Abstract
Objective
To establish baseline T2* values in healthy knee joint cartilage at 3 T.
Materials and Methods
Thirty-four volunteers (mean age: 24.6 ± 2.7 years) with no history or clinical findings indicative of any knee joint disease were enrolled. The protocol included a double-echo steady-state (DESS) sequence for morphological cartilage evaluation and a gradient-echo multi-echo sequence for T2* assessment. Bulk and zonal T2* values were assessed in eight regions: posterior lateral femoral condyle; central lateral femoral condyle; trochlea; patella; lateral tibial plateau; posterior medial femoral condyle; central medial femoral condyle; and medial tibial plateau. Statistical evaluation comprised a two-tailed t test and a one-way analysis of variance to identify zonal and regional differences.
Results
T2* mapping revealed higher T2* values in the superficial zone in all regions (P values ≤ 0.001) except for the posterior medial femur condyle (P = 0.087), and substantial regional differences demonstrating superior values in trochlear cartilage, intermediate values in patellar and central femoral condylar cartilage, and low T2* values in posterior femoral condylar cartilage and tibial plateau cartilage.
Conclusion
Substantial regional differences in T2* measures should be taken into consideration when conducting T2* mapping of knee joint cartilage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA), which is characterized by structural and biochemical cartilage abnormalities, may eventually become symptomatically and functionally debilitating [1]. Therefore, with hope and expectations for early intervention, there is a growing interest in reliable biomarkers sensitive to these structural and biochemical changes at the various stages of the disease [2].
Biochemically sensitive magnetic resonance imaging (MRI) techniques, such as delayed gadolinium-enhanced MRI (dGEMRIC) and T2/T2* mapping, that are sensitive to alterations in the collagen fiber network, water content, and glycosaminoglycan (GAG) content, which occur early in the course of OA, have the potential to provide robust biomarkers for disease onset and progression, and therefore, may be meaningful assessment tools for the diagnosis, treatment, and follow-up of cartilage abnormalities [3].
Similar to the T2 mapping technique, T2* mapping reflects water content and interactions between water molecules and collagen fibers in which high T2 or T2* values are indicators of a high water content with superior water molecule mobility. Therefore, in healthy articular cartilage, there is a decrease in the T2 and T2* values towards the deep cartilage zones, where the uniform perpendicular collagen fiber orientation and high glycosaminoglycan content promote water molecule restriction and T2 decay [4]. In keeping with this characteristic feature, T2* mapping may be treated as a valuable instrument for visualizing the physiological anisotropy of articular cartilage and cartilage repair tissue [5–7].
Although several in vitro and in vivo studies have confirmed the potential of T2* mapping for assessing articular cartilage [5–17], the spectrum of normative T2* values in various regions of the knee joint, which is critical for differentiating between “normal” and “abnormal”, has not been investigated.
The purpose of this prospective study was to obtain baseline T2* values of healthy knee joint cartilage at 3 T. Therefore, we performed a cross-sectional analysis with a cohort of healthy, asymptomatic, young adult volunteers with no suspected cartilage abnormalities. Furthermore, to explore if there were zonal and regional differences in the T2* values due to topographical variations in MRI parameters or biochemical composition, a region of interest (ROI) analysis was conducted.
Materials and methods
This study was performed in compliance with the regulations of the local ethics committee and institutional review board approval was obtained prior to the study.
Study population
Thirty-five healthy asymptomatic volunteers with no history or clinical findings indicative of any knee joint disorder were enrolled in this study. The study population included 22 men and 13 women with a mean age of 24.5 ± 2.6 years (range: 21–31 years). Subjects older than 35 years of age and those who were engaged in high level sports were not included in this study to reduce the age effects [18] and the confounding selection bias of undiagnosed cartilage damage in these cases. The nature of the procedure was thoroughly explained and all participants provided written informed consent prior to participation. Furthermore, all participants were instructed to adhere to normal physical activity levels on the day of the MRI. Demographic data of the participants, including age, height, and weight were collected on the day of imaging and all volunteers were screened for the exclusion criteria. Subjects were randomized for MRI of either of the knees. Thus, 18 right and 17 left knees were imaged. Collection of clinical findings and volunteer history was supervised by an orthopedic consultant with 8 years of experience in knee joint surgery.
Because MRI was conducted on a clinical scanner that was used for busy daily routine scanning, the majority of study participants underwent imaging at weekends in a consecutive fashion. Therefore, this study did not allow controlling for potential diurnal variation in the characteristics of articular cartilage [19].
Magnetic resonance imaging
Magnetic resonance imaging was performed with a 3-T scanner (Magnetom Trio; Siemens Medical Solutions, Erlangen, Germany) using an eight-channel transmit/receive knee array coil (In Vivo, Gainesville, FL, USA). All subjects were examined in the supine position with the knee joint in neutral rotation and extended in the center of the coil. The knee joint was carefully stabilized by sponges and adjustable straps to avoid motion artifacts.
The MRI protocol included a three-dimensional (3D) double-echo steady-state (DESS) sequence with water excitation for morphological cartilage assessment and a 3D multi-echo data image combination (MEDIC) sequence using six consecutive echoes for T2* mapping. T2* mapping was performed after ∼ 30 min of rest to minimize the effects of preceding physical activities on MR relaxation times of cartilage [20].
Both DESS and MEDIC delivered isotropic high-resolution 3D image data sets that facilitated retrospective high-quality multi-planar reformatting (MPR). Sequence setting and subsequent data analysis were specifically adjusted for superior cartilage image quality with high cartilage contrast to warrant optimal cartilage delineation rather than evaluation of multiple articular structures and features relevant to knee joint impairment and knee OA, which necessitates further pulse sequences in coronal, sagittal, and axial planes [21, 22]. Details on the imaging parameters used in this study are provided in Table 1.
Image analysis
The T2* maps were derived using an inline processing package (SyngoMapIt, Siemens Medical Solutions, Erlangen, Germany), which employs a non-linear least-squares curve-fitting algorithm. Image analysis was performed with a Leonardo® workstation (Siemens Medical Solutions, Erlangen, Germany).
From each 3D data set, sagittal reformats with a slice thickness of 1 mm parallel to the long axis of the femoral diaphysis and perpendicular to a line tangent to the posterior cortices of the femoral condyles were generated by using MPR (Figs. 1, 2). The reformats obtained were evaluated for any knee joint abnormality and artifacts. Of those, mid-sagittal reformats through the medial and lateral tibiofemoral joints and through the femoropatellar joint, which displayed the largest amount of cartilage in these regions, were selected to assess the medial, lateral, and patellofemoral compartments of each knee. This enabled us to assess knee joint cartilage of various regions in a large sample size within a reasonable amount of time with reasonable accuracy.
As performed previously [23, 24], knee joint cartilage was assessed in eight regions:
-
1.
Posterior lateral femoral condyle
-
2.
Central lateral femoral condyle
-
3.
Trochlea
-
4.
Patella
-
5.
Lateral tibial plateau
-
6.
Posterior medial femoral condyle
-
7.
Central medial femoral condyle
-
8.
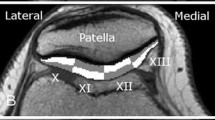
Medial tibial plateau (Fig. 3).
Fig. 3 Cartilage regions of interest representing a the patellofemoral and lateral tibiofemoral joint and b the medial tibiofemoral joint. The anterior and posterior margins of the menisci were used as landmarks in which the anterior margin of the meniscus separated the trochlea from the central femoral cartilage and the posterior margin of the meniscus separated the central femoral cartilage from the posterior femoral condyle
Bulk (full-thickness) and zonal (the zone adjacent to the subchondral bone is the deep zone and the zone adjacent to the articular surface is the superficial zone) T2* values (in milliseconds, ms) were then obtained in these cartilage regions by undertaking an ROI analysis (Figs. 4, 5). Each ROI was defined by multiple marker points that facilitated accurate T2* assessment even in curved cartilage regions, keeping volume averaging at both the cartilage/bone interface and the cartilage/fluid interface at a minimum.
Bulk cartilage T2* relaxation time assessment by means of region of interest (ROI) analysis in a DESS and b corresponding color-coded T2* reformats. Each ROI was defined by multiple marker points for accurate T2* assessment even in curved cartilage regions, keeping volume averaging and chemical shift artifacts at both the cartilage/bone interface and the cartilage/fluid interface at a minimum. The DESS reformats served as a reference for accurate placement of the ROI squares within the cartilage
The morphological cartilage evaluation was performed by two musculoskeletal radiologists with 7 and 12 years of experience to identify and exclude knee joints with morphologically evident cartilage damage. The T2* data collection was performed by two orthopedic surgeons with a special interest in biochemical imaging and 7 years of segmenting experience. One of these observers performed the T2* mapping in all subjects, while (for purposes of intra- and inter-reader reliability assessment for T2* analysis of knee joint cartilage) both observers repeated the T2* mapping in designated reformats in ten randomly selected knee joints. Both these observers were blinded to each other’s interpretations and observer 1 was specifically blinded to his first set of observations. The two sets of readings for observer 1 were performed more than 4 weeks apart.
Statistical analyses
In this study, IBM SPSS software (version 21.0; IBM Corp., Armonk, NY, USA) was used for statistical analyses. T2* values for different zones and regions of knee joint cartilage are reported as mean ± standard deviations (SDs). A two-tailed t test was used to identify zonal differences in the T2* values, while regional differences (bulk T2* values) were assessed pair-wise using a one-way analysis of variance (ANOVA) with Bonferroni correction for multiple comparisons. Intra- and inter-observer agreement was calculated by intra-class correlation (ICC) analysis (pair-wise correlation, absolute agreement). P values below 0.05 were considered to be statistically significant.
Results
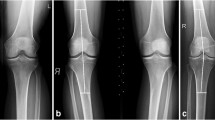
Double-echo steady-state imaging and T2* relaxation time mapping for knee joint cartilage assessment was successfully performed in all 35 volunteers. One volunteer was excluded from further analysis as morphological cartilage assessment revealed evident cartilage damage in this participant, although he was clinically asymptomatic (Fig. 6). Therefore, a total of 816 ROIs (34 volunteers, 8 regions per joint, 3 zones [superficial, deep, and bulk] per region) underwent assessment. The mean size of these ROIs was 0.54 ± 0.25 cm2 (148 ± 68 pixels), ranging from 0.11 cm2 (30 pixels) to 1.74 cm2 (480 pixels).
a, b Double-echo steady-state (DESS) and corresponding color-coded T2* reformats revealing a focal full-thickness retropatellar cartilage lesion (white arrows) in one asymptomatic volunteer. c, d Adjacent cartilage irregularities are clearly depicted on the T2* image while signal changes demonstrated on the DESS image are fairly undefined
Region of interest (ROI) analysis revealed higher T2* values in the superficial cartilage zones compared with those in the deep zones, which were, except for the posterior medial femoral condyle (P value = 0.087), statistically significant in all regions (P values ≤ 0.001; Table 2). There were also substantial regional differences in the T2* measurements, demonstrating superior values in trochlear cartilage, intermediate values in patellar and central femoral condylar cartilage, and low values in posterior femoral condylar cartilage and tibial plateau cartilage (Fig. 7). Notably, T2* values in the central femoral condylar cartilage regions were higher than those in the posterior femoral condylar cartilage regions, whereas cartilage T2* values in the medial and lateral knee joint compartments were similar (Table 3). ICC analysis proved high intra-reader reliability (ICC value = 0.934, P value < 0.001) and high inter-reader reliability (ICC = 0.886, P value < 0.001) for the T2* analysis in this study.
Discussion
This cross-sectional study of knee joint cartilage of asymptomatic young adult volunteers with no obvious clinical and imaging suspicion of cartilage abnormalities revealed a spatial distribution of cartilage T2* that has to be taken into consideration when conducting T2* mapping of knee joint cartilage, because changes in T2* values may be related to cartilage damage or physiological variations in the intrinsic cartilage structure.
Several studies utilizing T2* mapping in the evaluation of articular cartilage have been published [5–17]. As far as the evaluation of healthy knee joint cartilage is concerned, five previously reported studies do provide substantial contributions [5–7, 15, 16]. In a study on three T2 phantoms with altered T2 values and 10 healthy knee joints at 3 T, Pai et al. evaluated a T2* mapping technique based on 3D spoiled gradient recalled (SPGR) acquisition and compared the results with currently available two-dimensional sequences [16]. In their sample of 10 healthy volunteers, a mean bulk T2* value of 31.6 ± 4.5 ms for patellar cartilage was noted, which is similar to our results in the patellar region (mean T2* value: 28.3 ± 3.5 ms).
In a feasibility study on in vivo dGEMRIC, T2 and T2* relaxation measurements at 7 T that included 12 healthy volunteers (mean age 26.7 ± 3.4 years) and 4 patients (mean age 38.0 ± 14.0 years), Welsch et al. observed similar T2* values for healthy femoral and patellar cartilage (mean T2* value: 19.7 ± 6.4 ms versus 19.6 ± 5.2 ms), but lower values for tibial cartilage (mean T2* value: 16.6 ± 5.6 ms) [6]. Furthermore, all healthy cartilage regions revealed a significant increase from deep to superficial cartilage (P value < 0.001). Our findings closely reflect their observations, with higher T2* values in femoral condyle cartilage compared with T2* values in tibial plateau cartilage, and higher values at the superior cartilage zones.
The same study group also reported cartilage T2 and T2* mapping at 3 T after matrix-associated autologous chondrocyte transplantation (MACT) treatment of middle to large full-thickness cartilage defects located on the femoral condyle in 30 patients (mean age 34.6 ± 9.9 years) [7]. ROI analysis was performed for full-thickness and for deep and superficial aspects of the cartilage repair tissue. Ipsilateral cartilage sites with morphologically normal cartilage were used as internal controls. In this study, the mean T2* value for control cartilage was significantly higher than that of repair cartilage (mean T2* value: 30.9 ± 6.6 ms versus 24.5 ± 8.1 ms, P value < 0.001). Notably, the zonal stratification (lower T2/T2* values at the deep zone) was higher for the T2* parameter, which can be related to the additional contribution of microscopic susceptibility fields at the cartilage/bone interface. The T2* values in the study by Welsch et al. closely resemble our results in weight-bearing femoral cartilage. However, the risk of confounding bias because of potentially undiagnosed cartilage damage has to be considered in this study sample of patients.
Mamisch et al., who performed T2 and T2* mapping in weight-bearing femoral condylar cartilage of 15 healthy volunteers (mean age 27.4 ± 4.9 years) and 15 patients (mean age 39.6 ± 13.7 years) after micro-fracture (MFX) therapy at 3 T, noted a significant correlation between the two relaxation measures (Pearson coefficient = 0.828, P value < 0.001) and a zonal dependency with higher T2 and T2* values in the superior cartilage zone in the control group of healthy volunteers [5]. In contrast, no spatial variation was observed for cartilage repair tissue after MFX. The reported full-thickness T2* values (mean T2* value 22.6 ± 3.8 ms) for femoral condylar cartilage were substantially lower in their study compared with corresponding T2* values in our study. Differences in the time span between unloading and T2* mapping are a possible cause of this discrepancy. However, this aspect is not elucidated in the report by Mamisch et al. [5].
Newbould et al. have reported their observations on reproducibility and cross-sectional differences in T2* mapping of knee joint cartilage at 3 T in 33 patients with OA and 21 asymptomatic age-matched controls [15]. In their study, the high reproducibility of T2* mapping of knee joint cartilage was confirmed in 13 OA patients (mean age 64.4 ± 10.3 years) and 5 asymptomatic controls (mean age: 61.6 ± 8.7 years). Twenty OA patients (mean age 63.5 ± 9.2 years) and 16 controls (mean age 61.3 ± 6.9 years) were included in the cross-sectional analysis. ROI analysis was performed in various regions through the knee joint, including medial and lateral femoral cartilage, medial and lateral tibial cartilage, and patellar cartilage. Similar to our observations, regional T2* alterations in control cartilage (example: higher values in patellar cartilage [mean T2* value 25.3 ± 1.88 ms] than those in medial tibial cartilage [mean T2* value 17.1 ± 1.13 ms]) were noted. However, the control T2* values in this study were derived from age-matched asymptomatic subjects in their 60s, with some inadvertent possibility of cartilage degeneration. High Kellgren–Lawrence scores [25] indicative of joint space narrowing point toward this explanation. Therefore, these values may not be used as a reference for healthy cartilage.
This study has its limitations. We did not control for loading conditions and diurnal variations in articular cartilage. Therefore, a certain degree of inconsistency in the T2* measurements must be taken into account as physiological loading can alter T2* relaxation times for articular cartilage (example: decrease in T2* due to the exudation of water from cartilage and derangement of the collagen fiber network under compression) [26]. This also applies for the magic angle effect that is characterized by increasing T2* values as the collagen fibers approach an angle of ∼ 55° to the main magnetic field [27]. On the other hand, the methodology applied in this study closely reflects the circumstances in daily clinical routine, and hence, we believe that these normative data on T2* for knee joint cartilage are robust and clinically relevant. Furthermore, having assessed the same regions in each individual under standard conditions, the influence of the magic angle effect on the T2* values in articular cartilage is likely uniform for all subjects.
Image generation and image segmentation for undertaking ROI analyses in this study were performed manually, which is certainly operator-dependent and time-consuming. Automated image registration systems may be useful, in particular, in longitudinal/follow-up studies to compare cartilage T2* values at different time points. Notably, in this work ROI analysis took about 20 min per knee and proved to be reliable. In this study, the cartilage T2* was assessed in only one mid-sagittal reformat per knee joint compartment that included the area with the thickest cartilage in these regions. As demonstrated in previous studies [28–30], this method bears the risk of confounding selection bias related to an heterogeneous cartilage T2* distribution within these compartments. The rationale for our methodology was based on the high prevalence of cartilage damage in these main load-bearing regions and the intention to assess knee joint cartilage of various regions in a reasonable amount of time with equitable accuracy.
Our study sample was relatively small. This may have led to statistical power issues in some parts of this comparative study (example: trend of higher T2* values in the superficial zone compared with the deep zone for the posterior medial femoral condyle, which was borderline non-significant, P value = 0.087). Another limitation is that only young adults were enrolled in this study to keep age effects and the risk of undiagnosed cartilage damage to a minimum. Although we believe that this age group would obtain the greatest benefit from early cartilage damage diagnosis and corresponding intervention, the T2* values obtained in this study may not be representative for an older control group.
This study lacks surgical or histological confirmation of the cartilage status, which obviously cannot be performed for ethical reasons. Thus, it is possible that we have missed regions with cartilage degeneration, which could explain the outliers of T2* in this study.
Finally, there is also a theoretical possibility of variations in reference values based on the MRI scanner or sequence, which needs to be considered while using this information as normative data.
In conclusion, this study noted substantial regional differences in T2* values while mapping knee joint cartilage in asymptomatic healthy young adults. These regional differences (either based on physiological variations or functional demand) not only provide normative data for longitudinal studies but may have relevance for therapeutic strategies in various clinical scenarios.
References
Altman RD. The syndrome of osteoarthritis. J Rheumatol. 1997;24(4):766–7.
Burstein D, Gray M, Mosher T, Dardzinski B. Measures of molecular composition and structure in osteoarthritis. Radiol Clin N Am. 2009;47(4):675–86.
Crema MD, Roemer FW, Marra MD, Burstein D, Gold GE, Eckstein F, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics 2011;31(1):37–61.
Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8(4):355–68.
Mamisch TC, Hughes T, Mosher TJ, Mueller C, Trattnig S, Boesch C, et al. T2 star relaxation times for assessment of articular cartilage at 3 T: a feasibility study. Skeletal Radiol. 2012;41(3):287–92.
Welsch GH, Mamisch TC, Hughes T, Zilkens C, Quirbach S, Scheffler K, et al. In vivo biochemical 7.0 Tesla magnetic resonance: preliminary results of dGEMRIC, zonal T2, and T2* mapping of articular cartilage. Invest Radiol. 2008;43(9):619–26.
Welsch GH, Trattnig S, Hughes T, Quirbach S, Olk A, Blanke M, et al. T2 and T2* mapping in patients after matrix-associated autologous chondrocyte transplantation: initial results on clinical use with 3.0-Tesla MRI. Eur Radiol. 2010;20(6):1515–23.
Bittersohl B, Hosalkar HS, Hughes T, Kim YJ, Werlen S, Siebenrock KA, et al. Feasibility of T2* mapping for the evaluation of hip joint cartilage at 1.5 T using a three-dimensional (3D), gradient-echo (GRE) sequence: a prospective study. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of. Magn Reson Med. 2009;62(4):896–901.
Bittersohl B, Miese FR, Dekkers C, Senyurt H, Kircher J, Wittsack HJ, et al. T2* mapping and delayed gadolinium-enhanced magnetic resonance imaging in cartilage (dGEMRIC) of glenohumeral cartilage in asymptomatic volunteers at 3 T. Eur Radiol. 2013;23(5):1367–74.
Bittersohl B, Miese FR, Hosalkar HS, Herten M, Antoch G, Krauspe R, et al. T2* mapping of hip joint cartilage in various histological grades of degeneration. Osteoarthritis Cartilage. 2012;20(7):653–60.
Bittersohl B, Miese FR, Hosalkar HS, Mamisch TC, Antoch G, Krauspe R, et al. T2* mapping of acetabular and femoral hip joint cartilage at 3 T: a prospective controlled study. Invest Radiol. 2012;47(7):392–7.
Buchbender C, Scherer A, Kropil P, Korbl B, Quentin M, Reichelt D, et al. Cartilage quality in rheumatoid arthritis: comparison of T2* mapping, native T1 mapping, dGEMRIC, DeltaR1 and value of pre-contrast imaging. Skelet Radiol. 2012;41(6):685–92.
Marik W, Apprich S, Welsch GH, Mamisch TC, Trattnig S. Biochemical evaluation of articular cartilage in patients with osteochondrosis dissecans by means of quantitative T2- and T2-mapping at 3 T MRI: a feasibility study. Eur J Radiol. 2012;81(5):923–7.
Miese FR, Zilkens C, Holstein A, Bittersohl B, Kropil P, Mamisch TC, et al. Assessment of early cartilage degeneration after slipped capital femoral epiphysis using T2 and T2* mapping. Acta Radiol. 2011;52(1):106–10.
Newbould RD, Miller SR, Toms LD, Swann P, Tielbeek JA, Gold GE, et al. T2* measurement of the knee articular cartilage in osteoarthritis at 3 T. J Magn Reson Imaging. 2012;35(6):1422–9.
Pai A, Li X, Majumdar S. A comparative study at 3 T of sequence dependence of T2 quantitation in the knee. Magn Reson Imaging. 2008;26(9):1215–20.
Williams A, Qian Y, Chu CR. UTE-T2 * mapping of human articular cartilage in vivo: a repeatability assessment. Osteoarthritis Cartilage. 2011;19(1):84–8.
Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2–preliminary findings at 3 T. Radiology. 2000;214(1):259–66.
Waterton JC, Solloway S, Foster JE, Keen MC, Gandy S, Middleton BJ, et al. Diurnal variation in the femoral articular cartilage of the knee in young adult humans. Magn Reson Med. 2000;43(1):126–32.
Liess C, Lusse S, Karger N, Heller M, Gluer CC. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage. 2002;10(12):907–13.
Crema MD, Roemer FW, Marra MD, Guermazi A. Magnetic resonance imaging assessment of subchondral bone and soft tissues in knee osteoarthritis. Rheum Dis Clin N Am. 2009;35(3):557–77.
Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433–41.
Stehling C, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM. Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3 T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage. 2010;18(6):776–86.
Stehling C, Liebl H, Krug R, Lane NE, Nevitt MC, Lynch J, et al. Patellar cartilage: T2 values and morphologic abnormalities at 3.0-T MR imaging in relation to physical activity in asymptomatic subjects from the osteoarthritis initiative. Radiology. 2010;254(2):509–20.
Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502.
Rubenstein JD, Kim JK, Henkelman RM. Effects of compression and recovery on bovine articular cartilage: appearance on MR images. Radiology. 1996;201(3):843–50.
Xia Y. Magic-angle effect in magnetic resonance imaging of articular cartilage: a review. Invest Radiol. 2000;35(10):602–21.
Clark JM. Variation of collagen fiber alignment in a joint surface: a scanning electron microscope study of the tibial plateau in dog, rabbit, and man. J Orthop Res. 1991;9(2):246–57.
Goodwin DW, Wadghiri YZ, Zhu H, Vinton CJ, Smith ED, Dunn JF. Macroscopic structure of articular cartilage of the tibial plateau: influence of a characteristic matrix architecture on MRI appearance. AJR Am J Roentgenol. 2004;182(2):311–8.
Shiomi T, Nishii T, Nakata K, Tamura S, Tanaka H, Yamazaki Y, et al. Three-dimensional topographical variation of femoral cartilage T2 in healthy volunteer knees. Skelet Radiol. 2013;42(3):363–70.
Acknowledgements
This study was funded by a research grant from the “German Research Foundation” (Deutsche Forschungsgemeinschaft). The authors have full control of all primary data.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bittersohl, B., Hosalkar, H.S., Sondern, M. et al. Spectrum of T2* values in knee joint cartilage at 3 T: a cross-sectional analysis in asymptomatic young adult volunteers. Skeletal Radiol 43, 443–452 (2014). https://doi.org/10.1007/s00256-013-1806-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-013-1806-1