Abstract
Objectives
To establish baseline T2* and T1Gd values of glenohumeral cartilage at 3 T.
Methods
Forty asymptomatic volunteers (mean age: 24.8 ± 2.2 years) without shoulder abnormalities were included. The MRI protocol comprised a double-echo steady-state (DESS) sequence for morphological cartilage evaluation, a gradient-echo multiecho sequence for T2* assessment, and a gradient-echo dual-flip-angle sequence for T1Gd mapping. Statistical assessment involved a one-way analysis of variance (ANOVA) to identify the differences between various regions of the glenohumeral joint and intraclass correlation (ICC) analysis comparing repetitive T2* and T1Gd measures to assess intra- and interobserver reliability.
Results
Both techniques revealed significant differences between superior and inferior glenohumeral cartilage demonstrating higher T2* (26.2 ms vs. 23.2 ms, P value < 0.001) and T1Gd (750.1 ms vs. 720.2 ms, P value = 0.014) values in the superior regions. No trend was observed in the anterior-posterior measurement (P value range: 0.279–1.000). High intra- and interobserver agreement (ICC value range: 0.895–0.983) was noted for both T2* and T1Gd mapping.
Conclusions
T2* and T1Gd mapping are reliable in the assessment of glenohumeral cartilage. The values from this study can be used for comparison to identify cartilage degeneration in patients suffering from shoulder joint abnormalities.
Key Points
• T2* mapping and dGEMRIC are sensitive to collagen degeneration and proteoglycan depletion.
• This study aimed to establish baseline T2*/dGEMRIC values of glenohumeral cartilage.
• Both techniques revealed significant differences between superior and inferior glenohumeral cartilage.
• High intra-/interreader agreement was noted for both T2* mapping and dGEMRIC.
• These baseline normal values should be useful when identifying potential degeneration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) histologically expresses a progressive disorganisation of collagen fibres, a decrease in water content and glycosaminoglycan (GAG) depletion, and multiple phases and grades of degeneration may occur in the same joint at the same time [1]. Magnetic resonance imaging (MRI) is a well-established diagnostic instrument for the morphological assessment of articular cartilage and its phases in the process of degeneration. However, its ability to visualise subtle articular cartilage matrix alterations that occur early in the course of OA remains limited [2].
Parametric MRI mapping techniques, such as delayed gadolinium-enhanced MRI of cartilage (dGEMRIC, T1Gd mapping) and T2 or T2* relaxation time mapping, which are sensitive to collagen fibre orientation, change in water content and proteoglycan depletion, can be meaningful additions to the diagnostic routine to support decision making, treatment monitoring and follow-up of cartilage abnormalities.
The dGEMRIC index (T1Gd relaxation time in milliseconds) reflects the uptake of negatively charged gadolinium-containing contrast agent within cartilage wherein the uptake is inversely proportional to the negatively charged tissue GAG content [3]. Because gadolinium reduces the T1 relaxation time, higher T1Gd relaxation values will be measured in healthy cartilage contrary to those in degenerated cartilage because of the high amount of gadolinium in the latter. The dGEMRIC approach is validated and has been used in several in vivo and in vitro studies [3–14].
T2 and T2* relaxation time mapping is sensitive to water content and interactions between water molecules and collagen fibres [15, 16]. In simplified terms, high T2 or T2* values are indicators of a high water content and superior water molecule mobility. Accordingly, in healthy articular cartilage, a decrease in T2 and T2* values can be noted toward the deep cartilage zones because of the uniform perpendicular collagen fibre orientation and high proteoglycan content that promote water molecule restriction and T2 decay. Based on the stage of cartilage degeneration, both T2 and T2* values can increase or decrease. However, because T2* is the relaxation time obtained with a gradient-echo (GRE) pulse sequence that contains the transverse relaxation time T2 and coherent de-phasing effects, which arise from spins within a voxel having different precession frequencies owing to local field variations, within the net T2* decay, T2* mapping will be influenced by both the transverse relaxation (T2) and by local susceptibility fields [17]. The lower T2* mapping values in general reflect the additional contribution of microscopic susceptibility fields to T2 relaxation. T2* mapping with its short acquisition time by using GRE MRI and the prospect of 3D biochemically sensitive cartilage evaluation is a potential alternative to the multi-spin-echo-derived T2 mapping technique.
Numerous studies have demonstrated the potential of dGEMRIC and T2* mapping in knee and hip joint cartilage [4, 6, 7, 9, 11–16, 18–30]. However, despite one dGEMRIC study that reports the contrast dynamics in hyaline and fibrous cartilage after intra-articular gadolinium injection in cadaveric shoulders [31], to the best of our knowledge, dGEMRIC and T2* mapping have not yet been performed in the shoulder joint. Furthermore, currently normative data are lacking for both dGEMRIC and T2* mapping in the glenohumeral joint; these are critical for defining the “abnormal” state in order to develop a standard of care for radiologists and referring orthopaedic surgeons and rheumatologists.
The purpose of this prospective study was to evaluate the feasibility of T2* and T1Gd mapping to assess glenohumeral joint cartilage and to establish baseline values of healthy glenohumeral cartilage at 3 T. Therefore, we performed a cross-sectional analysis including a cohort of healthy, asymptomatic, young adult volunteers between 20 and 30 years of age with no obvious suspected cartilage abnormalities.
Materials and methods
Study population
The study group compromised 40 healthy, asymptomatic medical students from the local university between 20 and 30 years of age. This included 18 men and 22 women in whom 20 right shoulders and 20 left shoulders were imaged. The mean age was 24.8 ± 2.2 years ranging from 21.6 years to 29.1 years. Exclusion criteria were history and/or clinical observations indicating any shoulder joint abnormalities or contraindication for undergoing intravenous gadolinium contrast-enhanced MRI.
This study met all regulations of the local ethics committee and all volunteers provided written informed consent before the study. Clinical findings and patient history were obtained by an orthopaedic consultant with a special interest in shoulder surgery.
Magnetic resonance imaging
Magnetic resonance imaging was performed in a 3-T system (Magnetom Trio, Siemens Medical Solutions, Erlangen, Germany) using a flexible four-channel body matrix phased-array coil. All volunteers were examined in supine position with the involved arm at their side in neutral rotation, which was supported by sponges and adjustable straps.
The MRI protocol included a pre- and a post-contrast examination. The pre-contrast protocol consisted of a 3D double-echo steady state (DESS) sequence with water excitation for morphological cartilage assessment and a 3D multiecho data image combination (MEDIC) sequence using six consecutive echoes in order to assess the T2* decay. This was followed by the intravenous injection of FDA-approved Gd-DOTA− (0.4 ml/kg, 0.2 mmol Gd/kg, Dotarem, Guerbet, Roissy, France). Subsequently, the subjects were asked to move around until MRI was continued 45 min after the contrast agent injection. The post-contrast MRI protocol included a B1 unenhanced sequence to assess field heterogeneity correction and a dual-flip angle (FA) 3D GRE sequence with volumetric interpolated breathhold examination (VIBE) for T1Gd assessment. T1Gd and T2* maps were derived by an inline processing package (SyngoMapIt, Siemens Medical Solutions, Erlangen, Germany), which utilises a non-linear least-squares fitting routine. Geometric imaging parameters were similar for DESS, MEDIC and VIBE imaging.
Of note, the sequence setting in this prospective study favoured superior glenohumeral image quality with high cartilage contrast rather than evaluation of soft tissue structures, such as the labrum or rotator cuff, which necessitate various pulse sequences with T1-, T2- and PD-weighting in coronal, sagittal and axial planes. Further details on the imaging parameters are provided in Table 1.
Image analyses
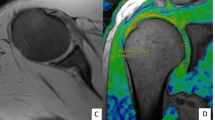
The 3D DESS, T2* and T1Gd data sets were transferred to a Leonardo® workstation (Siemens Medical Solutions, Erlangen, Germany) to perform further analyses. From each 3D data set, coronal oblique reformats with a slice thickness of 0.5 mm perpendicular to the glenoid surface were generated by using multiplanar reconstruction (MPR; Fig. 1). Of those DESS, T2* and T1Gd reformats, four corresponding reformats were selected to assess the glenohumeral cartilage at four sections of the joint: (1) anterior, (2) anterior–central, (3) posterior–central and (4) posterior.
Within each section, region of interest (ROI) analysis was performed defining the glenohumeral cartilage between the superior and inferior glenoidal margin. Because of low cartilage thickness and a high degree of congruency between the articular surfaces of the glenoid and the humeral head, glenoidal and humeral cartilage layers were not reliably distinguishable despite the use of a 3-T MRI system and the ability to perform high-resolution imaging (isotropic resolution of 0.5 mm3). Therefore, ROI analysis included glenoidal and humeral cartilage as one combined entity.
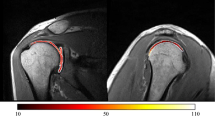
The glenohumeral cartilage was further divided into two similar portions with the portion close to the superior rim being the superior region and the portion close to the inferior rim being the inferior region. Thus, eight regions (one superior region and one inferior region per section) were analysed within each shoulder. In those, bulk T2* and T1Gd values were assessed by means of ROI analysis. The geometric shape of the ROI squares was defined by multiple marker points that facilitated accurate ROI placement even in curved cartilage regions wherein the corresponding DESS reformats served as reference for accurate placement of the ROI squares within cartilage bounds for bulk T2* and T1Gd assessment (Fig. 2).
Within each coronal oblique reformat, bulk T2* and T1Gd values were assessed in two regions (superior and inferior) by means of region of interest (ROI) analysis. Corresponding DESS reformats served as reference for accurate placement of the ROI squares within cartilage bounds. Of note, because of low cartilage thickness and a high degree of congruency between the articular surfaces, glenoidal and humeral cartilage layers were not reliably distinguishable. Therefore, ROI analysis included glenoidal and humeral cartilage as one combined entity
All primary T2* and T1Gd measurements were performed by one orthopaedic surgeon with a special interest in biochemical-sensitive MRI, whereas, for reliability assessment, T2* and T1Gd measurements were repeated by the latter and by a second observer (orthopaedic consultant) in ten randomly selected volunteers. The morphological cartilage evaluation was performed in consensus by two consultant radiologists, both of whom are specialists in musculoskeletal imaging. Of note, all ROIs with any morphologically evident cartilage changes were excluded.
Statistical analyses
In this study, IBM SPSS@ software (Version 20.0; IBM Corp., Armonk, NY, USA) was used for statistical analyses. T2* and T1Gd measurements are reported as mean values ± standard deviations. The one-way analysis of variance (ANOVA) including Bonferroni correction for multiple comparisons was applied in order to identify statistically significant differences between the T2* and T1Gd values of various regions of the glenohumeral joint. Inter- and intraobserver agreement was calculated by intraclass correlation (ICC) analysis (pair-wise correlation, absolute agreement). P values below 0.05 were considered to demonstrate statistical significance.
Results
A total of 320 regions underwent assessment (8 regions in each of the 40 volunteers). The complete T2* data set of two volunteers (16 regions) and one complete T1Gd data set (8 regions) were excluded because they were compromised by motion artefacts. In addition, one volunteer was excluded because morphological cartilage assessment revealed evident cartilage damage. Therefore, 296 ROIs (T2* mapping) and 304 ROIs (T1Gd mapping) were further analysed.
The mean size of the ROIs for T2* assessment was 0.14 ± 0.05 cm2 (range: 0.04–0.43 cm2), which corresponds to 56.2 ± 19.7 pixels (range: 15–149 pixels). The mean size of the ROIs for T1Gd assessment was 0.14 ± 0.05 cm2 (range: 0.04–0.35 cm2), corresponding to 54.9 ± 18.2 pixels (range: 17–141 pixels).
The total T2* mean value, factoring in the T2* values of all ROIs, was 24.7 ± 3.7 ms (range: 14.4–36.4 ms). The total T1Gd value was 735.3 ± 106.2 ms (range: 487.4–1020.2 ms). The T2* and T1Gd distributions in glenohumeral cartilage of asymptomatic volunteers with morphologically normal appearing cartilage are demonstrated in Tables 2 and 3 and in a bar diagram (Fig. 3).
Bar diagram demonstrating the T2* and T1Gd distribution in morphologically normal-appearing glenohumeral cartilage in various sections: (1) anterior, (2) anterior–central, (3) posterior–central and (4) posterior, superior and inferior. Notably, both techniques revealed significantly higher values in the superior regions, whereas no trend was observed in the anterior–posterior measurement in which the T2* and T1Gd mapping values were similar
Both techniques revealed statistically significant differences between the superior and the inferior regions demonstrating higher T2* values (26.2 vs. 23.2 ms, P value < 0.001) and higher T1Gd values (750.1 vs. 720.2 ms, P value = 0.014) in the superior regions. On the other hand, no trend was observed in the anterior-posterior measurement in which the T2* mapping values (P value = 0.747) and T1Gd mapping values (P value = 0.153) were similar (P value range interregion comparison: 0.279–1.000).
High intra- and interobserver agreement was noted for both T2* (ICC values: 0.937 and 0.895, P < 0.001) and T1Gd (ICC values: 0.983 and 0.950, P < 0.001) mapping.
Discussion
With rapid advances in shoulder joint preservation surgery and cartilage transplantation, and surgical outcomes being directly related to the degree of pre-existing OA, adequate preoperative detection of early cartilage degeneration is becoming increasingly important. Where standard cartilage MR imaging may be sensitive to gross macroscopic alterations, e.g. cartilage aberrations and fragmentations or defects, which are signs of fairly advanced OA, biochemically sensitive MR imaging techniques such as T2* mapping and dGEMRIC hold great promise as they aim to detect subtle cartilage matrix alterations that occur early in the course of OA.
Although T2* mapping and dGEMRIC have been extensively investigated in knee and hip joint cartilage, only one preliminary dGEMRIC study has analysed the contrast medium dynamics in cadaveric shoulders after intra-articular gadolinium injection, and no studies have been performed in the shoulder joint for T2* mapping and dGEMRIC. In addition, the current literature lacks normative data for both of these modalities in the glenohumeral joint that are necessary to differentiate between “normal” and “abnormal”. Considering that MRI of shoulder joint cartilage constitutes its own technical challenges related to the low cartilage thickness and high degree of congruency between the articulating surfaces, and the fact that the cartilage ultrastructure and/or molecular composition are known to vary topographically, there was a need to further elaborate on the potential of T2* mapping and dGEMRIC in shoulder joint cartilage.
Therefore, the purpose of this cross-sectional study on healthy, asymptomatic, young adult volunteers with no obvious suspected cartilage abnormalities was to evaluate the feasibility of T2*- and T1Gd mapping (dGEMRIC) to assess glenohumeral joint cartilage and to elaborate baseline T1Gd and T2* values in the glenohumeral cartilage at 3 T.
Based on our results demonstrating high intra- and interobserver agreement for both T2* and dGEMRIC, we consider both techniques to be reliable tools for assessing glenohumeral joint cartilage. Furthermore, we believe that our study of 39 asymptomatic volunteers (one dropout), which revealed mean T2* and T1Gd values of glenohumeral cartilage that, despite differences in the methodology and study population, are similar to those of morphologically normal-appearing cartilage in other joints (reported mean T2* values ranging from 11.8 ms to 32.7 ms and T1Gd values ranging from 540 ms to 710 ms) [7, 8, 13, 22, 25, 28–30, 32], provides sufficient baseline data on the pattern of T2* and T1Gd in the glenohumeral joint cartilage for future studies.
Notably, a statistically significant drop in both T2* and T1Gd values was noted in the inferior regions (P values: <0.001 and 0.014). This may be related to differences in the biochemical composition (collagen content and its orientation, water and GAG content), likely due to increased cartilage loading in the superior regions. A similar T1Gd pattern has been noted in the hip joint with superior T1Gd values in the weight-bearing superior regions [22, 23], which correlates with the GAG increase in this region [33, 34]. However, the present study is preliminary, and further studies including a gold standard such as intraoperative findings or histological analyses are required to confirm our results. Furthermore, the magic-angle effect characterised by increasing T2/T2* values towards an angle of ∼55° to the main magnetic field [35] is another factor that has to be monitored closely, particularly in spherically shaped joints like the shoulder.
This study has limitations. Despite using a 3-T MRI system to enhance image resolution, spatial image resolution was not sufficient to reliably differentiate between glenoidal and humeral head cartilage. Therefore, ROI analysis in this study alluded to glenoidal and humeral cartilage as one entity wherein joint fluid may have overestimated the T2* values in certain areas. This is contrary to the T1Gd assessment in which inclusion of gadolinium-containing joint fluid mapping reduces the T1 value. Of note, when mapping glenoidal and humeral cartilage as one entity, inclusion of joint fluid will increase with proceeding cartilage degeneration and cartilage thinning generating further overestimation (T2* measures) or underestimation (T1Gd measures). In addition, the evaluation of glenohumeral cartilage by standard MRI does not verify the real status of cartilage. Therefore, we may have missed cartilage lesions, which could explain the variability of T2* and T1Gd measures in this study.
In conclusion, we were able to outline the feasibility of T2* mapping and dGEMRIC for assessing glenohumeral joint cartilage at 3 T. T2* and T1Gd mapping values were similar to those of previously reported studies assessing morphologically normal-appearing articular cartilage. Notably, we recognised a consistent trend of T2* and T1Gd with significantly higher values in the superior regions, which has to be considered when interpreting cartilage changes in patients suffering from shoulder joint abnormalities. Furthermore, these observations form a reliable basis for designing appropriate comparative correlation studies that aim to analyse the pattern of cartilage degeneration, which may be specific for various types of shoulder joint pathology. This cross-sectional analysis of healthy, asymptomatic, young adult volunteers also yields sufficient reference values for upcoming studies on therapeutic strategies in which cutoff values for either conservative or surgical treatment can be elaborated. These studies would comprise controlled longitudinal follow-up measurements that consider both baseline (preoperative) T2* / T1Gd and outcome measures in those treated patients. In consideration of the high intra- and interobserver agreement, we believe that both T2* mapping and dGEMRIC are reproducible methods to evaluate glenohumeral joint cartilage.
Abbreviations
- OA:
-
Osteoarthritis
- GAG:
-
Glycosaminoglycan
- dGEMRIC:
-
Delayed gadolinium-enhanced MRI of cartilage
- GRE:
-
Gradient echo
- DESS:
-
Double-echo steady state
- MEDIC:
-
Multiecho data image combination
- FA:
-
Flip angle
- VIBE:
-
Volumetric interpolated breathhold examination
- ROI:
-
Region of interest
- ANOVA:
-
Analysis of variance
- ICC:
-
Intraclass correlation
References
Lohmander LS (2004) Markers of altered metabolism in osteoarthritis. J Rheumatol Suppl 70:28–35
Link TM, Stahl R, Woertler K (2007) Cartilage imaging: motivation, techniques, current and future significance. Eur Radiol 17:1135–1146
Burstein D, Bashir A, Gray ML (2000) MRI techniques in early stages of cartilage disease. Invest Radiol 35:622–638
Tiderius CJ, Olsson LE, Leander P, Ekberg O, Dahlberg L (2003) Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) in early knee osteoarthritis. Magn Reson Med 49:488–492
Williams A, Gillis A, McKenzie C et al (2004) Glycosaminoglycan distribution in cartilage as determined by delayed gadolinium-enhanced MRI of cartilage (dGEMRIC): potential clinical applications. AJR Am J Roentgenol 182:167–172
Burstein D, Velyvis J, Scott KT et al (2001) Protocol issues for delayed Gd(DTPA)(2-)-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med 45:36–41
Kim YJ, Jaramillo D, Millis MB, Gray ML, Burstein D (2003) Assessment of early osteoarthritis in hip dysplasia with delayed gadolinium-enhanced magnetic resonance imaging of cartilage. J Bone Joint Surg Am 85-A:1987–1992
Tiderius CJ, Jessel R, Kim YJ, Burstein D (2007) Hip dGEMRIC in asymptomatic volunteers and patients with early osteoarthritis: the influence of timing after contrast injection. Magn Reson Med 57:803–805
Cunningham T, Jessel R, Zurakowski D, Millis MB, Kim YJ (2006) Delayed gadolinium-enhanced magnetic resonance imaging of cartilage to predict early failure of Bernese periacetabular osteotomy for hip dysplasia. J Bone Joint Surg Am 88:1540–1548
Jessel RH, Zilkens C, Tiderius C, Dudda M, Mamisch TC, Kim YJ (2009) Assessment of osteoarthritis in hips with femoroacetabular impingement using delayed gadolinium enhanced MRI of cartilage. J Magn Reson Imaging 30:1110–1115
Trattnig S, Marlovits S, Gebetsroither S et al (2007) Three-dimensional delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) for in vivo evaluation of reparative cartilage after matrix-associated autologous chondrocyte transplantation at 3.0 T: Preliminary results. J Magn Reson Imaging 26:974–982
Mamisch TC, Dudda M, Hughes T, Burstein D, Kim YJ (2008) Comparison of delayed gadolinium enhanced MRI of cartilage (dGEMRIC) using inversion recovery and fast T1 mapping sequences. Magn Reson Med 60:768–773
Bittersohl B, Hosalkar HS, Haamberg T et al (2009) Reproducibility of dGEMRIC in assessment of hip joint cartilage: a prospective study. J Magn Reson Imaging 30:224–228
Bittersohl B, Hosalkar HS, Hughes T et al (2009) Feasibility of T2* mapping for the evaluation of hip joint cartilage at 1.5 T using a three-dimensional (3D), gradient-echo (GRE) sequence: a prospective study. Magn Reson Med 62:896–901
Nieminen MT, Rieppo J, Toyras J et al (2001) T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med 46:487–493
Mosher TJ, Dardzinski BJ (2004) Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol 8:355–368
Haacke E, Brown R, Thompson M, Venkatesan R (1999) MRI Physical Principles and Sequence Design. Wiley-Liss, New York
Bashir A, Gray ML, Boutin RD, Burstein D (1997) Glycosaminoglycan in articular cartilage: in vivo assessment with delayed Gd(DTPA)(2-)-enhanced MR imaging. Radiology 205:551–558
Miese FR, Zilkens C, Holstein A et al (2011) Assessment of early cartilage degeneration after slipped capital femoral epiphysis using T2 and T2* mapping. Acta Radiol 52:106–110
Welsch GH, Mamisch TC, Domayer SE et al (2008) Cartilage T2 assessment at 3-T MR imaging: in vivo differentiation of normal hyaline cartilage from reparative tissue after two cartilage repair procedures–initial experience. Radiology 247:154–161
Williams A, Sharma L, McKenzie CA, Prasad PV, Burstein D (2005) Delayed gadolinium-enhanced magnetic resonance imaging of cartilage in knee osteoarthritis: findings at different radiographic stages of disease and relationship to malalignment. Arthritis Rheum 52:3528–3535
Bittersohl B, Hosalkar HS, Werlen S, Trattnig S, Siebenrock KA, Mamisch TC (2011) dGEMRIC and subsequent T1 mapping of the hip at 1.5 Tesla: normative data on zonal and radial distribution in asymptomatic volunteers. J Magn Reson Imaging 34:101–106
Bittersohl B, Steppacher S, Haamberg T et al (2009) Cartilage damage in femoroacetabular impingement (FAI): preliminary results on comparison of standard diagnostic vs delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC). Osteoarthr Cartil/OARS Osteoarthr Res Soc 17:1297–1306
Mamisch TC, Hughes T, Mosher TJ et al (2011) T2 star relaxation times for assessment of articular cartilage at 3 T: a feasibility study. Skelet Radiol
Pollard TC, McNally EG, Wilson DC et al (2010) Localized cartilage assessment with three-dimensional dGEMRIC in asymptomatic hips with normal morphology and cam deformity. J Bone Joint Surg Am 92:2557–2569
Marik W, Apprich S, Welsch GH, Mamisch TC, Trattnig S (2011) Biochemical evaluation of articular cartilage in patients with osteochondrosis dissecans by means of quantitative T2- and T2*-mapping at 3 T MRI: A feasibility study. Eur J Radiol
Welsch GH, Mamisch TC, Hughes T et al (2008) In vivo biochemical 7.0 Tesla magnetic resonance: preliminary results of dGEMRIC, zonal T2, and T2* mapping of articular cartilage. Invest Radiol 43:619–626
Bittersohl B, Miese FR, Hosalkar HS et al (2012) T2 * mapping of hip joint cartilage in various histological grades of degeneration. Osteoarthr Cartil / OARS Osteoarthr Res Soc
Bittersohl B, Miese FR, Hosalkar HS et al (2012) T2* mapping of acetabular and femoral hip joint cartilage at 3 T: A prospective controlled study. Invest Radiol
Zilkens C, Miese F, Kim YJ et al (2012) Three-dimensional delayed gadolinium-enhanced magnetic resonance imaging of hip joint cartilage at 3 T: A prospective controlled study. Eur J Radiol
Wiener E, Hodler J, Pfirrmann CW (2009) Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) of cadaveric shoulders: comparison of contrast dynamics in hyaline and fibrous cartilage after intraarticular gadolinium injection. Acta Radiol 50:86–92
Williams A, Mikulis B, Krishnan N, Gray M, McKenzie C, Burstein D (2007) Suitability of T(1Gd) as the dGEMRIC index at 1.5 T and 3.0 T. Magn Reson Med 58:830–834
Yoshida K, Azuma H (1982) Contents and compositions of glycosaminoglycans in different sites of the human hip joint cartilage. Ann Rheum Dis 41:512–519
Venn MF (1978) Variation of chemical composition with age in human femoral head cartilage. Ann Rheum Dis 37:168–174
Xia Y (2000) Magic-angle effect in magnetic resonance imaging of articular cartilage: a review. Invest Radiol 35:602–621
Acknowledgements
This study was funded by a research grant from the “German Osteoarthritis Aid” (Deutsche Arthrose-Hilfe e.V.). The authors have full control of all primary data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bittersohl, B., Miese, F.R., Dekkers, C. et al. T2* mapping and delayed gadolinium-enhanced magnetic resonance imaging in cartilage (dGEMRIC) of glenohumeral cartilage in asymptomatic volunteers at 3 T. Eur Radiol 23, 1367–1374 (2013). https://doi.org/10.1007/s00330-012-2718-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-012-2718-5