Abstract
The objective is to develop low cost wastewater treatment systems for the efficient removal of toxic heavy metal ions including arsenic (As). For this, two bacterial strains, one gram negative and other gram positive dubbed as IT6 and S12, were isolated from arsenic contaminated wastewater and soil samples from Sheikhupura, Pakistan. The bacterial isolates were checked for their ability to resist various metal ions and antibiotics and were identified on the basis of 16S rRNA ribotyping. The strains were also checked for their potential to decontaminate arsenite at lab scale. Both strains were identified as Pseudomonas monteilii (IT6) and Bacillus infantis (S12) with minimum inhibitory concentration of 26.5 and 33 mM arsenite, respectively. Apart from arsenite, both bacterial strains showed fair resistance against other metal ions including chromium, lead, cobalt, selenium, zinc, cadmium, and mercury. Both IT6 and S12 showed high potential of arsenite oxidation of 92% and 96% at 37 °C and pH of 7 with 100 µg/mL arsenite after 96 h of incubation. The strains have also shown strong resistance against commonly used antibiotics including amikacin, imipenem, and ciprofloxacin. These bacterial strains are potential candidates to exterminate toxic metal ions from the wastewater for green chemistry due to presence of multiple metal resistance and efficient arsenite oxidizing potential.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Arsenic (As) contamination increases on earth due to anthropogenic activities such as industrialization (Sher and Rehman 2019; Sher et al. 2020a) and due to igneous activity (Mukhopadhyay et al. 2002). The wastewater release from industries is increasing contamination of drinking water and ground water resources. Arsenic is found in various environments (Sher et al. 2019). Due to its toxicity, it is at the top of a superfund list of hazardous substances (Lin et al. 2006; Sher et al. 2020a). When humans drink this water and ingest arsenic containing crops, the risk of lethal diseases increases (Rahman et al. 2014). Drinking of arsenic containing water causes cancer also known as arsenicosis and was observed in South Asia (Cai et al. 2009). In short, arsenic is carcinogenic in nature. The occupational exposure, e.g., in copper smelters, increases the risk for lung cancer (Vahter 2000). It also causes cancer of the urinary bladder, lungs, liver, skin and kidneys in people exposed to arsenic via drinking water. Other arsenic related disorders are hyperkeratosis, pigmentation changes, effects on the circulation, and liver and nervous system (Sher and Rehman 2019; Ji et al. 2019).
Living cells have specific mechanisms for the maintenance of specific concentration of essential (Cu and Zn) and non-essential (As, Cd, and Cr) metal ions (Finney and O’halloran 2003; Solioz and Stoyanov 2003). Living cells have metallothioneins (small proteins) that bind with heavy metal ions for their transportation (Romero-Isart and Vašák 2002). In metabolism of prokaryotes, arsenite can be eletron donors and arsenate an electron acceptor (Santini et al. 2007). Adenosine triphosphate (ATP) synthesis is inhibited by arsenate; arsenite inactivates proteins due to attachment with sulfhydryl groups. ThankGod et al. (2019) reported that various cell death pathways are activated on exposure to heavy metals, and the pathway depends on the heavy metal type, concentration and the metabolic state of the cell. Irem et al. (2019) reported that high concentration of arsenic (≥ 12 mg kg− 1) in soil is responsible for decrease in basmati rice grain yield up to 40–50%.
Over the last few decades, microorganisms are gaining more attention to eradicate toxic metal ions from the environment due to low cost, treatment efficiency, and eco-friendly strategy (Elahi and Rehman 2019a; Bhakat et al. 2019; Sher et al. 2020a, b; Altowayti et al. 2020). Through bacteria and archaea, arsenic can be transformed from one form to another (Edwards et al. 2000; Sehlin and Lindström 1992). The first arsenite oxidizing bacterium was observed in 1918 (Green 1918). Later in 1992, Alcaligenes faecalis arsenic oxidizing bacterium was isolated (Lett et al. 2012). The two forms of arsenic commonly found in the environment are arsenite and arsenate (Naidu and Bhattacharya 2009). The oxidation of arsenite into arsenate makes it less toxic (Naidu and Bhattacharya 2009) and less mobile (Battaglia-Brunet et al. 2006a; Sher et al. 2020a, b) because As5+ is more easily removed from water than As3+.
Mesophilic arsenic oxidizing bacteria are found in Proteobacteria (α-, β- and γ-). Thermus/Deinococcus lineage are thermophilic arsenite oxidizing bacteria (Santini et al. 2007). So, arsenite oxidizing bacteria obtain their energy from the oxidation of arsenite (Santini et al. 2000; Battaglia-Brunet et al. 2006b). All arsenite oxidizing bacteria contain arsenite oxidase enzyme that converts arsenite to a less toxic state called arsenate (Donahoe-Christiansen et al. 2004; Gihring and Banfield 2001). Oxygen is the terminal electron acceptor and arsenite is the electron donor in chemolithoautotrophic arsenite oxidizing bacteria (Santini et al. 2002).
Accurate, low cost and eco-friendly technologies to decontaminate arsenic polluted soils and water would help to decrease risks associated to this toxic element. Bioremediation is a possible way to clean the contaminated water and soil from heavy toxic metals compared to physical and chemical methods which are expensive and not eco-friendly. The main objective of the current research was to isolate the arsenic resistant bacterial strains which can be further used to decontaminate groundwater and drinking water containing arsenic.
2 Materials and Methods
2.1 Study Area
Arsenic resistant bacteria were isolated from soil and wastewater samples which were collected from Ittehad Chemical Industry, Sheikhupura District, Punjab-Pakistan. The selected area has been contaminated with metal ions including arsenic because it contains a large number of industries. The area of selection is within 31.7167° north, 73.9850° east. Wastewater samples were collected in sterilized bottles and soil samples were taken in polyethylene bags and transferred to the microbiology laboratory for further analysis. For this, samples were kept at 4 °C in the fridge.
2.2 Characterization of Samples
The pH and temperature of samples were measured by pH indicator strip and thermometer. pH and temperature of soil samples were measured by making soil and distilled water suspension in a ratio 1:10 (w/v) according to procedure described in Dey et al. (2017).
2.3 Isolation and Purification of Arsenic Resistant Bacteria
For the isolation of arsenic resistant bacteria (ARB), samples were diluted with autoclaved distilled water and spread on LB-agar plates already supplemented with 5 mM arsenite. The plates were incubated for 24 h at 37 °C and colonies were further purified by spreading on LB-agar plates.
2.4 Characterization of Arsenic Resistant Bacteria
The isolated arsenic resistant bacteria were phenotypically characterized with Gram staining, capsule and spore staining, and biochemically with catalase, oxidase, urease, indole, methyl red, VP test, nitrate reductase, salt tolerance, citrate, H2S production, and carbohydrate utilization tests according to Cappuccino and Sherman (2001). The molecular characterization of the bacteria was done by isolating DNA according to Sambrook et al. (2001), and 16S rRNA gene amplification was done according to procedure described in Rehman et al. (2007) using universal primer pair RS1 and RS3. Fermentas purification kit (#K0513) was used to clean PCR products, and sequenced with Genetic analysis system model CEQ-800 (Beckman) Coulter Inc. Fullerton, CA, USA. The data obtained after sequencing was submitted to GenBank to get the accession number.
2.5 Cross Metal Resistance
Minimum inhibitory concentrations (MICs) of bacterial isolates against other heavy metals, i.e., lead (Pb), cadmium (Cd), cobalt (Co), selenium (Se), chromium (Cr), zinc (Zn), and mercury (Hg), were also checked. LB-agar plates with different concentrations of heavy metals were prepared i.e., 100, 500, 1000, up to 5000 µg/mL (Pattanapipitpaisal et al. 2001), and inoculated with log phase cultures of bacteria. Cultures were allowed to incubate at 37oC for 24 h, and then growth (OD600) was determined.
2.6 Optimum Bacterial Growth Conditions
For determination of growth conditions two parameters were ascertained, i.e., temperature and pH according to procedure described in Elahi and Rehman (2019a).
2.6.1 Optimal of Temperature and pH
Tubes of three sets (each containing 5 mL LB-broth) with the following temperature, i.e., 20 °C, 28 °C, 37 °C and 42 °C and pH 5, 6, 7, 8, and 9 were used separately. The OD600nm was measured as function of bacterial growth with the help of spectrophotometer.
2.6.2 Effect of NaCl on Bacterial Growth
Isolated bacterial strains were grown in NaCl (100 to 500 mM) in LB-broth medium for 24 h at optimal growth conditions and OD600nm was measured with the help of spectrophotometer.
2.7 AgNO3 Assay
Acetate minimal agar supplemented with (100 µg/NaH2AsO3 after 48 h growth of bacterial strains was flooded with 0.1 M silver nitrate solution and the result was noted according to Simeonova et al. (2004).
2.8 Growth Curves
In the presence of 100 µg/mL of arsenite and in the absence of arsenite, growth curves were determined for the isolated bacterial strains in mineral salt medium (MSM) broth (FeSO4.7H2O 0.015 g, KH2PO4 4.7 g/L, MgSO4.7H2O 1.0 g/L, CaCl2.2H2O 0.01 g/L, Na2HPO4 0.12 g/L, NH4NO3 4.0 g/L, MnSO4.4H2O 0.01 g/L, glucose 10.0 g/L and yeast extract 5.0 g/L; pH:7–7.2) (Elahi and Rehman 2019a. For this purpose, 250 mL flasks containing 100 mL MSM-broth were inoculated with arsenic resistant bacterial cultures (100 µL) and incubated overnight. Growth was measured by taking optical densities at OD600nm at 4 h, and after a regular interval of 4 h up to 24 h, as described in Elahi and Rehman (2019a).
2.9 As3+ Oxidation Potential
The oxidation ability of the bacterium was determined at different temperatures, pH and arsenic concentrations. For this purpose, 5 mL of autoclaved MSM-broth was taken in test tubes and 24-h grown cultures of the isolated bacteria were added separately. The tubes were placed in a shaking incubator for 96 h, after 2 days 1 mL of broth was taken from shaking culture and centrifuged at 5000 rpm for 5 min. The supernatant was taken and checked for arsenite concentration by Safranin O dye method. After 4 days of incubation, a similar process was repeated. Finally, the supernatant was used for arsenite determination by the spectrophotometer process. The As + 3 oxidation potential was determined at 25 °C, 30 °C, 37 °C, and 42 °C after 48 h. Likewise, As + 3 oxidation ability was checked at various pH values (i.e., 3, 5, 7, and 9). The As + 3 oxidation potential was also determined at concentrations 100, 300, 500, and 1000 µg/mL (Naureen and Rehman 2016). Each experiment was done in triplicate.
2.9.1 As3+ Determination
The reaction mixture of each sample was supplemented with As3+ (100 µg/mL) along with 1 mL of 2% potassium iodate, and 1 mL of 1M hydrochloric acid. The mixture was mixed gently until the color turned bright yellow. Then, 0.02% safranin O (0.5 mL) was added, and the volume was made by adding distilled water up to 100 mL, and mixed gently for 2–3 min. The pH of the solution was adjusted at 4 with the help of 2 mL acetate buffer, and the flask was shaken well. OD532nm of the reactions was measured against reagent blank (Pasha and Narayana 2008).
2.10 Antibiotic Resistance
The strains were tested for resistance to antibiotics using 8 different antibiotic discs, norfloxacin (30 µg), imipenem (10 µg), amoxicillin/clavulanic acid (2:1), tetracycline (30 µg), ceftriaxone (30 µg), ciprofloxacine (5 µg) and nalidixic acid (30 µg). Muller Hinton-agar plates were prepared with the lawn of bacteria, and then antibiotic discs were placed onto the MH-agar plates. Results were observed after 24 h of incubation at 37 °C (Rehman et al. 2007).
2.11 Statistical Analysis
For each experiment at least three separate flasks were maintained. Each time three readings were taken, and the mean and standard error of the mean were calculated.
3 Results
3.1 Isolation and Purification of Arsenic Resistant Bacteria
A total of 18 bacterial isolates were isolated from both wastewater and soil samples. Six bacterial strains were isolated from wastewater taken from The Ittehad chemical industry including strain IT1 to IT6. While 12 strains were isolated from soil samples, i.e., S1 to S12. Then, the isolated strains were purified by quadrant spreading until obtaining a single and pure colony. On the basis of arsenic resistance and oxidation ability IT6 and S12 were further selected for arsenic bioremediation work.
3.2 Bacterial Identification
Both bacterial strains were rod, catalase positive and oxidase negative. Bacterial strain IT6 was gram negative while strain S12 was gram positive. Morphological and biochemical characteristics are given in Table 1. The bacterial isolates IT6 and S12 showed maximum homology with the genus of Pseudomonas and Bacillus. The data obtained from 16S rRNA gene sequencing showed 98% similarity with Pseudomonas monteilii (IT6) and 95% similarity with Bacillus infantis (S12) and was submitted to GenBank database under accession numbers of KX785170 and KX785172.
3.3 MIC Against Heavy Metal Ions
The MIC of IT6 and S12 against arsenite was found at 2000 µg/mL (26.5 mM) and 2500 (33 mM), respectively. The IT6 strain also showed resistance against metal ions, i.e., Cr (2200 µg/mL), Hg (100 µg/mL), Se (3000 µg/mL), Pb (600 µg/mL), Co (100 µg/mL), Cd (50 µg/mL), and Zn (500 µg/mL). The order of resistance of IT6 strain against different metal ions is Se > Cr > As > Pb > Zn > Co = Hg > Cd. The metal resistance of S12 was Cr (150 µg/mL), Hg (50 µg/mL), Se (3500 µg/mL), Pb (2000 µg/mL), Co (100 µg/mL), Cd (50 µg/mL), and Zn (600 µg/mL). The order of resistance of S12 strain against different metal ions is Se > As > Pb > Zn > Cr > Co > Hg = Cd.
3.4 Effect of pH, Temperature and Salinity on Bacterial Growth
Temperature and pH are the most important factors which affect the growth of the bacteria. Both IT6 and S12 can grow at a wide range of pH and temperature. The pH range was 5 to 9 and temperature range was 20 to 45 °C cteria, and then antibiotic discs were pl. The maximum growth (OD600nm) of both isolates was obtained at pH 7 (Fig. 1a) and at 37 °C (Fig. 1b). Arsenic resistant bacteria were grown in arsenic with increasing concentration of NaCl and it was found that the maximum growth was determined at 200 mM NaCl. After this, further increase in NaCl concentration retarded the growth of arsenic resistant bacteria (Fig. 1c).
3.5 Arsenite Oxidizing Ability Test
The appearance of brownish precipitates after the application of 0.1 M AgNO3 on the streak growth plates indicates that the isolated strains have arsenic oxidation potential. The AgNO3 reaction depends upon the arsenic ion either arsenite or arsenate. The reaction between AgNO3 and As + 3 generates yellow precipitates. Both strains had oxidation ability; as a result they produced brown precipitates as shown in Fig. 2.
3.6 Influence of Arsenic Stress on Growth
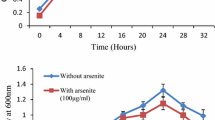
The optimum pH for both strains was 7 while optimum temperature was 37 °C. The growth curves were studied under As-stress and non-stress condition. It was noted that the lag phase was slightly extended in the presence of arsenic in both bacterial strains as compared to the control, i.e., without metal stress (Fig. 3a, b).
3.7 Effect of Temperature, pH and Arsenic on Oxidation Potential
Arsenic oxidation ability of the strains was determined at 25 °C, 30 °C, 37 °C and 42 °C with the 100 µg/mL arsenite after 96 h of incubation. Both strains (IT6 and S12) showed promising potential of arsenite oxidation of 92 and 96% at 37 °C and pH of 7 with 100 µg/mL arsenite after 96 h of incubation. The oxidation potential of both strains at 25 °C (56%, 58%), at 30 °C (72%, 76%), and at 42 °C (33%, 38%) was determined after 96 h incubation (Fig. 4a).
Likewise, As + 3 oxidation potential was determined at pH 3, 5, 7 and 9 with the 100 µg/mL arsenite concentration after 96 h of incubation. The maximum oxidation potential was obtained at pH 7. Strains IT6 and S12 had oxidation potential of 14%, 18% (pH 3), 45%, 54% (pH 5), and 77%, 83% (pH 9) after 96 h incubation at 37 °C (Fig. 4b).
The As + 3 oxidation potential was also checked at arsenite concentrations 100 µg/mL, 300 µg/mL, 500 µg/mL, and 1000 µg/mL after 96 h of incubation. The highest oxidation potential was obtained at 100 µg/mL arsenite. The oxidation ability of isolated strains, IT6 and S12, was 72% and 78% (300 µg/mL), 65% and 71% (500 µg/mL), and 28% and 34% (1000 µg/mL) after 96 h of incubation at optimum growth conditions (Fig. 4c).
3.8 Antibiotic Resistance Profile of the Isolated Strains
As mentioned, nine antibiotics discs were used, against the arsenic resistant bacterial strains. The strain IT6 showed weak resistance against cefuroxime sodium, tetracycline, norfloxacin, amoxicillin/clavulanic acid (2:1) 30 µg, ceftriaxone 30 µg discs and nalidixic acid, and strong resistance against, amikacin, imipenem and ciprofloxacin. The strain S12 was sensitive to cefuroxime sodium and ceftriaxone, showed weak resistance to tetracycline, nalidixic acid, and amoxicillin, but showed strong resistance to norfloxacin, amikacin, imipenem and ciprofloxacin (Fig. 5a, b).
4 Discussion
Multiple heavy metal-resistant bacterial strains are usually present in heavy metals affected wastewater and soil that convert toxic metal to less toxic form (Silver and Phung 2005; Qin et al. 2006; Dey et al. 2016; Sher et al. 2020a, b). In the present study, samples were taken from Sheikhupura district, Punjab, Pakistan for the isolation of ARB. Physical parameters were determined. Initially the diversity of bacteria in the samples was high, as already reported that the higher the concentration of organic carbon the higher the microbial diversity (Dey et al. 2015). But later on, IT6 and S12 was selected because of high resistance to arsenic, isolated from wastewater and soil samples, respectively.
A variety of arsenic resistant bacterial strains (Table 2) has been described by several researchers (Naureen and Rehman 2016; Wu et al. 2018; Aguilar et al. 2020; Sher et al. 2020a). The minimum inhibitory concentration of arsenite was 26.5 for isolate IT6 and 33 mM for isolate S12. Among these two bacterial strains, isolate IT6 showed highest resistance against arsenate and other toxic heavy metals. Another study by the same laboratory reported that Brevibacterium sp. CS2 and Micrococcus luteus strain AS2 has MIC against arsenite of 40 and 50 mM, respectively (Sher et al. 2019). While MIC against other heavy metals were: for arsenate (280 and 275 mM), lead (4 and 5 mM), cadmium (3 and 3 mM), chromium (3 and 4 mM), mercury (1 and 1.5 mM), selenium (5 and 5 mM), cobalt (5 and 5 mM) and nickel (4 and 3 mM), respectively (Sher et al. 2020a). In another study, it was reported that Klebsiella pneumoniae showed resistance against arsenite upto 21 mM (Mujawar et al. 2019).
Dey et al. (2016) reported that both bacterial strains, i.e., Bacillus sp. SW2 and Aneurinibacillus aneurinilyticus SW4, were able to resist arsenite up to 7.5 mM. Shi et al. (2019) reported that MacAB gene in E. coli is responsible to confer resistance against penicillin-like antibiotics and As3+ efflux from the cell. Yan et al. (2019) reported that the paralogous arsenic resistant genes possess different evolutionary paths leading to adapt to the environment containing toxic metal ions. A study reported that a bacterium isolated from industrial wastewater, Stenotrophomonas MB339, was able to utilize aromatic compounds and resist various heavy metal ions, i.e., Cu2+, As3+, Pb2+, Hg2+ and Ni2+. It was also reported that bacteria resist antibiotics, including ofloxacin, streptomycin, rifampicillin, erythromycin, ampicillin, and clindamycin (Aslam et al. 2018).
Both the isolated strain showed optimum growth at pH 7 and 37 °C. M. luteus strain AS2, isolated from industrial wastewater also showed optimum growth at pH 7 and temperature 37 °C (Sher et al. 2020a). In the present study, As + 3-oxidation ability of the bacterial strains was 92% and 96% after 4 days of incubation when the medium was maintained with 1 mM arsenite. Another study reported that Bacillus cereus and Acinetobacter jejuni could oxidize As + 3 at 92% and 88%, after 6 days when the initial As + 3-concentration was 1 mM (Naureen and Rehman 2016). One of the bacterial strains Thermus HR13 can oxidize 100% As + 3 containing 1 mM As + 3 in the medium within 16 h of incubation (Gihring and Banfield 2001). It has also been reported that Stenotrophomonas panacihumi can oxidize 500 µM within 12 h (Bahar et al. 2012; Aguilar et al. 2020) reported that Lysinibacillus boronitolerans has potential to convert 71.88% arsenite (10 mM) into arsenate.
With an As + 3-oxidizing microbial population growing in autotrophic medium CAsO1, As + 3 oxidation was observed in the range of 3–10 but the maximum arsenite oxidation potential was determined at pH 5–7 (Battaglia-Brunet et al. 2002). The optimum pH range for Stenotrophomonas panacihumi was 5–7 (Bahar et al. 2012). In the present investigation, all the isolated bacterial strains showed good arsenite oxidation potential at 250 mM initial arsenite concentration. The arsenic oxidation potential of the isolated bacterial strains was decreasing by increasing the initial arsenite concentration. Same observation was made in CAS01, which showed optimum arsenite oxidation at 6.5 mM but decreasing arsenite oxidation rate by increasing arsenic concentration (Battaglia-Brunet et al. 2002). The growth of isolated arsenic resistant bacteria was restricted due to increasing concentration of sodium chloride because high salt concentration creates a hypertonic environment which may restrict the growth of bacteria (Sher et al. 2020a).
Ke et al. (2018) reported that E. coli (arsRRP2) showed the maximum As resistance, selectivity, and adsorption potential within a wide pH range of 5.0 to 9.0 and salinities of 0 to 15.0 g/L NaCl range, and had the ability to remove As3+ from water containing low arsenite concentration. Dey et al. (2016) reported that Bacillus sp. SW2 and Aneurinibacillus aneurinilyticus SW4, isolated from arsenic contaminated groundwater, were able to remove 51.45% and 51.99%, of arsenite (1 mM) and 53.29% and 50.37% of arsenate (1 mM), respectively, from the growth medium.
All these studies suggest that As3+-oxidizing bacteria could be useful to treat As-polluted waters. Our strains, highly resistant to As and heavy metals, could be used to develop bio-processes for the treatment of industrial or mining wastewater containing important concentrations in metals and metalloids. Moreover, As3+-oxidizing strain may be useful for helping the phytoremediation of polluted soils. Guarino et al. (2020) reported that bio-phytoremediation technique by using Arundo donax L., and plant growth promoting bacteria including Stenotrophomonas maltophilia and Agrobacterium sp. was used, and the results clearly revealed that epigenetic modifications are involved in stress and arsenic detoxification. Moens et al. (2020) reported that Ochrobactrum tritici, a hyperaccumulator rhizobacterial strain, decreases the presence of arsenic inside the tissue of rice plants and also reduces the inhibitory effect of the metalloid on the plant’s growth.
5 Conclusions
In conclusion, both strains Pseudomonas monteilii (IT6) and Bacillus infantis (S12) showed MIC of 26.5 and 33 mM arsenite and were resistant to other heavy metal ions at significant level. Both bacterial strains showed efficient potential of arsenite oxidation at 37 °C and pH of 7 with 100 µg/mL arsenite after incubation of 96 h. Both strains were resistant against commonly used antibiotics including amikacin, imipenem, and ciprofloxacin. The multiple heavy metals resistant bacterial strains can potentially be used to remediate metal-polluted sites. For practical use, further research work is needed to investigate arsenite oxidizing potential of these strains with experiments using real polluted water in batch and continuous bioreactors.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Aguilar NC, Faria MCS, Pedron T, Batista BL, Mesquita JP, Bomfeti CA, Rodrigues JL (2020) Isolation and characterization of bacteria from a brazilian gold mining area with a capacity of arsenic bioaccumulation. Chemosphere 240:124871
Altowayti WAH, Almoalemi H, Shahir S, Othman N (2020) Comparison of culture-independent and dependent approaches for identification of native arsenic-resistant bacteria and their potential use for arsenic bioremediation. Ecotoxicol Environ Saf 250:111267
Aslam F, Yasmin A, Thomas T (2018) Essential gene clusters identified in Stenotrophomonas MB339 for multiple metal/antibiotic resistance and xenobiotic degradation. Curr Microbiol 75:1484–1492
Bahar MM, Megharaj M, Naidu R (2012) Arsenic bioremediation potential of a new arsenite-oxidizing bacterium Stenotrophomonas sp. MM-7 isolated from soil. Biodegradation 23:803–812
Battaglia-Brunet F, Dictor MC, Garrido F, Crouzet C, Morin D, Dekeyser K, Baranger P (2002) An arsenic (III) oxidizing bacterial population: selection, characterization, and performance in reactors. J Appl Microbiol 93:656–667
Battaglia-Brunet F, Itard Y, Garrido F, Delorme F, Crouzet C, Greffié C, Joulian C (2006a) A simple biogeochemical process removing arsenic from a mine drainage water. Geomicrobiol J 23:201–211
Battaglia-Brunet F, Joulian C, Garrido F (2006b) Oxidation of arsenite by Thiomonas strains and characterization of Thiomonas arsenivorans sp. nov. Antonie Van Leeuwenhoek 89:99–108
Bhakat K, Chakraborty A, Islam E (2019) Characterization of arsenic oxidation and uranium bioremediation potential of arsenic resistant bacteria isolated from uranium ore. Environ Sci Pollut Res 26:12907–12919 ()
Cai L, Liu G, Rensing C, Wang G (2009) Genes involved in arsenic transformation and resistance associated with different levels of arsenic-contaminated soils. BMC Microbiol 9:4–13
Cappucino JG, Sherman N (2001) Microbiology: a laboratory manual, 6th edn. Pearson education, San Francisco
Dey U, Das K, Roy P, Chatterjee SN, Mondal NK (2015) Searching of microbial agent for bioremediation of arsenic. Int J Extentsive Res 5:60–64
Dey U, Chatterjee S, Mondal NK (2016) Isolation and characterization of arsenic-resistant bacteria and possible application in bioremediation. Biotechnol Rep 10:1–7
Dey U, Chatterjee S, Mondal NK (2017) Investigation of bioremediation of arsenic by bacteria isolated from an arsenic contaminated area. Environ Process 4:183–199
Donahoe-Christiansen J, D’Imperio S, Jackson CR, Inskeep WP, McDermott TR (2004) Arsenite-oxidizing Hydrogenobaculum strain isolated from an acid-sulfate-chloride Geothermal spring in Yellowstone National Park. Appl Environ Microbiol 70:1865–1868
Edwards KJ, Bond PL, Gihring TM, Banfield JF (2000) An archaeal iron-oxidizing extreme acidophile important in acid mine drainage. Science 287:1796–1799
Elahi A, Rehman A (2019a) Multiple metal resistance and Cr6+ reduction by bacterium, Staphylococcus sciuri A-HS1, isolated from untreated tannery effluent. J King Saud Uni Sci 31:1005–1013
Finney LA, O’halloran TV (2003) Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science 300:931–936
Gihring TM, Banfield JF (2001) Arsenite oxidation and arsenate respiration by a new Thermus isolate. FEMS Microbiol Lett 204:335–340
Green HH (1918) Isolation and description of a bacterium causing oxidation of arsenite to arsenate in cattle dipping baths. Union S. Africa Dept. Agrie. 5th and 6th Repts. Direc. Vet. Research, pp 595–610
Guarino F, Miranda A, Castiglione S, Cicatelli A (2020) Arsenic phytovolatilization and epigenetic modifications in Arundo donax L. assisted by a PGPR consortium. Chemosphere 251:126310
Irem S, Islam E, Maathuis FJM, Niazi NK, Li T (2019) Assessment of potential dietary toxicity and arsenic accumulation in two contrasting rice genotypes: Effect of soil amendments. Chemosphere 225:104–114
Ji PY, Li ZY, Wang H, Dong JT, Li XJ, Yi HI (2019) Arsenic and sulfur dioxide co-exposure induce renal injury via activation of the NF-κB and caspase signaling pathway. Chemosphere 224:280–288
Ke C, Zhao C, Rensing C, Yang S, Zhang Y (2018) Characterization of recombinant E. coli expressing arsR from Rhodopseudomonas palustris CGA009 that displays highly selective arsenic adsorption. Appl Microbiol Biotechnol 102:6247–6255
Lett MC, Muller D, Lièvremont D, Silver S, Santini J (2012) Unified nomenclature for genes involved in prokaryotic aerobic arsenite oxidation. J Bacteriol 194:207–208
Lin YF, Walmsley AR, Rosen BP (2006) An arsenic metallochaperone for an arsenic detoxification pump. Proceed Natl Acad Sci USA 103:15617–15622
Moens M, Branco R, Morais PV (2020) Arsenic accumulation by a rhizosphere bacterial strain Ochrobactrum tritici reduces rice plant arsenic levels. World J Microbiol Biotechnol 36:23
Mujawar SY, Shamim K, Vaigankar DC, Dubey SK (2019) Arsenite biotransformation and bioaccumulation by Klebsiella pneumoniae strain SSSW7 possessing arsenite oxidase (aioA) gene. BioMetals 32:65–76
Mukhopadhyay R, Rosen BP, Phung LT, Silver S (2002) Microbial arsenic: from geocycles to genes and enzymes. FEMS Microbiol Rev 26:311–325
Naidu R, Bhattacharya P (2009) Arsenic in the environment-risks and management strategies. Environ Geochem Health 31:1–8
Naureen A, Rehman A (2016) Arsenite oxidizing multiple metal resistant bacteria isolated from industrial effluent: their potential use in wastewater treatment. World J Microbiol Biotechnol 32:133–144
Pasha C, Narayana B (2008) Determination of arsenic in environmental and biological samples using toluidine blue or safranine O by simple spectrophotometric method. Bull Environ Contam Toxicol 81:47–51
Pattanapipitpaisal P, Brown NL, Macaskie LE (2001) Chromate reduction and 16S rRNA identification of bacteria isolated from a Cr(VI)-contaminated site. Appl Microbiol Biotechnol 57:257–261
Qin J, Rosen BP, Zhang Y, Wang G, Franke S, Rensing C (2006) Arsenic detoxification and evolution of trimethylarsine gas by a microbial arsenite S-adenosylmethionine methyltransferase. Proceed Natl Acad Sci USA 103:2075–2080
Rahman A, Nahar N, Nawani NN, Jass J, Desale P, Kapadnis BP, Hossain K, Saha AK, Ghosh S, Olsson B, Mandal A (2014) Isolation and characterization of Lysinibacillus strain B1-CDA showing potential for bioremediation of arsenics from contaminated water. J Environ Sci Hlth Part A 49:1349–1360
Rehman A, Ali A, Muneer B, Shakoori AR (2007) Resistance and biosorption of mercury by bacteria isolated from industrial effluents. Pakistan J Zool 39:137–146
Romero-Isart N, Vašák M (2002) Advances in the structure and chemistry of metallothioneins. J Inorg Biochem 88:388–396
Sambrook J, MacCallum P, Russell D (2001) Molecular cloning: a laboratory manual. 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Santini JM, Sly LI, Schnagl RD, Macy JM (2000) A new chemolithoautotrophic arsenite oxidizing bacterium isolated from a gold mine: phylogenetic, physiological, and preliminary biochemical studies. Appl Environ Microbiol 66:92–97
Santini JM, Sly LI, Wen A, Comrie D, Wulf-Durand PD, Macy JM (2002) New arsenite oxidizing bacteria isolated from Australian Gold Mining Environments-Phylogenetic relationships. Geomicrobiol J 19:67–76
Santini JM, Kappler U, Ward SA, Honeychurch MJ, vanden RN, Bernhardt PV (2007) The NT-26 cytochrome c 552 and its role in arsenite oxidation. BBA Bioenergetics 1767:189–196
Sehlin HM, Lindström EB (1992) Oxidation and reduction of arsenic by Sulfolobus acidocaldarius strain BC. FEMS Microbiol Lett 93:87–92
Sher S, Rehman A (2019) Use of heavy metals resistant bacteria-a strategy for arsenic bioremediation. Appl Microbial Biotechnol 103:6007–6021
Sher S, Rehman A, Hansen LH, Nielsen TK (2019) Complete genome sequences of highly arsenite-resistant bacteria Brevibacterium sp. strain CS2 and Micrococcus luteus AS2. Microbiol Resour Announc 31:e00531–e00519. https://doi.org/10.1128/MRA.00531-19
Sher S, Hussain SZ, Rehman A (2020a) Phenotypic and genomic analysis of multiple heavy metal–resistant Micrococcus luteus strain AS2 isolated from industrial waste water and its potential use in arsenic bioremediation. Appl Microbial Biotechnol 104:2243–2254
Sher S, Hussain SZ, Rehman A (2020b) Multiple resistance mechanisms in Staphylococcus sp. strain AS6 under arsenite stress and its potential use in amelioration of wastewater. J King Saud Uni Sci 32:3050–3058
Shi K, Cao M, Li C, Huang J, Zheng S, Wang (2019) Efflux proteins MacAB confer resistance to arsenite and penicillin/macrolide-type antibiotics in Agrobacterium tumefaciens 5A. World J Microbiol Biotechnol 35:115
Silver S, Phung LT (2005) Genes and enzymes involved in bacterial oxidation and reduction of inorganic arsenic. Appl Environ Microbiol 71:599–608
Simeonova DD, Lièvremont D, Lagarde F, Muller DA, Groudeva VI, Lett M-C (2004) Microplate screening assay for the detection of arsenite-oxidizing and arsenate-reducing bacteria. FEMS Microbiol Lett 237:249–253
Solioz M, Stoyanov JV (2003) Copper homeostasis in Enterococcus hirae. FEMS Microbiol Rev 27:183–195
ThankGod CE, Michelangeli F, Otitoloju AA (2019) In vitro cyto-toxic assessment of heavy metals and their binary mixtures on mast cell-like, rat basophilic leukemia (RBL-2H3) cells. Chemosphere 223:686–693
Vahter M (2000) Genetic polymorphism in the biotransformation of inorganic arsenic and its role in toxicity. Toxicol Lett 112:209–217
Wu D, Zhang Z, Gao Q, Ma Y (2018) Isolation and characterization of aerobic, culturable, arsenic-tolerant bacteria from lead-zinc mine tailing in southern China. World J Microbiol Biotechnol 34(12):177
Yan Z, Li M, Wang J, Pan J (2019) Genome analysis revealing the potential mechanisms for the heavy metal resistance of Pseudomonas sp. P11, isolated from industrial wastewater sediment. Curr Microbiol 76:1361–1368
Author information
Authors and Affiliations
Contributions
SS1 performed experiments. AG helped in data analysis. SS2 and AR conceived and designed the study. The first draft of the manuscript was written by Shahid Sher and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sher, S., Ghani, A., Sultan, S. et al. Bacterial Strains Isolated from Heavy Metals Contaminated Soil and Wastewater with Potential to Oxidize Arsenite. Environ. Process. 8, 333–347 (2021). https://doi.org/10.1007/s40710-020-00488-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40710-020-00488-7