Abstract
During a 28-year field survey in India (1988–2016), groundwater arsenic contamination and its health effects were registered in the states of West Bengal, Jharkhand, Bihar and Uttar Pradesh in the Ganga River flood plain, and the states of Assam and Manipur in the flood plain of Brahamaputra and Imphal rivers. Groundwater of Rajnandgaon village in Chhattisgarh state, which is not in a flood plain, is also arsenic contaminated. More than 170,000 tubewell water samples from the affected states were analyzed and half of the samples had arsenic >10 μg/L (maximum concentration 3,700 μg/L). Chronic exposure to arsenic through drinking water causes various health problems, like dermal, neurological, reproductive and pregnancy effects, cardiovascular effects, diabetes mellitus, diseases of the respiratory and gastrointestinal systems, and cancers, typically involving the skin, lungs, liver, bladder, etc. About 4.5% of the 8,000 children from arsenic-affected villages of affected states were registered with mild to moderate arsenical skin lesions. In the preliminary survey, more than 10,000 patients were registered with different types of arsenic-related signs and symptoms, out of more than 100,000 people screened from affected states. Elevated levels of arsenic were also found in biological samples (urine, hair, nails) of the people living in affected states. The study reveals that the population who had severe arsenical skin lesions may suffer from multiple Bowens/cancers in the long term. Some unusual symptoms, such as burning sensation, skin itching and watering of eyes in the presence of sun light, were also noticed in arsenicosis patients.
Résumé
Au cours d’une étude sur le terrain, menée en Inde pendant 28 ans (1988–2016), la contamination des eaux souterraines en arsenic et ses effets sur la santé ont été enregistrés dans les états du Bengale occidental, du Jharkhand, du Bihar et de l’Uttar Pradesh dans la plaine d’inondation du Ganges, et dans les états de l’Assam et de Manipur dans la plaine d’inondation du Brahmapoutre et de l’Imphal. Les eaux souterraines sont également contaminées en arsenic dans le village de Rajnandgaon dans l’état de Chhattisgarh, qui n’est pas localisé dans une plaine d’inondation. Plus de 170,000 échantillons d’eau prélevés dans des forages tubés des états affectés par la contamination ont été analysés et la moitié de ces échantillons d’eau ont des concentrations en arsenic supérieures à 10 μg/L (concentration maximum 3,700 μg/L). L’exposition chronique à l’arsenic par l’eau potable cause de nombreux problèmes de santé, comme des problèmes dentaires, neurologiques, des effets sur la reproduction et la grossesse, des effets cardiovasculaires, le diabète sucré, las maladies des systèmes respiratoires et gastro-intestinal, et cancers dont ceux de la peau, des poumons, du foie, de la vessie, etc. Pour environ 4.5% des 8,000 enfants des villages affectés par l’arsenic des états concernés, on enregistre des problèmes faibles à modérés de lésions cutanées liées à l’arsenic. Lors de l’enquête préliminaire, plus de 10,000 des 100,000 personnes étudiées dans les états concernés ont été enregistrées avec différents types de signes et symptômes liés à l’arsenic. Des niveaux élevés en arsenic ont également été trouvés dans les différents échantillons biologiques (urine, ongles, cheveux) des populations vivant dans les états affectés. L’étude révèle que la population qui a de fortes lésions cutanées du fait de l’arsenic souffre de multiples cancers (type maladie de Bowen) sur le long terme. Des symptômes inhabituels, comme des sensations de brulure, des démangeaisons cutanées et des larmoiements en présence de la lumière du soleil, ont été également notés sur les patients atteints d’arsenicose.
Resumen
La contaminación del arsénico del agua subterránea y sus efectos sobre la salud se registraron durante un relevamiento de campo de 28 años en la India (1988–2016) en los estados de Bengala Occidental, Jharkhand, Bihar y Uttar Pradesh en la llanura de inundación del río Ganges y en los estados de Assam y Manipur en la llanura de inundación de los ríos Brahamaputra e Imphal. El agua subterránea de la aldea de Rajnandgaon en el estado de Chhattisgarh, que no está en una llanura de inundación, también está contaminada con arsénico. Se analizaron más de 170,000 muestras de agua de pozos de los estados afectados y la mitad de las muestras tenían arsénico >10 μg/L (concentración máxima 3,700 μg/L). La exposición crónica al arsénico a través del agua potable causa varios problemas de salud, como efectos dérmicos, neurológicos, reproductivos y del embarazo, los efectos cardiovasculares, la diabetes mellitus, las enfermedades de los sistemas respiratorio y gastrointestinal y cánceres que afectan típicamente la piel, los pulmones, etc. Alrededor del 4.5% de los 8,000 niños de las aldeas afectadas por el arsénico de los estados afectados fueron registrados con lesiones arsenicales de leve a moderada. En el relevamiento preliminar, más de 10,000 pacientes fueron registrados con diferentes tipos de signos y síntomas relacionados con el arsénico, de más de 100,000 personas examinadas de los estados afectados. Los niveles elevados de arsénico también se encontraron en muestras biológicas (orina, pelo, uñas) de las personas que viven en los estados afectados. El estudio revela que la población que tenía lesiones arsenicales severas de la piel puede sufrir de múltiples cánceres de la enfermedad de Bowens en el largo plazo. También se observaron en los pacientes con arsenicosis, algunos síntomas inusuales, como sensación de ardor, picazón de la piel y riesgo en ojos en presencia de luz solar.
摘要
在印度28年的野外调查期间(1988–2016年),记录了恒河冲积平原的西孟加拉邦、贾坎德邦、比哈尔邦和北方邦以及雅鲁藏布江和英帕尔河冲积平原的阿萨姆邦和曼尼普尔邦的地下水砷污染及其健康影响。未处于冲积平原的蒂斯加尔邦Rajnandgaon村的地下水也受到了砷的污染。分析了受影响地区的17多万的管井水样,一半的水样中砷含量> 10 μg/L(最大浓度3,700 μg/L)。通过饮用水慢性接触砷引起了各种各样的健康问题,诸如皮肤、神经、生殖和怀孕方面的影响、心血管影响、糖尿病、呼吸和肠胃系统疾病以及主要涉及皮肤、肺、肝和膀胱的癌症等等。在受砷影响的村庄和受影响的邦中8000多名儿童中,大约4.5%的儿童患有轻度到中度的砷化合物皮肤损伤。在初步的调查中,从受影响的邦中筛选了100000名人员,发现有10000多名患者呈现各种与砷相关的症候。在受影响的邦中居住的人们的生物样品(尿、头发和指甲)中也发现了砷含量升高。研究揭示,长期来看有严重砷化合物皮肤损伤的人们可能会遭受多种Bowens/癌症的困扰。在地方性砷 中毒病人中,还发现了一些不寻常的症状如灼热感、皮肤搔痒及阳光下眼睛流泪。
Resumo
Durante um levantamento de campo de 28 anos na Índia (1988–2016), contaminação por arsênio em águas subterrâneas e seus efeitos foram registrados nos estados de Bengala Ocidental, Jharkhand, Bihar e Uttar Pradesh na planície de inundação do Rio Ganges, e nos estados de Assam e Manipur nas planícies de inundação dos rios Brahamaputra e Imphal. A água subterrânea do vilarejo de Rajnandgaon no estado de Chhattisgarh, que não é uma planície de inundação, também está contaminada por arsênio. Mais de 170.000 amostras de água de poços tubulares dos estados afetados foram analisadas e metade das amostras continham arsênio >10 μg/L (concentração máxima de 3,700 μg/L). Exposição crônica a arsênio através de água potável causa vários problemas de saúde, tais como problemas dérmicos, neurológicos, reprodutivos e de gestação, problemas cardiovasculares, diabetes mellitus, doenças dos sistemas gastrointestinal e respiratório, e câncer, tipicamente envolvendo a pele, pulmões, fígado, bexiga, e etc. Aproximadamente 4.5% das 8,000 crianças dos vilarejos nos estados afetados por contaminação por arsênio foram registradas apresentando lesões de pele leves a moderadas. No levantamento preliminar, mais de 10,000 pacientes foram registrados com diferentes sinais e sintomas relacionados a contaminação por arsênio, entre 100,000 pessoas pesquisadas nos estados afetados. Níveis elevados de arsênio foram também encontrados em amostras biológicas (urina, cabelo, unhas) das pessoas vivendo nos estados afetados. O estudo revela que a população que apresenta lesões de pele causadas por exposição ao arsênio possam sofrer de doença de Bowen e diferentes tipos de câncer no decorrer dos anos. Alguns sintomas menos usuais, tais como sensação de queimadura, coceira na pele e olhos lacrimejando na presença de luz solar também foram notadas em pacientes envenenados por arsênio.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the last 100 years, high concentrations of arsenic of natural geochemical origin have been detected in groundwater in many countries around the world. To date, arsenic in groundwater has been identified in 105 countries, with an estimate of the exposed population of >200 million worldwide at concentrations greater than the World Health Organization (WHO) guideline value for arsenic of 10 μg/L (Murcott 2012; Naujokas et al. 2013). Arsenic is a metalloid element which is generally present in natural deposits and is exposed through weathering, erosion of rocks/soils and volcanic emissions. The anthropogenic sources of arsenic contamination in groundwater may be due to mining activities, metal smelting, and electronic manufacturing processes (Naujokas et al. 2013). The major route for arsenic exposure to humans is through drinking water where it is typically present in inorganic forms of either arsenite [As (III)] or arsenate [As (V)], while another significant pathway of arsenic contamination is through the food chain (Bhattacharya et al. 2012; Halder et al. 2014). When contaminated water is used for crop irrigation, it enters the food chain (Rasheed et al. 2016); thus, when the crops are being consumed, the people are exposed to arsenic. Arsenic-related major health hazards through chronic exposure of contaminated groundwater for drinking and cooking have been registered in several countries and the Asian countries are found to be the most affected. The health effects of arsenic in Argentina were first reported in 1917 (Ayeza 1917). Skin disorders were found among residents drinking water from wells in the province of Cordoba; this incidence was well known as ‘Bell Ville disease’ (Goyenechea. 1917). During the 1920s, in south-western Taiwan, villagers used groundwater for drinking to avoid the microbiological contamination found in surface waters. The presence of arsenic in the groundwater eventually led to arsenic poisoning of those who ingested the water. In the affected areas, villagers also suffered from a peripheral vascular disease that can lead to gangrene, known as Blackfoot disease (Chen et al. 1985, 1986). During the 1960s, groundwater arsenic contamination and its health effects were noticed in Lagunera region of Mexico (Cebrian et al. 1983). These are the three well-known countries where large numbers of people have suffered from various diseases related to arsenic toxicity—in fact, a significant portion of the database generated on health effects of arsenic in humans is based on these studies. Currently, all these three countries have provided alternative safe sources of drinking water.
In India, the first groundwater arsenic contamination was reported in 1976 from Chandigarh, North India, when some people were identified as suffering from arsenic toxicity (Datta 1976). Eight years later, in 1984, groundwater arsenic contamination and its health effects in the lower Ganga plain of West Bengal was reported (Garai et al. 1984). Researchers from the School of Environmental Studies (SOES), Jadavpur University, joined in the efforts to assess the levels of groundwater arsenic contamination in this region at the beginning of 1988. During the SOES field survey of India, lasting 28 years, groundwater arsenic contamination and its health effects have been reported from West Bengal, Jharkhand, Bihar, and Uttar Pradesh in the flood plain of the Ganga River, and Assam and Manipur in the flood plain of Brahamaputra and Imphal rivers. Groundwater of Rajnandgaon village in Chhattisgarh state is also arsenic contaminated and some people had arsenical skin lesions including cancer but the source of arsenic in Chhattisgarh is not from the flood plains of Newer Alluvium (Holocene) as in Ganga, Brahmaputra, and Imphal rivers. Arsenic groundwater contamination in Chhattisgarh is due to natural deposition of arsenic-rich pyrite and its mobilization (Acharyya et al. 2005). The magnitude of arsenic contamination in Chhattisgarh state is much less compared to flood plain contamination. Arsenic contamination of groundwater in gold mining areas is known, but people suffering from arsenic toxicity including cancer has only been confirmed from Karnataka state (Chakraborti et al. 2013). Groundwater arsenic contamination from industrial waste release in India has also been reported (Sekhar et al. 2003); however, people suffering seriously from arsenical toxicity due to the consumption of groundwater contaminated by a pesticide ‘Paris green (copper acetoarsenite)’ has been recorded in Kolkata city also (Chatterjee et al. 1993; Mazumder et al. 1992).
Geology, geomorphology, and hydrogeology of the study area
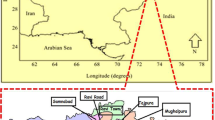
The present study area consists of five states in the Ganga–Brahmaputra plain in India (Uttar Pradesh, Bihar, Jharkhand, West Bengal and Assam), and one North Eastern Hill state, Manipur. Figure 1 shows the geological map of the Ganges and Brahmaputra drainage basins. The Ganga plain is geomorphological characterized and predominated by alluvial deposit of Quaternary age. Three states (Uttar Pradesh, Bihar and West Bengal) of the current study area are located in this zone. It has been postulated that the great alluvial tract of the Brahmaputra River, which is by nature a geo-synclinal basin, formed concomitantly with the Himalayas to the north and is composed of alluvial and more recent Pleistocene sediment deposits (Goswami et al. 2014; Singh and Goswami 2011). A study based on mineralogical and stratigraphic data indicates that the two rivers (Ganges and Brahmaputra) changed their positions several times during the Holocene (Heroy et al. 2003), resulting in deposition of the sediments, which are considered very fertile (Kumar et al. 2010). The area of Jharkhand which was formed in the Paleozoic age consists of mainly granites, gneisses and charnockites (Heroy et al. 2003). The Manipur state comprises geologically young rock formations that were uplifted by the Tertiary orogeny of the Himalayas from the shallow bed of the Tethys Sea. The rocks are dominantly Tertiary and Cretaceous sediments with minor igneous and metamorphic rocks (Chakraborti et al. 2008).
Geological map of the Ganges and Brahmaputra drainage basins, focusing on states: Uttar Pradesh, Bihar, Jharkhand, Chhattisgarh, West Bengal, Assam and Manipur (modified from Heroy et al. 2003)
Groundwater arsenic-contaminated states and magnitude of contamination in India
Figure 2 shows the major affected states and the magnitude of groundwater arsenic contamination across India. The chronological years of discovery and places of incidents of groundwater arsenic contamination in India is given in Table 1. Table 2 shows the groundwater arsenic concentration status in the different states considered in the present study. More than 170,000 water samples from tubewells were analysed from all surveyed states of India and half of the samples had arsenic greater than 10 μg/L. The maximum arsenic concentration was detected in a tubewell from West Bengal as 3,700 μg/L, which was 370 times higher than the WHO guideline value (10 μg/L) and 74 times higher than the Indian standard of arsenic (50 μg/L) in drinking water. Elevated concentrations of arsenic in groundwater were also detected in other states such as Bihar and Uttar Pradesh. Altogether 13.85 and 6.96 million people from all surveyed states were exposed to arsenic greater than 10 and 50 μg/L, respectively.
Sources of arsenic and mechanisms of arsenic leaching to groundwater
In nature, arsenic occurs as a component of over 245 minerals, usually ores containing sulfide, along with trace metals (Mandal and Suzuki 2002). The weathering of rocks and minerals appears to be the main source of arsenic in the soils, rocks, natural waters and organisms; mobilized is through a combination of natural processes such as weathering reactions, biological activity, transport, precipitation, volcanic emissions as well as through a number of other anthropogenic activities (Smedley and Kinniburgh 2002). It has been reported, based on the arsenic contamination scenario in Asia, that the floodplain of the rivers originating from the Himalayan Mountains and Tibetan Plateau region are also having groundwater arsenic contamination issues (Chakraborti et al. 2004). Based on this, arsenic contamination has been noticed in West Bengal, Bihar, Jharkhand, Uttar Pradesh in the Gangetic plain, Manipur in the north eastern Hill states, Brahmaputra plain in Assam, and PMB (Padma-Meghna-Brahmaputra) plain in Bangladesh. The presence of groundwater arsenic contamination found in Iran, Pakistan, China, Lao PDR, Vietnam, Myanmar, Cambodia, and Thailand also can be linked with the floodplain of the rivers originating from the same region. These places have been shown as red dots in Fig. 3. Numerous studies have been conducted to identify the leaching mechanism of arsenic from the sediment to aquifer and a number, such as oxidation, reduction, and recent inflow of carbon, have been proposed which possibly substantiate individually or collectively depending upon the geochemistry of the aquifer and organic matter present in the sediments (Ahmed et al. 2004; Anawar et al. 2003; Bhattacharya et al. 1997; Chakraborti et al. 2001; Chowdhury et al. 1999; Das et al. 1996; Harvey et al. 2002; Mukherjee and Bhattacharya 2001; Nickson et al. 1998, 2000; Ravenscroft et al. 2001). A study from West Bengal reported that metal-reducing bacteria play a crucial role in arsenic release from sediments (Islam et al. 2004). It was also reported from Bangladesh that microbially or geochemically mediated reductive dissolution of arsenic associated with Fe oxyhydroxides is the primary reason for arsenic release, and that these reducing conditions are caused by decomposition of organic matter (Anawar et al. 2011). The existing strong correlation between the arsenic level and prevalence of high concentrations of iron, phosphate, and ammonium ions, along with the weathering of carbonate and silicate minerals and surface-water/groundwater interactions, ion exchange, and anthropogenic activities (such as excessive groundwater withdrawal for agricultural irrigation) seem to be the primary processes causing arsenic contamination in groundwater (Kumar et al. 2010). From various studies of the Bengal delta, it is now well recognized that heavy withdrawal of groundwater is also responsible for arsenic contamination in groundwater (Harvey et al. 2002).
Arsenic-affected children in affected states of India
Infants and children are far more susceptible to the adverse effects of toxic substances than adults (NRC 1999). From this study on arsenic-affected states in India, it was noticed that children (usually) under 11 years of age do not show arsenical skin lesions although their biological samples contain high levels of arsenic; however, exceptions were observed when (1) arsenic content in water consumed by children is very high (≥1,000 μg/L) and (2) arsenic content in drinking water is not alarmingly high (500 μg/L) but the children’s nutrition is poor (Rahman et al. 2001). High arsenic content in their biological samples prove that children in the arsenic affected areas of the Ganga-Meghna-Brahmaputra plains may not show physical manifestations but are sub-clinically affected (Chakraborti et al. 2004).
Children are at double risk as their bodies are frailer and when their system tries to expel the poison their internal organs are affected severely. This in turn retards their further growth, both physical and mental. During the last ∼20 years of the preliminary survey, about 8,000 children from arsenic affected villages of affected states were screened and the survey registered about 4.5% of children with arsenical skin lesions; thus, the future generation is in significant danger. Researchers have witnessed the unimaginable sufferings of children in arsenic affected villages in many states of India, which is carrying forward generation after generation. Madanpur is a remote village in the Bhagabangola block in the Murshidabad district, West Bengal, where researchers visited two times at an interval of 10 years and witnessed apathy towards this situation. The first time, in 1992, among the 300 villagers of Madanpur, half of them had symptoms of arsenical skin lesions. Most of them were suffering from malnutrition and 40% of the children between 6 and 11 years had visible symptoms of arsenical skin lesions. The three tube-wells that villagers of Madanpur used for withdrawing drinking water had an average arsenic level of 700 μg/L. During the second visit, in 2002, researchers noted that nothing had improved at all. Some of the previously identified children had died or lost their strength to work; they had become very thin young adults of very poor health. Table 3 shows the dermatological symptoms of 12 children from Murshidabad district.
Arsenic-related health effects in the chronically exposed population in the affected states of India
Mechanisms of arsenic toxicity in humans
Arsenic poisoning falls into two types, acute and chronic. Acute toxicity immediately results in nausea, vomiting, abdominal pain, severe diarrhoea and soon after peripheral neuropathy and other symptoms (NRC 1999). Chronic arsenic toxicity is usually felt after a prolonged period of ingestion of contaminated water/source, resulting in multisystem diseases. Arsenic is now a well-documented human carcinogen; however, there is no medicine to treat chronic arsenic poisoning. The focus of management is to reduce arsenic at the source.
In the gastrointestinal tract of humans, 70–90% of arsenic from the source, e.g. drinking water, is absorbed irrespective of the arsenite, As (III), or arsenate, As (V), state. Once ingested, inorganic arsenic is readily absorbed from the gastrointestinal tract and transported by blood to various organs in the human body (IPCS 2001). After entering the cell, As(V) is reduced to As(III) by glutathione (GSH). Arsenite has high affinity for the thiol (-SH) group; thus, arsenite deactivates enzymes, other functional proteins, and low molecular weight compounds such as GSH and cysteine blocking the active -SH group. Arsenic can exert its toxic effects through impairment of cellular respiration by inhibition of various mitochondrial enzymes and uncoupling of oxidative phosphorylation (IPCS 2001).
The As3+ species can react with the -SH group of protein and enzymes, thereby making them inactive, and increases reactive oxygen species in the cells causing cell damage. It is also reported that arsenic could inhibit 200 enzymes in the body (Chakraborti 2011). It has been regarded that multisystem noncancerous effects could be due to deactivation of essential enzymatic functions by trivalent arsenic compounds and subsequent oxidative stress to cells. A few studies have detected in urine, along with all the four arsenic species [As (III), As(V), MMA(V) and DMA(V)], the presence of very unstable monomethylarsonous acid [MMA(III)] and dimethylarsinous acid [DMA(III)]. It is also considered that inorganic As3+ and the reduced forms—MMA(III) and DMA(III)—formed during methylation are highly reactive and contribute to the observed toxicity of inorganic arsenic. Arsenate (AsO4 –) has similar structure as phosphate (PO4 3–) and thus can substitute PO4 3– in adenosine diphosphate (ADP). This substitution prevents conversion of ADP to ATP (adenosine triphosphate) that produces energy to cell. The affinity of arsenic deposition in tissues depends on the nature of the tissue and the type of arsenic species. Hair and nails are cysteine-containing proteins with an active -SH group. Trivalent arsenic has affinity for the SH group. In keratin tissues such as hair, nails, sole, and palm, cumulative deposition of arsenic occurs. During the past two decades, five monographs (IARC 2012; 2004; IPCS 2001; NRC 1999, 2001) along with a large number of reports and special issues have been published to include the research activities related to chronic arsenic exposure and various carcinogenic and non-carcinogenic health effects.
It is evident now that inorganic arsenic exposure deactivates the function of enzymes, some important anions, cations, transcriptional events in cells and causes other direct or indirect effects. Such activities of inorganic arsenic result in numerous illnesses. Repeated epidemiological investigations confirmed such illness—examples are (1) dermal effects, (2) cardiovascular effects, (3) respiratory effects, (4) gastrointestinal effects, (5) endocrinological effects (diabetes mellitus), (6) neurological effects, (7) reproductive and developmental effects, (8) cancers, and (9) other effects.
Dermal effects-A
Arsenic poisoning is mainly caused by ingestion of contaminated underground water. Signs and symptoms of arsenicosis depend on (1) arsenic concentration in drinking water; (2) the volume of water consumed; (3) duration of consumption; (4) nutritional status (immunity) of an individual. To get a clinical symptom it may take about 6 months to 10 years or even more.
The stages of arsenicosis can be divided into (1) the pre-clinical stage, (2) clinical stage, (3) stage of internal complications and (4) the stage of malignancy. Clinical manifestations can be divided into minor and major dermatological signs. The minor signs are: Mee’s line on nail, pigmentation on tongue, conjunctival congestion, non-pitting oedema (Fig. 4a). The major signs are: diffuse and spotted melanosis or “raindrop pigmentation”, leucomelanosis, spotted keratosis, diffuse and spotted keratosis on palm, dorsal keratosis, gangrene, multiple squamous carcinoma, Bowens. Malignancy from these diseases may occur after about 15–20 years of clinical onset of the disease as mono centric or multi centric squamous cell carcinoma. Malignancy in other organs—lungs, bladder, liver, uterus etc.—may also develop.
Dermal effects-B
To identify an arsenicosis patient, cutaneous manifestations are the most prominent characteristic. Normally, diffuse melanosis, that is darkening of skin in the body or palm, is the earliest symptom (Fig. 4b); however, it is not necessary that those suffering from arsenic toxicity will always have symptoms of diffuse melanosis. Usually, spotted pigmentation (Fig. 4c, spotted melanosis) is the second stage, appearing on the chest, back, or a limb, which is a very common symptom. Leucomelanosis (Fig. 4d), which is white and black spots side by side, is common in persons who have stopped drinking arsenic-contaminated water but had spotted melanosis earlier. Mucus membrane melanosis on gums, lips, and tongue may also be due to arsenic toxicity. Diffuse with nodular keratosis on the palms (Fig. 4e) and soles (Fig. 4f) is a sign of severe arsenic toxicity as is also rough dry skin often with palpable nodules (spotted keratosis) in the dorsum of hands, feet, and legs (Fig. 4g).
There are many diseases, however, that mimic arsenical dermatosis—for example, Addison’s disease, which mimics diffuse melanosis; xeroderma pigmentosum is similar to spotted melanosis, and Verruca vulgaris mimics spotted keratosis. A combination of pigmentation (spotted melanosis) and nodular rough skin (spotted palmoplantar keratosis) almost points to arsenic toxicity excluding hundreds of causes of isolated pigmentation and nodular rough skin. In the case of any doubt, hair and nail analysis for arsenic will reveal the truth. There is always the question that at what concentration and drinking for how long will one show arsenical skin lesions? From the literature survey, some cases of arsenic-related symptoms have been reported at 100 or 200 μg/L concentration, but 300 μg/L exposure for a couple of years is considered adequate for the appearance of arsenical skin lesions. With high arsenic concentrations in water (say 1,000 μg/L), the skin lesions may appear within 6 months. It has also been noted that severe symptoms of arsenical skin lesions might serve as markers for other outcomes including skin cancer and internal cancer. Figure 4h shows a patient with severe diffuse and nodular keratosis who died of lung cancer. More common hyperkeratosis appears earlier than skin cancer. It is the opinion of toxicologists that the appearance of arsenical skin lesions indicates severe internal damage. As there is no medicine for arsenic toxicity, arsenic-safe water and nutritious food are considered the only remedy. It has also been reported that mild diffuse melanosis (+, mild) and mild spotted melanosis (+) may disappear with use of safe water and nutritious food. For persons affected with moderate skin lesions (spotted melanosis (++, moderate) and/or spotted keratosis (++), the skin lesions may reduce but may not vanish. For severe cases, even after several years, skin lesions especially keratosis continue even when hair, nails, and skin indicate a safe level of arsenic in the body. The spotted melanosis usually turns to leucomelanosis. Another unique feature is where the keratosis reappears even after it has been removed by ointment or surgery. It was also noticed that males had higher prevalence of skin lesions than females exposed at similar levels of arsenic through ingestion.
Further, it has been noticed that in a few cases when all members of a big family had arsenic-affected manifestations and were drinking water from hand tube-wells contaminated with a high concentration of arsenic, one or two members of the family had no skin lesions although hair, nails, and urine had high concentrations of arsenic the same as all family members.
Neurological effects
Neurological effects of acute arsenic exposure are more serious and long lasting compared to those of sub-acute or chronic exposure. Acute arsenic toxicity affects both central and peripheral nervous systems, whereas chronic arsenic toxicity affects predominantly the peripheral nervous system. The severity usually decreases with time but may last for several years or even lifelong. Neurological effects from chronic arsenic exposure through drinking groundwater have been reported from many countries; however, more incidents of it are from Asian countries where more than 100 million people are considered to be exposed chronically to arsenic-contaminated groundwater (Chakraborti 2011).
The researchers associated with the project reported here have studied several groups of groundwater arsenic-exposed subjects from several arsenic-contaminated districts in states of India (Ahamed et al. 2006a; Chakraborti et al. 1999, 2016a, 2016b; Mukherjee et al. 2003, 2005; Nayak et al. 2008; Rahman et al. 2001) suffering from arsenic related neurological toxicity. Most of the districts investigated are highly contaminated with arsenic and people have arsenical skin lesions. The arsenic-exposed subjects were studied for their dermatological, neurological, and other non-neurological systemic involvements, including malignancies and obstetric outcomes.
Neurological observations were recorded in all subjects for items considered consistent with peripheral sensory and motor neuropathy and for other neurologic abnormalities. Items included, to characterize neuropathy, were (1) pain and paresthesia (e.g. burning) in a ‘stocking and glove’ distribution, (2) numbness, (3) hyperpathia/allodynia, (4) distal hypesthesia (perception of pinprick, vibration, joint-position, touch sensations), (5) calf tenderness, (6) weakness/atrophy of distal limb muscles or gait disorder and (7) reduction/absence of tendon reflexes. The features of autonomic instability and central nervous system (CNS) involvement were also investigated (Ahamed et al. 2006a; Chakraborti et al. 1999, 2016a, 2016b; Mukherjee et al. 2003, 2005; Nayak et al. 2008; Rahman et al. 2001).
Electrodiagnostic studies were performed in many subjects to evaluate peripheral nerve and central pathway functions by interpreting nerve conduction (sensory and motor nerve), electromyography (muscle function), and evoked potentials (visual, brain stem auditory and somatosensory). Quantitative sensory testing (QST) was performed in some subjects for determination of perception thresholds of different modalities of sensation, e.g. vibration, thermal stimulus and pain.
The diagnosis of arsenic neuropathy was confirmed in arsenic-exposed subjects with features of neuropathy by the presence of arsenic typical skin lesions (arsenic dermopathy) and analysis of arsenic in hair, nails, urine and the water they were drinking, and after exclusion of other possible causes and alternative explanations (Ahamed et al. 2006a; Chakraborti et al. 1999, 2016a, 2016b; Mukherjee et al. 2003, 2005; Nayak et al. 2008; Rahman et al. 2001).
The neuropathy in arsenic toxicity was further categorized into sensory or sensorimotor types and graded according to severity. The presenting neuropathic features of the study groups of chronically arsenic-exposed subjects included: sensory features of distal paresthesia ranging from a low of 18.4% to a high of 57.2%, limb pains from 9 to 18.7%, and distal hypesthesia from 22 to 46.9%, outnumbering motor features of distal limb weakness or atrophy ranging from 3 to 15.3%.
The prevalence of neuropathy ranged from a low of 37.3% to a high of 60.24%, and sensory neuropathy outnumbered motor nerve involvement (Ahamed et al. 2006a, b; Chakraborti et al. 1999, 2016a, 2016b; Mukherjee et al. 2003, 2005; Nayak et al. 2008; Rahman et al. 2001). There was a sub-acutely affected group of arsenicosis with high prevalence of neuropathy in Bardhaman district of West Bengal, India, where 33 out of 38 cases neurologically examined, i.e. 86.8%, revealed features of neuropathy, of which 76.3% had sensory and 10.5% sensorimotor neuropathy (Mukherjee et al. 2003). In the study population even the children were found to be suffering neurological complications from chronic arsenic toxicity. The prevalence of neuropathy in children up to 15 years varied from 12.5 to 33.3% (Ahamed et al. 2006a, b; Chakraborti et al. 2016a; Nayak et al. 2008), which is less compared to adults.
A cross-sectional study investigated the intellectual function among 351 children age 5–15 years from West Bengal in 2001–2003 (von Ehrenstein et al. 2007). Intellectual function was assessed with six subtests from the Wechsler Intelligence Scale for Children as well as with the Total Sentence Recall test, the Colored Progressive Matrices test, and a pegboard test. Arsenic in urine and lifetime water sources (including during the pregnancy period) were assessed using measurements of samples from 409 wells. The study found associations between arsenic and reductions in the adjusted scores of the vocabulary test, the object assembly test and the picture completion test, although they did not find associations between long-term water arsenic concentrations and intellectual function (von Ehrenstein et al. 2007).
Electrophysiological study findings revealed that arsenic toxicity damaged sensory more than motor nerves and defined clinical as well as subclinical neuropathy. Abnormal sensory nerve functions were observed in 27–60% of patients of arsenicosis with neuropathy; motor nerve dysfunctions was observed in 16.7–40% of these patients and 10% of asymptomatic subjects tested positive by the standard nerve conduction studies (Mukherjee et al. 2003, 2005; Nayak et al. 2008). Limitations of standard nerve conduction studies prompted the use of quantitative sensory testing (QST) to study not only the large myelinated but also small myelinated and unmyelinated nerve fibers. In 60–95% of clinical neuropathy cases, one or more of the sensory parameters of vibration, thermal (cold, warmth) and pain perception thresholds were increased. Subclinical sensory neuropathy due to arsenic was detected in 30–60% of asymptomatic cases by QST compared to only 10% by standard nerve conduction studies, QST is thus proved to be a helpful screening tool.
Arsenic neuropathy is a common occurrence. The occurrence may be clinical or subclinical, predominantly sensory with small fiber involvement, sensorimotor and/or mixed fiber neuropathy, or it may be the precipitating factor for associated complications like non-healing ulcers in distal parts of extremities.
Central nervous system (CNS) and other neurological features in arsenicosis
The features of CNS involvement were important findings in the subjects involved in this study, whether neuropathic or not. Mood changes, with depression, irritability, anxiety, and/or reduced concentration, were common and affected their occupational and family activities (Mukherjee et al. 2003). Sleep abnormalities and headaches (2%) were more common than in control subjects (Mukherjee et al. 2003, 2005). Other neurological findings ranging from 9 to 13.2% comprised of tremors, proximal limb wasting, decreased vision not due to ophthalmic causes, decreased hearing (aural cause excluded), and decreased libido (Mukherjee et al. 2003, 2005).
Reproduction and developmental effects
Studies in both humans and animals have confirmed that inorganic arsenic and methylated species of arsenic can cross the placenta (ATSDR 2007). This information is an indication that arsenic may affect reproductive and developmental health effects.
Chronic human exposure to arsenic can adversely affect reproductive performance apart from other health hazards (ATSDR 2007). Several epidemiological studies have revealed the association between chronic arsenic exposure and adverse pregnancy outcome (IARC 2004; IPCS 2001). As part of this project, studies were carried out in different villages of Murshidabad district of West Bengal (Mukherjee et al. 2005), in villages in Bihar (Chakraborti et al. 2003, 2016a, 2016b), villages in Uttar Pradesh state (Ahamed et al. 2006a), and one village in the Sahibganj district of Jharkhand state (Nayak et al. 2008).
The study population was composed of married women of the reproductive age group 18–45 years who previously had at least one pregnancy. Researchers enlisted the respondents through house to house visits, and the majority were of lower socio-economic status. The respondents had been drinking arsenic-contaminated water for 5 years or more. Information was collected on each respondent’s lifetime pregnancy history, which included the number of pregnancies, spontaneous miscarriage, stillbirth, preterm birth, lower birth weight and neonatal death. These women were examined clinically and their obstetric history was analyzed in detail. These studies were carried out over a 14-year period. The subjects were categorized into different groups according to the level of contamination of arsenic in drinking water; high contamination (90–800 μg/L) was associated with a high rate of stillbirths and neonatal mortality compared to the group of women supplied with arsenic-safe drinking water. From the arsenic-contaminated district of Murshidabad, West Bengal, women exposed to high arsenic-contaminated groundwater (284–1,474 μg/L) for 5–10 years had more spontaneous abortions (miscarriages), stillbirths, preterm birth, low birth weight, and neonatal deaths. Most of the women had arsenical skin lesions. During the survey in the Murhsidabad district of West Bengal, a woman was found with arsenical skin lesions whose pregnancies had been severely affected (the first pregnancy had preterm birth, the second pregnancy resulted in spontaneous abortion, and the third resulted in early neonatal death; arsenic content in the drinking water was 1,617 μg/L and arsenic content in the woman’s urine was 1,474 μg/L. The WHO guideline value for arsenic in drinking water is 10 μg/L. The average concentration of arsenic in urine in the unexposed population is usually less than 100 μg/L (ASTDR U 2007).
von Ehrenstein et al. ( 2006) studied pregnancy outcomes and infant mortality among 202 married women in West Bengal between 2001 and 2003. Reproductive histories were ascertained using structured interviews. Arsenic exposure during each pregnancy was assessed, and involved 409 water-source wells. Odds ratios for spontaneous abortion, stillbirth, neonatal mortality, and infant mortality were estimated with logistic regression based on the method of generalized estimating equations. Exposure to high concentrations of arsenic (≥200 μg/L) during pregnancy was associated with a six-fold increased risk of stillbirth after adjustment for potential confounders. Arsenic-related skin lesions were found in 12 women who had a substantially increased risk of stillbirth. No association was found between arsenic exposure and spontaneous abortion or overall infant mortality. This study adds to the limited evidence that exposure to high concentrations of arsenic during pregnancy increases the risk of stillbirth; however, there was no indication of the increased rates of spontaneous abortion and overall infant mortality that have been reported in some studies (von Ehrenstein et al. 2006).
Cancer: the future danger
At present, the International Agency for Research on Cancer (IARC), WHO, US Environmental Protection Agency (US EPA), and other health protection authorities consider arsenic to be causing skin, lung, liver, urinary bladder, and kidney cancer. It is reported that life-time consumption of arsenic-contaminated water at 1 L/day having arsenic at 50 μg/L concentration could cause cancer in 13 people out of 1,000 population (Smith et al. 2002). Hossain et al. (2013) reported that, in a tropical country, an adult drinks about 6 L of water per day; hence, the risk of cancer is much higher. In the cohort study reported here, 1,194 registered arsenic patients with skin lesions were re-examined between January 2009 and January 2010, out of the 2,384 villagers that had been screened earlier (1995–2000) from 33 villages and 16 blocks/thanas from some districts of West-Bengal (India) and Bangladesh. Only these patients were re-surveyed because the study’s longer-term database contained the concentrations of arsenic in the hand-driven tube-wells they had used for drinking along with arsenic data on their biological samples and details of their skin lesions. Findings of this cohort study indicate that 14% of the patients examined earlier (who had arsenical skin lesions) had died with non-healing ulcers and 48% are currently suffering from Bowens and arsenic-related cancers. On the basis of this study (SOES 2010), it was concluded “Are Millions in Ganga-Meghna-Brahmaputra Plain already exposed to arsenic-contaminated water potentially at risk from cancer”? Figure 5 shows some of the patients with Bowens and cancer from arsenic affected villages of West Bengal during this cohort study.
In the Chak-Khor Gachi village of Baduria block, North 24 Pargana district of West Bengal, over a 15-year period, the researcher’s medical group identified more than 35 arsenic-affected patients suffering from multiple Bowens and 28 of them had died from liver cancer by the time of the re-survey. Figure 6a shows a cancer patient and Fig. 6b shows a patient with keratosis, from Rajnandangaon, Chattisgarh state of India.
Even during the first survey, 10–15 years earlier, the researchers were not aware of arsenic-affected villages with incidences of cancer other than suspected Bowens, squamous or Basel cell carcinoma—of course other types of cancers were present but patients were not tested for other cancers. In the course of time, it has been established that arsenic can cause other types of cancers. Now, in arsenic-affected villages of West Bengal and other arsenic-affected states of India, lung cancer and liver cancer are more common. It is noted that the appearance of severe keratosis and Bowens indicate the possibility of other forms of cancer. The local authorities and community health service providers need to address this issue to prevent future cancer ‘massacres’ in arsenic-affected states of India.
Cardiovascular effects from arsenic toxicity
Apart from the highly prevalent neoplastic outcomes, development of vascular disease is also common among the arsenic endemic population. Vascular disorder refers to the dysfunction of vessels—arteries, veins, capillaries, including the heart. This dysfunction may be of two types: peripheral vascular disease such as dysfunctioning of small arteries and capillaries in the peripheral areas as the skin, feet, arms, etc., and cardiovascular disease (CVD) associated directly with the heart and larger arteries. The cardiovascular diseases generally manifested are arteriosclerosis, atherosclerosis, ischemia heart disease (which results in hypertension, HTN), heart blockage, cardiac arrest, stroke, and infarction, and in the periphery, arteriosclerosis, black foot disease, gangrene, etc. Epidemiological studies have revealed high arsenic exposure (>300 μg/L) associated with the manifestation of both peripheral and CVD from different parts of the world (Srivastava et al. 2009). Arteriosclerosis is the thickening of ‘intima’, the interior most walls of arteries. Atherosclerosis is a variation of arteriosclerosis, where the thickening is due to the deposition of fat or high-cholesterol plaque formation, which might be manifested due to arsenic toxicity (Simeonova and Luster 2004). A unique exhibition of peripheral arteriosclerosis associated with high arsenic concentration in drinking water may be shown in ‘Blackfoot disease’ which was common in southwestern Taiwan. The biological mechanism behind the arsenic-induced cardiovascular deformities may be as follows. Arsenic can produce reactive oxygen like hydrogen peroxide and hydroxyl radicals which can induce alterations of nitric oxide metabolism and endothelial function (Chakraborti 2011a). Arsenic-induced cardiovascular disease in humans is now regarded as interrelated with genetic, nutritional, and environmental factors. The adverse cardiovascular manifestation in humans resulting from long-term arsenic exposure, is an independent risk factor and may be persistent and irreversible (Wang et al. 2007). Recent studies also indicate the cardiovascular implications of low to moderate arsenic exposure by ingestion, in the form of high death rates due to CVD (Medrano et al. 2010), nonlinear blood pressure (Chen et al. 2007), and high pulse pressure (Chen et al. 2007), though there is no evidence of risk of hypertension (Abdul et al. 2015). Tchounwou et al. (2003) reported that individuals exposed to elevated inorganic arsenic levels in drinking water showed a range of health effects including peripheral vascular disease, non-cirrhotic portal fibrosis, nasal septum perforation, bronchitis, and polyneuropathy.
Very limited studies have been conducted on cardiovascular effects for arsenic poisoning in India. Guha Mazumder et al. (2012) reported the association of hypertension (HTN) among an arsenic-exposed population of 280 people, compared to a control of 100 in West Bengal. The study found a dose-effect relationship between arsenic exposure and arsenic levels in hair and HTN. No further association was found for HTN among the exposed population, with or without skin lesions. A study conducted by Das et al. (2012) on 103 exposed people from Murshidabad, West Bengal, compared to the population of 107 from uncontaminated areas of Medinipur, West Bengal, found elevated levels of inflammatory cytokines (IL6, IL8, LCP1) in the exposed population, associated with the high risk of atherosclerosis.
Respiratory effects from arsenic toxicity
Inorganic arsenic shows a major adverse manifestation on the human respiratory system; the longer the exposure, the more grave the problem. One of the earliest studies on the effects of inorganic arsenic exposure toward pulmonary health was from a preliminary investigation conducted in Chile during 1970 (Borgoño et al. 1977). Several studies on arsenic-impacted areas of West Bengal have reported that ingestion of inorganic arsenic for a prolonged period causes respiratory problems, including coughs, chest sound, bronchitis, and shortness of breath.
A cross-sectional survey was carried out in 1996 involving 7,683 participants in one arsenic-affected district of West Bengal, who were chronically exposed to arsenic-contaminated groundwater (Mazumder et al. 1998). One of the study objectives was to establish prevalence of pulmonary effects on the people that already have arsenic dermatological features. There was prevalence of coughs, shortness of breath, and chest sound in both males and females, and these symptoms rose with increasing arsenic content in water. The respiratory effects were more pronounced in those exposed to high concentrations of arsenic (≥300 μg/L). The study further concluded that chronic exposure to arsenic-contaminated water could cause respiratory effects (Mazumder et al. 1998).
Mazumder et al. (2005) conducted a study on the severity of chronic non-malignant bronchiectasis among the population of the arsenic-exposed region of West Bengal. Among the study’s total population of 38 people (27 having arsenical skin lesions, and 11 having no skin lesions) they found a bronchiectasis severity score of 3.4 (±3.6) for the exposed population having skin lesions, compared to 0.9 (±1.6) in the population having no skin lesions. In a cross sectional study, De et al. (2004) assessed chronic arsenic poisoning on pulmonary dysfunction among the affected population in West Bengal compared with a control population. Among 107 exposed subjects, they found respiratory involvement in 33 (33.8%) of them. Most of the manifestation was obstructive lung disease, in 20 people (68.9%). Others suffered restrictive lung disease (1 person, 3.5%), mixed symptoms (8 people, 27.6%), and malignancy (4 people, 12.2%). Ghosh (2013) showed the prevalent of nonproductive cough (49.31% subjects) and change in spirometry (23.28% subjects) as the most common symptoms in a study population of 73 from Arsenic Clinic, Institute of Postgraduate Medical Education and Research, Kolkata, West Bengal, over a period of 1 year and 4 months during 2008–2009. In a more recent study conducted in West Bengal comparing the respiratory effect of chronic low level (≤50 μg/L) arsenic exposure, Das et al. (2014) documented that the exposed population (11–50 μg/L; n = 446) had higher prevalence of upper and lower respiratory symptoms, dyspnea, asthma, eye irritation and headache than the control population (<50 μg/L; n =388). The study further substantiated negative association between arsenic level and spirometric parameters. Bhattacharyya et al. (2014) investigated the pulmonary arterial system performance relative to the arsenic endemic population through high-resolution computerized tomography, among 194 arsenic exposed and 196 unexposed people. The exposed population showed higher prevalence of cough (odds ratio, OR, 3.23) and shortness of breath (OR 1.76), compared to the control population. Similar results were found in cases of other respiratory manifestations like bronchiectasis [mean ± SD (standard deviation): 2.41 ± 2.32 vs. 1.22 ± 1.48 (P <0.001)], pulmonary artery branch dilation—2.48 ± 2.33 vs. 0.78 ± 1.56, (P <0.001)— and pulmonary trunk dilation (0.26 ± 0.45 vs. nil) when compared to the control.
Gastrointestinal effect of arsenic toxicity
The gastrointestinal effect of arsenic toxicity may be broadly categorized by acute or subacute arsenic exposure and chronic arsenic exposure. Acute or subacute arsenic exposure, which is generally immediate but highly concentrated exposure, could result in overt gastrointestinal disturbances starting from mild to severe abdominal pain, cramping, and diarrhoea and most importantly to submucosal capillary damage from the absorbed arsenic (Tchounwou et al. 2003). The overall effects may lead to more serious conditions, like life-threatening haemorrhagic gastroenteritis and severe shock (Chakraborti 2011a). With chronic exposure when it is of low concentration, overt gastrointestinal symptoms may not be noticed or absent but some of the gastrointestinal disturbances could occur like mild esophagitis, gastritis, or colitis with respective upper and lower abdominal discomfort, anorexia, malabsorption, and weight loss (Tchounwou et al. 2003). In the West Bengal study area, 60 out of the 156 affected villagers chronically exposed to arsenic-contaminated groundwater showed dyspepsia (Mazumder et al. 1998). One of the detailed studies was conducted on 25,274 people screened from Murshidabad district of West Bengal; among the registered 4,813 patients having arsenical skin lesions, more than 50% of the patients showed gastrointestinal symptoms of anorexia, nausea, dyspepsia, altered taste, abdominal pain, enlarged liver and spleen, and ascites (collection of fluid in abdomen; Mukherjee et al. 2005). In another epidemiological study in West Bengal, nothing other than abdominal pain was observed in both the affected population drinking chronic arsenic-contaminated water (50–3,400 μg/L) and the control population (>50 μg/L; Mazumder et al. 2001). An investigation into the gastrointestinal impact of a population exposed to elevated arsenic concentrated water (>50 μg/L) but with the population not having any skin lesions (Majumdar et al. 2009) found only moderate prevalence of diarrhoea.
Endocrinological effects (diabetes mellitus) of arsenic toxicity
Ingestion of chronic levels of arsenic in drinking water is considered to be the reason for an increased risk of type 2 diabetes mellitus. To explain this, researchers are looking at the effects of arsenic on the endocrine system. It has been postulated that arsenic might act as an endocrine disruptor at doses as low as 0.4 μg/L arsenite (Kapaj et al. 2006). One study has substantiated that accumulation of arsenic in the pancreas inhibits the secretion of insulin and decreases the viability of the cells (Lu et al. 2011). There are numerous reports of the prevalence of diabetes in arsenic endemic areas of Taiwan (Tsai et al. 1999; Tseng et al. 2000), Bangladesh (Rahman et al. 1998), Millad County of Uttar Pradesh, India (Lewis et al. 1999), Cyprus (Makris et al. 2012), and Mexico (Coronado-González et al. 2007); however there is lack of prominent study to show the prevalence of diabetes mellitus among the arsenic endemic population in India overall.
Other health effects
Haematological effects are observed from both acute and chronic arsenic exposure and their results are anaemia, leukopenia, and thrombocytopenia. Reports are scanty for the population chronically exposed through drinking arsenic-contaminated groundwater and subsequent haematological effects. Most of the population suffering from arsenic skin lesions is from a poor socio-economic background. The following features were commonly observed during the 24-year field survey in the arsenic-endemic areas of India where people have been drinking arsenic-contaminated groundwater and had arsenical skin lesions:
-
1.
Skin itching in response to sun light, burning and watering of eyes, weight loss, loss of appetite, weakness, lethargy and easily fatigued, thus limiting physical activities and work capacity.
-
2.
Chronic respiratory complaints were also common. A chronic cough with or without expectoration was evident in more than 50% of the studied people. As reported by the villagers, the unique sound of ‘cough of arsenicosis’ from adjacent homes at night was reported to create an unusual atmosphere. The cough may be painful and sputum may contain blood to be misdiagnosed as pulmonary tuberculosis. In late stages, shortness of breath predominates.
-
3.
Gastrointestinal symptoms of anorexia, nausea, dyspepsia, altered taste, abdominal pain, enlarged liver and spleen, and ascites were also observed in more than 50% of patients.
-
4.
Moderate-to-severe anaemia was evident in nearly 30% of cases.
-
5.
Conjunctival congestion and leg oedema were less common and found in 10% of the cases.
Is the WHO guideline value for arsenic in drinking water (10 μg/L) in the developing countries adequate?
The WHO guideline value for arsenic in drinking water is 10 μg/L. At present, in most of the arsenic-affected Asian countries, the permissible limit of arsenic in drinking water is 50 μg/L. Considering the large area and population potentially at risk from arsenic danger within world’s arsenic scenario, India appears to be at the top of the list. It is important to note that the WHO guideline value (10 μg/L) was allocated on the basis of 2 L of drinking water per day, whereas in tropical arsenic-affected states in India, the average direct drinking water consumption is about 4 L of water per day, and total water intake (direct and indirect) is about 6 L/day (Hossain et al. 2013). In arsenic-affected areas of India, arsenic-contaminated groundwater is in use for agriculture and thus food is also arsenic contaminated, an additional source of arsenic to the consumer. It is also reported that a better nutritional status means that the population can resist arsenic toxicity (Chowdhury et al. 2000; Milton et al. 2004). By comparison, the WHO permissible limit for fluoride (F) in drinking water has long been 1.5 mg/L. For India, on the basis of the factors discussed above, the WHO has suggested reducing the permissible limit for F in drinking water in India from 1.5 to 1.0 mg/L and India has accepted this reduction; however, the permissible limit of arsenic in drinking water in India remains 50 μg/L. On the basis of the reasons discussed in the previous, the permissible limit for arsenic in drinking water in India logically should be less than 5 μg/L. Further, it is reported that the cancer risk to those drinking arsenic-contaminated water at 50 μg/L at 1.0 L/day is 1.3 people per 100 (Smith et al. 2002). A group of scientists has also opined that the WHO limit may not be safe for pregnant mothers and children (Osborn 2012). In the meantime, some parts of the world, considering the danger of the guideline value of arsenic 10 μg/L, already have reduced their permissible level of arsenic. New Jersey and South Carolina in the USA has accepted a 5-μg/L limit for their drinking and cooking, while for Australia it is 7 μg/L.
Conclusion
It appears from the overall study that the groundwater of some states in India is very contaminated with arsenic. Current statistics show that the groundwater of 7 states of India are affected with arsenic and this poses risk to 70 million people in terms of various arsenic health effects, including various forms of cancer. Children are most susceptible to arsenic poisoning. People who had been found in previous surveys to have arsenic keratosis, now suffer from multiple Bowens/cancers in the long term. Even with higher arsenic standards (50 μg/L instead of 10 μg/L), authorities and communities are apparently not able to generally enforce the standards and some urgent intervention is needed to address the search for alternative sources of water or to implement small-scale/rural water treatment. Inhabitants of the Ganga-Meghna-Brahmaputra plains and other river flood plains have, for thousands of years, used the traditional sources of water like rivers, ponds, lakes, dug wells, and other water bodies for consumption. Even in the extreme southern part of India, rainwater harvest is a traditional procedure for drinking water. These water sources should be considered as alternative safe-water options to the affected communities, as opposed to deeper groundwater. Effective management of the vast surface water resources, and good nutrition, along with greater population awareness, could potentially minimize the impact of groundwater arsenic contamination in India.
References
Abdul KSM, Jayasinghe SS, Chandana EP, Jayasumana C, De Silva PMC (2015) Arsenic and human health effects: a review. Environ Toxicol Pharmacol 40:828–846
Acharyya S, Shah B, Ashyiya I, Pandey Y (2005) Arsenic contamination in groundwater from parts of Ambagarh-Chowki block, Chhattisgarh, India: source and release mechanism. Environ Geol 49:148–158
Ahamed S, Sengupta MK, Mukherjee A, Hossain MA, Das B, Nayak B, Pal A, Mukherjee SC, Pati S, Dutta RN (2006a) Arsenic groundwater contamination and its health effects in the state of Uttar Pradesh (UP) in upper and middle Ganga plain, India: a severe danger. Sci Total Environ 370:310–322
Ahamed S, Sengupta MK, Mukherjee SC, Pati S, Mukherjee A, Rahman MM, Hossain MA, Das B, Nayak B, Pal A (2006b) An eight-year study report on arsenic contamination in groundwater and health effects in Eruani village, Bangladesh and an approach for its mitigation. J Health Popul Nutr 129–141
Ahmed KM, Bhattacharya P, Hasan MA, Akhter SH, Alam SM, Bhuyian MH, Imam MB, Khan AA, Sracek O (2004) Arsenic enrichment in groundwater of the alluvial aquifers in Bangladesh: an overview. Appl Geochem 19:181–200
Anawar HM, Akai J, Komaki K, Terao H, Yoshioka T, Ishizuka T, Safiullah S, Kato K (2003) Geochemical occurrence of arsenic in groundwater of Bangladesh: sources and mobilization processes. J Geochem Explor 77:109–131
Anawar HM, Akai J, Mihaljevič M, Sikder AM, Ahmed G, Tareq SM, Rahman MM (2011) Arsenic contamination in groundwater of Bangladesh: perspectives on geochemical, microbial and anthropogenic issues. Water 3:1050–1076
Atsdr U (2007) Toxicological profile for arsenic. Agency for Toxic Substances and Disease Registry, Atlanta, GA
Ayeza A (1917) Arsenicismo regional indemico [Regional arsenic]. Bol Acad Nac Med Buenos Aires 1:11–13
Bhattacharya P, Chatterjee D, Jacks G (1997) Occurrence of arsenic-contaminated groundwater in alluvial aquifers from delta plains, eastern India: options for safe drinking water supply. Int J Water Resour Dev 13:79–92
Bhattacharya S, Gupta K, Debnath S, Ghosh UC, Chattopadhyay D, Mukhopadhyay A (2012) Arsenic bioaccumulation in rice and edible plants and subsequent transmission through food chain in Bengal basin: a review of the perspectives for environmental health. Toxicol Environ Chem 94:429–441
Bhattacharyya P, Sen P, Ghosh A, Saha C, Pp B, Das A, Majumdar K, Mazumder DG (2014) Chronic lung disease and detection of pulmonary artery dilatation in high resolution computerized tomography of chest in chronic arsenic exposure. J Environ Sci Health A 49:1453–1461
Borgoño JM, Vicent P, Venturíno H, Infante A (1977) Arsenic in the drinking water of the city of Antofagasta: epidemiological and clinical study before and after the installation of a treatment plant. Environ Health Perspect 19:103
Cebrian ME, Albores A, Aguilar M, Blakely E (1983) Chronic arsenic poisoning in the north of Mexico. Hum Toxicol 2:121–133
Chakraborti D (2011) Arsenic: occurrence in groundwater. In: Nriaju JO (ed) Encyclopaedia of environmental health. Elsevier, New York, pp 165–180
Chakraborti D, Biswas B, Roy Chowdhury T, Basu G, Mandal B, Chowdhury U, Mukherjee S, Gupta J, Chowdhury SF, Rathore K (1999) Arsenic groundwater contamination and suffering of people in Rajnandgaon district. Madhya Pradesh Curr Sci 77:502–504
Chakraborti D, Basu GK, Biswas BK, Chowdhury UK, Rahman MM, Paul K, Chowdhury TR, Chanda CR, Lodh D, Ray SL (2001) Characterization of arsenic bearing sediments in Gangetic delta of West Bengal-India: arsenic exposure and health effects. Elsevier, New York, pp 27–52
Chakraborti D, Mukherjee SC, Pati S, Sengupta MK, Rahman MM, Chowdhury UK, Lodh D, Chanda CR, Chakraborti AK, Basu GK (2003) Arsenic groundwater contamination in Middle Ganga Plain, Bihar, India: a future danger? Environ Health Perspect 111:1194
Chakraborti D, Sengupta MK, Rahman MM, Ahamed S, Chowdhury UK, Hossain MA, Mukherjee SC, Pati S, Saha KC, Dutta RN, Quamruzzaman Q (2004) Groundwater arsenic contamination and its health effects in the Ganga-Meghna-Brahmaputra plain. J Environ Monit 6:74N–83N
Chakraborti D, Singh EJ, Das B, Shah BA, Hossain MA, Nayak B, Ahamed S, Singh NR (2008) Groundwater arsenic contamination in Manipur, one of the seven north-eastern hill states of India: a future danger. Environ Geol 56:381–390
Chakraborti D, Ghorai SK, Das B, Pal A, Nayak B, Shah BA (2009) Arsenic exposure through groundwater to the rural and urban population in the Allahabad-Kanpur track in the upper Ganga plain. J Environ Monit 11:1455–1459
Chakraborti D, Rahman MM, Murrill M, Das R, Patil S, Sarkar A, Dadapeer H, Yendigeri S, Ahmed R, Das KK (2013) Environmental arsenic contamination and its health effects in a historic gold mining area of the Mangalur greenstone belt of northeastern Karnataka, India. J Hazard Mater 262:1048–1055
Chakraborti D, Rahman MM, Ahamed S, Dutta RN, Pati S, Mukherjee SC (2016a) Arsenic groundwater contamination and its health effects in Patna district (capital of Bihar) in the middle Ganga plain, India. Chemosphere 152:520–529
Chakraborti D, Rahman MM, Ahamed S, Dutta RN, Pati S, Mukherjee SC (2016b) Arsenic contamination of groundwater and its induced health effects in Shahpur block, Bhojpur district, Bihar state, India: risk evaluation. Environ Sci Pollut Res 23(10):9492–9504
Chatterjee A, Das D, Chakraborti D (1993) A study of ground water contamination by arsenic in the residential area of Behala, Calcutta due to industrial pollution. Environ Pollut 80:57–65
Chen C-J, Chuang Y-C, Lin T-M, Wu H-Y (1985) Malignant neoplasms among residents of a blackfoot disease-endemic area in Taiwan: high-arsenic artesian well water and cancers. Cancer Res 45:5895–5899
Chen C, Chuang Y, You S, Lin T, Wu H (1986) A retrospective study on malignant neoplasms of bladder, lung and liver in blackfoot disease endemic area in Taiwan. Br J Cancer 53:399
Chen C-J, Wang S-L, Chiou J-M, Tseng C-H, Chiou H-Y, Hsueh Y-M, Chen S-Y, Wu M-M, Lai M-S (2007) Arsenic and diabetes and hypertension in human populations: a review. Toxicol Appl Pharmacol 222:298–304
Chowdhury TR, Basu GK, Mandal BK, Biswas BK, Samanta G, Chowdhury UK, Chanda CR, Lodh D, Roy SL, Saha KC (1999) Arsenic poisoning in the Ganges delta. Nature 401:545–546
Chowdhury UK, Biswas BK, Chowdhury TR, Samanta G, Mandal BK, Basu GC, Chanda CR, Lodh D, Saha KC, Mukherjee SK (2000) Groundwater arsenic contamination in Bangladesh and West Bengal, India. Environ Health Perspect 108:393
Coronado-González JA, Del Razo LM, García-Vargas G, Sanmiguel-Salazar F, Escobedo-de la Peña J (2007) Inorganic arsenic exposure and type 2 diabetes mellitus in Mexico. Environ Res 104:383–389
Das D, Samanta G, Mandal BK, Chowdhury TR, Chanda CR, Chowdhury PP, Basu GK, Chakraborti D (1996) Arsenic in groundwater in six districts of West Bengal, India. Environ Geochem Health 18:5–15
Das N, Paul S, Chatterjee D, Banerjee N, Majumder NS, Sarma N, Sau TJ, Basu S, Banerjee S, Majumder P (2012) Arsenic exposure through drinking water increases the risk of liver and cardiovascular diseases in the population of West Bengal, India. BMC Public Health 12:1
Das D, Bindhani B, Mukherjee B, Saha H, Biswas P, Dutta K, Prasad P, Sinha D, Ray MR (2014) Chronic low-level arsenic exposure reduces lung function in male population without skin lesions. Int J Public Health 59:655–663
Datta D (1976) Arsenic and non-cirrhotic portal hypertension. Lancet 307:433
De B, Mazumdar D, Sen S, Guru S, Kundu S (2004) Pulmonary involvement in chronic arsenic poisoning from drinking contaminated ground water. JAPI 52:395–400
Garai R, Chakraborty A, Dey S, Saha K (1984) Chronic arsenic poisoning from tube-well water. J Indian Med Assoc 82:34–35
Ghosh A (2013) Evaluation of chronic arsenic poisoning due to consumption of contaminated ground water in West Bengal, India. Int J Prev Med 4:976
Goswami R, Rahman MM, Murrill M, Sarma KP, Thakur R, Chakraborti D (2014) Arsenic in the groundwater of Majuli: the largest river island of the Brahmaputra—magnitude of occurrence and human exposure. J Hydrol 518:354–362
Goyenechea M (1917) Sobre la nueva enfermedad descubierta en Bell-Ville [On the new disease discovered in Bell-Ville]. Rev Med Rosario 7:485
Guha Mazumder D, Purkayastha I, Ghose A, Mistry G, Saha C, Nandy AK, Das A, Majumdar KK (2012) Hypertension in chronic arsenic exposure: a case control study in West Bengal. J Environ Sci Health A 47:1514–1520
Halder D, Biswas A, Šlejkovec Z, Chatterjee D, Nriagu J, Jacks G, Bhattacharya P (2014) Arsenic species in raw and cooked rice: implications for human health in rural Bengal. Sci Total Environ 497:200–208
Harvey CF, Swartz CH, Badruzzaman A, Keon-Blute N, Yu W, Ali MA, Jay J, Beckie R, Niedan V, Brabander D (2002) Arsenic mobility and groundwater extraction in Bangladesh. Science 298:1602–1606
Heroy DC, Kuehl SA, Goodbred SL (2003) Mineralogy of the Ganges and Brahmaputra Rivers: implications for river switching and Late Quaternary climate change. Sediment Geol 155:343–359
Hossain MA, Rahman MM, Murrill M, Das B, Roy B, Dey S, Maity D, Chakraborti D (2013) Water consumption patterns and factors contributing to water consumption in arsenic affected population of rural West Bengal, India. Sci Total Environ 463:1217–1224
IARC (2004) Some drinking-water disinfectants and contaminants, including arsenic. Working Group on the Evaluation of Carcinogenic Risks to Humans. World Health Organization. International Agency for Research on Cancer, Lyon, France
IARC (2012) Evaluation of carcinogenic risks to human: arsenic, metals, fibres, and dusts, vol 100 C—a review of human carcinogens. IARC, Lyon, France, pp 11–465
IPCS (2001) Environmental health criteria 224 arsenic and arsenic compounds. World Health Organization, Geneva
Islam FS, Gault AG, Boothman C, Polya DA, Charnock JM, Chatterjee D, Lloyd JR (2004) Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature 430:68–71
Kapaj S, Peterson H, Liber K, Bhattacharya P (2006) Human health effects from chronic arsenic poisoning: a review. J Environ Sci Health A 41:2399–2428
Kumar P, Kumar M, Ramanathan A, Tsujimura M (2010) Tracing the factors responsible for arsenic enrichment in groundwater of the middle Gangetic Plain, India: a source identification perspective. Environ Geochem Health 32:129–146
Lewis DR, Southwick JW, Ouellet-Hellstrom R, Rench J, Calderon RL (1999) Drinking water arsenic in Utah: a cohort mortality study. Environ Health Perspect 107:359
Lu T-H, Su C-C, Chen Y-W, Yang C-Y, Wu C-C, Hung D-Z, Chen C-H, Cheng P-W, Liu S-H, Huang C-F (2011) Arsenic induces pancreatic β-cell apoptosis via the oxidative stress-regulated mitochondria-dependent and endoplasmic reticulum stress-triggered signaling pathways. Toxicol Lett 201:15–26
Majumdar K, Mazumder DG, Ghose N, Ghose A, Lahiri S (2009) Systemic manifestations in chronic arsenic toxicity in absence of skin lesions in West Bengal. Indian J Med Res 129:75
Makris KC, Christophi CA, Paisi M, Ettinger AS (2012) A preliminary assessment of low level arsenic exposure and diabetes mellitus in Cyprus. BMC Public Health 12:1–8
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58:201–235
Mazumder D, Gupta JD, Chakraborty A, Chatterjee A, Das D, Chakraborti D (1992) Environmental pollution and chronic arsenicosis in south Calcutta. Bull World Health Organ 70:481
Mazumder D, Das GJ, Santra A, Pal A, Ghose A, Sarkar S (1998) Chronic arsenic toxicity in west Bengal: the worst calamity in the world. J Indian Med Assoc 96(4–7):18
Mazumder DG, Ghosh N, De BK, Santra A, Das S, Lahiri S, Haque R, Smith AH, Chakraborti D (2001) Epidemiological study on various noncarcinomatous manifestations of chronic arsenic toxicity in a district of West Bengal. In: Chappell WR, Abernathy CO, Calderon RL (eds) Arsenic exposure and health effects. Elsevier, Amsterdam, pp 153–164
Mazumder DNG, Steinmaus C, Bhattacharya P, von Ehrenstein OS, Ghosh N, Gotway M, Sil A, Balmes JR, Haque R, Hira-Smith MM (2005) Bronchiectasis in persons with skin lesions resulting from arsenic in drinking water. Epidemiology 16:760–765
Medrano MJ, Boix R, Pastor-Barriuso R, Palau M, Damián J, Ramis R, Del Barrio JL, Navas-Acien A (2010) Arsenic in public water supplies and cardiovascular mortality in Spain. Environ Res 110:448–454
Milton AH, Hasan Z, Shahidullah S, Sharmin S, Jakariya M, Rahman M, Dear K, Smith W (2004) Association between nutritional status and arsenicosis due to chronic arsenic exposure in Bangladesh. Int J Environ Health Res 14:99–108
Mukherjee AB, Bhattacharya P (2001) Arsenic in groundwater in the Bengal Delta Plain: slow poisoning in Bangladesh. Environ Rev 9:189–220
Mukherjee SC, Rahman MM, Chowdhury UK, Sengupta MK, Lodh D, Chanda CR, Saha KC, Chakraborti D (2003) Neuropathy in arsenic toxicity from groundwater arsenic contamination in West Bengal, India. J Environ Sci Health A 38:165–183
Mukherjee SC, Saha KC, Pati S, Dutta RN, Rahman MM, Sengupta MK, Ahamed S, Lodh D, Das B, Hossain MA (2005) Murshidabad: one of the nine groundwater arsenic-affected districts of West Bengal, India, part II—dermatological, neurological, and obstetric findings. Clin Toxicol 43:835–848
Murcott S (2012) Arsenic contamination in the world: an international sourcebook 2012. IWA, London
Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, Suk WA (2013) The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect 121(3):295–302. doi:10.1289/ehp.1205875
Nayak B, Das B, Chandra Mukherjee S, Pal A, Ahamed S, Amir Hossain M, Maity P, Dutta RN, Dutta S, Chakraborti D (2008) Groundwater arsenic contamination in the Sahibganj district of Jharkhand state, India in the middle Ganga plain and adverse health effects. Toxicol Environ Chem 90:673–694
Nickson R, McArthur J, Burgess W, Ahmed KM, Ravenscroft P, Rahmann M (1998) Arsenic poisoning of Bangladesh groundwater. Nature 395:338–338
Nickson R, McArthur J, Ravenscroft P, Burgess W, Ahmed K (2000) Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Appl Geochem 15:403–413
NRC (1999) Arsenic in drinking water. National Research Council,, Washington, DC
NRC (2001) Arsenic in drinking water. National Research Council, Washington, DC
Osborn K (2012) Shout it out loud: arsenic—your customers need to understand the dangers of arsenic. Water Technol 2012, Nov 7, NRC, Washington, DC
Rahman M, Tondel M, Ahmad SA, Axelson O (1998) Diabetes mellitus associated with arsenic exposure in Bangladesh. Am J Epidemiol 148:198–203
Rahman MM, Chowdhury UK, Mukherjee SC, Mondal BK, Paul K, Lodh D, Biswas BK, Chanda CR, Basu GK, Saha KC (2001) Chronic arsenic toxicity in Bangladesh and West Bengal, India: a review and commentary. J Toxicol Clin Toxicol 39:683–700
Rasheed H, Slack R, Kay P (2016) Human health risk assessment for arsenic: a critical review. Crit Rev Environ Sci Technol. doi:10.1080/10643389.2016.1245551
Ravenscroft P, McArthur J, Hoque B (2001) Geochemical and palaeohydrological controls on pollution of groundwater by arsenic. Arsenic Exposure Health Eff IV:53–77
Sekhar KC, Chary N, Kamala C, Rao JV, Balaram V, Anjaneyulu Y (2003) Risk assessment and pathway study of arsenic in industrially contaminated sites of Hyderabad: a case study. Environ Int 29:601–611
Simeonova PP, Luster MI (2004) Arsenic and atherosclerosis. Toxicol Appl Pharmacol 198:444–449
Singh B, Goswami R (2011) Influence of landform and geomorphic process on topographic evolution of a river island. Int J Eng Sci Technol 2011:5562–5571
Smedley P, Kinniburgh D (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Smith AH, Lopipero PA, Bates MN, Steinmaus CM (2002) Arsenic epidemiology and drinking water standards. Science 296:2145–2146
SOES (2010) Are Millions in Ganga-Meghna-Brahmaputra Plain already exposed to arsenic contaminated water potentially at risk from cancer? A preliminary follow up study on arsenic patients registered during 1995 to 2000. School of Environmental Studies, Jadavpur University, Kolkata, India and Dhaka Community Hospital, Bangladesh
Srivastava S, Chen Y, Barchowsky A (2009) Arsenic and cardiovascular disease. Toxicol Sci 107:312–323
Tchounwou PB, Patlolla AK, Centeno JA (2003) Carcinogenic and systemic health effects associated with arsenic exposure: a critical review. Toxicol Pathol 31:575–588
Tsai S-M, Wang T-N, Ko Y-C (1999) Mortality for certain diseases in areas with high levels of arsenic in drinking water. Arch Environ Health 54:186–193
Tseng C-H, Tai T-Y, Chong C-K, Tseng C-P, Lai M-S, Lin BJ, Chiou H-Y, Hsueh Y-M, Hsu K-H, Chen C-J (2000) Long-term arsenic exposure and incidence of non-insulin-dependent diabetes mellitus: a cohort study in arseniasis-hyperendemic villages in Taiwan. Environ Health Perspect 108:847
von Ehrenstein O, Mazumder DG, Hira-Smith M, Ghosh N, Yuan Y, Windham G, Ghosh A, Haque R, Lahiri S, Kalman D (2006) Pregnancy outcomes, infant mortality, and arsenic in drinking water in West Bengal, India. Am J Epidemiol 163:662–669
von Ehrenstein OS, Poddar S, Yuan Y, Mazumder DG, Eskenazi B, Basu A, Hira-Smith M, Ghosh N, Lahiri S, Haque R (2007) Children’s intellectual function in relation to arsenic exposure. Epidemiology 18:44–51
Wang C-H, Hsiao CK, Chen C-L, Hsu L-I, Chiou H-Y, Chen S-Y, Hsueh Y-M, Wu M-M, Chen C-J (2007) A review of the epidemiologic literature on the role of environmental arsenic exposure and cardiovascular diseases. Toxicol Appl Pharmacol 222:315–326
Acknowledgements
We thank all participants involved in this study for their extensive cooperation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the office of the Principal and Chairman, Institutional Ethics Committee (IEC) Medical College Kolkata, India and the Ethics committee, Jadavpur University, Kolkata, and informed consent was obtained from study participants.
Additional information
Published in the special issue “Hydrogeology and Human Health”
Rights and permissions
About this article
Cite this article
Chakraborti, D., Rahman, M.M., Das, B. et al. Groundwater arsenic contamination and its health effects in India. Hydrogeol J 25, 1165–1181 (2017). https://doi.org/10.1007/s10040-017-1556-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10040-017-1556-6