Abstract
Thraustochytrids have been applied for industrial production of the omega-3 fatty acid docosahexaenoic (DHA) since the 1990s. During more than 20 years of research on this group of marine, heterotrophic microorganisms, considerable increases in DHA productivities have been obtained by process and medium optimization. Strains of thraustochytrids also produce high levels of squalene and carotenoids, two other commercially interesting compounds with a rapidly growing market potential, but where yet few studies on process optimization have been reported. Thraustochytrids use two pathways for fatty acid synthesis. The saturated fatty acids are produced by the standard fatty acid synthesis, while DHA is synthesized by a polyketide synthase. However, fundamental knowledge about the relationship between the two pathways is still lacking. In the present review, we extract main findings from the high number of reports on process optimization for DHA production and interpret these in the light of the current knowledge of DHA synthesis in thraustochytrids and lipid accumulation in oleaginous microorganisms in general. We also summarize published reports on squalene and carotenoid production and review the current status on strain improvement, which has been hampered by the yet very few published genome sequences and the lack of tools for gene transfer to the organisms. As more sequences now are becoming available, targets for strain improvement can be identified and open for a system-level metabolic engineering for improved productivities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thraustochytrids are unicellular, eukaryote, heterotrophic, and obligate marine microorganisms, commonly found in seawater and sediments, with the highest abundance in nutrient-rich areas, such as mangrove forests (Raghukumar 2002; Singh et al. 2014). Thraustochytrid strains are able to accumulate high levels of lipids as triacylglycerols, with a high content of the long-chain omega-3 (ω3) fatty acid docosahexaenoic acid (DHA). High-productivity strains can be cultivated to cell densities above 100 g/l dry weight in 4 days and accumulate lipid levels in the range of 50–70 % of cell dry weight (CDW) with DHA constituting 30–70 % of the total fatty acids (Chang et al. 2013a; Li et al. 2015; Raghukumar 2008). The first reports on use of thraustochytrids for production of DHA appeared early in the 1990s and were initiated by the rapidly increasing understanding of the benefits of long-chain polyunsaturated (LC-PUFA) ω3 fatty acids for human health. Production of DHA-rich oils based on thraustochytrids was commercialized a few years later by the US company OmegaTech, which later was acquired by Martek, and is now a part of DSM. The history of the development of the industrial production of DHA-rich oils from thraustochytrids by OmegaTech has been reviewed by Barclay et al. (2010), describing their strategies for strain isolation and further developments to the 150-m3 production scale.
The taxonomy and phylogeny of thraustochytrids have been extensively described by others, and DHA-producing species of thraustochytrids have been identified within all the thraustochytrid genera (Honda et al. 1999; Yokoyama and Honda 2007; Yokoyama et al. 2007). Taxonomic reclassifications within the thraustochytrids have resulted in the establishment of several new genera. For instance, Aurantiochytrium was established as a separate genus in 2007, and some species previously classified as Schizochytrium were moved to the new genus (Yokoyama and Honda 2007). The highest cell densities and DHA productivities have been reported for species of Schizochytrium, Aurantiochytrium, and Ulkenia. The ability of thraustochytrids to produce high levels of DHA is attributed to the presence of an alternative pathway for DHA synthesis, catalyzed by a polyketide synthase (PKS) enzyme complex. The PKS also generates another LC-PUFA, docosapentaenoic acid (DPA; C22:5 ω6), which is a characteristic fatty acid for thraustochytrids (Hauvermale et al. 2006; Lippmeier et al. 2009; Metz et al. 2001).
The extensive research on thraustochytrids has mainly been motivated by their properties as DHA producers. However, during the strain screening programs, also other commercially interesting products have been identified. For instance, some strains produce high levels of carotenoids, others squalene. These products have established, and growing, markets in food, feed, and pharma, currently produced from other raw materials. As saprophytic organisms, thraustochytrids also produce extracellular enzymes, and some strains have been reported to produce extracellular polysaccharides, see, e.g., Gupta et al. (2012) and Singh et al. (2014). However, based on the reported production levels, the intracellular, lipid-related compounds squalene and carotenoids seem to have the highest potential for a future industrial production by thraustochytrids. Figure 1 shows the biosynthetic pathways for DHA, squalene, and carotenoids.
Metabolic network for the biosynthesis of DHA, squalene, steroids, and carotenoids from glucose and glycerol. DHA is produced by the PKS pathway (see Fig. 2), while squalene, steroids, and carotenoids are produced via the mevalonate pathway (Sun et al. 2014). Dashed arrows indicate multiple enzymatic steps, and dashed, grey arrows in addition indicate reactions where energy carriers have been omitted
The most recent reviews on thraustochytrids thoroughly cover isolation methods and analytical methods (Gupta et al. 2012), ecological impacts, distribution and role in marine habitats (Singh et al. 2014), and metabolic pathways and enzymes involved in DHA synthesis (Xie and Wang 2015). Results from the high number of process optimization studies are also referred, but not as a main topic. In the present review, we extract the main findings from the reports on process optimization for DHA production and interpret these in light of the current knowledge of DHA synthesis in thraustochytrids and lipid accumulation in oleaginous microorganisms in general. We also summarize the published reports on squalene and carotenoid production and review the current status on strain improvement and available genetic tools for thraustochytrids.
DHA—yet the only commercial product from thraustochytrids
Applications of DHA and market prospects
DHA-rich oils from thraustochytrids are currently on the market as dietary supplements. The main source of the marine ω3 fatty acids eicosapentaenoic acid (EPA) and DHA are fish oils. Approximately 200,000 t fish oils are used in products for the human markets, while the production of microbial ω3-rich oils constituted only 5000 t in 2011 with thraustochytrids and the heterotrophic microalgae Chrypthecodinium cohnii as production organisms. Dietary supplements constitute the largest market share of 55 % for ω3 products, followed by functional food and beverages, and pharmaceuticals. The ω3-PUFA market is projected to show an annual growth rate of 12.8 % between 2014 and 2019 and is expected to be worth USD 4300 millions by 2019 (www.marketsandmarkets.com).
EPA and DHA are also important constituents in the feed for marine aquaculture. The annual production of fish oil is in the order of 1 × 106 t, of which 71 % was used for aquafeed in 2010 (FAO 2014). The global catches of the fish stocks used for oil production have reached its maximum limit. A future growth of the marine aquaculture will therefore require new sources for these fatty acids in substantial volumes, and microbial oils have been proposed as a solution (Olsen 2011). This will represent a large-volume market but with far lower prices than for the current human products.
DHA synthesis in thraustochytrids
In oleaginous yeast, phototrophic microalgae, and bacteria (e.g., Rhodococcus spp.), saturated fatty acids dominate in the storage lipids, mainly C16:0 but also C14:0 and C18:0 (Alvarez and Steinbüchel 2002; Goold et al. 2015; Wang 2015). The only known oleaginous microorganisms that produce LC-ω3-PUFA as a major part of their storage lipids are thraustochytrids, the closely related labyrinthulids, and the heterotrophic dinoflagellate C. cohnii. However, EPA and DHA frequently occur in the membrane lipids of marine microorganisms, both bacteria and microalgae (Mühlroth et al. 2013; Valentine and Valentine 2004).
The thraustochytrids use a standard fatty acid synthase (FAS) enzyme complex for synthesis of the shorter, saturated fatty acids, mainly C14:0 and C16:0 (Hauvermale et al. 2006). The thraustochytrids also express some desaturases and elongases, since low levels of C16:1, C18:1, C18:2, arachidonic acid (ARA), EPA, and other unsaturated fatty acids can be found (Yokoyama and Honda 2007; Yokoyama et al. 2007). Although proven in only a few strains, it is likely that all high-level DHA-producing strains use the PKS pathway as the main generator of DHA. In this pathway, DHA is synthesized by successive elongation steps with malonyl-CoA, similar to those carried out by FAS, but omitting the removal of the double bond introduced from malonyl-CoA in most of the cycles, thereby saving reducing power (NADPH) and eliminating the need for molecular oxygen and NADPH required for the desaturases. The pathway is not fully unraveled, but hypothetical models have been proposed (Fig. 2) (Metz et al. 2001; Ratledge 2004). The PKS pathway also generates DPA (C22:5, ω6), in the order of 10 % of the fatty acids (Chaisawang et al. 2012; Hauvermale et al. 2006; Matsuda et al. 2012). This indicates some plasticity early in the DHA biosynthesis pathway allowing for the synthesis of C6:0 instead of the usual C6:1 intermediate. When new two-carbon units are added to C6:0 following the same set of reactions as shown in Fig. 2, the result will be DPA.
Alternative pathways for DHA biosynthesis. a The FAS pathway followed by elongation and desaturation. Only the ω3 pathway to DHA is depicted, while the branching point to the ω6 pathway is indicated. b The PKS pathway (Metz et al. 2001; Ratledge 2004). The number of NADPH needed at each step, or sequence of steps, is displayed to the left of the arrows, while the enzyme activities involved are indicated to the right. KS ketoacyl synthase; KR ketoreductase; DH dehydratase; DH/i bifunctional dehydratase and trans-cis isomerase, the isomerase may also move the double bond; ER enoyl reductase; Δ desaturase, the number indicates which bond (counted from the carboxyl end) is desaturated; and ELO elongase, a multifunctional enzyme with KS, KR, DH, and ER activities
FAS and PKS use the same precursors, acetyl-CoA and NADPH (Fig. 1). Reducing power in the form of NADPH is assumed to be limiting for lipid accumulation (Ratledge 2014). Malic enzyme (ME) is a main generator of NADPH for fatty acid synthesis in most, but not all, studied oleaginous microorganisms (Dulermo et al. 2015; Garay et al. 2014; Ratledge 2014). In order for ME to act as an NADPH generator for FA synthesis, it has been proposed that the enzyme has to form an integrated complex with the ATP:citrate lyase enzyme (ACL) and the FAS complex to ensure a direct channeling of acetyl-CoA into fatty acids (Ratledge 2002). It is not known whether the PKS in thraustochytrids would require a specific ME. ME activity has been demonstrated in cell-free extracts of thraustochytrids (Chaisawang et al. 2012; Chang et al., 2013b; Ren et al. 2013; Ren et al. 2009; Song et al. 2013). In time course studies, the activity of ME increased rapidly at the initiation of triacylglycerol (TAG) accumulation and continued to increase in parallel with the lipid accumulation (Ren et al. 2009; Song et al. 2013). The first NADPH-generating enzyme of the pentose phosphate pathway, glucose-6-phosphate dehydrogenase (G6PD), had its maximum activity during the active cell growth. However, more than 50 % of the maximum activity was still maintained at the end of the lipid accumulation phase (Ren et al., 2009, 2013). In a study by Song et al. (2013) using Aurantiochytrium sp. SD116, the FAS was inhibited by supplementing the growth medium with valeric acid (pentanoic acid) added continuously as pH control. Despite far less total lipids when valeric acid was added, the DHA concentration was unaffected (20 g/l at the end in both cases). The ME activity was significantly lower than in the control, but when the supply of valeric acid was terminated, the ME activity and the synthesis of C16:0 and other FAS products were restored. This study indicated that the measured ME activity was linked to the FAS, and the authors suggested that PKS does not depend on ME for NADPH generation. However, a study by Ren et al. (2009) supports a role of ME in DHA production in Schizochytrium sp. HX-308. Addition of malic acid in the rapid lipid accumulation phase increased the fraction of DHA of total fatty acids (TFAs) from 35 to 60 % and from ~8 to ~15 % of the biomass. The TFA content of the biomass increased from ~23 to ~26 %, meaning that the synthesis of other fatty acids was reduced.
Generally, oleaginous microorganisms accumulate lipids as TAGs at conditions where an essential nutrient, often nitrogen, is limiting cell division and organic carbon is in excess. The underlying mechanisms have been extensively reviewed by Ratledge (2004) and Ratledge and Wynn (2002), mainly based on studies on yeasts and filamentous fungi. Briefly, nitrogen limitation leads to a low level of AMP, reduced activity of the AMP-dependent isocitrate dehydrogenase of the tri-carboxylic acid (TCA) cycle, and accumulation of citrate, which is transported to the cytosol. ATP:citrate lyase splits citrate to oxaloacetic acid and acetyl-CoA, thereby providing a continuous supply of the fatty acid precursor acetyl-CoA in the cytosol. This enzyme has been found in all oil-accumulating microorganisms investigated, including the thraustochytrids (Chaisawang et al. 2012; Chang et al., 2013b; Janthanomsuk et al. 2015; Ren et al. 2009). Few thorough studies on the initiation of lipid accumulation in thraustochytrids have been reported. However, from studies where a defined nitrogen source has been used, it is evident that lipid accumulation is initiated when N is depleted (Jakobsen et al. 2008; Janthanomsuk et al. 2015; Qu et al., 2013b; Ren et al. 2010). When grown on media with a high N content supplied as yeast extract or peptones, TAGs accumulate also when N is still available (Chang et al., 2013a; Chang et al. 2014; Huang et al. 2012). Any explanations were not discussed by the authors but could be that the depletion of the most easily utilized amino acids and concomitant reduction in growth rate onsets the lipid accumulation, despite still high concentrations of nitrogen in the medium. Also, in continuous cultures with N as the growth-limiting compound, lipid accumulation occurred (Ethier et al. 2011; Ganuza and Izquierdo 2007). Phosphorus (P) limitation also initiated TAG accumulation in thraustochytrids (Jakobsen et al. 2008; Ren et al. 2013), but the effect of P limitation on DHA synthesis and lipid accumulation is far less studied than the effects of N limitation.
In fermentation studies where N was completely depleted, the lipid production rates decreased 1–2 days (20–50 h) after onset of the TAG accumulation. The production rates of the FAS products decreased at an earlier stage than the DHA production rates, thereby increasing the fraction of DHA of the total fatty acids towards the end of the fermentation (Chaisawang et al. 2012; Jakobsen et al. 2008; Li et al. 2015; Ren et al. 2010). A similar reduction in lipid accumulation rates in oleaginous fungi was shown to be due to inactivation of ME, and varying stability of ME was assumed to determine the maximum lipid levels obtained in different species and strains (Ratledge and Wynn 2002). In a study where high concentrations of yeast extract and peptone were fed continuously, no reduction in production rates occurred (Huang et al. 2012). In other studies where nitrogen was supplied during the lipid accumulation phase, also oxygen was limiting (see below), complicating the data interpretation. Lower DHA fraction of TFA during the lipid accumulation phase than during exponential growth has often been observed (Chaung et al. 2012; Ren et al. 2014a, b). This can, at least partly, be explained by phospholipids (PLs) as the dominating lipid class during exponential growth with nitrogen in excess, since the PLs have a higher DHA fraction than the storage lipids (Fan et al. 2007; Qu et al. 2013a; Ren et al. 2014a). During exponential growth with N in excess, PLs are the dominating or only lipid class, constituting in the order of 10 % of CDW of thraustochytrids. The PLs constitute a decreasing fraction of the total lipids (TLs) during the lipid accumulation phase. At TL contents above 60 % of CDW, neutral lipids, dominated by TAGs, constituted 90–95 % of the TL (Fan et al. 2007; Ren et al. 2014a; Yaguchi et al. 1997).
The fatty acid yields on the carbon source are generally not reported but can be calculated to 0.29 g/g glucose in the lipid accumulation phase after N depletion in the study by Chaisawang et al. (2012). This is close to the theoretical yield, which is approximately 0.3 g/g glucose (Ratledge, 2014).
Other parameters influencing the DHA levels and production rates
Reduced oxygen supply, resulting in zero dissolved oxygen (DO) in the medium, has been shown to increase the relative fraction of DHA of TFA but also to a certain extent of the fraction of the biomass. A direct effect of oxygen limitation is that the content of unsaturated fatty acids produced by the oxygen-dependent desaturases is decreased, thereby increasing the relative fraction of the other fatty acids (Jakobsen et al. 2008). More importantly, low oxygen transfer rate (OTR) also reduced the fraction of the FAS products of TFA, thereby increasing the fraction of DHA. This was more evident the lower the OTR (Chang et al. 2014; Jakobsen et al. 2008; Qu et al. 2011; Ren et al. 2010) and indicates that the FAS activity is more affected by the oxygen supply than the PKS activity.
Results from experiments where the temperature was reduced within a range where the cell yields and lipid contents were not significantly affected (between 15 - 20 and 30 °C) indicate increasing DHA fractions with decreasing temperatures. For instance, the DHA fraction of TFA in Aurantiochytrium mangrovei Sk-02 increased from 29 to 42 % in the late lipid accumulation phase when the temperature was decreased from 30 to 12 °C; however, the DHA content of the cell mass was the same (~12 %) since the TFA content (mainly C16:0) of CDW decreased at lower temperatures (Chodchoey and Verduyn 2012). Several other studies also show a higher DHA fraction at lower temperatures but with smaller effects and not always clear trends. The temperature effect was even more evident for DPA than for DHA but with an opposite response. The DPA fraction of TFA, as well as the cell mass, decreased with decreasing temperature (Chodchoey and Verduyn 2012; Taoka et al. 2009; Unagul et al. 2005; Zeng et al. 2011). Hence, the synthesis of the two PKS products seems to react differently to temperature.
In order to maintain the maximum lipid accumulation rate, the concentration of the carbon source should be above 15–20 g/l. When the concentration drops below this level, for instance, towards the end of fermentations, the rates decrease. This effect was also evident when the carbon source was added by pulsing, allowing the concentration to decrease to ~5 g/l (Qu et al. 2013a). Limiting the carbon source by slow feeding gave similar, or slightly reduced, lipid contents of CDW as obtained with carbon in excess, however with reduced lipid production rates (Janthanomsuk et al. 2015; Qu et al. 2013a). At decreasing feeding rates, the production rates of the FAS products decreased more than the DHA production rates, resulting in a higher DHA fraction of the fatty acids.
Summary of the main factors affecting the DHA production rates
Total lipid contents of the cell mass above 80 % (Li et al. 2015) and DHA contents above 80 % of TFA (Huang et al. 2012) have been reported. However, such extremes have never been obtained simultaneously. The variations in DHA fraction of TFA observed when oxygen transfer rates and feeding strategies (N, P, and C) are changed seem to a larger degree to be caused by variations in the production rates for the fatty acids generated by FAS than the DHA production rates. In order to improve the DHA productivity, factors specifically increasing the rates of the PKS need to be identified. For a comparison of reported effects, the specific productivity (q P ) of DHA related to the “fat-free” cell mass have been calculated (Table 1). The highest q P values were obtained when very high concentrations of complex nitrogen sources were applied. When nitrogen is available during the lipid accumulation phase, new synthesis of the rate-limiting enzymes is possible, and high enzyme activities can be maintained throughout the fermentation. However, more direct comparisons of different strategies for N supply, using the same strain, are needed. The stability of ME is assumed to be important to maintain a high lipid production rate in yeast and fungi. However, for thraustochytrids, it is yet unclear whether FAS and PKS use the same ME for NADPH generation, if they have their specific MEs, or even if the NADPH for PKS is generated by other enzymes. More knowledge about the number of MEs and possible associations with the two enzyme complexes will be provided by genome analyses. The distribution of the carbon flow between the two pathways will also be affected by the K m values for the enzymes belonging to the respective systems. No such studies have been reported.
The overall volumetric productivity of the processes has not been emphasized in the sections above. However, the highest reported DHA productivities in the scientific literature are 7–8 g/l day. These were obtained with Schizochytrium sp. S31 at 150 g/l CDW (Chang et al., 2013a) and with Aurantiochytrium limacinum SR21 at 88 g/l CDW (Li et al. 2015). In a patent, 13 g/l day at 190 g/l CDW has been reported (Bailey et al. 2003).
Squalene production in thraustochytrids
Applications of squalene and market prospects
Some thraustochytrids, in particular some species belonging to the genus Aurantiochytrium, produce squalene in quantities of more than 30 % of the CDW. Squalene is an intermediate in the biosynthesis of sterols like cholesterol and ergosterol (Fig. 1) and is widespread in nature. In the livers of deep-sea sharks, it may constitute more than 80 % of the oil (Bakes and Nichols 1995). Squalene is extensively used as an excipient in pharmaceutical emulsions for the delivery of vaccines, drugs, and other medicinal substances. It improves the immune system and is therefore used as a protective agent in cancer treatment, and it is also used as a hydrating and antioxidant agent in cosmetics (Huang et al. 2009; Reddy and Couvreur 2009). The squalene market is currently growing and is expected to reach 4000 t and a value of USD 177 million by 2019 (www.marketsandmarkets.com). The shark liver oil has been the traditional source for squalene, but the uncontrolled killing of these animals has caused growing environmental concerns. Combined with governmental regulations, it has restricted the growth of this segment. Alternative squalene sources include vegetable oils, where the highest quantities are found in amaranth and olive oils, on average 7–8 % (w/w) and 1 %, respectively (Popa et al. 2015). Due to the higher demand of processing plants when extracting squalene from vegetable oils compared to shark livers, the potential of biotechnological production in microbial cell factories has attracted increasing attention (Ghimire et al. 2016).
Squalene-producing strains and reported production data
Squalene is produced from farnesyl diphosphate (FPP) via the mevalonate pathway (Fig. 1). The enzymes preceding FPP have not been mapped in thraustochytrids. However, several enzymes related to squalene and sterol synthesis have been shown to be expressed in Aurantiochytrium sp. SD116, including sterol 24-C-methyltransferase, cycloartenol synthase, cholesterol transport protein, and squalene synthetase (Ma et al. 2015). The squalene synthase of Aurantiochytrium sp. KRS101 has been produced recombinantly and shown to catalyze the conversion of two molecules of FPP into squalene in the presence of NADPH and Mg2+ (Hong et al. 2013b).
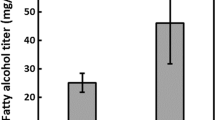
The highest reported squalene levels so far are 32 and 20 % of CDW and were obtained with the strains Aurantiochytrium spp., Yonez5–1 and 18 W-13a (Table 2). In strain 18 W-13a, squalene constituted 69 % of TL and in Yonez5–1 ~94 % (Nakazawa et al., 2012, 2014). The kinetics of the accumulation is not known, as the first sampling was made when the maximum level of biomass, total lipids, and squalene already was reached (Kaya et al. 2011). Also, strains primarily selected for their TAG and/or DHA contents have been shown to produce high levels of squalene, up to 6.6 % of TL or 3.3 % of the cell mass (Hoang et al. 2014; Qu et al. 2013a). In A. mangrovei PQ6 (formerly classified as Schizochytrium mangrovei PQ6), squalene was accumulated during the lipid accumulation phase, but faster than the increase in lipids, and reached a maximum of 33 mg/g of CDW (6 % of TL) and 1.0 g/l after 4 days (Hoang et al. 2014). In contrast, Schizochytrium sp. CCTCC M209059 produced relatively high amounts of squalene in the growth and early lipid accumulation phase, comprising 38 % of TL (30–40 % TL of CDW) and 11 % of CDW (~3 g/l). The TL continued to increase to 70 %, but the squalene content decreased in actual concentrations (g/l), and had disappeared at the end of the TAG accumulation phase (Ren et al. 2014a). The reported volumetric productivities are in the order of 0.2–1.4 g/l day (Table 2). The cell dry weights for the two strains accumulating 20–30 % squalene of CDW were only 3.4 and 6 g/l, respectively, indicating that a considerable potential for improved productivities if the cell density can be increased.
In conclusion, thraustochytrids are promising candidate production organisms for squalene. However, more work is needed on strain selection and studies of the production kinetics. For instance, to which degree the squalene production is growth associated or related to the lipid accumulation seems to vary between strains. A strain accumulating high levels with high volumetric productivities would be the selection for a process targeting squalene as the main product, while squalene associated with the biomass residues after oil extraction could be a valuable co-product to DHA production.
Thraustochytrids as carotenoid producers
Application of carotenoids and market prospects
Thraustochytrids are often pigmented and have been found to synthesize carotenoids (Aki et al. 2003; Burja et al. 2006; Raghukumar 2008; Singh et al. 2014). Carotenoids are a diverse group of natural pigments, mainly produced by plants and also by some microorganisms. The carotenoids can be divided into xanthophylls and carotenes. The two groups have similar molecular structures, but xanthophylls contain oxygen, while carotenes are purely hydrocarbons. The commercially most important carotenoids are the xanthophylls astaxanthin, cantaxanthin, lutein, and zeaxanthin and the carotenes β-carotene and lycopene. The main application of carotenoids is as colorant in food and feed but with an increasing market in nutrition, pharmaceuticals, and cosmetics (Berman et al. 2015). The dominating carotenoid products in both quantities and value are chemically synthesized astaxanthin and cantaxanthin for aquaculture feed. Astaxanthin is the economically most important carotenoid globally, with a market size of above 350 million USD. In 2010, the global market for carotenoids was reported to be 1200 million USD and with an estimated annual growth of 2.3 % (Cutzu et al. 2013). In 2014, the Global Information (http://www.giiresearch.com/report/bc199439-global-market-carotenoids.html) reported the market value to 1500 million USD, with an estimate to reach 1800 million USD in 2019, indicating an annual growth rate of 3.9 %.
Today, most carotenoids are either chemically synthesized (e.g., astaxanthin, β-carotene, and cantaxanthin) or obtained from plant extracts. Up to now, microbially produced carotenoids have not been cost competitive with the chemically synthesized products, thus only serving higher-price, niche applications (Schmidt et al. 2011).
Carotenoid-producing strains and reported production data
Carotenoids, both xanthophylls and carotenes, are synthesized by the mevalonate pathway via the common intermediate lycopene (Fig. 1). No studies on the involved enzymes and genes in thraustochytrids have been reported.
A Thraustochytrium strain-denoted ONC-T18 was found to produce β-carotene and the xanthophylls astaxanthin, zeaxanthin, cantaxanthin, phoenicoxanthin, and echinenone, with total levels in the order of 30 μg/g CDW (Armenta et al. 2006; Burja et al. 2006). Higher carotenoid levels were reported for Thraustochytrium CHN-1, which produced 160 μg/g astaxanthin and 450 μg/g total carotenoids (Carmona et al. 2003), while Aurantiochytrium sp. KH105 produced 1.5–3.4 mg/g total carotenoids depending on media and cultivation conditions, with astaxanthin levels up to ~1.4 mg/g (Aki et al. 2003; Yamasaki et al. 2006). Highest carotenoid levels so far are reported for the strain Thraustochytriidae sp. AS4-A1 (Ulkenia sp.), producing up to 40 mg/g astaxanthin or 4 % of CDW. The cell densities were 8–12 g/l and the astaxanthin concentrations 0.30–0.45 g/l after 6 days. The astaxanthin production followed the DHA accumulation profile with the maximum concentration obtained somewhat later than the maximum DHA concentration (Quilodran et al. 2010). Interestingly, recombinant expression of the Vitreoscilla hemoglobin protein resulted in ninefold increased astaxanthin content in Aurantiochytrium sp. SK4 under microaerobic conditions, and this engineering approach also positively affected cell growth and PUFA production (Suen et al. 2014).
The maximum reported astaxanthin level of 4 % of CDW by thraustochytrids is far higher than the 6–7 mg/g obtained by the yeast Phaffia rhodozyma (Schmidt et al. 2011), and in the same order as for the phototropic microalgae Haematococcus pluvialis, both used for commercial production of astaxanthin. Thraustochytrids are therefore highly interesting production organisms for this carotenoid and for a simultaneous production of DHA and astaxanthin for salmon feed. Due to the low cell densities, the reported volumetric productivities are yet low for thraustochytrids. If high-cell-density fermentations can be developed, it is not unlikely that also the volumetric productivities will exceed the productivities reported for other potential production organisms. Further, current progress in development of genetic tools and genome sequencing of thraustochytrids should open for engineering of thraustochytrids as future production hosts for a range of carotenoids, utilizing the knowledge generated from the ongoing engineering efforts on other microorganisms, such as the yeasts P. rhodozyma and Xanthophyllomyces dendrorhous (Schmidt et al. 2011; Gassel et al. 2013).
Available genome sequences and tools for engineering of thraustochytrids
The scarcity of thraustochytrids genome data has so far limited the identification of targets for strain development. A partial genome of a thraustochytrid (QPX) identified as a parasite on the hard clam quahog has been published (Garcia-Vedrenne et al. 2013). More recently, the genome sequence of a good DHA producer Schizochytrium sp. CCTCC M209059 was published (Ji et al. 2015). Draft genome data of two thraustochytrids, A. limacinum SR21 and S. aggregatum ATCC 28209, are available from the Joint Genome Institute (JGI; http://genome.jgi.doe.gov/), and a genome scale metabolic model of A. limacinum SR21 was recently built and analyzed for DHA production (Ye et al. 2015). A few transcriptome studies of thraustochytrids have also been published (Ma et al. 2015; Rubin et al. 2014).
However, even if more genome sequences now are becoming available, efficient methods for gene transfer are still scarce for thraustochytrids. To date, there are only 12 thraustochytrid strains, belonging to 4 different genera, which have been genetically manipulated, and only a few methods have been successfully employed for delivering transgenes into thraustochytrid cells. Except for one published study, where the authors established Agrobacterium-mediated transformation for Schizochytrium sp. (Cheng et al. 2012), electroporation or particle bombardment using linear DNA appear to be the choice (Table 3), but the reported methods do not necessarily work for any thraustochytrid strain. However, other methods that have been successfully applied for photosynthetic microalgae may be applicable in thraustochytrids as well. Kim et al. (2014) reported that Chlamydomonas reinhardtii with an intact cell wall could be transformed by the use of positively charged aminoclay nanoparticles, and last year a bacterial conjugation-based method was established that directly transfers episomes from Escherichia coli to the diatoms Phaeodactylum tricornutum and Thalassiosira pseudonana with high transformation efficiency (Karas et al. 2015). These novel approaches might open new opportunities for thraustochytrid transformation. Recently, Sun et al. (2015) demonstrated that the cre/loxP system could be utilized to generate markerless mutations in A. limacinum, and this might enable the construction of strains with several mutations.
Besides the DNA delivery method, the selection method and the control of gene expression need to be taken into consideration when establishing genetic toolkits for thraustochytrids. Only a limited range of selection markers has been employed to select thraustochytrid transformants (Table 3). The concentrations needed for growth inhibition are strain dependent, indicating that the resistance to antibiotic selection of thraustochytrids need to be tested on a case-by-case basis (Cheng et al. 2011; Lippmeier et al. 2009; Suen et al. 2014). It has also been reported that mutating the gene encoding ribosomal protein L44 (P56E) renders Aurantiochytrium sp. KRS101 cycloheximide resistant (Hong et al. 2013a). In addition, it has been demonstrated that the enhanced fluorescence protein (EGFP) can be functionally expressed in A. limacinum, T. aureum, and Schizochytrium sp. (Cheng et al. 2012; Sakaguchi et al. 2012; Sun et al. 2015). In order to control transgene expression, promoters from highly expressed endogenous genes are often used. Table 3 also summarizes which promoters were used for controlling transgene expression in the cited studies.
The methods described above have mainly been used to elucidate the role of enzymes related to fatty acid synthesis, yet to a lesser degree for strain improvement. This will change when genome sequences now become available, enabling identification of targets for genetic engineering. In parallel with the efforts in sequencing, there is a need to further develop genetic toolkits.
Future prospects
Despite more than 20 years of research on DHA production by thraustochytrids, basic knowledge of biochemistry, genetics, and regulation of the synthesis is still lacking. Of particular importance for future strain and process improvements is the understanding of how the two main pathways used for fatty acid synthesis is regulated and how higher rates of the PKS pathway can be obtained. The few reported studies on squalene and carotenoids indicate a high production potential of these compounds. However, the information about the production kinetics is scarce, for instance, to which degree the production is growth associated or related to the lipid accumulation. The genome sequences that now are becoming available will help to generate hypotheses that can be verified experimentally by various omic analyses, biochemical studies of enzyme activities, and the use of labeled substrates. This will open for a system-level metabolic engineering for strain improvement.
DHA-rich oils produced by thraustochytrids are already commercialized for the human market. Production of DHA for other, lower-price markets, such as feed ingredients, as well as the production of squalene and carotenoids, will depend on the availability and price development for the current feedstocks for these products. Squalene and carotenoids could also be added-value co-products from a potential future large-volume production of DHA as a fish feed ingredient and thereby improve the process economy.
References
Abe E, Ikeda K, Nutahara E, Hayashi M, Yamashita A, Taguchi R, Doi K, Honda D, Okino N, Ito M (2014) Novel lysophospholipid acyltransferase PLAT1 of Aurantiochytrium limacinum F26-b responsible for generation of palmitate-docosahexaenoate-phosphatidylcholine and phosphatidylethanolamine. PLoS One 9:e102377
Aki T, Hachida K, Yoshinaga M, Katai Y, Yamasaki T, Kawamoto S, Kakizono T, Maoka T, Shigeta S, Suzuki O, Ono K (2003) Thraustochytrid as a potential source of carotenoids. J Am Oil Chem Soc 80:789–794
Alvarez HM, Steinbüchel A (2002) Triacylglycerols in prokaryotic microorganisms. Appl Microbiol Biotechnol 60:367–376
Armenta RE, Burja A, Radianingtyas H, Barrow CJ (2006) Critical assessment of various techniques for the extraction of carotenoids and co-enzyme Q 10 from the thraustochytrid strain ONC-T18. J Agric Food Chem 54:9752–9758
Bailey RB, DiMasi D, Hansen JM, Mirrasoul PJ, Ruecker CM, Veeder GT, Kaneko T, Barclay WR (2003) Enhanced production of lipids containing polyunsaturated fatty acids by very high density cultures of eukaryotic microbes in fermentors. US Patent 6:607,900
Bakes MJ, Nichols PD (1995) Lipid, fatty acid and squalene composition of liver oil from six species of deep-sea sharks collected in southern Australian waters. Comp Biochem Physiol B 110:267–275
Barclay W, Weaver C, Metz C, Hansen J (2010) Development of a docosahexaenoic acid production technology using Schizochytrium: historical perspective and update. In: Ratledge C, Cohen Z (eds) Single cell oils: microbial and algal oils, 2nd edn. AOCS Press, Urbana, pp. 75–96
Bayne ACV, Boltz D, Owen C, Betz J, Maia G, Azadi P, Archer-Hartmann S, Zirkle R, Lippmeier JC (2013) Vaccination against influenza with recombinant hemagglutinin expressed by Schizochytrium sp. confers protective immunity. PLoS One 8:e61790
Berman J, Zorrilla-Lopez U, Farre G, Zhu CF, Sandmann G, Twyman RM, Capell T, Christou P (2015) Nutritionally important carotenoids as consumer products. Phytochem Rev 14:727–743
Burja AM, Radianingtyas H, Windust A, Barrow CJ (2006) Isolation and characterization of polyunsaturated fatty acid producing Thraustochytrium species: screening of strains and optimization of omega-3 production. Appl Microbiol Biotechnol 72:1161–1169
Carmona ML, Naganuma T, Yamaoka Y (2003) Identification by HPLC-MS of carotenoids of the Thraustochytrium CHN-1 strain isolated from the Seto Inland Sea. Biosci Biotechnol Biochem 67:884–888
Chaisawang M, Verduyn C, Chauvatcharin S, Suphantharika M (2012) Metabolic networks and bioenergetics of Aurantiochytrium sp. B-072 during storage lipids formation. Braz J Microbiol 43:1192–1205
Chang G, Gao N, Tian G, Wu Q, Chang M, Wang X (2013a) Improvement of docosahexaenoic acid production on glycerol by Schizochytrium sp. S31 with constantly high oxygen transfer coefficient. Bioresour Technol 142:400–406
Chang G, Luo Z, Gu S, Wu Q, Chang M, Wang X (2013b) Fatty acid shifts and metabolic activity changes of Schizochytrium sp. S31 cultured on glycerol. Bioresour Technol 142:255–260
Chang G, Wu J, Jiang C, Tian G, Wu Q, Chang M, Wang X (2014) The relationship of oxygen uptake rate and kLa with rheological properties in high cell density cultivation of docosahexaenoic acid by Schizochytrium sp. S31. Bioresour Technol 152:234–240
Chaung KC, Chu CY, Su YM, Chen YM (2012) Effect of culture conditions on growth, lipid content, and fatty acid composition of Aurantiochytrium mangrovei strain BL10. AMB Express 2:42
Cheng RB, Lin XZ, Wang ZK, Yang SJ, Rong H, Ma Y (2011) Establishment of a transgene expression system for the marine microalga Schizochytrium by 18S rDNA-targeted homologous recombination. World J Microbiol Biotechnol 27:737–741
Cheng R, Ma R, Li K, Rong H, Lin X, Wang Z, Yang S, Ma Y (2012) Agrobacterium tumefaciens mediated transformation of marine microalgae Schizochytrium. Microbiol Res 167:179–186
Chodchoey K, Verduyn C (2012) Growth, fatty acid profile in major lipid classes and lipid fluidity of Aurantiochytrium mangrovei SK-02 as a function of growth temperature. Braz J Microbiol 43:187–200
Cutzu R, Coi A, Rosso F, Bardi L, Ciani M, Budroni M, Zara G, Zara S, Mannazzu I (2013) From crude glycerol to carotenoids by using a Rhodotorula glutinis mutant. World J Microbiol Biotechnol 29:1009–1017
Dulermo T, Lazar Z, Dulermo R, Rakicka M, Haddouche R, Nicaud JM (2015) Analysis of ATP-citrate lyase and malic enzyme mutants of Yarrowia lipolytica points out the importance of mannitol metabolism in fatty acid synthesis. Biochim Biophys Acta 1851:1107–1117
Ethier S, Woisard K, Vaughan D, Wen Z (2011) Continuous culture of the microalgae Schizochytrium limacinum on biodiesel-derived crude glycerol for producing docosahexaenoic acid. Bioresour Technol 102:88–93
Fan KW, Jiang Y, Faan YW, Chen F (2007) Lipid characterization of mangrove thraustochytrid — Schizochytrium mangrovei. J Agric Food Chem 55:2906–2910
FAO (2014) The state of world fisheries and aquaculture. Food and Agriculture Organization of the United Nations, Rome, 2014. E-ISBN 978–92-5-108276-8 (PDF)
Ganuza E, Izquierdo MS (2007) Lipid accumulation in Schizochytrium G13/2S produced in continuous culture. Appl Microbiol Biotechnol 76:985–990
Garay LA, Boundy-Mills KL, German JB (2014) Accumulation of high-value lipids in single-cell microorganisms: a mechanistic approach and future perspectives. J Agric Food Chem 62:2709–2727
Garcia-Vedrenne AE, Groner M, Page-Karjian A, Siegmund GF, Singhal S, Sziklay J, Roberts S (2013) Development of genomic resources for a thraustochytrid pathogen and investigation of temperature influences on gene expression. PLoS One 8:e74196
Gassel S, Schewe H, Schmidt I, Schrader J, Sandmann G (2013) Multiple improvement of astaxanthin biosynthesis in Xanthophyllomyces dendrorhous by a combination of conventional mutagenesis and metabolic pathway engineering. Biotechnol Lett 35:565–569
Ghimire GP, Thuan NH, Koirala N, Sohng JK (2016) Advances in biochemistry and microbial production of squalene and its derivatives. J Microbiol Biotechnol 26:441–451
Goold H, Beisson F, Peltier G, Li-Beisson Y (2015) Microalgal lipid droplets: composition, diversity, biogenesis and functions. Plant Cell Rep 34:545–555
Gupta A, Barrow CJ, Puri M (2012) Omega-3 biotechnology: thraustochytrids as a novel source of omega-3 oils. Biotechnol Adv 30:1733–1745
Hauvermale A, Kuner J, Rosenzweig B, Guerra D, Diltz S, Metz JG (2006) Fatty acid production in Schizochytrium sp.: involvement of a polyunsaturated fatty acid synthase and a type I fatty acid synthase. Lipids 41:739–747
Hoang MH, Ha NC, Thom le T, Tam LT, Anh HT, Thu NT, Hong DD (2014) Extraction of squalene as value-added product from the residual biomass of Schizochytrium mangrovei PQ6 during biodiesel producing process. J Biosci Bioeng 118:632–639
Honda D, Yokochi T, Nakahara T, Raghukumar S, Nakagiri A, Schaumann K, Higashihar T (1999) Molecular phylogeny of labyrinthulids and thraustochytrids based on the sequencing of 18S ribosomal RNA gene. J Eukaryot Microbiol 46:637–647
Hong WK, Heo SY, Oh BR, Kim CH, Sohn JH, Yang JW, Kondo A, Seo JW (2013a) A transgene expression system for the marine microalgae Aurantiochytrium sp. KRS101 using a mutant allele of the gene encoding ribosomal protein L44 as a selectable transformation marker for cycloheximide resistance. Bioprocess Biosyst Eng 36:1191–1197
Hong WK, Heo SY, Park HM, Kim CH, Sohn JH, Kondo A, Seo JW (2013b) Characterization of a squalene synthase from the thraustochytrid microalga Aurantiochytrium sp. KRS101. J Microbiol Biotechnol 23:759–765
Huang ZR, Lin YK, Fang YE (2009) Biological and pharmacological activities of squalene and related compounds: potential uses in cosmetic dermatology. Molecules 14:540–554
Huang TY, Lu WC, Chu IM (2012) A fermentation strategy for producing docosahexaenoic acid in Aurantiochytrium limacinum SR21 and increasing C22:6 proportions in total fatty acid. Bioresour Technol 123:8–14
Jakobsen AN, Aasen IM, Josefsen KD, Strøm AR (2008) Accumulation of docosahexaenoic acid-rich lipid in thraustochytrid Aurantiochytrium sp. strain T66: effects of N and P starvation and O2 limitation. Appl Microbiol Biotechnol 80:297–306
Janthanomsuk P, Verduyn C, Chauvatcharin S (2015) Improved docosahexaenoic acid production in Aurantiochytrium by glucose limited pH-auxostat fed-batch cultivation. Bioresour Technol 196:592–599
Ji XJ, Mo KQ, Ren LJ, Li GL, Huang JZ, Huang H (2015) Genome sequence of Schizochytrium sp. CCTCC M209059, an effective producer of docosahexaenoic acid-rich lipids. Genome Announc 3:e00819–e00815
Karas BJ, Diner RE, Lefebvre SC, McQuaid J, Phillips AP, Noddings CM, Brunson JK, Valas RE, Deerinck TJ, Jablanovic J, Gillard JT, Beeri K, Ellisman MH, Glass JI, Hutchison CA, Smith HO, Venter JC, Allen AE, Dupont CL, Weyman PD (2015) Designer diatom episomes delivered by bacterial conjugation. Nat Commun 6:692
Kaya K, Nakazawa A, Matsuura H, Honda D, Inouye I, Watanabe MM (2011) Thraustochytrid Aurantiochytrium sp. 18 W-13a accumulates high amounts of squalene. Biosci Biotechnol Biochem 75:2246–2248
Kim S, Lee YC, Cho DH, Lee HU, Huh YS, Kim GJ, Kim HS (2014) A simple and non-invasive method for nuclear transformation of intact-walled Chlamydomonas reinhardtii. PLoS One 9:e101018
Li J, Liu R, Chang G, Li X, Chang M, Liu Y, Jin Q, Wang X (2015) A strategy for the highly efficient production of docosahexaenoic acid by Aurantiochytrium limacinum SR21 using glucose and glycerol as the mixed carbon sources. Bioresour Technol 177:51–57
Lippmeier JC, Crawford KS, Owen CB, Rivas AA, Metz JG, Apt KE (2009) Characterization of both polyunsaturated fatty acid biosynthetic pathways in Schizochytrium sp. Lipids 44:621–630
Ma Z, Tan Y, Cui G, Feng Y, Cui Q, Song X (2015) Transcriptome and gene expression analysis of DHA producer Aurantiochytrium under low temperature conditions. Sci Report 5:14446
Matsuda T, Sakaguchi K, Kobayashi T, Abe E, Kurano N, Sato A, Okita Y, Sugimoto S, Hama Y, Hayashi M, Okino N, Ito M (2011) Molecular cloning of a Pinguiochrysis pyriformis oleate-specific microsomal delta12-fatty acid desaturase and functional analysis in yeasts and thraustochytrids. J Biochem 150:375–383
Matsuda T, Sakaguchi K, Hamaguchi R, Kobayashi T, Abe E, Hama Y, Hayashi M, Honda D, Okita Y, Sugimoto S, Okino N, Ito M (2012) Analysis of Δ12-fatty acid desaturase function revealed that two distinct pathways are active for the synthesis of PUFAs in T. aureum ATCC 34304. J Lipid Res 53:1210–1222
Metz JG, Roessler P, Facciotti D, Levering C, Dittrich F, Lassner M, Valentine R, Lardizabal K, Domergue F, Yamada A, Yazawa K, Knauf V, Browse J (2001) Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science 293:290–293
Mühlroth A, Li K, Røkke G, Winge P, Olsen Y, Hohmann-Marriott MF, Vadstein O, Bones AM (2013) Pathways of lipid metabolism in marine algae, co-expression network, bottlenecks and candidate genes for enhanced production of EPA and DHA in species of Chromista. Mar Drugs 11:4662–4697
Nakazawa A, Matsuura H, Kose R, Kato S, Honda D, Inouye I, Kaya K, Watanabe MM (2012) Optimization of culture conditions of the thraustochytrid Aurantiochytrium sp. strain 18 W-13a for squalene production. Bioresour Technol 109:287–291
Nakazawa A, Kokubun Y, Matsuura H, Yonezawa N, Kose R, Yoshida M, Tanabe Y, Kusuda E, Van Thang D, Ueda M, Honda D, Mahakhant A, Kaya K, Watanabe MM (2014) TLC screening of thraustochytrid strains for squalene production. J Appl Phycol 26:29–41
Olsen Y (2011) Resources for fish feed in future mariculture. Aquac Environ Int 1:187–200
Popa O, Babeanu NE, Popa I, Nita S, Dinu-Parvu CE (2015) Methods for obtaining and determination of squalene from natural sources. Biomed Res Int 2015:367202
Qu L, Ji XJ, Ren LJ, Nie ZK, Feng Y, Wu WJ, Ouyang PK, Huang H (2011) Enhancement of docosahexaenoic acid production by Schizochytrium sp. using a two-stage oxygen supply control strategy based on oxygen transfer coefficient. Lett Appl Microbiol 52:22–27
Qu L, Ren LJ, Li J, Sun GN, Ji XJ, Nie ZK, Huang H (2013a) Biomass composition, lipid characterization, and metabolic profile analysis of the fed-batch fermentation process of two different docosahexaenoic acid producing Schizochytrium sp. strains. Appl Biochem Biotechnol 171:1865–1876
Qu L, Ren LJ, Sun GN, Ji XJ, Nie ZK, Huang H (2013b) Batch, fed-batch and repeated fed-batch fermentation processes of the marine thraustochytrid Schizochytrium sp. for producing docosahexaenoic acid. Bioprocess Biosyst Eng 36:1905–1912
Quilodran B, Hinzpeter I, Hormazabal E, Quiroz A, Shene C (2010) Docosahexaenoic acid (C22:6n-3, DHA) and astaxanthin production by Thraustochytriidae sp. AS4-A1, a native strain with high similitude to Ulkenia sp.: evaluation of liquid residues from food industry as nutrient sources. Enzym Microb Technol 47:24–30
Raghukumar S (2002) Ecology of the marine protists, the Labyrinthulomycetes (thraustochytrids and labyrinthulids). Eur J Protistol 38:127–145
Raghukumar S (2008) Thraustochytrid marine protists: production of PUFAs and other emerging technologies. Mar Biotechnol 10:631–640
Ratledge C (2002) Regulation of lipid accumulation in oleaginous microorganisms. Biochem Soc T 30:1047–1050
Ratledge C (2004) Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie 86:807–815
Ratledge C (2014) The role of malic enzyme as the provider of NADPH in oleaginous microorganisms: a reappraisal and unsolved problems. Biotechnol Lett 36:1557–1568
Ratledge C, Wynn JP (2002) The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv Appl Microbiol 51:1–51
Reddy LH, Couvreur P (2009) Squalene: a natural triterpene for use in disease management and therapy. Adv Drug Deliv Rev 61:1412–1426
Ren LJ, Huang H, Xiao AH, Lian M, Jin LJ, Ji XJ (2009) Enhanced docosahexaenoic acid production by reinforcing acetyl-CoA and NADPH supply in Schizochytrium sp. HX-308. Bioprocess Biosyst Eng 32:837–843
Ren LJ, Ji XJ, Huang H, Qu L, Feng Y, Tong QQ, Ouyang PK (2010) Development of a stepwise aeration control strategy for efficient docosahexaenoic acid production by Schizochytrium sp. Appl Microbiol Biotechnol 87:1649–1656
Ren LJ, Feng Y, Li J, Qu L, Huang H (2013) Impact of phosphate concentration on docosahexaenoic acid production and related enzyme activities in fermentation of Schizochytrium sp. Bioprocess Biosyst Eng 36:1177–1183
Ren LJ, Sun GN, Ji XJ, Hu XC, Huang H (2014a) Compositional shift in lipid fractions during lipid accumulation and turnover in Schizochytrium sp. Bioresour Technol 157:107–113
Ren LJ, Sun LN, Zhuang XY, Qu L, Ji XJ, Huang H (2014b) Regulation of docosahexaenoic acid production by Schizochytrium sp.: effect of nitrogen addition. Bioprocess Biosyst Eng 37:865–872
Rubin E, Tanguy A, Perrigault M, Pales Espinosa E, Allam B (2014) Characterization of the transcriptome and temperature-induced differential gene expression in QPX, the thraustochytrid parasite of hard clams. BMC Genomics 15:245
Sakaguchi K, Matsuda T, Kobayashi T, Ohara J, Hamaguchi R, Abe E, Nagano N, Hayashi M, Ueda M, Honda D, Okita Y, Taoka Y, Sugimoto S, Okino N, Ito M (2012) Versatile transformation system that is applicable to both multiple transgene expression and gene targeting for thraustochytrids. Appl Environ Microbiol 78:3193–3202
Schmidt I, Schewe H, Gassel S, Jin C, Buckingham J, Hümbelin M, Sandmann G, Schrader J (2011) Biotechnological production of astaxanthin with Phaffia rhodozyma/Xanthophyllomyces dendrorhous. Appl Microbiol Biotechnol 89:555–571
Singh P, Liu Y, Li LS, Wang GY (2014) Ecological dynamics and biotechnological implications of thraustochytrids from marine habitats. Appl Microbiol Biotechnol 98:5789–5805
Song X, Tan Y, Liu Y, Zhang J, Liu G, Feng Y, Cui Q (2013) Different impacts of short-chain fatty acids on saturated and polyunsaturated fatty acid biosynthesis in Aurantiochytrium sp. SD116. J Agric Food Chem 61:9876–9881
Suen YL, Tang H, Huang J, Chen F (2014) Enhanced production of fatty acids and astaxanthin in Aurantiochytrium sp. by the expression of Vitreoscilla hemoglobin. J Agric Food Chem 62:12392–12398
Sun L, Ren L, Zhuang X, Ji X, Yan J, Huang H (2014) Differential effects of nutrient limitations on biochemical constituents and docosahexaenoic acid production of Schizochytrium sp. Bioresour Technol 159:199–206
Sun H, Chen H, Zang X, Hou P, Zhou B, Liu Y, Wu F, Cao X, Zhang X (2015) Application of the cre/loxP site-specific recombination system for gene transformation in Aurantiochytrium limacinum. Molecules 20:10110–10121
Taoka Y, Nagano N, Okita Y, Izumida H, Sugimoto S, Hayashi M (2009) Influences of culture temperature on the growth, lipid content and fatty acid composition of Aurantiochytrium sp. strain mh0186. Mar Biotechnol 11:368–374
Unagul P, Assantachai C, Phadungruengluij S, Suphantharika M, Verduyn C (2005) Properties of the docosahexaenoic acid-producer Schizochytrium mangrovei Sk-02: effects of glucose, temperature and salinity and their interaction. Bot Mar 48:387–394
Valentine RC, Valentine DL (2004) Omega-3 fatty acids in cellular membranes: a unified concept. Prog Lipid Res 43:383–400
Wang CW (2015) Lipid droplet dynamics in budding yeast. Cell Mol Life Sci 72:2677–2695
Xie Y, Wang G (2015) Mechanisms of fatty acid synthesis in marine fungus-like protists. Appl Microbiol Biotechnol 99:8363–8375
Yaguchi T, Tanaka S, Yokochi T, Nakahara T, Higashihara T (1997) Production of high yields of docosahexaenoic acid by Schizochytrium sp. strain SR21. J Am Oil Chem Soc 74:1431–1434
Yamasaki T, Aki T, Shinozaki M, Taguchi M, Kawamoto S, Ono K (2006) Utilization of Shochu distillery wastewater for production of polyunsaturated fatty acids and xanthophylls using thraustochytrid. J Biosci Bioeng 102:323–327
Yan J, Cheng R, Lin X, You S, Li K, Rong H, Ma Y (2013) Overexpression of acetyl-CoA synthetase increased the biomass and fatty acid proportion in microalga Schizochytrium. Appl Microbiol Biotechnol 97:1933–1939
Ye C, Qiao W, Yu X, Ji X, Huang H, Collier JL, Liu L (2015) Reconstruction and analysis of the genome-scale metabolic model of Schizochytrium limacinum SR21 for docosahexaenoic acid production. BMC Genomics 16:799
Yokoyama R, Honda D (2007) Taxonomic rearrangement of the genus Schizochytrium sensu lato based on morphology, chemotaxonomic characteristics, and 18S rRNA gene phylogeny (Thraustochytriaceae, Labyrinthulomycetes): emendation for Schizochytrium and erection of Aurantiochytrium and Oblongichytrium gen. nov. Mycoscience 48:199–211
Yokoyama R, Salleh B, Honda D (2007) Taxonomic rearrangement of the genus Ulkenia sensu lato based on morphology, chemotaxonomical characteristics, and 18S rRNA gene phylogeny (Thraustochytriaceae, Labyrinthulomycetes): emendation for Ulkenia and erection of Botryochytrium, Parietichytrium, and Sicyoidochytrium gen. nov. Mycoscience 48:329–341
Zeng Y, Ji XJ, Lian M, Ren LJ, Jin LJ, Ouyang PK, Huang H (2011) Development of a temperature shift strategy for efficient docosahexaenoic acid production by a marine fungoid protist, Schizochytrium sp. HX-308. Appl Biochem Biotechnol 164:249–255
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors confirm that ethical principles have been followed in the manuscript preparation.
Funding
The work on thraustochytrids and microbial oil production at SINTEF and NTNU is funded by grants from The Research Council of Norway.
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Aasen, I.M., Ertesvåg, H., Heggeset, T.M.B. et al. Thraustochytrids as production organisms for docosahexaenoic acid (DHA), squalene, and carotenoids. Appl Microbiol Biotechnol 100, 4309–4321 (2016). https://doi.org/10.1007/s00253-016-7498-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7498-4