle and sheep meats. Intl Food Res J 22(4):1719–1726
Das KK, Das SN, Dhundasi SA (2008) Nickel, its adverse health effects and oxidative stress. Indian J Med Res 128:412–425
De J, Ramaiah N, Vardanyan L (2008) Detoxification of toxic heavy metals by marine bacteria highly resistant to mercury. Mar Biotechnol 10(4):471–477
Dey S, Gupta S, Mahanty U (2014) Study of particulate matters, heavy metals and gaseous pollutants at Gopalpur (23°29′52.67″ N, 87°23′46.08″E), a tropical industrial site in eastern India. IOSR-JESTFT 8(2):01–13
Ding M, Shi X (2002) Molecular mechanisms of Cr(VI)-induced carcinogenesis. Mol Cell Biochem 234:293–300
Dinis MDL, Fiuza A (2011) Exposure assessment to heavy metals in the environment: measures to eliminate or reduce the exposure to critical receptors. In: Simeonov LI et al (eds) Environmental heavy metal pollution and effects on child mental 27 development: risk assessment and prevention strategies. Springer, Dordrecht, pp 27–50
Djukic D, Mandic L (2018) Microorganisms as indicators of soil pollution with heavy metals. Acta Agric Serb XI(22):45–55
Drira Z, Sahnoun H, Ayadi H (2017) Spatial distribution and source identification of heavy metals in surface waters of three coastal areas (Gulf of Gabes, Tunisia). Pol J Environ Stud 26:1–13
Draszawka-Bolzan (2014) The contents of cadmium in perennial ryegrass (Lolium perenne L.) as affected by application of multicomponent fertilizers. Int Lett Chem Phys Astron 12:134–138
Duffus JH (2002) Heavy metal-A meaningless term? Pure Appl Chem 74:793–807
Durham TR, Snow ET (2006) Metal ions and carcinogenesis. In: Bignold LP (ed) Cancer: cell structures, carcinogens, and genomic instability. Birkhauser Verlag, Switzerland, pp 97–130
Duruibe J, Ogwuegbu MOC, Egwurugwu J (2007) Heavy metal pollution and human biotoxic effects. Int J Phys Sci 2:112–118
Ebrahimpour MP, Rahimeh AB, Babaei H, Mohammadreza R (2011) Bioaccumulation of heavy metals in freshwater fish species, Anzali, Iran. Bull Environ Contam Toxicol 87:386–392
EFSA (2006) Metals as contaminants in food: European Commission Regulation 315/93/EEC by European Food Safety Authority
Eichler A, Gramlich G, Kellerhals T, Tobler L, Schwikowski M (2015) Pb pollution from leaded gasoline in South America in the context of a 2000-year metallurgical history. Sci Adv 1(2):e1400196
El-Helow ER, Sabry SA, Amer RM (2000) Cadmium biosorption by a cadmium resistant strain of Bacillus thuringiensis: regulation and optimization of cell surface affinity for metal cations. Biometals 13:273–280
EPA (2001) Parameters of water quality: interpretation and standards. Published by the Environmental Protection Agency, Ireland. ISBN: 1-84096-015-3
Eqani SAMAS, Bhowmik A, Qamar S, Shah STA, Muhammad S, Mulla S, Fasola M, Shen H (2016) Mercury contamination in deposited dust and its bioaccumulation patterns throughout Pakistan. Sci Total Environ 569–570:585–593
Evans CW, Hills JM, Dickson JM (2000) Heavy metal pollution in Antarctica: a molecular ecotoxicological approach to exposure assessment. J Fish Biol 57:8–19
Faisal M, Hasnain S (2006) Growth stimulatory effect of Ochrobactrum intermedium and Bacillus cereus on Vigna radiata plants. Lett Appl Microbiol 43:461–466
Farnese FS, Oliveira JA, Gusman GS, Leão GA, Silveira NM, Silva PM, Ribeiro C, Cambria J (2014) Effects of adding nitroprusside on arsenic stressed response of Pistia stratiotes L. under hydroponic conditions. Int J Phytoremed 16(2):123–137
Fernandez-Luqueno F, López-Valdez F, Gamero P, Luna S, Aguilera-González EN, Martinez A, Pérez R (2013) Heavy metal pollution in drinking water-a global risk for human health: a review. Afr J Environ Sci Technol 7:567–584
Flora SJ, Mittal M, Mehta A (2008) Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J Med Res 128(4):501–523
Florea AM, Busselberg D (2006) Occurrence use and potential toxic effects of metals and metal compounds. Biometals 19:419–427
Forster WA (1954) Toxic effects of heavy metals on crop plants grown in soil culture. Ann Appl Biol 41:637–651
Fraga CG (2005) Relevance, essentiality, and toxicity of trace elements in human health. Mol Aspects Med 26(4–5):235–244
Gadd GM (2009) Biosorption: a critical review of scientific rationale, environmental importance, and significance for pollution treatment. J Chem Technol Biotechnol 84:13–28
Galaris D, Evangelou A (2002) The role of oxidative stress in mechanisms of metal-induced carcinogenesis. Crit Rev Oncol Hematol 42:93–103
Ganesan V (2008) Rhizoremediation of cadmium soil using a cadmium-resistant plant growth-promoting rhizopseudomonad. Curr Microbiol 56:403–407
Garg VK, Yadav P, Mor S (2014) Heavy metals bioconcentration from soil to vegetables and assessment of health risk caused by their ingestion. Biol Trace Elem Res 157:256
Ghorbani H, Moghaddas NH, Kashi H (2015) Effects of land use on the concentrations of some heavy metals in soils of Golestan province. Iran J Agr Sci Tech 17(4):1025–1040
Gidlow DA (2004) Lead toxicity. Occup Med (Lond) 54(2):76–81
Gikas P, Sengor S, Ginn T, Moberly J, Peyton B (2009) The effects of heavy metals and temperature on microbial growth and lag. Global Nest J 11:325–332
Giller KE, Witter E, McGrath SP (1998) Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils. Soil Biol Biochem 30(10–11):1389–1414
Godwill E, Cynthia JI, Ilo US, Marcellus U, Eugene A, Osuji GA (2015) Determination of some soft drink constituents and contamination by some heavy metals in Nigeria. Toxicol Rep 2:384–390
Goel R, Suyal DC, Kumar V, Jain J, Soni R (2017) Stress-tolerant beneficial microbes for sustainable agricultural production. In: Panpatte DG et al (eds) Microorganisms for green revolution, microorganisms for sustainability. Springer, Singapore
Golding J, Steer CD, Hibbeln JR, Emmett PM, Lowery T, Jones RE (2013) Dietary predictors of maternal prenatal blood mercury levels in the ALSPAC birth cohort study. Environ Health Perspect 121(10):1214–1218
Guala SD, Vega Flora A, Covelo, Emma F (2010) The dynamics of heavy metals in plant-soil interactions. Ecol Model 221(8):1148–1152
Gulz PA, Gupta SK, Schulin R (2005) Arsenic accumulation of common plants from contaminated soils. Plant Soil 272:337–347
Gupta A, Meyer JM, Goel R (2002) Development of heavy metal-resistant mutants of phosphate solubilizing Pseudomonas sp. NBRI 4014 and their characterization. Curr Microbiol 45(5):323–327
Gupta A, Rai V, Bagdwal N, Goel R (2005) In situ characterization of mercury-resistant growth-promoting fluorescent pseudomonads. Microbiol Res 160:385–388
Hamsa NA, Yogesh G, Koushik U, Patil L (2017) Nitrogen transformation in soil: effect of heavy metals. Int J Curr Microbiol Appl Sci 6(5):816–832
Hamzah A, Wong KK, Hasan FN (2013) Determination of total arsenic in soil and arsenic-resistant bacteria from selected groundwater in Kandal Province, Cambodia. J Radioanal Nucl Chem 297:291
Harris GK, Shi X (2003) Signaling by carcinogenic metals and metal-induced reactive oxygen species. Mutat Res 533(1-2):183-200
Hart BT, Lake PS (1987) Studies of heavy metal pollution in Australia with particular emphasis on aquatic systems. In: Hutchinson TC, Meema KM (eds) Lead, mercury, cadmium, and arsenic in the environment. Wiley, New York, pp 187–216
Hartikainen ES, Lankinen P, Rajasärkkä J (2012) Impact of copper and zinc on the growth of saprotrophic fungi and the production of extracellular enzymes. Boreal Environ Res 17:210–218
Hawkes SJ (1997) What is a “Heavy Metal”. J Chem Educ 74(11):1374
He ZL, Yang XE, Stoffella PJ (2005) Trace elements in agroecosystems and impacts on the environment. J Trace Elem Med Biol 19:125–140
He Z, Shentu J, Yang X, Baligar VC, Zhang T, Stoffella PJ (2015) Heavy metal contamination of soils: sources, indicators, and assessment. Int Environ Indic 9:17–18
Hemambika B, Balasubramanian V, Kannan VR, James RA (2013) Screening of chromium-resistant bacteria for plant growth-promoting activities. Soil Sediment Contam 22:717–736
Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DA (2011) Arsenic exposure and toxicology: a historical perspective. Toxicol Sci 123(2):305–332
Humbatov FY, Ahmadov MM, Balayev VS, Suleymanov BA (2015) Trace metals in water samples taken from Azerbaijan Sector of Caspian Sea. J Chem Chem Eng 9:288–295
Hutton M (1983) Sources of cadmium in the environment. Ecotoxicol Environ Saf 7:9–24
Hutton M, Symon C (1986) The quantities of cadmium, lead, mercury and arsenic entering the UK environment from human activities. Sci Total Environ 57:129–150
Ilyin et al (2004) Heavy metals. In: Lovblad G, Tarrason L, Torseth K, Dutchak S (eds) EMEP assessment report–part I, convention on long-range transboundary air pollution. pp 107–128
Imperato M, Paola A, Naimo D, Arienzo M, Stanzione D, Violante P (2003) Spatial distribution of heavy metals in urban soils of Naples city (Italy). Environ Pollut 124:247–256
Inoue K (2013) Heavy metal toxicity. J Clin Toxicol S(3):1–2
INSA (2011) Hazardous metals and minerals pollution in India: sources, toxicity, and management. Published by Shri SK Sahni, Executive Secretary on behalf of Indian National Science Academy, Bahadurshah Zafar Marg, New Delhi
Israr M, Jewell A, Kumar D, Sahi SV (2011) Interactive effects of lead, copper, nickel, and zinc on growth, metal uptake and antioxidative metabolism of Sesbania drummondii. J Hazard Mat 186:1520–1526
Jadoon S, Malik A (2017) DNA damage by heavy metals in animals and human beings: an overview. Biochem Pharmacol 6(3):1–8
Jaffe D, Prestbo E, Swartzendruber P, Penzias PW, Kato S, Takami A, Hatakeyama S, Kajii Y (2005) Export of atmospheric mercury from Asia. Atmos Environ 28:3029–3038
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7(2):60–72
Jan AT, Azam M, Siddiqui K, Ali A, Choi I, Haq QM, Dallinger R (2015) Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Int J Mol Sci 16(12):29592–29630
Jha Y, Subramanian RB, Mishra KK (2017) Role of plant growth promoting rhizobacteria in accumulation of heavy metal in metal contaminated soil. Emerg Life Sci Res 3(1):48–56
Ji-yun N, Kuang L, Li Z, Xu W, Wang C, Chen Q, Li A, Zhao X, Xie H, Zhao D, Wu Y, Cheng Y (2016) Assessing the concentration and potential health risk of heavy metals in China’s main deciduous fruits. J Integr Agric 15(7):1645–1655
John R, Ahmad P, Gadgil K, Sharma S (2009) Heavy metal toxicity: effect on plant growth, biochemical parameters and metal accumulation by Brassica juncea L. IJPP 3(3):65–76
Jomova K, Valko M (2011) Advance in metal-induced oxidative stress and human disease. Toxicology 283:65–87
Kaur S, Kamli MR, Ali A (2011) Role of arsenic and its resistance in nature. Can J Microbiol 57:769–774
Khan M, Zaidi A, Goel R, Musarrat J (2011) Biomanagement of metal-contaminated soils. Environmental pollution, vol 20. Springer, Dordrecht
Khan N, Mishra A, Chauhan Sharma YK, Nautiyal CS (2012) Paenibacillus lentimorbusenhances the growth PS, of chickpea (Cicer arietinum L.) in the chromium-amended soil. Antonie Van Leeuwenhoek 101:453–459
Khan K, Lu Y, Khan H, Ishtiaq M, Khan S, Waqas M (2013) Heavy metals in agricultural soils and crops and their health risks in Swat District, northern Pakistan. Food Chem Toxicol 58:449–458
Krupa Z, Siedlecka A, Skórzynska-Polit E, Maksymiec W (2002) Heavy metal interactions with plant nutrients. In: Prasad MNV, Strzałka K (eds) Physiology and biochemistry of metal toxicity and tolerance in plants. Springer, Dordrecht, pp 287–301
Kuffner M, De Maria S, Puschenreiter M, Fallmann K, Wieshammer G, Gorfer M, Strauss J, Rivelli AR, Sessitsch A (2010) Culturable bacteria from Zn- and Cd-accumulating Salix caprea with differential effects on plant growth and heavy metal availability. J Appl Microbiol 108:1471–1484
Kumar P, Gupta SB, Anurag SR (2019) Bioremediation of cadmium by mixed indigenous isolates Serratia liquefaciens BSWC3 and Klebsiella pneumoniae RpSWC3 isolated from Industrial and mining affected water samples. Pollution 5(2):351–360
Kuzniar A, Banach A, Stępniewska Z, Frąc M, Oszust K, Gryta A, Kłos M, Wolińska A (2018) Community-level physiological profiles of microorganisms inhabiting soil contaminated with heavy metals. Int Agrophys 32(1):101–109
Lampis S, Santi C, Ciurli A, Andreolli M, Vallini G (2015) Promotion of arsenic phytoextraction efficiency in the fern Pteris vittata by the inoculation of As-resistant bacteria: a soil bioremediation perspective. Front Plant Sci 6:80
Lee CC, Huang HT, Wu YC, Hsu YC, Kao YT, Chen HL (2018) The health risks of lead and cadmium in foodstuffs for the general population of Taiwan. J Exp Food Chem 3:137
Leonard SS, Harris GK, Shi XL (2004) Metal-induced oxidative stress and signal transduction. Free Radic Biol Med 37:1921–1942
Li S, Huang W, Duan Y, Xing J, Zhou Y (2015) Human fatality due to thallium poisoning: autopsy, microscopy, and mass spectrometry assays. J Forensic Sci 60(1):247–251
Liang Y, Yi X, Dang Z, Wang Q, Luo H, Tang J (2017) Heavy metal contamination and health risk assessment in the vicinity of a tailing pond in Guangdong, China. Int J Environ Res Public Health 14(12):1557
Liao X, Zhang C, Sun G, Li Z, Shang L, Fu Y, Yang Y (2018) Assessment of metalloid and metal contamination in soils from Hainan, China. Int J Environ Res Public Health 15(3):454
Liu X, Song Q, Tang Y, Li W, Xu J, Wu J, Wang F, Brookes PC (2013) Human health risk assessment of heavy metals in the soil-vegetable system: a multi-medium analysis. Sci Total Environ 463–464:530–540
Mahaffey KR, Corneliussen EP, Jelinek CF, Fiorino JA (1975) Heavy metal exposure from foods. Environ Health Perspect 12:63–69
Mahmood Q, Rashid A, Ahmad A (2012) Current status of toxic metals addition to environment and its consequences. In: Anjum NA, Ahmad I, Pereira ME, Duarte AC, Umar S (eds) The plant family Brassicaceae: contribution towards phytoremediation, environmental pollution, vol 21. Springer, Dordrecht, pp 35–69
Mahurpawar M (2015) Effects of heavy metals on human health. Int J Res Granthaalayah (IJRG):1–7
Malekzadeh E, Alikhani HA, Savaghebi FGR, Zarei M (2012) Bioremediation of cadmium-contaminated soil through cultivation of maize inoculated with plant growth-promoting Rhizobacteria. Bioremed J 16(4):204–211
Malik A (2004) Metal bioremediation through growing cells. Environ Int 30:261–278
Mamtani R, Penny S, Ismail D, Cheema S (2011) Metals and disease: a global primary health care perspective. J Toxicol 2011:1–11
Manios T, Stentiford E, Millner P (2002) The effect of heavy metals on the total protein concentration of Typha latifolia plants, growing in a substrate containing sewage sludge compost and watered with metalliferous wastewater. J Environ Sci Health A Tox Hazard Subst Environ Eng 37:1441–1451
Maqbool F, Niaz K, Hassan FI, Khan F, Abdollahi M (2017) Immunotoxicity of mercury: pathological and toxicological effects. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 35(1):29–46
Mazumder DN (2008) Chronic arsenic toxicity & human health. Indian J Med Res 128(4):436–447
Meharg AA, Rahman MM (2003) Arsenic contamination of Bangladesh Paddy field soils: implications for rice contribution to arsenic consumption. Environ Sci Technol 37(2):229–234
Meliani A, Bensoltane A (2016) Biofilm-mediated heavy metals bioremediation in PGPR Pseudomonas. J Bioremed Biodegr 7(5):1–9
Mesa V, Navazas A, González-Gil R, González A, Weyens N, Lauga B, Gallego JLR, Sánchez J, Peláez AI (2017) Use of endophytic and rhizosphere bacteria to improvephytoremediation of arsenic-contaminated industrial soils by autochthonous Betula celtiberica. Appl Environ Microbiol 83(8):1–18
Mielke HW, Powell ET, Shah A, Gonzales CR, Mielke PW (2001) Multiple metal contamination from house paints: consequences of power sanding and paint scraping in New Orleans. Environ Health Perspect 109(9):973–978
Min J, Min K (2016) Blood cadmium levels and Alzheimer’s disease mortality risk in older US adults. Environ Health 15(69):1–6
Mishra A, Tripathi BD (2008) Heavy metal contamination of soil, and bioaccumulation in vegetables irrigated with treated wastewater in the tropical city of Varanasi, India. Toxicol Environ Chem 90(5):861–871
Moller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481
Montgomery EB (1995) Heavy metals and the etiology of Parkinson’s disease and other movement disorders. Toxicology 97:3–9
Mudgal V, Madaan N, Mudgal A, Singh RB, Mishra S (2010) Effect of toxic metals on human health. Open Nutraceuticals J 3(1):94–99
Naja GM, Volesky B (2009) Toxicity and sources of Pb, Cd, Hg, Cr, As, and radionuclides in the environment. In: Wang LK, M-HS W, Hung Y-T, Shammas NK, Chen JP (eds) Handbook of advanced industrial and hazardous wastes management. CRC, Boca Raton, pp 13–59
Nazar R, Iqbal N, Masood A, Khan MIR, Syeed S, Khan NA (2012) Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am J Plant Sci 3:1476–1489
Notarachille G, Arnesano F, Calò V, Meleleo D (2014) Heavy metals toxicity: effect of cadmium ions on amyloid beta protein 1–42. Possible implications for Alzheimer’s disease. Biometals 27:371–388
Nriagu JO, Pacyna JM (1988) Quantitative assessment of worldwide contamination of air, water, and soils by trace metals. Nature 333:134–139
Okareh OT, Oladipo TA (2015) Heavy metals in selected tissues and organs of slaughtered goats from Akinyele Central Abattoir, Ibadan, Nigeria. JBAH 5:2224–3208
Ovecka M, Takac T (2014) Managing heavy metal toxicity stress in plants: biological and biotechnological tools. Biotechnol Adv 32:73–86
Oves M, Khan SM, Qari HA, Felemban NM, Almeelbi T (2016) Heavy metals: biological importance and detoxification strategies. J Bioremed Biodegr 7:334
Pal P, Sen M, Manna A, Pal J, Pal P, Roy S, Roy P (2009) Contamination of groundwater by arsenic: a review of occurrence, causes, impacts, remedies and membrane-based purification. J Integr Environ Sci 6(4):295–316
Panagos P, Liedekerke MV, Yigini Y, Montanarella L (2013) Contaminated sites in Europe: review of the current situation based on data collected through a European network. J Environ Public Health 2013:1–11
Park S, Ely RL (2008) Candidate stress genes of Nitrosomonas europaea for monitoring inhibition of nitrification by heavy metals. Appl Environ Microbiol 74:5475–5482
Patocka KK (2016) Lead exposure and environmental health. Mil Med Sci Lett 85(4):147–163
Peralta-video J, de la Guadalupe R, Gonzalez JH, Jorge GT (2004) Effects of the growth stage on the heavy metal tolerance of alfalfa plants. Adv Environ Res 8:679–685
Peter AL, Viraraghavan T (2005) Thallium: a review of public health and environmental concerns. Environ Int 31:493–501
Piade JJ, Jaccard G, Dolka C, Belushkin M, Wajrock S (2015) Differences in cadmium transfer from tobacco to cigarette smoke, compared to arsenic or lead. Toxicol Rep 2:12–26
Pierce BL, Argos M, Chen Y, Melkonian S, Parvez F, Islam T, Ahmed A, Hasan R, Rathouz PJ, Ahsan H (2010) Arsenic exposure, dietary patterns, and skin lesion risk in Bangladesh: a prospective study. Am J Epidemiol 173(3):345–354
Plum LM, Rink L, Haase H (2010) The essential toxin: impact of zinc on human health. Int J Environ Res Public Health 7(4):1342–1365
Pourahmad J, Brien PJO, Jokar F, Daraei B (2003) Carcinogenic metal induced sites of reactive oxygen species formation in hepatocytes. Toxicol In Vitro 17(5–6):803–810
Qu C, Wang S, Ding L, Zhang M, Wang D, Giesy JP (2018) Spatial distribution, risk and potential sources of lead in soils in the vicinity of a historic industrial site. Chemosphere 205:244–252
Rafique A, Amin A, Latif Z (2015) Screening and characterization of mercury-resistant nitrogen-fixing bacteria and their use as biofertilizers and for mercury bioremediation. Pak J Zool 47(5):1271–1277
Rajaganapathy V, Xavier F, Sreekumar D, Mandal PK (2011) Heavy metal contamination in soil, water and fodder and their presence in livestock and products: a review. J Environ Sci Technol 4(3):234–249
Rajapaksha RMCP, Tobor-Kapłon MA, Bååth E (2004) Metal toxicity affects fungal and bacterial activities in soil differently. Appl Environ Microbiol 70(5):2966–2973
Rajindiran S, Dotaniya ML, Coumar MV, Panwar NR, Saha JK (2015) Heavy metal polluted soils in India: status and counter measures. JNKVV Res J 49:320–337
Rani A, Goel R (2009) Strategies for crop improvement in contaminated soils using metal-tolerant bioinoculants. In: Khan MS, Zaidi A, Musarrat J (eds) Microbial strategies for crop improvement. Springer, Berlin, pp 85–104
Rani A, Shouche YS, Goel R (2008) Declination of copper toxicity in pigeon pea and soil system by growth-promoting Proteus vulgaris KNP3 strain. Curr Microbiol 57(1):78
Ravenscroft P, William B, Matin AK, Melanie B, Jerome P (2005) Arsenic in groundwater of the Bengal Basin, Bangladesh: distribution, field relations, and hydrogeological setting. Hydrogeol J 13:727–751
Ravenscroft P, Brammer H, Richards KS (2009) Chapter 1, Introduction. In: Arsenic pollution: a global synthesis. Wiley-Blackwell, Chichester, pp 1–24
Rice K, Kathryn C, Hornberger M, George (2002) Anthropogenic sources of arsenic and copper to sediments in a Suburban Lake, Northern Virginia. Environ Sci Technol 36:4962–4967
Rodrigues S, Pereira ME, Sarabando L, Lopes LD, Cachada A, Duarte A (2006) Spatial distribution of total Hg in urban soils from an Atlantic coastal city (Aveiro, Portugal). Sci Total Environ 368:40–46
Roozbahani MM, Ardakani SS, Karimi H, Sorooshnia R (2015) Natural and anthropogenic source of heavy metals pollution in the soil samples of an industrial complex a case study. IJT 9(29):1336–1341
Rosemary F, Vitharana UWA, Indraratne SP, Weerasooriya SVR (2014) Concentrations of trace metals in selected land use of a dry zone soil catena of Sri Lanka. Trop Agric Res 25(4):512–522
Roslan R, Omar RC, Baharuddin INZ, Zulkarnain MS, Hanafiah MIM (2016) Erosion and soil contamination control using coconut flakes and plantation of Centella Asiatica and Chrysopogon Zizanioides. IOP Conf Ser Mater Sci Eng 160(1):1–6
Rossman TG (2003) Mechanism of arsenic carcinogenesis: an integrated approach. Mutat Res 533:37–65
Rousseau M-C, Parent M-E, Nadon L, Latreille B, Siemiatycki J (2007) Occupational exposure to lead compounds and risk of cancer among men: a population-based case-control study. Am J Epidemiol 166(9):1005–1014
Sadler WR, Trudinger PA (1967) The inhibition of microorganisms by heavy metals. Miner Deposita 2:158–168
Saha R, Nandi R, Saha B (2011) Sources and toxicity of hexavalent chromium. J Coord Chem 64(10):1782–1806
Salnikow K, Su W, Blagosklonny MV, Costa M (2000) Carcinogenic metals induce hypoxiainducible factor-stimulated transcription by reactive oxygen species-independent mechanism. Cancer Res 60:3375–3388
Saluja B, Sharma V (2014) Cadmium resistance mechanism in acidophilic and alkalophilic bacterial isolates and their application in bioremediation of metal-contaminated soil. Soil Sedim Contamin 23:1–17
Saluja B, Gupta A, Goel R (2011) Mechanism of arsenic resistance prevalent in Bacillus species isolated from soil and groundwater sources of India. Ekologija 57(4):155–161
Santos IR, Silva-Filho EV, Schaefer CE, Albuquerque-Filho MR, Campos LD (2005) Heavy metal contamination in coastal sediments and soils near the Brazilian Antarctic Station, King George Island. Mar Pollut Bull 50(2):185–194
Santra S, Subhas C, Samal A, Bhattacharya P, Banerjee S, Biswas A, Majumdar J (2013) Arsenic in foodchain and community health risk: a study in Gangetic West Bengal. Procedia Environ Sci 18:2–13
Sathawara NG, Parikh DJ, Agarwal YK (2004) Essential heavy metals in environmental samples from Western India. Bull Environ Contam Toxicol 73:756–761
Sbihi K, Cherifi O, El-gharmali A, Oudra B, Aziz F (2012) Accumulation and toxicological effects of cadmium, copper, and zinc on the growth and photosynthesis of the freshwater diatom Planothidium lanceolatum (Brébisson) Lange-Bertalot: a laboratory study. JMES 3:497–506
Scarano G, Morelli E (2003) Properties of phytochelatin-coated CdS nanocrystallites formed in a marine phytoplanktonic alga (Phaeodactylum tricornutum, Bohlin) in response to Cd. Plant Sci 165:803–810
Selvaraj K (2018) Effect of nickel chloride on the growth and biochemical characteristics of Phaseolus Mungo. JOJ Scin 1(1):1–6
Sengor SS, Barua S, Gikas P, Ginn TR, Peyton B, Sani RK, Spycher N (2009) Influence of heavy metals on microbial growth kinetics including lag time: mathematical modelling and experimental verification. Environ Toxicol Chem 28(10):2020–2029
Sethy SK, Ghosh S (2013) Heavy metal toxicity in seeds. JNSBM 4:272–275
Shahid M, Khalid S, Abbas G, Shahid N, Nadeem M, Aslam M (2015) Heavy metal stress and crop productivity. In: Hakeem KR (ed) Crop production and global environmental issues. Springer, New York, pp 1–25
Sharma RK, Agrawal M, Marshall FM (2007) Heavy metals contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicol Environ Saf 66:258–266
Sharma B, Singh S, Siddiqi NJ (2014) Biomedical implications of heavy metals induced imbalances in redox systems. Biomed Res Int 2014:1–26
Sharp RM, Brabander DJ (2017) Lead (Pb) bioaccessibility and mobility assessment of urban soils and composts: fingerprinting sources and refining risks to support urban agriculture. GeoHealth 1:333–345
Shukla GS, Singhal R (1984) The present status of biological effects of toxic metals in the environment: lead, cadmium, and manganese. Can J Physiol Pharmacol 62(8):1015–1031
Sidhu GPS (2016) Heavy metal toxicity in soils: sources, remediation technologies and challenges. Adv Plants Agric Res 5(1):1–2
Signes PA, Mitra K, Sarkhel S, Hobbes M, Burló F, de Groot W, Carbonell-Barrachina A (2008) Arsenic speciation in food and estimation of the dietary intake of inorganic arsenic in a Rural Village of West Bengal, India. J Agric Food Chem 56:9469–9474
Silva ALO, Barrocas PRG, Jacob SC, Moreira JC (2005) Dietary intake and health effects of selected toxic elements. Braz J Plant Physiol 17:79–93
Singh J, Kalamdhad AS (2011) Effects of heavy metals on soil, plants, human health and aquatic life. Int J Res Chem Environ 1:15–21
Singh N, Deepak K, Sahu A (2007) Arsenic in the environment: effects on human health and possible prevention. J Environ Biol 28:359–365
Singh S, Shrivastava A, Barla A, Bose S (2015) Isolation of arsenic-resistant bacteria from Bengal delta sediments and their efficacy in arsenic removal from soil in association with Pteris vittata. Geomicrobiol J 32(8):712–723
Sinha B, Bhattacharyya K (2014) Arsenic accumulation and speciation in transplanted autumn rice as influenced by source of irrigation and organic manures. Int J Bioresour Environ Agric Sci 5(3):336–368
Sinha S, Mukherjee SK (2008) Cadmium-induced siderophore production by a high Cd-resistant bacterial strain relieved Cd toxicity in plants through root colonization. Curr Microbiol 56:55–60
Smith AH, Lingas EO, Rahman MM (2000) Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull World Health Organ 78(9):1093–1103
Smolders A, Lock ACR, Van der VG, Medina HIR, Roelofs J (2003) Effects of mining activities on heavy metal concentrations in water, sediment, and macroinvertebrates in different reaches of the Pilcomayo River, South America. Arch Environ Contam Toxicol 44:314–323
Sobariu DL, Tudorache Fertu DL, Diaconu M, Pavel LV, Hlihor RM, Dragoi EN, Curteanu S, Lenz M, Corvini PF, Gavrilescu M (2017) Rhizobacteria and plant symbiosis in heavy metal uptake and its implications for soil bioremediation. New Biotechnol 39:125–134
de Souza MP, Chu D, Zhao M, Zayed AM, Ruzin SE, Schichnes D, Terry N (1999) Rhizosphere bacteria enhance selenium accumulation and volatilization by Indian mustard. Plant Physiol 119(2):565–574
Srivastava NK, Majumder CB (2008) Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J Hazard Mater 151:1–8
Su C, Jiang L, Zhang W (2014) A review of heavy metal contamination in the soil worldwide: situation, impact, and remediation techniques. Environ Skept Crit 3:24–38
Sugita M, Izuno T, Tatemichi M, Otahara Y (2001) Cadmium absorption from smoking cigarettes: calculation using recent findings from Japan. Environ Health Prev Med 6(3):154–159
Tchounwou PB, Patlolla AK, Centeno JA (2003) Carcinogenic and systemic health effects associated with arsenic exposure – a critical review. Toxicol Pathol 31(6):575–588
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metals toxicity and the environment. EXS 101:133–164
Teitzel GM, Parsek MR (2003) Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. Appl Environ Microbiol 69:2313–2320
Titah H, Abdullah S, Idris M, Anuar N, Basri H, Mukhlisin M (2014) Identification of rhizobacteria from Ludwigia octovalvis grown in arsenic. Aust J Basic Appl Sci 8(8):134–139
Toth G, Hermann T, Da Silva MR, Montanarella L (2016) Heavy metals in agricultural soils of the European Union with implications for food safety. Environ Int 88:299–309
Tripathi M, Munot HP, Shouche YS, Meyer JM, Goel R (2004) Isolation and functional characterization of siderophore-producing lead- and cadmium-resistant Pseudomonas putida KNP9. Curr Microbiol 50:233–237
Trivedi P, Pandey A, Sa T (2007) Chromate reducing and plant growth promoting activities of psychrotrophic Rhodococcus erythropolis MtCC 7905. J Basic Microbiol 47:513–517
Tunegova M, Toman R, Tancin V (2016) Heavy metals–environmental contaminants and their occurrence in different types of milk. Slovak J Anim Sci 49(3):122–131
Turer D, Maynard JB, Sansalone JJ (2001) Heavy metal contamination in soils of urban highways: comparison between runoff and soil concentration at Cincinnati, Ohio. Wat Air Soil Pollut 132:293–314
UNEP (2013) Global mercury assessment 2013: sources, emissions, releases and environmental transport. UNEP Chemicals Branch, Geneva
UNICEF (2008) Arsenic primer: guidance for Unicef country offices on the investigation and mitigation of arsenic contamination. Programme Division UNICEF, New York
Upadhyay N, Vishwakarma K, Singh J, Mishra M, Kumar V, Rani R, Sharma S (2017) Tolerance and reduction of chromium (VI) by Bacillus sp. MNU16 isolated from contaminated coal mining soil. Front Plant Sci 8(778):1–13
Van TN, Ozaki A, Tho HN, Duc AN, Thi YT, Kurosawa K (2016) Arsenic and heavy metal contamination in soils under different land use in an estuary in Northern Vietnam. Int J Environ Res Public Health 13(11):1091
Venkatesh T (2009) Global perspective of lead poisoning. AJMS 2(2):1–4
Vijayadeep C, Sastry PS (2014) Effect of heavy metal uptake by E. coli and Bacillus steps. J Bioremed Biodegr 5(5):1–3
Wang S, Shi X (2001) Molecular mechanisms of metal toxicity and carcinogenesis. Mol Cell Biochem 222(1–2):3–9
Wang Q, Xiong D, Zhao P, Yu X, Tu B, Wang G (2011) Effect of applying an arsenic-resistant and plant growth–promoting rhizobacterium to enhance soil arsenic phytoremediation by Populus deltoidesLH0517. J Appl Microbiol 111:1065–1074
Welbaum G, Sturz AV, Dong Z, Nowak J (2004) Fertilizing soil microorganisms to improve productivity of agroecosystems. Crit Rev Plant Sci 23:175–193
Whiting SN, de Souza MP, Terry N (2001) Rhizosphere bacteria mobilize Zn for hyperaccumulation by Thlaspi caerulescens. Environ Sci Technol 35(15):3144–3150
WHO (2017) Guidelines for drinking-water quality: fourth edition incorporating the first addendum. World Health Organization, Geneva
WHO-FAO (1995) General standard for contaminants and toxins in food and feed. CODEX Alimentarius, International Food Standards; Jointly published by FAO and WHO
Wu B, Wang G, Wu J, Fu Q, Liu C (2014) Sources of heavy metals in surface sediments and an ecological risk assessment from two adjacent plateau reservoirs. PLoS One 9(7):1–14
Wu H, Liao Q, Chillrud SN, Yang Q, Huang L, Bi J, Yan B (2016a) Environmental exposure to cadmium: health risk assessment and its associations with hypertension and impaired kidney function. Sci Rep 6(29989):1–9
Wu X, Cobbina SJ, Mao G, Xu H, Zhang Z, Yang L (2016b) A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res Int 23(9):8244–8259
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol 11:1–20
Xiao T, Yang F, Li S, Zheng B, Ning Z (2012) Thallium pollution in China: a geo-environmental perspective. Sci Total Environ 421–422:51–58
Xie Y, Fan J, Zhu W, Amombo E, Lou Y, Chen L, Fu J (2016) Effect of heavy metals pollution on soil microbial diversity and bermudagrass genetic variation. Front Plant Sci 7:755
Xu X, Huang Q, Huang Q, Chen W (2012) Soil microbial augmentation by an EGFP-tagged Pseudomonas putida X4 to reduce phyto available cadmium. Int Biodeterior Biodegrad 71:55–60
Xu Y, Sun Q, Yi L, Yin X, Wang A, Li Y, Chen J (2014) The source of natural and anthropogenic heavy metals in the sediments of the Minjiang River Estuary (SE China): implications for historical pollution. Sci Total Environ 493:729–736
Yabe J, Ishizuka M, Umemura T (2010) Current levels of heavy metal pollution in Africa. J Vet Med Sci 72:1257–1263
Yadav P, Singh B, Garg VK, Mor S, Pulhani V (2017) Bioaccumulation and health risks of heavy metals associated with consumption of rice grains from croplands in Northern India. Hum Ecol Risk Assess 23(1):14–27
Yamaji K, Watanabe Y, Masuya H, Shigeto A, Yui H, Haruma T (2016) Root fungal endophytes enhance heavy-metal stress tolerance of Clethra barbinervis growing naturally at mining sites via growth enhancement, promotion of nutrient uptake and decrease of heavy-metal concentration. PLoS One 11(12):1–15
Yao XF, Zhang JM, Tian L, Guob JH (2017) The effect of heavy metal contamination on the bacterial community structure at Jiaozhou Bay, China. Braz J Microbiol 48:71–78
Yeo B, Langley-Turnbaugh S (2010) Trace element deposition on Mount Everest. Soil Horiz 51:72–78
Zaharescu DG, Hooda PS, Soler AP, Fernandez J, Burghelea CI (2009) Trace metals and their source in the catchment of the high altitude Lake Respomuso, Central Pyrenees. Sci Total Environ 407:3546–3553
Zaltauskaite J, Sliumpaite I (2013) Single and combined toxicity of copper and cadmium to H. vulgare growth and heavy metal bioaccumulation. E3S Web Conf 1:15013
Zeitoun MM, Mehana ES (2014) Impact of water pollution with heavy metals on fish health: overview and updates. Glob Vet 12(2):219–231
Zengin F, Munzuroglu O (2005) Effect of some heavy metals on the content of chlorophyll, proline and some antioxidant chemicals in bean (Phaseolus vulgaris L.) seedlings. Acta Biol Cracov 47(2):157–164
Zhang X, Zhong T, Liu L, Ouyang X (2015) Impact of soil heavy metal pollution on food safety in China. PLoS One 10(8):1–14
Zurek G, Rybka K, Pogrzeba M, Krzyżak J, Prokopiuk K (2014) Chlorophyll a fluorescence in evaluation of the effect of heavy metal soil contamination on perennial grasses. PLoS One 9(3):1–10
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Dash, B., Soni, R., Goel, R. (2019). Rhizobacteria for Reducing Heavy Metal Stress in Plant and Soil. In: Sayyed, R., Arora, N., Reddy, M. (eds) Plant Growth Promoting Rhizobacteria for Sustainable Stress Management . Microorganisms for Sustainability, vol 12. Springer, Singapore. https://doi.org/10.1007/978-981-13-6536-2_10
Download citation
DOI: https://doi.org/10.1007/978-981-13-6536-2_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-6535-5
Online ISBN: 978-981-13-6536-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)