Abstract
Purpose
To assess the relationship between knee alignment and T2 values of femorotibial cartilage and menisci in healthy subjects at 3 T.
Materials and methods
Thirty-six healthy subjects divided into three subgroups of 12 neutral, 12 varus, and 12 valgus alignment of the femorotibial joint were investigated on 3-T MR scanner using a 2D multi-echo turbo spin-echo (TSE) sequence for T2 mapping. Wilcoxon signed-rank test and analysis of covariance (ANCOVA) were performed to determine any statistically significant differences in subregional T2 values of femorotibial cartilage and menisci among the three subgroups of healthy subjects.
Results
Lateral femoral anterior cartilage subregion (52 ± 3 ms, mean ± standard deviation; 53 ± 2 ms) had significantly higher T2 values (p < 0.05) than medial femoral anterior cartilage subregion (51 ± 2 ms; 51 ± 2 ms) in varus and valgus groups, respectively. There were statistically significant differences (p < 0.05) in T2 values of tibial central cartilage subregion between lateral and medical compartment among varus, valgus, and neutral subgroups. Lateral body segment of meniscus (41 ± 3 ms) had significantly higher (p < 0.05) T2 values than medial body segment (40 ± 2 ms) in the varus subgroup.
Conclusions
Some degree of correlation between knee alignment and subregional T2 values of femorotibial cartilage and menisci exists in healthy subjects. These findings indicate that T2 mapping may be sensitive in assessing the load distribution pattern of human cartilage and menisci with knee alignment abnormality, which may be used as reference baseline when understanding the occurrence and progression of knee osteoarthritis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cartilage and meniscus of diarthrodial joint have various important functions supportive of frictionless motion of the joint, weight distribution, and shock absorption etc. [1]. Knee osteoarthritis (OA) has different etiologies such as joint malalignment, obesity, knee joint trauma or repair, meniscal abnormality, or anterior cruciate ligament (ACL) tears [2,3,4,5,6,7,8,9,10,11]. Varus and valgus malalignment predominantly influences the load distribution pattern of femorotibial cartilage at the human knee joint, which has been shown to be a potential possible risk factor for OA onset and progression [4]. The medial compartment of the femorotibial joint is expected to bear about 60–80% of the compressive load in a knee with neutral alignment [12], though this load distribution is known to change drastically after even minute alterations in lower limb alignment [13].
Articular cartilage consists of roughly 65%–80% water and percentage of the dry weight with approximately 60% collagen and 12% sulfated proteoglycan (PG). Collagen fibrils are predominantly composed of type II collagen. Aggrecan is the major PG in articular cartilage, containing chondroitin sulfate and keratin sulfate (GAG) chains. The menisci of the knee joint have a lower content of PG with roughly 70% water and approximately 60–70% collagen, 2–8% PG of the dry weight. Different than that of articular cartilage, the collagen fibrils of menisci are mainly composed of type I collagen [14,15,16,17]. Prior to the structural changes, the cartilage tissue is subject to molecular modifications within the cartilage matrix. The degeneration or loss of GAGs and breakdown of collagen are typical characterization of early OA [2,3,4].
T2 mapping offers the information concerning the variation of water and collagen content, structure, and organization integrity of cartilage [2, 15, 18]. Damage to the collagen-PG matrix and the elevation of water content in degenerating cartilage have proved to be associated with the elevation of T2 values [15], and increased T2 values have been observed in patients with OA [2, 16]. Importantly, T2 mapping has been thought to be a useful indicator of cartilage degradation and repair tissues following surgical intervention in the knee, hip, and ankle joints [15]. Friedrich et al. [2] evaluated the correlation of T2 mapping of the femorotibial cartilage with knee alignment in OA patients, and found that the T2 values were significantly elevated in the medial knee of patients with varus alignment. Tsai and coworkers [19] confirmed the feasibility of quantitatively measuring the T2 values in the diverse zones of human knee menisci and suggested that zonal and sex differences in the MR T2 values in the posterior horns of the knee menisci in the young healthy population may be relevant to sex differences in the onset of knee OA. More recently, Takao and colleagues [20] also observed that zonal differences existed in T1rho and T2 relaxation times of the normal meniscus.
Although previous studies have used T2 mapping to investigate the variation of water content and collagen integrity in the degenerated or intact femorotibial cartilage and menisci [2, 18,19,20,21,22,23], to our knowledge, such investigations that employ quantitative T2 mapping of subregional femorotibial cartilage and menisci in healthy subjects have not been conducted as yet, especially when combining with different lower-limb alignment to evaluate the articular cartilage and meniscal load distribution pattern. Therefore, the main purpose of this study is to assess the relationship between knee joint alignment and quantitative T2 values of articular cartilage and menisci in healthy subjects. Specifically, our hypothesis is that quantitative T2 mapping of the compartment-specific subregional femorotibial cartilage and menisci might be a potential sensitive biomarker for non-invasively and non-destructively assessing the cartilage and meniscal load distribution pattern resulted from different lower-limb alignment in healthy subjects alone.
Materials and methods
Study population
The content and nature of the research study were fully explained to all the recruited volunteers. All included subjects provided written informed consent to participate in the study, which was approved by the local institutional review board (IRB). For all the recruited healthy subjects, the inclusion criteria were in good health condition according to medical history, physical examination, and the absence of contraindications to MR imaging. Furthermore, for the cohort with healthy asymptomatic knee joint, the inclusion criteria were: no known inflammation, trauma, or pain in the knee, and normal knee radiographs prior to MR scan. Exclusion criteria were inflammatory arthritis, knee OA, and knee OA secondary to some other causes (namely, acute or chronic infection, prior surgery, or previous fracture). An experienced radiologist (J.Z., over 15 years of subspeciality experience) determined whether the subjects had radiographically normal knees, and no subjects included in the study have any abnormal knee MRI findings.
Thirty-six subjects (n = 13 males and n = 23 females, ranging in age from 18 to 65 years, mean ± SD = 30.4 ± 13.4 years) (Table 1) with normal knees based on radiographs were recruited. The subjects’ body height and weight were obtained in order to calculate the body mass index (BMI). Subjects with a BMI of greater than 24.9 kg/m2 were classified as overweight, and subjects with a BMI of greater than 29.9 kg/m2 were classified as obese [2, 9, 23]. The demographics of the recruited subjects are listed in Table 1.
Radiographic analysis
The assessment of anatomical alignment angles for all the recruited healthy subjects were performed by an experienced observer (J.Z., over 15 years of radiological subspeciality experience). To quantify the inter-subject reproducibility of anatomical alignment angles measurement, ten healthy subjects (over 20% of all the study population) with varus alignment were specifically measured once again by another experienced observer (N.H., over 7 years of radiological subspeciality experience) using root mean square coefficient of variation percentage (RMS-CV%).
For the assessment of anatomical alignment, angles were measured on anteroposterior (AP), weight-bearing, short knee radiographs on a Neusoft PACS workstation (Neusoft Corporation, Shenyang, Liaoning, China) as shown in Fig. 1. The femoral and the tibial anatomical axes were obtained by using the approach in [2]. The medial angle of intersection of the femoral and tibial anatomical axes was measured (Fig. 1a, b, c). Knee alignment was considered neutral if the medial angle measured between 182 and 184°, valgus if > 184°, or varus if < 182°, respectively [24, 25].
Representative weight-bearing anteroposterior (AP) radiographs of both knees with alignment measurement made at the left knee obtained from a 22-year old female subject, the measured angle is 184° (neutral, Fig. 1a); the right knee from a 25-year old female subject, the measured angle is 181° (varus, Fig. 1b); and the right knee from an 23-year old female subject, the measured angle is 189° (valgus, Fig. 1c), respectively
MR imaging
All MRI experiments were performed on a 3.0-T clinical MR scanner (Ingenia, Philips, The Netherlands). An 18-cm diameter, eight-channel transmit-receive phased-array (PA) knee coil (Invivo Corp., Gainesville, FL, USA) was employed for all the MR imaging measurements. To limit human subject motion during the acquisitions, the knee was fixed with foam padding.
The following imaging protocol was performed: a sagittal 2D multi-echo turbo spin-echo (TSE) pulse sequence with fat saturation and with a repetition time (TR) of 4000 ms; echo times (TEs) of 16.5, 33, 49.5, 66, and 82.5 ms; a field of view (FOV) of 15 × 15 cm; a matrix of 188 × 185 (interpolated to 256 × 256); a receive bandwidth of 122 Hz/pixel; a number of signal averages (NSA) of 1; an in-plane spatial resolution of 0.59 × 0.59 mm2; and a slice thickness of 2 mm (inter-slice gap = 0). Total acquisition time (TA) for all five echo times (33 slices for each TE) was roughly 18 min and 11 s.
Image analysis
For quantitative T2 mapping assessment, all the MR images were analyzed based on the subregional femorotibial cartilage and meniscus in both lateral and medial compartments.
Twelve subregions for femorotibial cartilage and six subregions for the meniscus were defined in each subject: lateral femoral anterior (LFa), lateral femoral central (LFc), lateral femoral posterior (LFp), medial femoral anterior (MFa), medial femoral central (MFc), medial femoral posterior (MFp), lateral tibial anterior (LTa), lateral tibial central (LTc), lateral tibial posterior (LTp), medial tibial anterior (MTa), medial tibial central (MTc), medial tibial posterior (MTp) for femorotibial cartilage assessment; and lateral anterior (La), lateral central (Lc) [lateral-side body segment], lateral posterior (Lp), medial anterior (Ma), medial central (Mc) [medial-side body segment], medial posterior (Mp) for meniscus assessment, just similar to those defined in the literature [9, 26, 27] and as shown in Fig. 2 and Fig. 3 for the subregional demarcation of femorotibial cartilage and the meniscus.
Representative sagittal fat-saturated T2-weighted 2D turbo spin-echo (TSE) images with echo time (TE) = 16.5 ms illustrating how the regions of interest (ROIs) of cartilage subregions in the lateral (Fig. 2a) and medial (Fig. 2b) compartments were defined, respectively, obtained from a 25-year-old female subject with neutral knee alignment. White lines demarcate the anterior (a), central (c), and posterior (p) subregions of the lateral and medial femoral condyle (LF and MF) and lateral tibial and medial plateau (LT and MT) of the cartilage, respectively
Representative sagittal fat-saturated T2-weighted 2D turbo spin-echo (TSE) images with echo time (TE) = 16.5 ms illustrating how the ROIs of meniscal subregions in the lateral (a, b) and medial (c, d) compartments were defined, respectively, obtained from the same subject as in Fig. 2. White arrowheads point to the location of the meniscal subregions
The in-house coded routines in MATLAB (version 7.1, MathWorks Inc., Natick, MA, USA) and C++ were used for offline processing of the acquired MR images including cartilage and meniscus segmentation, and calculation of subregional T2 values. The signal intensities of the T2-weighted images were fitted on a pixel by pixel basis using a linear least-squares method as shown in Eq. 1 [2, 22, 23]:
where S(TE) is the measured signal intensity of the image at a particular echo time (TE), S0 is the signal intensity at the shortest TE and C is an intercept (i.e., point where the graph of a function or relation intercepts the axis of the coordinate system). For the calculation of the T2 values, the detailed computing process is the same as those in [2, 22, 23]. The ROIs used in this study analyses consisted of the aforementioned subregions of femorotibial cartilage and meniscus. All ROIs were constructed by segmenting these anatomical structures manually (by L.W.) at each slice of the acquired dataset.
The custom-built MATLAB routine ultimately yielded color-coded T2 maps, in which the manually segmented cartilage and meniscal ROIs were overlaid on the shortest echo time (TE = 16.5 ms) images.
Statistical analysis
Statistical analysis was performed using SPSS Statistics software (version 22.0, IBM, Armonk, NY, USA). As non-parametric analyses were conducted, results are summarized as mean ± SD and median ± inter-quartile range (IQR) (not listed). The inter-subject reproducibility of cartilage and meniscus T2 values measurement was quantified using RMS-CV%.
The healthy subjects were divided into three subgroups with respect to knee alignment as neutral, varus, and valgus. All comparisons were stratified by compartment. One-way analysis of covariance (ANCOVA) was utilized to obtain the p values comparing T2 measurements by regressing out effect of covariates, namely, age, gender, and BMI in each of the 12 cartilage subregions and six meniscal subregions for each pairing of subgroups (neutral vs. varus, neutral vs. valgus, and varus vs. valgus, respectively). T2 values recorded for a given compartment were first converted to ranks and the ranks were used as the dependent variable. Because gender and age were significantly different among subgroups of neutral and varus, varus and valgus (Table 1), we adjusted for these two factors in our ANCOVA models. ANCOVA model for each subregion included age and BMI as numeric covariates, gender as a nominal covariate, and subject subgroups as the between-subjects nominal factor of interest. Wilcoxon signed-rank test was also conducted to calculate p values when comparing mean T2 values for compartmental subregional cartilage and menisci, respectively, in each subject subgroup (varus, valgus, and neutral). All the reported p values were considered statistically significant when p < 0.05.
Results
As shown in Table 1, regarding knee alignment, 12 healthy subjects were neutral, 12 varus, and 12 valgus, respectively. The measured mean knee alignment angle (mean ± SD) for neutral, varus, and valgus subgroups were 183.1 ± 0.8°, 178.9 ± 2.1°, and 187.3 ± 2.4°, respectively. The re-measured mean knee alignment angle for extra ten varus healthy subjects was 178.2 ± 4.2°. The percentage coefficient of variation (CV%) of the knee alignment angle measurement for neutral, varus, and valgus subgroups were 0.57, 1.56, and 1.47, respectively. And the CV% of the knee alignment angle measurement for extra remeasurement of ten varus healthy subjects was 1.52.
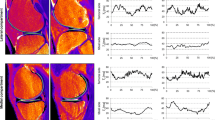
For neutral, varus, and valgus alignment subgroups, the mean and standard deviation (SD) of T2 values within each cartilage and meniscus subregion were listed in Table 2. The CV% of compartmental cartilage subregion T2 measurement of LFa, LFc, LFp, LTa, LTc, LTp, MFa, MFc, MFp, MTa, MTc, and MTp were 6.44, 10.39, 5.19, 14.1, 13.38, 7.8, 6.78, 9.53, 8.23, 12.74, 11.51, 9.89 for neutral subgroup; 6.33, 7.58, 7.9, 7.94, 8.54, 4.75, 3.86, 6.76, 7.74, 7.09, 8.23, 5.71 for varus subgroup; and 4.38, 7.99, 6.57, 11.59, 7.93, 5.56, 4.04, 7.89, 4.64, 9.53, 3.67, 5.75 for valgus subgroup, respectively. Representative T2 maps of cartilage on the lateral (a, c, e) and medial (b, d, f) side obtained from the same three healthy subjects as in Fig. 1 were displayed in Fig. 4 with neutral (Fig. 4a, b), varus (Fig. 4c, d), and valgus (Fig. 4e, f) knee alignment, respectively. The color bar scale on the right side shows the distribution of T2 values. Fig. 6 showed the barchart of T2 values within each compartmental cartilage and meniscus subregions for neutral, varus, and valgus alignment subgroups, respectively.
Representative T2 maps of cartilage from the same three subjects as in Fig. 1. T2 maps of cartilage on the lateral (a, c, e) and medial (b, d, f) side with neutral (a, b), varus (c, d), and valgus alignment (e, f), respectively. The color bar scale on the right shows the distribution of T2 values
The CV% of compartmental meniscus subregion T2 measurement of La, Lc, Lp, Ma, Mc, and Mp were 8.9, 9.63, 8.4, 5.06, 4.56, and 8.24 for neutral subgroup; 8.5, 7.75, 5.28, 5.02, 4.75, and 5.62 for varus subgroup; and 9.79, 6.94, 7.1, 5.48, 5.77, and 6.69 for valgus subgroup, respectively. Representative T2 maps of menisci on the lateral (a, b, e, f, i, j) and medial (c, d, g, h, k, l) side obtained from the same three healthy subjects as in Fig. 1 were displayed in Fig. 5 with neutral (Fig. 5a, b, c, d), varus (Fig. 5e, f, g, h), and valgus (Fig. 5i, j, k, l) knee alignment, respectively. Fig. 5b, f, and j showed the meniscal body segment on the lateral side, and Fig. 5d, h, and l on the medial side, respectively. The color-bar scale on the right side shows the distribution of T2 values.
Representative T2 maps of menisci from the same three subjects as in Fig. 1. T2 maps of menisci on the lateral (a, b, e, f, i, j) and medial (c, d, g, h, k, l) side with neutral (a, b, c, d), varus (e, f, g, h), and valgus alignment (i, j, k, l), respectively. b, f, j show the meniscal body segment on the lateral side, and (d, h, l) on the medial side, respectively. The color bar scale on the right shows the distribution of T2 values
When comparisons were made between the same cartilage subregions among the neutral, varus, and valgus subgroups (Tables 2, 3 and Fig. 6), the following findings were identified statistically significant: LFa cartilage subregion had significantly higher T2 values than MFa with p = 0.0304, and p = 0.0304 in the varus (52 ± 3 vs. 51 ± 2 ms) and valgus (53 ± 2 vs. 51 ± 2 ms) subgroup, respectively. There were statistically significant differences in T2 values of tibial central cartilage subregion between lateral and medial compartment among the neutral, varus, and valgus subgroups (51 ± 6 vs. 46 ± 6 ms; 52 ± 4 vs. 44 ± 4 ms; 53 ± 2 vs. 47 ± 4 ms) with p = 0.0009, p = 0.0014, and p = 0.0166, respectively as listed and shown in Tables 2, 3 and Fig. 6.
When comparisons were made between the same meniscal regions among the neutral, varus, and valgus subgroups (Tables 2, 3 and Fig. 6), the only statistically significant difference was that varus subgroup had significantly higher T2 value (41 ± 3 ms) at the central subregion (body segment) of the lateral meniscus than that (39 ± 2 ms) at the central subregion of the medial meniscus with p = 0.0464 as listed and shown in Tables 2, 3 and Fig. 6.
When comparisons were made between T2 values of femorotibial cartilage and those of anterior, central, and posterior meniscus subregions stratified by compartment (lateral and medial) and subjects’ knee alignment group (varus, valgus, and neutral), all comparison pairs were identified statistically significant as listed and shown in Tables 2, 4 and Fig. 6, respectively.
When comparison pairs of subjects’ knee alignment groups in terms of T2 values of cartilage and meniscus subregions based on ANCOVA were performed, no statistically significant difference was found among them as listed and shown in Table 5 and Fig. 6, respectively.
Discussion
There are only a handful of reports in prior literature dealing with the implementation of quantitative MR T2 mapping or T1rho mapping techniques in determining the possible correlation between knee alignment and associated cartilage or meniscus damage [2, 9]. In this study, T2 mapping as our first endeavor was used to perform quantitative analyses and to evaluate the possible relationship between knee joint alignment and T2 values of femorotibial cartilage and menisci on both lateral and medial sides in healthy subjects alone.
T2 relaxation time of cartilage is by nature derived from the interactions of cartilage water within the cartilage macromolecules (mainly from collagen integrity), which are modulated by their chemical and structural states [22]. Our T2 values computed from all the included healthy subjects in this work agree well with those reported in previous literature [2, 22, 28, 29]. A noteworthy inconsistency in our study compared to prior work is that the healthy cartilage T2 values in tibial central are generally higher than those in femoral central, although they are not statistically different, among varus, valgus, and neutral subgroup, respectively. This inconsistency is only on the medial side but not on the lateral side. Compared to femoral cartilage, tibial cartilage may bear slightly more body weight, which may give rise to the extrusion of more water from the matrix of tibial cartilage, causing relatively higher T2 values observed in femoral cartilage when compared to tibial cartilage as reported and suggested in prior literature [15, 22, 28, 29]. This guess for higher cartilage T2 values only applies to central cartilage subregions in lateral compartment but not in medial compartment among varus, valgus, and neutral subgroups, respectively. Interestingly, lateral femoral anterior cartilage in varus and valgus subgroup, respectively had significantly higher T2 values than those corresponding subregions in the medial compartment, which agrees with the aforementioned explanation.
OA is a multi-factorial diarthrodial cartilage degeneration disease, the pathogenesis of which is deemed to result primarily from mechanical factors acting within the context of systemic susceptibility. Tamanas et al. in their systematic review [30] found limited evidence for a relationship between knee alignment and the incidence of knee OA, although they did find strong evidence for knee malalignment as an independent risk factor for progression of knee OA on radiographs and MR imaging. Brouwer and coworkers [24] suggested that an increasing degree of varus alignment is associated not only with progression of knee OA but also with development of knee OA. However, this association seems particularly applicable only to overweight and obese persons. On the other hand, as a matter of fact, the de facto effect degree and pattern of knee alignment on the onset and progression of OA remains controversial and inconclusive in previous studies. Some investigators [31] implied that knee malalignment is related to OA progression, and that it also mediates the effects of other potential possible risk factors of OA including obesity, quadriceps strength, laxity, and stage of disease. While all the existing evidence is conflicting, knee malalignment may play a smaller role in incident disease. It also appears that knee malalignment varies with the disease progression and is per se possibly influenced by structural changes within the joint. Hunter et al. [31, 32] found that baseline knee alignment is not related to either incident radiographic femorotibial OA or medial femorotibial OA, and they even implied that knee malalignment is not a risk factor for OA, but rather is a marker of disease progression and/or its severity.
Interestingly enough, our preliminary findings in this study were different from the results in some prior publications [32, 33]. Sauerschnig et al. [33] observed that knee alignment has no influence on cartilage T2 values in asymptomatic knee joints at all. However, our study found that subregional cartilage T2 values of LTc were significantly lower compared to those of MTc in varus, valgus, and neutral subgroup, respectively. Moreover, there also exist significant differences in T2 values of anterior cartilage subregion between lateral and medial compartments (LFa vs. MFa) in varus and valgus subgroups, respectively. However, significant differences in T2 values of cartilage subregions between lateral and medial compartments were not observed in the neutral subgroup alone except for the tibial central cartilage subregion, which agrees with the common intuition toward the effect of femorotibial cartilage load distribution pattern on cartilage T2 values, namely, both varus subgroup alone and/or valgus subgroup alone instead of neutral subgroup alone should have more or less significant differences in T2 values between compartmental femorotibial cartilage due to seemingly unbalanced cartilage load distribution. We are not very clear on these results; Our speculation for these observations is that the walking gait due to individual discrepancy in healthy neutral knee may possibly have less deterministic impact on the cartilage T2 values due to the femorotibial cartilage load distribution compared to that in both healthy varus knee and healthy valgus knee.
The human knee menisci act as important functions for joint shock absorption, joint stability, and joint lubrication [20, 34]. Meniscal injury or degeneration may be a risk factor for the origination of knee OA [9, 22, 35,36,37]. T2 values of menisci computed from all the included healthy subjects in our current study agree well with those reported in previous literature [19, 20], with a range roughly from 30 to 46 ms considering many different influencing factors for the fitting calculation of T2 relaxation times [15]. An interestingly comparable consensus in our study with the aforementioned guess for T2 values is that the healthy meniscal T2 values in Mc subregion are significantly lower compared to those of Lc meniscal subregions in varus subgroup, although the T1rho instead of T2 mapping technique was performed in previous work, the changes of T1rho values in menisci may result from the macromolecular content (both collagen and PG), structure (collagen) and mechanical loading [7, 9].
Of note, compared to T2 values of femorotibial cartilage between lateral and medial compartment, only T2 values of central meniscus subregion (body segment) in varus subgroup had statistically significant difference relative to both neutral and valgus lower limb alignment. We are not clear why the valgus knee malalignment had less apparent impact on T2 values of meniscal subregions when compared to those in varus knee alignment, and our speculation is that the walking gait due to individual discrepancy in healthy varus knee possibly has more deterministic influence on the T2 values of meniscal subregions due to the complex menisci load distribution pattern than that in both healthy neutral knee and healthy valgus knee. More work and further investigation to clarify these speculations remain warranted.
This study surely has limitations in terms of measurement error coming from the manually drawn ROIs among the different subregions in both cartilage and meniscus. Given these measurement limitations in T2 values, further investigation is necessary. Our current work did not consider the confounding factor of the magic angle effects on the computing of cartilage T2 values, because there remain inconclusive and controversial, or even mutually contradictive conclusions in previous publications: some studies suggested that it is unlikely that the “magic angle effect” accounts for regional differences in cartilage signal intensity observed in clinical imaging [38]; and on the other hand, some work implied that magic-angle effect needs to be carefully considered when interpreting cartilage abnormalities in OA patients [39]. All these previous inconclusive research results indicate that further and more endeavors remain to be done to clarify on the interfering factors such as cartilage T2 values computing and the magic angel effects, our current study is still preliminary, further investigation is warranty to focus on fixing these interfering factors. Additionally, the acquisition and measurement of the mechanical axis is technically difficult and does not avoid pelvic radiation, as opposed to the short-film measurement technique. Another shortcoming in our present work is that our healthy subjects are relatively young; further and future work remains necessary concerning the healthy menisci T2 mapping of knee alignment when considering the influence from the demarcation in age, overweight, and obesity. A larger sample size is definitely indispensable as well.
Conclusions
Our study underscores the presence of statistically significant differences in T2 values of specific femorotibial cartilage and menisci subregions among healthy subjects with different knee alignment of neutral, varus, and valgus. Based on our preliminary results, T2 mapping appears to be a promising potential sensitive biomarker for evaluating the load distribution pattern of human cartilage and menisci with lower limb alignment abnormality, which may be used as reference baseline when understanding the onset and progression of knee OA.
References
Maizlin ZV, Clement JJ, Patola WB, Fenton DM, Gillies JH, Vos PM, et al. T2 mapping of articular cartilage of glenohumeral joint with routine MRI correlation—initial experience. HSS J. 2009;5:61–6.
Friedrich KM, Shepard T, Chang G, Wang L, Babb JS, Schweitzer M, et al. Does joint alignment affect the T2 values of cartilage in patients with knee osteoarthritis? Eur Radiol. 2010;20:1532–8.
Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–46.
Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286:188–95.
Sharma L. Local factors in osteoarthritis. Curr Opin Rheumatol. 2001;13:441–6.
Arokoski JP, Jurvelin JS, Vaatainen U, Helminen HJ. Normal and pathological adaptations of articular cartilage to joint loading. Scand J Med Sci Sports. 2000;10:186–98.
Wang L, Chang G, Bencardino J, Babb JS, Rokito A, Jazrawi L, et al. T1rho MRI at 3T of menisci in patients with acute anterior cruciate ligament (ACL) injury. J Magn Reson Imaging. 2015;41:544–9.
Wang L, Salibi N, Chang G, Bencardino JT, Babb JS, Rokito A, et al. Evaluation of subchondral bone marrow lipids of acute anterior cruciate ligament (ACL)-injured patients at 3T. Acad Radiol. 2014;21:758–66.
Wang L, Vieira RL, Rybak LD, Babb JS, Chang G, Krasnokutsky S, et al. Relationship between knee alignment and T1ρ values of articular cartilage and menisci in patients with knee osteoarthritis. Eur J Radiol. 2013;82:1946–52.
Wang L, Salibi N, Chang G, Vieira RL, Babb JS, Krasnokutsky S, et al. Assessment of subchondral bone marrow lipids in healthy controls and mild osteoarthritis patients at 3T. NMR Biomed. 2012;25:545–55.
Gong J, Pedoia V, Facchetti L, Link TM, Ma CB, Li X. Bone marrow edema-like lesions (BMELs) are associated with higher T1ρ and T2 values of cartilage in anterior cruciate ligament (ACL)-reconstructed knees: a longitudinal study. Quant Imaging Med Surg. 2016;6:661–70.
Schipplein OD, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991;9:113–9.
Tetsworth K, Paley D. Malalignment and degenerative arthropathy. Orthop Clin North Am. 1994;25:367–77.
Chang EY, Du J, Chung CB. UTE imaging in the musculoskeletal system. J Magn Reson Imaging. 2015;41:870–83.
Surowiec RK, Lucas EP, Ho CP. Quantitative MRI in the evaluation of articular cartilage health: reproducibility and variability with a focus on T2 mapping. Knee Surg Sports Traumatol Arthrosc. 2014;22:1385–95.
Liu F, Chaudhary R, Hurley SA, Munoz DRA, Alexander AL, Samsonov A, et al. Rapid multicomponent T2 analysis of the articular cartilage of the human knee joint at 3. 0T J Magn Reson Imaging. 2014;39:1191–7.
Goodwin DW, Wadghiri YZ, Zhu H, Vinton CJ, Smith ED, Dunn JF. Macroscopic structure of articular cartilage of the tibial plateau: influence of a characteristic matrix architecture on MRI appearance. Am J Roentgenol. 2004;182:311–8.
Prasad AP, Nardo L, Schooler J, Joseph GB, Link TM. T1ρ and T2 relaxation times predict progression of knee osteoarthritis. Osteoarthr Cartilage. 2013;21:69–76.
Tsai PH, Chou MC, Lee HS, Lee CH, Chung HW, Chang YC, et al. MR T2 values of the knee menisci in the healthy young population: zonal and sex differences. Osteoarthr Cartilage. 2009;17:988–94.
Takao S, Nguyen TB, Yu HJ, Hagiwara S, Kaneko Y, Nozaki T, et al. T1rho and T2 relaxation times of the normal adult knee meniscus at 3T: analysis of zonal differences. BMC Musculoskelet Disord 2017;18:202. https://doi.org/10.1186/s12891-017-1560-y.
Shapiro LM, McWalter EJ, Son MS, Levenston M, Hargreaves BA, Gold GE. Mechanisms of osteoarthritis in the knee: MR imaging appearance. J Magn Reson Imaging. 2014;39:1346–56.
Wang L, Regatte RR. Quantitative mapping of human cartilage at 3.0 T: parallel changes in T2, T1ρ, and dGEMRIC. Acad Radiol. 2014;21:463–71.
Friedrich KM, Shepard T, de Oliveira VS, Wang L, Babb JS, Schweitzer M, et al. T2 measurements of cartilage in osteoarthritis patients with Meniscal tears. Am J Roentgenol. 2009;193:W411–5.
Brouwer GM, van Tol AW, Bergink AP, Belo JN, Bernsen RM, Reijman M, et al. Association between valgus and varus alignment and the development and progression of radiographic osteoarthritis of the knee. Arthritis Rheum. 2007;56:1204–11.
Kraus VB, Vail TP, Worrell T, McDaniel G. A comparative assessment of alignment angle of the knee by radiographic and physical examination methods. Arthritis Rheum. 2005;52:1730–5.
Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthr Cartilage. 2004;12:177–90.
Eckstein F, Ateshian G, Burgkart R, Burstein D, Cicuttini F, Dardzinski B, et al. Proposal for a nomenclature for magnetic resonance imaging based measures of articular cartilage in osteoarthritis. Osteoarthr Cartilage. 2006;14:974–83.
Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3T MRI. Osteoarthr. Cartilage. 2007;15:789–97.
David-Vaudey E, Ghosh S, Ries M, Majumdar S. T2 relaxation time measurements in osteoarthritis. Magn Reson Imaging. 2004;22:673–82.
Tanamas S, Hanna FS, Cicuttini FM, Wluka AE, Berry P, Urquhart DM. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61:459–67.
Hunter DJ, Sharma L, Skaife T. Alignment and osteoarthritis of the knee. J Bone Joint Surg Am. 2009;91(Suppl 1):85–9.
Hunter DJ, Niu J, Felson DT, Harvey WF, Gross KD, McCree P, et al. Knee alignment does not predict incident osteoarthritis: the Framingham osteoarthritis study. Arthritis Rheum. 2007;56:1212–8.
Sauerschnig M, Bauer JS, Kohn L, Hinterwimmer S, Landwehr S, Woertler K, et al. Alignment does not influence cartilage T2 in asymptomatic knee joints. Knee Surg Sports Traumatol Arthrosc. 2014;22:1396–403.
Shoemaker SC, Markolf KL. The role of meniscus in the anterior-posterior stability of the loaded anterior cruciate-deficient knee. Effects of partial versus total excision. J Bone Joint Surg Am. 1986;68:71–9.
Wang L, Chang G, Xu J, Vieira RL, Krasnokutsky S, Abramson S, et al. T1rho MRI of menisci and cartilage in patients with osteoarthritis at 3T. Eur J Radiol. 2012;81:2329–36.
Nebelung S, Tingart M, Pufe T, Kuhl C, Jahr H, Truhn D. Ex vivo quantitative multiparametric MRI mapping of human meniscus degeneration. Skelet Radiol. 2016;45:1649–60.
Lohmander LS, Englund PM, LDahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–69.
Mosher TJ, Smith H, Dardzinski BJ, Schmithorst VJ, Smith MB. MR imaging and T2 mapping of femoral cartilage: in vivo determination of the magic angle effect. AJR Am J Roentgenol. 2001;177:665–9.
Wang L, Regatte RR. Investigation of regional influence of magic-angle effect on T2 in human articular cartilage with osteoarthritis at 3T. Acad Radiol. 2015;22:87–92.
Funding
This study was funded in part by Suzhou Bureau of Science and Technology, Suzhou, Jiangsu Province, China (Grant No.: sys2018058). This work was also partially supported by research grant K512801315 from the Scientific Research Foundation for the Returned Overseas Chinese Scholars from the Ministry of Education, China. This research study was supported in part by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), China, too. No separate funding was used specifically for this research work. The funding sources had no involvement in the study design, the collection, analysis, and interpretation of data, the writing of the report, and the decision to submit the paper for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, J., Hu, N., Liang, X. et al. T2 mapping of cartilage and menisci at 3T in healthy subjects with knee malalignment: initial experience. Skeletal Radiol 48, 753–763 (2019). https://doi.org/10.1007/s00256-019-3164-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-019-3164-0