Abstract
Purpose
To prospectively evaluate four non-invasive markers of cartilage quality—T2* mapping, native T1 mapping, dGEMRIC and ΔR1—in healthy volunteers and rheumatoid arthritis (RA) patients.
Materials and methods
Cartilage of metacarpophalangeal (MCP) joints II were imaged in 28 consecutive subjects: 12 healthy volunteers [9 women, mean (SD) age 52.67 (9.75) years, range 30–66] and 16 RA patients with MCP II involvement [12 women, mean (SD) age 58.06 (12.88) years, range 35–76]. Sagittal T2* mapping was performed with a multi-echo gradient-echo on a 3 T MRI scanner. For T1 mapping the dual flip angle method was applied prior to native T1 mapping and 40 min after gadolinium application (delayed gadolinium-enhanced MRI of cartilage, dGEMRIC, T1Gd). The difference in the longitudinal relaxation rate induced by gadolinium (ΔR1) was calculated. The area under the receiver operating characteristic curve (AROC) was used to test for differentiation of RA patients from healthy volunteers.
Results
dGEMRIC (AUC 0.81) and ΔR1 (AUC 0.75) significantly differentiated RA patients from controls. T2* mapping (AUC 0.66) and native T1 mapping (AUC 0.66) were not significantly different in RA patients compared to controls.
Conclusions
The data support the use of dGEMRIC for the assessment of MCP joint cartilage quality in RA. T2* and native T1 mapping are of low diagnostic value. Pre-contrast T1 mapping for the calculation of ΔR1 does not increase the diagnostic value of dGEMRIC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cartilage degradation has been recognized as a key feature of joint damage in rheumatoid arthritis (RA) [1]. T2* mapping, native T1 mapping and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) have been used for non-invasive assessment of cartilage quality.
T2* mapping is a non-invasive measure of cartilage quality reflecting cartilage hydration and collagen integrity [2–4]. T2* mapping has been demonstrated to be sensitive to cartilage damage in osteochondrosis dissecans of the talus [5] and in femoroacetabular impingement of the hip [6].
dGEMRIC uses the ionic properties of the contrast agent Gd(DTPA)2− for molecular imaging. In cartilage with reduced density of negatively charged glycosaminoglycan (GAG), a main component of hyaline cartilage, the penetration of Gd(DTPA)2− is increased. This way, the concentration of contrast agent in cartilage is a measure of GAG content [7] and can be assessed with T1Gd mapping [8].
Assuming that T1Gd after Gd(DTPA)2− administration corresponds to GAG content, one implicitly assumes that pre-contrast T1 relaxation time is constant. However, pre-contrast T1 values have been reported to be abnormal in damaged cartilage in vitro [9] and in vivo [10]. In particular, cartilage fibrillation and cartilage edema have been associated with an increase in pre-contrast T1 values [9, 10]. In the follow-up of surgical cartilage repair procedures, pre-contrast T1 values have been reported to be severely altered [11, 12]. For this reason, ΔR1 (the difference in relaxation rate between pre- and post-contrast T1 mapping) has been proposed as a more precise parameter of Gd-DTPA2− concentration in cartilage [10, 11, 13]
Operationally, it is desirable to perform dGEMRIC without the acquisition of pre-contrast images because the long waiting time (typically 30–90 min [8]) after contrast injection implies two consecutive imaging sessions for the patient. In a clinical study ΔR1 has been demonstrated to be only slightly superior to post-contrast T1Gd mapping in differentiating healthy volunteers from knee osteoarthritis (OA) patients [14]. In early hip OA, ΔR1 and post-contrast T1Gd (i.e. dGEMRIC) have been shown to correlate in a linear manner [15]. Both studies suggest post-contrast T1Gd may be sufficient for the evaluation of cartilage damage in clinical dGEMRIC studies of OA.
Recently dGEMRIC has been used to assess cartilage quality in finger joints of patients with RA, demonstrating significant differences compared to healthy controls [16]. No reports exist on the value of pre-contrast T1 mapping in dGEMRIC either in finger joints or in RA patients.
The purpose of the present study was to prospectively evaluate four non-invasive markers of cartilage quality—T2* mapping, native T1 mapping, dGEMRIC and ΔR1—in healthy volunteers and rheumatoid arthritis (RA) patients.
Materials and methods
Patients
This study was approved by the institutional review board and all patients and volunteers provided written informed consent.
A total of 28 consecutive subjects were enrolled in the present study: 12 healthy volunteers [9 women, mean (SD) age 52.7 (9.8) years, range 30–66] and 16 patients [12 women, mean (SD) age 58.1 (12.9) years, range 35–76] with established RA according to the 2010 ACR criteria [17] were imaged (Table 1). Inclusion criteria were MCP II involvement without visual cartilage loss in MRI. Signs of inflammation [joint swelling, pain and tenderness as well as Disease Activity Score 28 (DAS 28)] were recorded in clinical examination. CRP was determined within 5 days of MRI examination.
Imaging protocol
Images of MCP II were acquired on a 3 T MR scanner (Magnetom Trio, Siemens, Erlangen, Germany). The subjects were positioned prone with the hand extended overhead, palm down. One 4 cm loop coil was fixed on the palmar, and another coil on the dorsal side of MCP II.
Sagittal T2* mapping was performed with a multi-echo gradient echo sequence with a matrix of 384 × 312 , FOV 90 mm allowing for an in-plane resolution of 234 μm, a slice thickness of 2 mm, a TR of 600 ms and a train of five TEs (5.68, 15.82, 25.96, 36.10, 46.24 ms). Two averages were chosen, the bandwidth was 260 Hz/pixel and flip angle was 60°. Acquisition time was 3:47 min. T2* maps were calculated in-line with a pixel-wise monoexponential non-negative least squares fit analysis using MapIt software (Siemens, Erlangen, Germany).
For T1 mapping, the dual flip angle technique was applied [18]. T1 mapping was performed prior to intravenous administration of 0.4 ml/kg body weight of gadolinium contrast agent (Magnevist; Schering, Berlin, Germany) and after a 40 min delay (dGEMRIC, T1Gd). TE was 3.72 ms, TR was 15 ms, flip angles were 5° and 26°, slice thickness was 2 mm and the FOV was 73 × 90 mm with a matrix of 312 × 384 and an in-plane resolution of 234 μm. Acquisition time was 2:25 min. Slices were positioned perpendicular to the joint spaces.
Image processing
The image data of the dGEMRIC measurements were transferred to an external workstation running Windows XP® (Microsoft®, Redmond, WA). T1 maps were corrected for patient movement between measurements with the STROKETOOL software (http://www.digitalimagesolutions.de, Frechen, Germany) using an image registration method based on least squares measure [19].
T1 maps were generated from the native images (T1 map) and the gadolinium-enhanced images (T1Gd, i.e. dGEMRIC). The T1 maps were calculated according to Eq. 1:
where \( Q(x,y,z) = \frac{{{S_1}(x,y,z)}}{{{S_2}(x,y,z)}} \) and S 1(x,y,z), S 2(x,y,z) are the pixel intensities in the images acquired with flip angles of 5° and 26°.
Image analysis
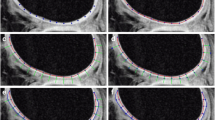
Cartilage thickness was measured including phalangeal and metacarpal cartilage in the same image section.
ROIs were selected to include the phalangeal and the metacarpal cartilage of MCP II in the same image section (Fig. 1). Mean T2*, native T1 values and T1Gd values were determined. To take into account the different sizes of the phalangeal and the metacarpal cartilage, weighted means of the two areas were calculated using the number of pixels as weights. ΔR1 was calculated with Eq. 2:
Statistical analysis
Student’s independent two-sample t-test was applied to determine if healthy subjects and RA patients differed significantly in their means for each of the four investigated parameters.
The area under the receiver operating characteristic curve (AROC) was used to reveal the parameter which is best qualified to identify RA patients. To determine the best cut off points to distinguish between RA patients and healthy subjects, the maximum of the Youden index was calculated (Youden index = sensitivity + specificity – 1). Exact lower confidence bounds for sensitivity and specificity were computed with the binomial distribution.
Correlations among T2*, native T1, T1Gd, ΔR1 and clinical signs of inflammation, DAS 28 and CRP were estimated with Spearman’s correlation coefficient. The correlations among ΔR1, native T1 and T1Gd were calculated with Pearson’s correlation coefficient. The statistical analysis was performed using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
There was no significant differences in age (P = 0.2365) and gender (P = 1.0) between healthy volunteers and patients. No significant difference in MCP II cartilage thickness between the groups (healthy controls: 1.31 ± 0.29 mm, RA patients: 1.37 ± 0.33 mm, P = 0.621) was noted.
T2* mapping
T2* values did not differ significantly between volunteers (18.79 ± 4.49 ms) and RA patients (16.26 ± 2.52 ms) (P = 0.099) (Fig. 1, Table 2). The best cut off point to separate healthy individuals and RA patients is 19.46 according to the Youden index, which leads to a sensitivity of 0.94 with a lower confidence limit of 0.74 and a specificity of 0.58 with a lower confidence limit of 0.32 (Fig. 2, Table 2).
Native T1 mapping
No statistically significant difference between native T1 values of volunteers (958.03 ± 90.22 ms) and RA patients (903.47 ± 164.90 ms) (P = 0.274) was noted. The best cut off point to separate healthy individuals and RA patients according to the Youden index is 864.35, which leads to a sensitivity of 0.50 with a lower confidence limit of 0.28 and a specificity of 0.92 with a lower confidence limit of 0.66 (Fig. 3).
T1Gd (dGEMRIC index)
There was a statistically significant difference in the means of dGEMRIC indexes between volunteers (482.55 ± 87.45 ms) and RA patients (367.15 ± 95.73 ms) (P = 0.003). The best cut off point to separate healthy individuals and RA patients according to the Youden index is 378.99, resulting in a sensitivity of 0.63 with a lower confidence limit of 0.39 and a specificity of 0.92 with a lower confidence limit of 0.66 (Fig. 4). The Youden index maximises the sum of sensitivity and specificity with equal weights for sensitivity and specificity. In our case a low sensitivity would lead to a high number of RA cases that would be diagnosed to have normal cartilage quality. If sensitivity is more important than specificity in this setting, we would rather suggest 384.33 as a cut off point, which has an only slightly lower Youden index, but leads to a sensitivity of 0.69 (lower confidence bound 0.45) and a specificity of 0.83 (lower confidence bound 0.56) (Table 3).
ΔR1
There was a statistically significant difference in the means of ΔR1 between volunteers (1.09 ±0.47 s−1) and RA patients (1.78 ± 0.93 s−1) (P = 0.018) (Fig. 5). The best cut off point according to the Youden index to separate healthy individuals and RA patients is 1.40 s−1, which leads to a sensitivity of 0.69 with a lower confidence limit of 0.45 and a specificity of 0.75 with a lower confidence limit of 0.47.
There was a significant correlation between ΔR1 and T1Gd (Pearson’s r = −0.92, P < 0.0001) and between ΔR1 and native T1 (Pearson’s r =0.41, P = 0.0290) (Fig. 6).
There was no significant correlation among T2*, T1, ΔR1 or T1Gd and clinical signs of inflammation, DAS 28 or CRP.
Discussion
In clinical imaging and research of RA, the exploitation of the potential of MRI is continuously increasing [20–22]. Bone marrow oedema has been identified as a possible precursor to erosions and as an early marker of RA [23]. Dynamic T1-weighted imaging can be used to quantify synovial hyperperfusion reflecting arthritis activity [20, 24], and MR angiography has been shown to depict abnormal vessels reflecting synovial neovascularisation [25]. Cartilage damage in RA has been demonstrated to be associated with physical disability, suggesting that effort should be invested in research in this field [26]. Cartilage damage in RA caused by proteases is a common finding. The release of neopeptides from cartilage has been discussed to maintain the chronic immune response [27]. The depletion of GAG has been demonstrated to be a factor in in vitro studies on cartilage degeneration in RA [28]. In vivo increased levels of anti-GAG autoantibodies have been demonstrated in RA patients [29]. Increased serum levels of cartilage matrix proteins have been reported to predict radiographic joint damage [30]. In the preclinical course of RA, increased markers of cartilage turnover have been reported, suggesting that cartilage damage may be a very early feature of the disease [31]. In conventional MRI, cartilage damage is made visible as a volume loss slowly developing over years [32]. Especially in the early course of RA, cartilage loss in morphological MRI may be absent [33]. The lack of sensitivity of conventional MRI to assess cartilage damage in RA has triggered the application of molecular MRI of cartilage [16, 34]. However, to the best of our knowledge, the value of different MRI techniques sensitive to molecular cartilage damage has not been evaluated in RA patients.
Non-invasive measures of cartilage quality such as T2* mapping, native T1 mapping, dGEMRIC and ΔR1 may have the potential to detect cartilage damage before irreversible volume loss occurs [8]. In order to assess their possible value, the correlation of these techniques with clinical features and their discrimination of healthy control subjects from RA patients are compared in the present paper.
T2* is considered to reflect cartilage hydration and collagen integrity [5, 6]. Cartilage thinning has been proposed to lead to an increased effect of susceptibility artefacts on T2* mapping, resulting in lower T2* values [6]. A favourable technical feature of T2* mapping is that it does not require contrast agent. Gadolinium-based contrast agents are reported to induce nephrogenic systemic fibrosis (NSF) in patients with pre-existing kidney disease [35]. Furthermore, allergic-like reactions are rare but possible reactions [36]. Although NSF may be completely avoidable, if patients with renal failure are restricted from using gadolinium [37], biochemical cartilage MRI without contrast agents seems desirable. Our results indicate that no alterations can be measured with T2* mapping in finger cartilage of RA patients and that T2* mapping may be of lower diagnostic value than dGEMRIC in differentiating RA patients from controls. In our patient group, no significant cartilage thinning was noted, reducing the probability of a significantly different effect of susceptibility artefacts in this group.
T2* mapping can be used to depict depth-dependent relaxation stratification in healthy cartilage, which may be lost in cartilage damage [3]. Although an in-plane resolution of 233 μm was achieved in the present study, an even higher resolution would be necessary to allow delineation of cartilage layer architecture in MCPs. This possible diagnostic virtue of T2* mapping could not be exploited in the present study, and further studies are needed to assess the diagnostic value of depth-dependent T2* relaxativity in RA.
Native T1 mapping has been proposed to be sensitive to cartilage degeneration in patients with knee OA with increased T1 values in OA [14]. However, compared to dGEMRIC, it has lower power to discriminate knee OA from healthy controls [14]. Our results corroborate the finding that native T1 mapping alone is of limited value in the assessment of cartilage quality.
dGEMRIC is an established quantitative imaging technique sensitive to cartilage degeneration [7, 8, 34, 38–40]. Our data support its sensitivity for cartilage degeneration in RA. In the present study, dGEMRIC was superior to T2* mapping and native T1 mapping in discriminating RA patients from healthy controls.
Low dGEMRIC values are considered to represent Gd(DTPA)2− accumulation within areas of GAG depletion [7]. However, the majority of publications using the dGEMRIC technique report on findings in patients with degenerative osteoarthritis [8]. In RA patients, highly active inflammatory changes can be seen with severely increased joint perfusion [24]. The effect of hyperperfusion and increased Gd(DTPA)2− concentration in joint inflammation on dGEMRIC values has not yet been systematically explored and awaits further evaluation. In chondromalacia and osteoarthritis of the patella, altered perfusion with MR-contrast agent has been demonstrated, possibly reflecting changes in intrachondral microvascularity and vascular permeability [41]. No reports on the microvascularity of the cartilage in RA are present in the literature, and further studies are needed to address this topic. However, factors other than GAG depletion alone need to be taken into account in the discussion of dGEMRIC in RA patients. In the absence of cartilage thinning, dGEMRIC abnormalities in RA may point towards cartilage damage on a molecular rather than on a macroscopic level.
The area under the curve (AUC) of ΔR1 in the discrimination of RA patients from healthy controls was lower than the AUC of dGEMRIC in the present study. Corroborating earlier findings [14, 15], there was a highly significant correlation between ΔR1 and T1Gd (dGEMRIC index). This correlation is inherent to the calculation of ΔR1 (Eq. 2): since T1Gd is lower than native T1 (due to the application of gadolinium), 1/T1Gd is larger than 1/T1 and the effect of T1Gd on ΔR1 is higher than of native T1. These results indicate a close correlation between ΔR1 and T1Gd which is not linear but reflects the reciprocal character of Eq. 2. However, the high value of Pearson’s correlation coefficient (r = −0.92) demonstrates that for native T1 and T1Gd values occurring in MCP joint cartilage of RA patients, the correlation between ΔR1 and T1Gd can be assumed to be almost linear.
In the evaluation of degenerative cartilage disorders of the knee, ΔR1 has been shown to be of slightly higher discriminative value than dGEMRIC [14]. In early OA of the hip, a linear correlation between ΔR1 and dGEMRIC has been demonstrated [15], and no additional value of ΔR1 has been assumed by the respective authors. In both studies the drawback of additional pre-contrast MRI has been discussed, and both groups conclude in accordance with our data that dGEMRIC is sufficient in the evaluation of cartilage damage.
Clinical signs of inflammation and CRP did not correlate significantly with T2*, native T1, dGEMRIC or ΔR1 values. However, the CIMESTRA study reports on a correlation between markers of cartilage catabolism (C-telopeptide of collagen II) and disease [42]. DAS 28 and CRP represent systemic inflammation beyond the single joint examined in MRI and are not specific; involvement of MCP II was an inclusion criterion in the present study and severe inflammation of other joints did not lead to exclusion. Perfusion-weighted MRI has been proposed as a tool to assess inflammatory activity [24] and may be useful to evaluate the influence of inflammation on cartilage quality in future MRI studies.
The present study has limitations. One is the limited number of subjects involved in this study. This is reflected by broad confidence intervals for sensitivity and specificity, for example. Non-invasive measures of cartilage quality were assessed and no cartilage biopsy, histology or biochemistry was available. As in the majority of in vivo studies on cartilage quality, a true gold standard reflecting the condition of the cartilage is lacking. Specificity and sensitivity values have been calculated under the assumption that cartilage degeneration was present in all RA patients. However, due to the lack of a gold standard, the true incidence of cartilage damage in our group remains unknown. Using the above stated cut off values, a number of patients could possibly be assumed to have normal cartilage. Further studies are needed to assess sensitivity and specificity of non-invasive evaluation of cartilage quality in RA using a true gold standard such as histology or arthroscopy. T2* stratification as one possible diagnostic feature of T2* mapping could not be exploited in the present study, and further studies are needed to assess the diagnostic value of T2* relaxativity stratification in RA. dGEMRIC used a 40 min delay after Gd administration. Since the positioning in the MR scanner can be uncomfortable or even painful for arthritis patients, the patients were allowed to stand up and move during the waiting period. This way, native T1 and dGEMRIC images were not coregistered the way they can be in in vitro studies, and no ΔR1 maps could be calculated.
In conclusion, our results support the use of post-contrast dGEMRIC for the assessment of MCP joint cartilage quality in RA. In our study, T2* and native T1 mapping were of low diagnostic value, compared to dGEMRIC. Pre-contrast T1 mapping for the calculation of ΔR1 is time consuming, inconvenient for the patient and did not increase the diagnostic value of dGEMRIC in our study population. Further study is needed to compare the different techniques of biochemical cartilage MRI in RA.
References
Dodge GR, Pidoux I, Poole AR. The degradation of type II collagen in rheumatoid arthritis: an immunoelectron microscopic study. Matrix. 1991;11(5):330–8.
Trattnig S, Mamisch TC, Welsch GH, Glaser C, Szomolanyi P, Gebetsroither S, et al. Quantitative T2 mapping of matrix-associated autologous chondrocyte transplantation at 3 Tesla: an in vivo cross-sectional study. Invest Radiol. 2007;42(6):442–8.
Welsch GH, Mamisch TC, Hughes T, Zilkens C, Quirbach S, Scheffler K, et al. In vivo biochemical 7.0 Tesla magnetic resonance: preliminary results of dGEMRIC, zonal T2, and T2* mapping of articular cartilage. Invest Radiol. 2008;43(9):619–26.
Mamisch TC, Hughes T, Mosher TJ, Mueller C, Trattnig S, Boesch C, et al. T2 star relaxation times for assessment of articular cartilage at 3 T: a feasibility study. Skeletal Radiol. 2011.
Marik W, Apprich S, Welsch GH, Mamisch TC, Trattnig S. Biochemical evaluation of articular cartilage in patients with osteochondrosis dissecans by means of quantitative T2- and T2*-mapping at 3T MRI: a feasibility study. Eur J Radiol. 2011.
Bittersohl B, Hosalkar HS, Hughes T, Kim YJ, Werlen S, Siebenrock KA, et al. Feasibility of T2* mapping for the evaluation of hip joint cartilage at 1.5T using a three-dimensional (3D), gradient-echo (GRE) sequence: a prospective study. Magn Reson Med. 2009;62(4):896–901.
Bashir A, Gray ML, Burstein D. Gd-DTPA2− as a measure of cartilage degradation. Magn Reson Med. 1996;36(5):665–73.
Burstein D, Gray M, Mosher T, Dardzinski B. Measures of molecular composition and structure in osteoarthritis. Radiol Clin North Am. 2009;47(4):675–86.
Gillis A, Gray M, Burstein D. Relaxivity and diffusion of gadolinium agents in cartilage. Magn Reson Med. 2002;48(6):1068–71.
Tiderius CJ, Olsson LE, Leander P, Ekberg O, Dahlberg L. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) in early knee osteoarthritis. Magn Reson Med. 2003;49(3):488–92.
Watanabe A, Wada Y, Obata T, Ueda T, Tamura M, Ikehira H, et al. Delayed gadolinium-enhanced MR to determine glycosaminoglycan concentration in reparative cartilage after autologous chondrocyte implantation: preliminary results. Radiology. 2006;239(1):201–8.
Trattnig S, Marlovits S, Gebetsroither S, Szomolanyi P, Welsch GH, Salomonowitz E, et al. Three-dimensional delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) for in vivo evaluation of reparative cartilage after matrix-associated autologous chondrocyte transplantation at 3.0T: preliminary results. J Magn Reson Imaging. 2007;26(4):974–82.
Tiderius CJ, Olsson LE, de Verdier H, Leander P, Ekberg O, Dahlberg L. Gd-DTPA2)-enhanced MRI of femoral knee cartilage: a dose-response study in healthy volunteers. Magn Reson Med. 2001;46(6):1067–71.
Li W, Du H, Scheidegger R, Wu Y, Prasad PV. Value of precontrast T(1) for dGEMRIC of native articular cartilage. J Magn Reson Imaging. 2009;29(2):494–7.
Bittersohl B, Hosalkar HS, Kim YJ, Werlen S, Siebenrock KA, Mamisch TC. Delayed gadolinium-enhanced magnetic resonance imaging (dGEMRIC) of hip joint cartilage in femoroacetabular impingement (FAI): are pre- and postcontrast imaging both necessary? Magn Reson Med. 2009;62(6):1362–7.
Miese FR, Ostendorf B, Wittsack HJ, Reichelt DC, Mamisch TC, Zilkens C, et al. Metacarpophalangeal joints in rheumatoid arthritis: delayed gadolinium-enhanced MR imaging of cartilage–a feasibility study. Radiology. 2010;257(2):441–7.
Aletaha D, Neogi T, Silman A, Funovits J, Felson D, Bingham Cr, et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69(9):1580–8.
Mamisch TC, Dudda M, Hughes T, Burstein D, Kim YJ. Comparison of delayed gadolinium enhanced MRI of cartilage (dGEMRIC) using inversion recovery and fast T1 mapping sequences. Magn Reson Med. 2008;60(4):768–73.
Miese F, Kropil P, Ostendorf B, Scherer A, Buchbender C, Quentin M. et al. Eur J Radiol: Motion correction improves image quality of dGEMRIC in finger joints. Eur J Radiol. 2011. doi:10.1016/j.ejrad.2011.01.006
Cimmino MA, Innocenti S, Livrone F, Magnaguagno F, Silvestri E, Garlaschi G. Dynamic gadolinium-enhanced magnetic resonance imaging of the wrist in patients with rheumatoid arthritis can discriminate active from inactive disease. Arthritis Rheum. 2003;48(5):1207–13.
Kirkhus E, Bjørnerud A, Thoen J, Johnston V, Dale K, Smith HJ. Contrast-enhanced dynamic magnetic resonance imaging of finger joints in osteoarthritis and rheumatoid arthritis: an analysis based on pharmacokinetic modeling. Acta Radiol. 2006;47(8):845–51.
Fouque-Aubert A, Chapurlat R, Miossec P, Delmas PD. A comparative review of the different techniques to assess hand bone damage in rheumatoid arthritis. Joint Bone Spine. 2010;77(3):212–7.
Ostendorf B, Mattes-György K, Reichelt DC, Blondin D, Wirrwar A, Lanzman R, et al. Early detection of bony alterations in rheumatoid and erosive arthritis of finger joints with high-resolution single photon emission computed tomography, and differentiation between them. Skeletal Radiol. 2010;39(1):55–61.
Ostergaard M, Lorenzen I, Henriksen O. Dynamic gadolinium-enhanced MR imaging in active and inactive immunoinflammatory gonarthritis. Acta Radiol. 1994;35(3):275–81.
Vasanth L, Foo L, Potter H, Adler R, Finzel K, Pavlov H, et al. Using magnetic resonance angiography to measure abnormal synovial blood vessels in early inflammatory arthritis: a new imaging biomarker? J Rheumatol. 2010;37(6):1129–35.
Aletaha D, Funovits J, Smolen JS. Physical disability in rheumatoid arthritis is associated with cartilage damage rather than bone destruction. Ann Rheum Dis. 2011;70(5):733–9.
Smolen JS, Aletaha D, Steiner G. Does damage cause inflammation? Revisiting the link between joint damage and inflammation. Ann Rheum Dis. 2009;68(2):159–62.
Hollander AP, Atkins RM, Eastwood DM, Dieppe PA, Elson CJ. Human cartilage is degraded by rheumatoid arthritis synovial fluid but not by recombinant cytokines in vitro. Clin Exp Immunol. 1991;83(1):52–7.
György B, Tóthfalusi L, Nagy G, Pásztói M, Géher P, Lörinc Z, et al. Natural autoantibodies reactive with glycosaminoglycans in rheumatoid arthritis. Arthritis Res Ther. 2008;10(5):R110.
Lindqvist E, Eberhardt K, Bendtzen K, Heinegård D, Saxne T. Prognostic laboratory markers of joint damage in rheumatoid arthritis. Ann Rheum Dis. 2005;64(2):196–201.
Turesson C, Bergström U, Jacobsson LT, Truedsson L, Berglund G, Saxne T. Increased cartilage turnover and circulating autoantibodies in different subsets before the clinical onset of rheumatoid arthritis. Ann Rheum Dis. 2011;70(3):520–2.
Gandy SJ, Brett AD, Dieppe PA, Keen MC, Maciewicz RA, Taylor CJ, et al. Measurement of cartilage volumes in rheumatoid arthritis using MRI. Br J Radiol. 2005;78(925):39–45.
McQueen F, Clarke A, McHaffie A, Reeves Q, Williams M, Robinson E, et al. Assessment of cartilage loss at the wrist in rheumatoid arthritis using a new MRI scoring system. Ann Rheum Dis. 2010;69(11):1971–5.
Tiderius CJ, Sandin J, Svensson J, Dahlberg LE, Jacobsson L. Knee cartilage quality assessed with dGEMRIC in rheumatoid arthritis patients before and after treatment with a TNF inhibitor. Acta Radiol. 2010;51(9):1034–7.
Girardi M, Kay J. Elston DM, Leboit PE, Abu-Alfa A, Cowper SE. Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J Am Acad Dermatol. 2011; in press.
Prince MR, Zhang H, Zou Z, Staron RB, Brill PW. Incidence of immediate gadolinium contrast media reactions. AJR Am J Roentgenol. 2011;196(2):W138–143.
Wang Y, Alkasab TK, Narin O, Nazarian RM, Kaewlai R, Kay J et al. Incidence of nephrogenic systemic fibrosis after adoption of restrictive gadolinium-based contrast agent guidelines. Radiology. 2011;260(1):105–11.
Burstein D, Velyvis J, Scott KT, Stock KW, Kim YJ, Jaramillo D, et al. Protocol issues for delayed Gd(DTPA)(2-)-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med. 2001;45(1):36–41.
Gillis A, Bashir A, McKeon B, Scheller A, Gray ML, Burstein D. Magnetic resonance imaging of relative glycosaminoglycan distribution in patients with autologous chondrocyte transplants. Invest Radiol. 2001;36(12):743–8.
Bashir A, Gray ML, Boutin RD, Burstein D. Glycosaminoglycan in articular cartilage: in vivo assessment with delayed Gd(DTPA)(2-)-enhanced MR imaging. Radiology. 1997;205(2):551–8.
Sanz R, Martí-Bonmatí L, Rodrigo JL, Moratal D. MR pharmacokinetic modeling of the patellar cartilage differentiates normal from pathological conditions. J Magn Reson Imaging. 2008;27(1):171–7.
Christensen AF, Hørslev-Petersen K, Christgau S, Lindegaard HM, Lottenburger T, Junker K, et al. Uncoupling of collagen II metabolism in newly diagnosed, untreated rheumatoid arthritis is linked to inflammation and antibodies against cyclic citrullinated peptides. J Rheumatol. 2010;37(6):1113–20.
Acknowledgements
We thankfully acknowledge the support of Mrs. E. Rädisch, Ms. L. Dor and Mr. Ch. Nowak in data acquisition and image post-processing. This study was supported by a grant of the Heinrich-Heine-University of Düsseldorf Research Committee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buchbender, C., Scherer, A., Kröpil, P. et al. Cartilage quality in rheumatoid arthritis: comparison of T2* mapping, native T1 mapping, dGEMRIC, ΔR1 and value of pre-contrast imaging. Skeletal Radiol 41, 685–692 (2012). https://doi.org/10.1007/s00256-011-1276-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-011-1276-2