Abstract
Objectives
Our aim was to investigate the role of age, sex, and location on MR T2* values of the knee cartilage in asymptomatic controls and patients with osteoarthritis (OA).
Methods
A total of 100 participants, including 40 with OA and 60 asymptomatic controls, were enrolled in this study. Patients with OA were compared to age- (≥ 41 years old) and sex-matched controls. Controls were divided by age (aged 21–40 years, 41–60, ≥ 61). T2* values were acquired using a T2*-weighted fast gradient-echo sequence and a 1.5-T MRI scanner. T2* values of the femoral and tibial cartilages at the weight-bearing areas were obtained for comparisons.
Results
The T2* values significantly increased with age and were significantly higher in the medial femoral cartilage (35.96 ± 4.06 and 31.85 ± 2.44 ms), medial tibial cartilage (30.95 ± 2.87 and 28.24 ± 1.74 ms), and lateral femoral cartilage (33.90 ± 3.15 and 31.51 ± 2.28 ms) in OA patients versus age- and sex-matched controls. Among OA patients, the T2* values for women exceed those in men in the medial femoral cartilage (37.59 ± 4.43 and 34.16 ± 2.63 ms) and medial tibial cartilage (32.17 ± 2.59 and 29.62 ± 2.53 ms; p < 0.01). Correlations were found between the Lequesne index and the T2* values for the medial femoral cartilage (r = 0.636, p < 0.001) and the medial tibial cartilage (r = 0.433, p = 0.005).
Conclusion
Cartilage T2* values tend to increase with age and are useful in assessing cartilage degeneration in early OA.
Key Points
• Age, sex, and location have important effects on cartilage T2* values at the knee.
• MR T2* measurements are useful toward assessing cartilage degeneration.
• The medial femoral and tibial cartilage T2* values correlate well with disease severity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cartilage affected by progressive osteoarthritis (OA) has proteoglycan loss and collagen fiber changes that act as surrogate biomarkers reflecting disease severity—these can be measured using quantitative magnetic resonance (MR) techniques such as contrast-enhanced T1 and mapping of T1ρ and T2 [1,2,3]. Of these techniques, MR T2 measurements are ideal for clinical applications because they do not require injection of contrast agents and advanced sequence programming. Previous studies have demonstrated that T2 values are reflective of subtle changes in water content and orientation of collagen fibers in knee cartilage; these indicators are signals of cartilage degradation [4] and correlate with OA severity [5].
The T2* relaxation time reveals additional information related to local field inhomogeneity, and these may be more sensitive to changes in tissue composition compared with T2 relaxation time [6]. Recently, T2* measurements have been used to further explore T2 relaxation and the local susceptibility effect on knee cartilage [7]. Indeed, T2* imaging can evaluate hip joint cartilage at 1.5 T with shorter acquisition times than T2 mapping using gradient-recalled echo (GRE) MR imaging [8]. Mamisch et al demonstrated that both T2 and T2* values have similar responses in the assessment of articular cartilage and cartilage repair tissues [9]; however, the relationship between altered T2* values and cartilage degradation remains controversial because previous studies used discrepant imaging parameters [6, 10]. The MR T2* measurements have revealed alterations in cartilage composition—as well as microstructural characteristics—which support its potential utility in T2* imaging for early OA detection.
The composition and orientation of collagen fibers in knee cartilage are location dependent and are subject to various biomechanical stresses. T2 values vary significantly between locations in the cartilage in both unaffected knees and knees affected by OA because of the magic angle effect [11, 12]. These regional discrepancies in cartilage T2* measurements can influence the diagnostic value of these tests. Moreover, there are significant changes in the proteoglycan content as a function of age and sex [13, 14]. Although the reason remains unclear, OA is epidemiologically more prevalent in women than in men [15]. Therefore, it might be useful to derive the regional MR T2* values in knee cartilage relative to age and sex.
There is a high prevalence of OA in the weight-bearing tibiofemoral (TF) joint in Asian populations [16, 17]. However, relatively few studies have investigated knee cartilage using fast T2* measurements [18]. The purpose of this study was to determine the effects of age, sex, and location on MR T2* values in TF joint cartilage in asymptomatic people and to characterize the early changes in T2* values as a function of age. In addition, we compared the T2* values between patients with early OA and age- and sex-matched asymptomatic controls.
Methods
Patient enrollment
Sixty unaffected people were enrolled as approved by the institutional review board of Taipei Medical University in Taipei, Taiwan (approval number 201108018); all participants provided informed consent. Inclusion criteria included the following: (1) body mass index (BMI) less than 30 kg/m2 [19]; (2) asymptomatic with normal Lequesne indices [20] of 0 for both knees; (3) no evidence of meniscal tears or meniscal intra-substance fluid on MR imaging; (4) no evidence of ligamentous abnormalities on MR imaging; and (5) no loss of the meniscus or a discoid meniscus. The Lequesne index is a verified questionnaire given to a patient to evaluate if there is any knee discomfort associated with knee osteoarthritis. It comprises five questions relevant to knee pain or discomfort including maximum distance walked and activities of daily living [20].
Here, a meniscal tear was defined as an abnormal linear or complex high signal intensity communicating with the articular surface [21]. The exclusion criteria for unaffected controls were (1) age of less than 20 years; (2) presence of OA risk factors; (3) history of knee injury, obesity, high-intensity exercise or sports, or loss of knee stability; (4) history of non-OA knee arthropathy, knee surgery, or chronic disease; (5) long-term medication or nutritional supplement use; (6) poor image quality; and (7) abnormal findings on MR images (e.g., cruciate ligament tears, meniscal tears, synovitis). In total, 60 normal participants fulfilled the study criteria (Fig. 1).
The institutional review board also approved the participation of OA patients and waived the need for informed consent due to the retrospective nature of the study (approval number N201704004). Patients with early OA symptoms were referred from a single orthopedic surgeon who performed routine standardized physical examinations and applied a MR T2* knee protocol between January 2012 and December 2015. These patients were diagnosed based on the American College of Rheumatology Classification Criteria of 1986 and the more recent European League Against Rheumatism recommendations of 2010 [22, 23], and then patients were eligible for inclusion if they met all of the following three criteria for definite early OA: pain in the knee, a Kellgren-Lawrence grade not greater than 2, and arthroscopic or MRI findings demonstrating degenerative changes of the knee [24]. Furthermore, to avoid the inclusion of definite OA, patients with the joint space width less than 3 mm were excluded [25].
All participants received three X-ray projections of their knees, including anteroposterior (AP) and lateral standing views and skyline (Merchant) view, as routine radiographic techniques. The Kellgren-Lawrence grading system was used to confirm diagnoses. This system considers several radiographic features of OA including joint space narrowing and osteophyte development [26]. Inclusion criteria included (1) aged more than or equal to 41 years, (2) diagnosis of tibia-femoral knee OA by radiography based on a Kellgren-Lawrence grade of 1 or 2, and (3) at least two episodes of symptomatic knee joint pain rated greater than 3 on a 0–10 visual analog scale for a period of 10 days.
Any patient with a history of systemic autoimmune rheumatoid disease, traumatic knee injury, septic arthritis, intra-articular fracture involving a knee joint, knee arthroscopy, meniscal extrusion and maceration, knee malalignment, BMI greater than 30 kg/m2, or whose MR images showed lateral cartilage degeneration and/or meniscal tears was excluded because medial knee OA is more prevalent than lateral OA [27]. Knee angles were obtained based on the effective measurements using AP knee radiographs [28]. Patients with malalignment of greater than 5° in the valgus or varus direction were excluded. In total, 40 OA patients (20 male) were enrolled (Fig. 1).
All 100 participants were evaluated for body height and mass, and each received a routine MR examination before undergoing the T2* imaging. In addition, each completed the Lequesne index questionnaire and was then evaluated by a trained interviewer using identical validated Lequesne index questionnaires. For the knee, the Lequesne index questionnaire consists of 10 items spanning three scales. The largest consists of five items and evaluates pain or discomfort. The smallest consists of one item and evaluates the maximum distance walked. The third scale consists of four items and evaluates the activities of daily living. Each scale provides a score from 0 (no pain or functional limitation) to 8 (extreme pain and functional limitation) resulting in a total score from 0 to 24—this is a direct aggregate of symptoms and functions as a singular global index.
The MR T2* values were determined for the right knee in the 60 asymptomatic controls who were then divided by age. Group 1 was aged 21 to 40 years and had a mean (± standard deviation [SD]) BMI of 22.0 ± 3.1 kg/m2. Group 2 was aged 41 to 60 years, and BMI was 22.5 ± 2.9 kg/m2. Group 3 was aged more than or equal to 61 years, and BMI was 25.0 ± 3.8 kg/m2. The age- and sex-matched controls (a combination of group 2 and group 3) were compared to the 40 OA patients (aged more than or equal to 41 years, and BMI was 24.6 ± 3.9 kg/m2). Although only controls above 40 years were used for further comparisons with OA patients, the asymptomatic participants aged 21 to 40 years were included for assessing the age effect on the T2* measurements. Detailed information about participant characteristics is shown in Table 1.
Data acquisition
All data were acquired on a 1.5-T clinical MR scanner (Magnetom Avanto; Siemens Healthineers). The right knee of each participant was centered in a single-channel knee coil (Siemens Healthineers). The “magic angle effect” on the cartilage T2* measurement was minimized [29] by straightening the leg so that the long axis was parallel to the main magnetic field (B0). The leg was then immobilized using an MR-compatible plastic pad. Pilot images were obtained in the three orthogonal planes using spin-echo sequences, including coronal proton density with and without fat saturation, sagittal T2-weighted with fat saturation, and axial proton density with fat saturation.
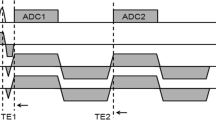
Subsequently, oblique sagittal T2*-weighted images were obtained using a fast, multi-slice, multi-echo, gradient-echo sequence prescribed to cover the medial and lateral menisci. The parameters were TR = 403 ms; TE = 4.38, 11.85, 19.32, 26.79, 33.88, and 40.58 ms; matrix size = 256 × 256 (zero-filled to 512 × 512); in-plane resolution = 0.23 × 0.23 mm; slice thickness = 3 mm; slice gap = 1 mm; NEX = 2; 17 slices; and acquisition time = 15 min 30 s.
Data analysis
Selection of regions of interest
Two raters (one musculoskeletal radiologist and one orthopedic surgeon) interpreted all MR images in selecting OA patients and controls, and a consensus of interpretation was reached. After applying an auto-correlation-based motion correction [30], the femoral and tibial cartilages were manually segmented from the sagittal view of the first-echo image of the knee (Fig. 2). The anterior and posterior margins of the menisci were used as landmarks to draw the ROIs in regions of the femoral and tibial cartilages, respectively. Two experienced operators (PHT and WPC with 8 and 20 years of experience, respectively) selected ROIs for three randomly selected participants separately, discussed their disagreements together, and reached a consensus on the ROI selection procedure stated above to minimize discrepancies. The upper and lower boundaries of the articular cartilage (approximate 2–4 pixels wide) were excluded from ROIs to avoid partial volume effects. The inter-operator disagreement in ROI selection was less than 8%, which limited discrepancies in the T2* estimates to less than 6%.
T2* calculation
Cartilage T2* analysis was conducted on a zone-by-zone basis improving data fitting precision in the presence of noise and minimizing the partial volume effect [31]. The mean signal intensity was derived in the femoral and tibial cartilages on each motion-corrected image. The T2* values were subsequently analyzed in MATLAB 2010b (version 7.11; MathWorks) based on the least squares single-exponential curve-fitting method. Values for R2 were assessed to confirm goodness of fit to the curve. R2 > 0.95 was used to define an acceptable fit. Averaged T2* values derived over all slices were used for further comparisons.
Statistical analysis
All data were analyzed using Statistical Package for the Social Science software, Version 20.0 (SPSS). Means and SDs for the femoral and tibial cartilage T2* values in each study group (OA patients and asymptomatic participants) were calculated first. A paired t test was used to compare differences between two compartments within a given group. The root-mean-square average coefficient of variation (CVRMS) and intra-class correlation coefficient (ICC, two-way random effects model, multiple raters/measurements) were calculated to assess method reproducibility. Reproducibility was considered good when the CVRMS is less than 10% and the ICC exceeds 0.75.
Two-way repeated measures analysis of variance (ANOVA) was used to examine differences in T2* values between age groups and to compare the cartilage T2* values between men and women in the asymptomatic participants and OA patients, respectively. Pearson’s correlation test was used to analyze the association between T2* values and Lequesne indices in the OA group. Multiple testing was corrected using the Benjamini-Hochberg method for false discovery rate [32]. The findings were considered statistically significant p < 0.05.
Results
Age-dependent differences in T2* values
The mean (± SD) of the femoral and tibial cartilage T2* values for each study group is demonstrated as follows. T2* imaging of the medial and lateral TF cartilages in the groups of 21–40, 41–60, and > 60-year-olds are compared in Fig. 3. The T2* values of the femoral cartilage in 21–40-year-olds were 27.9 ± 1.9 and 27.1 ± 1.9 ms in the medial and lateral compartments, respectively. These values were significantly greater in 41–60-year-olds (30.4 ± 1.4 and 30.3 ± 2.1 ms, respectively) and > 60-year-olds (33.3 ± 2.5 and 32.7 ± 1.9 ms, respectively; p < 0.001). Additionally, the T2* values of the tibial cartilage in 21–40-year-olds were 24.9 ± 1.3 and 24.8 ± 1.2 ms in the medial and lateral compartments, respectively. These values were significantly greater in 41–60-year-olds (27.6 ± 1.6 and 26.8 ± 1.6 ms, respectively) and > 60-year-olds (28.9 ± 1.7 and 28.2 ± 1.3 ms, respectively; p < 0.001). The T2* values did not differ significantly between the two compartments within each group. The CVRMS and ICC for the selected ROIs in the controls were less than 9% and greater than 0.8, respectively, indicating good reproducibility.
Plots of cartilage T2* values with 95% confidence interval measured at femoral (a) and tibial (b) compartments of asymptomatic participants in three different age groups. Asterisks indicate significant increases of T2* values from group 1 to group 3 (p < 0.001) via an ANOVA. Group 1, 21–40 years old; group 2, 41–60 years old; group 3, > 60 years old; MFC, medial femoral cartilage; LFC, lateral femoral cartilage
Comparisons of T2* values between OA patients and unaffected controls
Table 2 shows T2* values of the cartilage in patients with OA (age, 59.1 ± 7.8 years) and in age- and sex-matched controls (age, 56.9 ± 9.3 years). Although a significant difference was not found between T2* values for the lateral tibial cartilage (28.2 ± 2.5 and 27.5 ± 1.6 ms, respectively), this was not the case for any other compartment. Values were significantly greater in the OA group for the medial femoral cartilage (36.0 ± 4.1 and 31.9 ± 2.4 ms, respectively), medial tibial cartilage (31.0 ± 2.9 and 28.2 ± 1.7 ms, respectively), and lateral femoral cartilage (33.9 ± 3.2 and 31.5 ± 2.3 ms, respectively) relative to those of controls (p < 0.01).
Sex differences in T2* values
Table 3 shows comparisons of the cartilage T2* values between men and women in the asymptomatic participants and OA patients, respectively. The T2* values were significantly greater in the medial femoral cartilage (37.6 ± 4.4 and 34.2 ± 2.6 ms, respectively) and medial tibial cartilage (32.2 ± 2.6 and 29.6 ± 2.5 ms, respectively; p < 0.01), but not in the lateral femoral cartilage (33.7 ± 3.3 and 34.1 ± 3.0 ms, respectively) or lateral tibial cartilage (29.1 ± 2.6 and 27.6 ± 2.2 ms, respectively). No significant differences were seen in the T2* values between the sexes among the asymptomatic controls.
Correlation between T2* values and Lequesne index in OA patients
Figure 4 shows the relationship between the T2* values and the Lequesne index in patients with OA. Although the T2* values for the lateral femoral cartilage and the lateral tibial cartilage did not significantly correlate with the Lequesne index (r = 0.088, p = 0.589 and r = 0.089, p = 0.586, respectively), strong and moderate correlations were found between the Lequesne index and the T2* values for the medial femoral cartilage (r = 0.636; p < 0.001) and the medial tibial cartilage (r = 0.433; p = 0.005).
The distribution and correlation of the Lequesne index and T2* values measured at the medial femoral, medial tibial, lateral femoral, and lateral tibial compartments in OA patients. Significant positive correlations between the T2* values and the Lequesne index were found in the medial femoral and medial tibial compartments
Discussion
We investigated the feasibility of early detection of cartilage degeneration and differentiation of OA from normal cartilage using quantitative MR T2* measures in vivo. The findings show good reliability of cartilage T2* values as shown in the previous report [7]. Age-related increases in T2* values were found in both the medial and lateral compartments of the femoral and tibial cartilages suggesting possible associations between MR T2* values and age. A similar tendency has been reported elsewhere using MR cartilage T2 measurements suggesting an altered collagen network and an elevated water content in articular cartilage during advanced age or OA progression [33,34,35]. The cartilage T2* measurements offer further insight into altered cartilage geometry and its composition during OA progression while offering the benefit of a shorter acquisition time.
Two prior in vitro studies using three-dimensional GRE-based sequences with highly reduced TR values (less than 100 ms) showed significant decreases in T2* indicating increasing grades of cartilage degeneration [6, 36]. However, other studies showed that mean T2* values were significantly greater in injured cartilage compared to those of healthy human and animal tissue controls using a multi-echo GRE sequence where TR exceeded 400 ms [10, 37]. This controversy highlights the potential influence of imaging sequences and parameters.
In this study, multi-echo GRE images were acquired at TR = 403 ms. A higher water signal was preserved when TR is greater than 100 ms. This occurs because of the long T1 relaxation time of free water protons. Our findings reveal that the group with early OA has greater T2* values compared to controls. This finding is consistent with previous reports [7] and could have resulted from disrupted cartilage organization and an elevated ratio between free water and bound water during OA progression.
Osteoarthritis affects mostly women [37]—a meta-analysis demonstrated that knee OA is more severe in women than in men [38], and that study emphasized the need to understand sex-based effects on cartilage degradation. Prior work showed significant sex differences in asymptomatic controls consistent with a previous report [39]. In contrast, our results demonstrate that the T2* values in the medial femoral and medial tibial compartments are greater in women than those in men among those with early OA.
Both mechanical and biologic dysfunction in knee joints can trigger OA onset. Although the exact pathological mechanism of OA has not yet been elucidated, several previous studies have demonstrated either greater prevalence or greater severity of OA in the medial compartment of the knee [15, 40]. This might be the result of discrepant mechanical stresses between the medial and lateral cartilages originating from joint instability and/or mobility impairment.
While the T2* values in the medial compartment did not differ significantly from those in the tibial compartment in asymptomatic controls, significant differences were found between those with early OA and controls as well as between sexes in the OA group. Moreover, stronger correlations were found between T2* values and the Lequesne index in the medial compartment compared to the lateral compartment among early OA patients. This hints the potential for monitoring the severity of knee OA using quantitative MR T2* measurements.
This study does have some limitations. For one, a single-component exponential T2* fitting was performed on the cartilage. Although this method has been frequently used to detect early degeneration of knee cartilage, multi-component T2* mapping can provide more extensive information such as a signal from short T2 components of cartilage. This could be an important and novel OA biomarker [41]. Thus, ultra-short echo time imaging could be an alternative for assessing cartilage changes in future studies. Second, contamination by the partial volume effect cannot be completely ignored. Higher-resolution three-dimensional MR cartilage imaging might provide a solution. Finally, assessing the association between histological examinations and cartilage T2* values could facilitate further clinical interpretations.
Conclusion
This study demonstrates the feasibility of using MR T2* measurements at 1.5 T to detect the early changes of cartilage degeneration. T2* values of knee cartilage were correlated with age, sex, and location. Cartilage T2* values tend to increase with age and are useful in assessing cartilage degeneration in early OA.
Abbreviations
- GRE:
-
Gradient-recalled echo
- MR:
-
Magnetic resonance
- OA:
-
Osteoarthritis
- ROI:
-
Region of interest
- TF:
-
Tibiofemoral
References
Andreisek G, White LM, Yang Y, Robinson E, Cheng HL, Sussman MS (2009) Delayed gadolinium-enhanced MR imaging of articular cartilage: three-dimensional T1 mapping with variable flip angles and B1 correction. Radiology 253:865–873
Huang GS, Lee HS, Chou MC et al (2010) Quantitative MR T2 measurement of articular cartilage to assess the treatment effect of intra-articular hyaluronic acid injection on experimental osteoarthritis induced by ACLX. Osteoarthritis Cartilage 18:54–60
van Tiel J, Kotek G, Reijman M et al (2016) Is T1ρ mapping an alternative to delayed gadolinium-enhanced MR imaging of cartilage in the assessment of sulphated glycosaminoglycan content in human osteoarthritic knees? An in vivo validation study. Radiology 279:523–531
Koff MF, Amrami KK, Kaufman KR (2007) Clinical evaluation of T2 values of patellar cartilage in patients with osteoarthritis. Osteoarthritis Cartilage 15:198–204
Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S (2004) T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology 232:592–598
Bittersohl B, Hosalkar HS, Miese FR et al (2015) Zonal T2* and T1Gd assessment of knee joint cartilage in various histological grades of cartilage degeneration: an observational in vitro study. BMJ Open e006895:5. https://doi.org/10.1136/bmjopen-002014-006895
Newbould RD, Miller SR, Toms LD et al (2012) T2* measurement of the knee articular cartilage in osteoarthritis at 3T. J Magn Reson Imaging 35:1442–1429
Bittersohl B, Hosalkar HS, Hughes T et al (2009) Feasibility of T2* mapping for the evaluation of hip joint cartilage at 1.5T using a three-dimensional (3D), gradient-echo (GRE) sequence: a prospective study. Magn Reson Med 62:896–901
Mamisch TC, Hughes T, Mosher TJ et al (2012) T2 star relaxation times for assessment of articular cartilage at 3T: a feasibility study. Skeletal Radiol 41:287–292
Marik W, Apprich S, Welsch GH, Mamisch TC, Trattnig S (2012) Biochemical evaluation of articular cartilage in patients with osteochondrosis dissecans by means of quantitative T2- and T2*-mapping at 3 T MRI: a feasibility study. Eur J Radiol 81:923–927
Smith HE, Mosher TJ, Daradzinski BJ et al (2001) Spatial variation in cartilage T2 of the knee. J Magn Reson Imaging 14:50–55
Stahl R, Blumenkrantz G, Carballido-Gamio J et al (2007) MRI-derived T2 relaxation times and cartilage morphometry of the tibio-femoral joint in subjects with and without osteoarthritis during a 1-year follow-up. Osteoarthritis Cartilage 15:1225–1234
Loeser RF (2009) Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage 17:971–979
Larbre JP, Da Silva JA, Moore AR, James IT, Scott DL, Willoughby DA (1994) Cartilage contribution to gender differences in joint disease progression. A study with rat articular cartilage. Clin Exp Rheumatol 12:401–408
Lange AK, Fiatarone Singh MA, Smith RM et al (2007) Degenerative meniscus tears and mobility impairment in women with knee osteoarthritis. Osteoarthritis Cartilage 15:701–708
Inoue K, Hukuda S, Fardellon P et al (2001) Prevalence of large-joint osteoarthritis in Asian and Caucasian skeletal populations. Rheumatology (Oxford) 40:70–73
Neogi T, Zhang Y (2013) Epidemiology of osteoarthritis. Rheum Dis Clin North Am 39:1–19. https://doi.org/10.1016/j.rdc.2012.1010.1004
Bittersohl B, Hosalkar HS, Sondern M et al (2014) Spectrum of T2* values in knee joint cartilage at 3 T: a cross-sectional analysis in asymptomatic young adult volunteers. Skeletal Radiol 43:443–452
Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK (2008) Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord 9. https://doi.org/10.1186/1471-2474-1189-1132
Dawson J, Linsell L, Doll H et al (2005) Assessment of the Lequesne index of severity for osteoarthritis of the hip in an elderly population. Osteoarthritis Cartilage 13:854–860
Stroller DW, Martin C, Crues JV 3rd, Kaplan L, Mink JH (1987) Meniscal tears: pathologic correlation with MR imaging. Radiology 163:731–738
Altman R, Asch E, Bloch D et al (1986) Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 29:1039–1049
Zhang W, Doherty M, Peat G et al (2010) EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis 69:483–489
Luyten FP, Denti M, Filardo G, Kon E, Engebretsen L (2012) Definition and classification of early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 20:401–406
Messent EA, Ward RJ, Tonkin CJ, Buckland-Wright C (2005) Cancellous bone differences between knees with early, definite and advanced joint space loss; a comparative quantitative macroradiographic study. Osteoarthritis Cartilage 13:39–47
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16:494–502
Heidari B (2011) Knee osteoarthritis prevalence, risk factors, pathogenesis and features: part I. Caspian J Intern Med 2:205–212
Colebatch AN, Hart DJ, Zhai G, Williams FM, Spector TD, Arden NK (2009) Effective measurement of knee alignment using AP knee radiographs. Knee 16:42–45
Bydder M, Rahal A, Fullerton GD, Byder GM (2007) The magic angle effect: a source of artifact, determinant of image contrast, and technique for imaging. J Magn Reson Imaging 25:290–300
Chiang SW, Tsai PH, Chang YC et al (2013) T2 values of posterior horns of knee menisci in asymptomatic subjects. PLoS One 8:e59769
Miller AJ, Joseph PM (1993) The use of power images to perform quantitative analysis on low SNR MR images. Magn Reson Imaging 11:1051–1056
Storey JD (2002) A direct approach to false discovery rates. J R Stat Soc Series B Stat Methodol 64:479–498
Mosher TJ, Liu Y, Yang QX et al (2004) Age dependency of cartilage magnetic resonance imaging T2 relaxation times in asymptomatic women. Arthritis Rheum 50:2820–2828
Mosher TJ, Dardzinski BJ, Smith MB (2000) Human articular cartilage: influence ofaging and early symptomatic degeneration on the spatial variation of T2—preliminary findings at 3 T. Radiology 214:259–266
Chou MC, Tsai PH, Huang GS et al (2009) Correlation between the MR T2 value at 4.7 T and relative water content in articular cartilage in experimental osteoarthritis induced by ACL transection. Osteoarthritis Cartilage 17:441–447
Bittersohl B, Miese FR, Hosalkar HS et al (2012) T2* mapping of hip joint cartilage in various histological grades of degeneration. Osteoarthritis Cartilage 20:653–660
O’Connor MI (2007) Sex difference in osteoarthritis of the hip and knee. J Am Acad Orthop Surg 15:S22–S25
Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G (2005) A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage 13:769–781
Mosher TJ, Collins CM, Smith HE et al (2004) Effect of gender on in vivo cartilage magnetic resonance imaging T2 mapping. J Magn Reson Imaging 19:323–328
Eckstein F, Collins JE, Nevitt MC et al (2015) Brief report: cartilage thickness change as an imaging biomarker of knee osteoarthritis progression: data from the Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis Rheumatol 67:3184–3189
Qian Y, William AA, Chu CR, Boada FE (2010) Multicomponent T2* mapping of knee cartilage: technical feasibility ex vivo. Magn Reson Med 64:1427–1432
Funding
This study was funded by grant (no. 104SWF-08) at Taipei Medical University Wan Fang Hospital Inter-institute Research Project and grant (no. MOST102-2221-E-038-009-) at Ministry of Science and Technology, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Professor Wing P. Chan.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
All subjects signed the study informed consent form for normal subjects.
Written informed consent was waived by the Institutional Review Board for OA patients.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Prospective (normal subjects)/retrospective (OA patients)
• Observational
• Performed at one institution
Rights and permissions
About this article
Cite this article
Tsai, PH., Wong, CC., Chan, W.P. et al. The value of MR T2* measurements in normal and osteoarthritic knee cartilage: effects of age, sex, and location. Eur Radiol 29, 4514–4522 (2019). https://doi.org/10.1007/s00330-018-5826-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5826-z