Abstract
The effect of aeration on the performance of docosahexaenoic acid (DHA) production by Schizochytrium sp. was investigated in a 1,500-L bioreactor using fed-batch fermentation. Six parameters, including specific growth rate, specific glucose consumption rate, specific lipid accumulation rate, cell yield coefficient, lipid yield coefficient, and DHA yield coefficient, were used to understand the relationship between aeration and the fermentation characteristics. Based on the information obtained from the parameters, a stepwise aeration control strategy was proposed. The aeration rate was controlled at 0.4 volume of air per volume of liquid per minute (vvm) for the first 24 h, then shifted to 0.6 vvm until 96 h, and then switched back to 0.4 vvm until the end of the fermentation. High cell density (71 g/L), high lipid content (35.75 g/L), and high DHA percentage (48.95%) were achieved by using this strategy, and DHA productivity reached 119 mg/L h, which was 11.21% over the best results obtained by constant aeration rate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyunsaturated fatty acids (PUFA), especially docosahexaenoic acid (DHA; 22:6, n−3), arachidonic acid (ARA; 20:4, n−6), and eicosapentaenoic acid (EPA; 22:5, n−3), have received worldwide attention due to their beneficial effects for humans (Gill and Valivety 1997). EPA has beneficial effects on the cardiovascular system and has been used specifically for treatment of atherosclerosis and hyperlipemia, schizophrenia, and certain cancers (Ward and Singh 2005). DHA and ARA are usually incorporated into infant formula because of their promotion effect for visual acuity and neural development (Gill and Valivety 1997). In addition, DHA also plays a key role in lowering the incidence of certain cardiovascular diseases (Horrocks and Yeo 1999). The traditional commercial source of DHA is fish oil. However, DHA from fish oil contains undesirable fishy smell and inevitable contamination by marine pollution (Sijtsma and de Swaaf 2004), which is incompatible with the concept of healthy eating. During the past 20 years, DHA production by microorganism has been well developed (Ganuza et al. 2008; Morita et al. 2006; Nakahara et al. 1996; Rosa et al. 2010). Among numerous strains, Schizochytrium sp. is noteworthy and often considered as a satisfactory alternative to fish oil due to the advantages of fast growth rate and high productivity.
Industrial production of DHA usually requires two prerequisites, low cost and high productivity. Use of cheap raw materials, such as soymilk residue (Fan et al. 2001), crude glycerol (Chi et al. 2007), coconut water (Unagul et al. 2007), sweet sorghum juice (Liang et al. 2010), etc., have been considered in DHA production, which make the cost reduction possible. High productivity can be achieved only with high cell density and high DHA content. High cell density was often realized by fed-batch cultivation, which could supply sufficient carbon source without the negative effect of substrate inhibition (Yamanè and Shimizu 1984). High DHA content can be accomplished by two-stage fermentation. For two-stage fermentation, oxygen supply is one of the key factors limiting DHA content since Schizochytrium species synthesize long chain PUFAs via a polyunsaturated fatty acid synthase (Metz et al. 2001), which is O2 independent. Jakobsen et al. (2008) studied the effect of oxygen limitation on DHA-rich lipid accumulation in Aurantiochytrium sp. T66, which was also a genus of Schizochytrium. They found that the content of DHA reached 52% in O2-limited cells while only reaching 25% under nitrogen limitation conditions. However, cell dry weight decreased to 25–27 from 90–100 g/L, showing the contradiction between cell proliferation and lipid accumulation. Subsequently, in order to avoid such a contradiction, Chi et al. (2009) studied a two-stage growth of Schizochytrium limacinum SR21 with shifting dissolved oxygen level in shake flask scale. Using this process, DHA content increased from 3.65 to 6.56 g/L. Although these studies showed that oxygen supply had an obvious effect on cell growth and DHA synthesis, the overall fermentation performance, such as the kinetics of cell growth and substrate consumption, were not studied sufficiently to reveal the relationship between oxygen supply and DHA production. Further, these cultivation methods would not be usable in the large-scale production.
In this study, the effect of different oxygen supply level, which was implemented by setting different aeration rate, was investigated in a 1,500-L bioreactor using fed-batch fermentation. The kinetic parameters of cell growth, substrate consumption, and product accumulation were used to understand the relationship between oxygen supply and lipid accumulation. Based on the analysis, a stepwise aeration control strategy was proposed and successfully employed in the fed-batch fermentation, which would provide guidance for DHA industrial production and the other single cell oil production.
Materials and methods
Microorganism
Schizochytrium sp. CCTCC M209059, preserved in the China Centre for Type Culture Collection (CCTCC), was used in the present study.
Culture conditions
The seed culture medium and the conditions were the same as those used in our previous study (Ren et al. 2009). The culture preserved in the glycerin tube was inoculated into a 250-mL flask with 50 mL medium and cultivated for 24 h. After three generations of cultivation, the preculture was inoculated into a 150-L seed fermentor with an inoculum size of 1% (v/v) and cultivated for 24 h. The seed culture (10%, v/v) was then transferred to a 1,500-L fermentor with a working volume of 1,000 L. The impeller speed was set at 100 rpm. The aeration was controlled at 24 and 36 m3/h to achieve the aeration rate of 0.4 (00Experiment I) and 0.6 (experiment II) volume of air per volume of liquid per minute (vvm), respectively. Experiment III was carried out according to our proposed stepwise aeration control strategy. During the fermentation process, in addition to adding glucose at the initial fermentation stage, intermittent glucose feeding was also supplied to keep its concentration above 15 g/L.
Cell dry weight, total lipids, and fatty acid analysis
Broth (50 mL) was used to determine cell dry weight by gravimetric method. The methods of lipid extraction and fatty acid methyl esters (FAMEs) preparation were the same as those used in our previous study (Ren et al. 2009). The FAME samples were analyzed by GC system (GC-2010, Shimadzu, Japan). The GC was equipped with a capillary column (DB-23, 60 m × 0.22 mm) and flame ionization detector (FID). Nitrogen was used as the carrier gas. The injector was maintained at 250°C with an inject volume of 1 μL. The column was raised from 100°C to 200°C at 25°C/min and then increased to 230°C at 4°C/min, keeping this temperature for 9 min. The FID detector temperature was set at 280°C. Fatty acids were identified through comparison with related external standards (Sigma, USA). The quantities of individual FAMEs and squalene were estimated from the peak areas on the chromatogram using nonadecanoic acid (C19:0) as the internal standard.
Performance analysis of fermentation broth
Glucose and glutamate were measured enzymatically using a bioanalyzer simultaneously (SBA-40C, Institute of Biology, Shandong Academy of Sciences, China). Nitrogen concentration in the broth was determined using alkaline potassium persulfate digestion and UV spectrophotometry according to the China Standard GB 11894-89 method. The phosphate concentration was determined by molybdenum blue colorimetric method (Murphy and Riley 1962). pH was determined every 4 h by using a pH meter.
Calculation of kinetic parameters
The specific cell growth rate (μ, h−1), specific glucose consumption rate (q s , h−1), and specific lipid accumulation rate (q p , h−1) were estimated from experimental data of cell growth (x, g/L), residual glucose concentration (s, g/L), and lipid accumulation (p, g/L) according to the method mentioned in our previous study (Ji et al. 2009). Cell yield coefficient (Y x/s, %), lipid yield coefficient (Y p/s, %), and DHA yield coefficient (Y DHA/s , %) were calculated from experimental data by Eqs. 1–3, respectively.
Results
Fermentation disparity in response to different aeration conditions
To study the physiological responses and the fermentation disparity to different oxygen supply conditions, Schizochytrium sp. CCTCC M209059 was cultivated in a 1,500-L bioreactor with different aeration rate. Representative data were shown in Figs. 1, 2, 3, and 4.
To better analyze the process of cell growth and lipid accumulation and understand the effect of dissolved oxygen on DHA production, the fermentation process was divided into the following three stages according to the cell growth characteristics. Stage 1 represents the process from the beginning till nitrogen exhaustion, stage 2 refers to the phase of lipid accumulation, and stage 3 was the stationary phase with slight cell dry weight increase.
In stage 1 (from 0 to 44 h) of experiment I, glucose was consumed to below 20 g/L after feeding once. Monosodium glutamate (MSG) was exhausted at 40 h. With affluent nitrogen and carbon source, the cell dry weight increased sharply at this stage from 7 to 35 g/L. In stage 2 (from 44 to 108 h), total lipid concentration increased from 8.5 to 23.7 g/L after nitrogen exhaustion, which indicated that cell metabolisms shift to lipid accumulation from cell growth. Meanwhile, a slight increase of cell dry weight was also noticed at this stage, which could be explained by the lipid accumulation or diauxic growth of CCTCC M209059. In stage 3 (from 108 to 152 h), cell dry weight reached the maximum and maintained at a constant level, indicating the coming of stationary phase. Lipids increase at this stage was only 3.49 g/L. However, consumed glucose was 30 g/L, which might be explained by the consumption of cell maintenance.
When cell was cultivated at high aeration (as shown in Fig. 2), glucose consumption rate increased greatly, and MSG exhausted at 32 h with cell dry weight reaching 25 g/L at that time. Compared with experiment I, cells consumed equal amounts of glucose and MSG but produced less biomass. The main increase in cell dry weight was concentrated on stage 2 (from 32 to 92 h) at which point biomass reached 58 from 25 g/L. At this stage, glucose consumption rate was so fast that glucose needed to be fed every 8 h. Lipids also increased sharply with the increase of cell dry weight. Glucose consumption rate began to decline at 92 h. After the eighth batch of glucose feeding, cells started autolysis, and the cultivation was ended immediately.
The time course of pH in these two experiments showed a similar tendency, i.e., first increasing, then keeping a constant level (Fig. 3).The metabolism of nitrogen source containing amino group might contribute to the alkalinization of the medium and thus increasing the broth pH. The carbon source consumption could induce Schizochytrium sp. to secrete some acids including malic acid, citric acid, pyruvic acid, succinic acid, and fumaric acid, decreasing the broth pH (Wu et al. 2005). In addition, the pH at high aeration condition was much higher compared to than that in low aeration conditions, indicating that low aeration might induce the production of acids at higher concentration.
Intracellular product disparity in response to different aeration conditions
Lipids were extracted from the sample every 8 h after the glucose fed for the first time and subsequently esterified for GC analysis. Variations of the main production of FAMEs and squalene were shown in Fig. 4. During the fermentation stages, squalene content decreased from 3.37 to 0.83 g/100 g lipids in experiment II and from 2.5 to 0.81 g/100 g lipids in experiment I. The squalene content in experiment II was much higher than that in experiment I, indicating that high aeration condition was beneficial to squalene synthesis. The final DHA and DPA content in low aeration were slightly higher than that in high aeration. The disparity in saturated fatty acids content between these two experiments was not so pronounced.
Kinetic parameters disparity in response to different aeration conditions
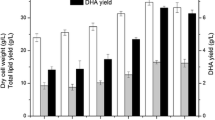
To analyze the kinetic characteristics of the above two processes, six kinetic parameters, including μ, q s , q p , Y x/s, Y p/s, and Y DHA/s , were calculated and shown in Fig. 5 and Table 1. The value of μ, q s , and q p all fluctuated with time because of glucose feeding. μ, q s , and q p in high aeration were much higher than that in low aeration condition at the early fermentation stage. This indicates that high aeration rate was beneficial to cell growth and glucose consumption. From 70 to 100 h, the value of q p in low aeration showed an increase, indicating that high lipid accumulation rate at the middle–late stage could be maintained at low aeration condition. After 100 h, q p in both experiments was relatively low; q p at high aeration condition was slightly higher than that at low aeration condition. Considering the phenomenon of cell autolysis in high aeration condition, lower aeration at the late stage might be more favorable for the whole cell activity.
Yx/s, Yp/s, and YDHA/s at different stages were also compared in Table 1. At stage 1, low aeration rate could ensure higher Yx/s than high aeration rates. At stage 2, Yx/s, Yp/s, and YDHA/s in experiment II were all higher than in experiment I, indicating that higher aeration could not only improve cell growth and glucose consumption but could also accelerate the conversion of glucose to biomass and lipids. At stage 3, although Yx/s at high aeration rate condition (13.04%) was 48.7% higher than that at low aeration rate condition, YDHA/s was 2.7 times lower than that at low aeration rate. This further confirmed the importance of reducing oxygen supply in the later period.
A stepwise aeration control strategy for efficient DHA production
Based on the above analysis, a stepwise aeration control strategy was proposed. In this strategy, the aeration rate was controlled at 0.4 vvm during the first 24 h to ensure high Y x/s. It was then shifted to 0.6 vvm until 96 h to maintain high cell growth rate and glucose consumption rate. Finally, it was switched back to 0.4 vvm again to avoid cell autolysis and obtain high Y DHA/s and PUFAs percentage.
Time course of cell growth, glucose consumption, MSG consumption, and total lipid production at the stepwise aeration control strategy were shown in Fig. 6. Table 2 summarized the main parameters of constant aeration and stepwise aeration control strategy. It was observed that the DHA content and DHA productivity reached 15.76 g/L and 119 mg/L h, which were 15.04% and 11.21% higher than the best results of constant aeration conditions, respectively. DHA content in total fatty acids (TFA) was 48.95%, which was a little lower than 51.48% at low aeration condition. However, the fermentation time was shortened to 132 h from 156 h. In conclusion, the DHA content and DHA productivity were both improved by using this aeration control strategy. The proposed stepwise aeration control strategy was therefore proved to be successful for efficient DHA production.
Discussion
In this paper, the effect of aeration on cell growth, lipid accumulation, and DHA production were investigated systemically, and a stepwise aeration control strategy was proposed to achieve high cell density, high lipid accumulation rate, and high PUFAs percentage. The final cell dry weight, total lipids, and DHA percentage reached 71 g/L, 35.17 g/L, and 48.95%, respectively. The highest DHA productivity obtained in this study was 119 mg/L h, which was achieved by the developed stepwise aeration control strategy. It is close to the highest published value of 134 mg/L h by Schizochytrium sp. SR21 (Yaguchi et al. 1997) and is much higher than 93 mg/L h by using Aurantiochytrium sp. T66. (Jakobsen et al. 2008).
High cell density is the first premise for high production of intracellular products. In terms of aeration effect on aerobic fermentation, it was obvious that high aeration was beneficial to cell growth by increasing cell growth rate (Alfenore et al. 2004; Dosoretz et al. 1990). Our study also showed this positive effect, but with a slight reduction of Y x/s at stage 1 (Table 1). This might be explained by the fact that high aeration rate intensified cell respiration and energy metabolism, which would trigger more carbon flux flow to tricarboxylic acid cycle. Therefore, appropriate aeration adjustment should be adopted to promote the efficiency of substrate utilization efficiency in pilot scale production.
High content of lipids in cell dry weight is the second premise for efficient DHA production. Many studies showed that nitrogen limitation, phosphorus limitation, or oxygen limitation all could enhance lipids accumulation (Jakobsen et al. 2008), but had negative effect on cell growth. Pursuing high lipid accumulation blindly while ignoring low biomass was not practical, especially in large-scale production. Under the conditions of nitrogen limitation, the intracellular AMP level could be reduced by activating AMP deaminase, which catalyzed the deamination of AMP to inosine 5′-monophosphate and ammonia. Low AMP level could inhibit isocitrate dehydrogenase that causes citrate to accumulate in the mitochondria. The accumulated citrate could be cleavaged to acetyl-CoA for fatty acid synthesis (Ratledge 2004). Phosphorus limitation might have a similar mechanism. In this study, the residual phosphorus in the broth were 0.2 and 0.1 g/L for low and high aeration experiments, respectively. Residual nitrogen were 0.2 and 0.4 g/L. In this paper, nitrogen and phosphorus limitation could be achieved automatically after 40 h when nitrogen and phosphorus were nearly exhausted with the C/N ratio above 50:1. This might be the reason for high lipid accumulation rate.
High DHA percentage in TFA is the premise for high oil quality. Low aeration was more favorable for efficient DHA synthesis (Chi et al. 2009; Jakobsen et al. 2008). It was reported by Lippmeier et al. (2009) that the PUFA synthase was the sole system responsible for de novo biosynthesis of PUFAs in Schizochytrium even though this microorganism owns several other fatty acid desaturase and elongase activities. Apart from PUFAs, C14:0 and C16:0 were the main saturated fatty acids synthesized by fatty acid synthetase (FAS). The reduction of saturated fatty acids content is the key for high DHA yield. Our results showed that oxygen limitation was an effective method to achieve high DHA content. This is similar to the observation of Jakobsen et al. (2008). Apart from oxygen limitation, other strategies, such as adding cerulenin (Morita et al. 2005), the inhibitor of FAS, to the broth, were also effective to reduce the percentage of saturated fatty acids. However, it is too expensive, and using antibiotics in the process of infant food additive production is always questioned.
This work provided an analytical method, which could detect squalene and fatty acids simultaneously using gas chromatogram equipped with a DB-23 capillary column. Squalene is an important intermediate in the cholesterol biosynthetic pathway and an essential natural antioxidant to protect the cells from free radicals and reactive oxygen species (Chang et al. 2008). Jiang et al. (2004) had reported that fatty acids and squalene could be produced simultaneously by Schizochytrium mangrovei. The strain used in this study could also produce this bioactive compound with the content of 0.8 g/100 g lipids. The existence of squalene makes the oil more stable and of particularity.
In conclusion, this study systematically examined the effect of aeration on DHA production by Schizochytrium sp. CCTCC M209059 and proposed a stepwise aeration control strategy to achieve high cell growth rate, high lipid accumulation rate, and high PUFA percentage. This strategy was applied in a 1,500-L bioreactor for DHA production. In addition, the idea of controlling different environmental conditions at different culture stages based on the kinetic characteristics of different processes could be applied for the production of other useful metabolites, especially other intracellular products.
References
Alfenore S, Cameleyre X, Benbadis L, Bideaux C, Uribelarrea JL, Goma G, Molina-Jouve C, Guillouet SE (2004) Aeration strategy: a need for very high ethanol performance in Saccharomyces cerevisiae fed-batch process. Appl Microbiol Biotechnol 63:537–542
Chang MH, Kim HJ, Jahng KY, Hong SC (2008) The isolation and characterization of Pseudozyma sp. JCC 207, a novel producer of squalene. Appl Microbiol Biotechnol 78:963–972
Chi ZY, Pyle D, Wen ZY, Frear C, Chen SL (2007) A laboratory study of producing docosahexaenoic acid from biodiesel-waste glycerol by microalgal fermentation. Process Biochem 42:1537–1545
Chi ZY, Liu Y, Frear C, Chen SL (2009) Study of a two-stage growth of DHA-producing marine algae Schizochytrium limacinum SR21 with shifting dissolved oxygen level. Appl Microbiol Biotechnol 81:1141–1148
Dosoretz CG, Chen AC, Grethlein HE (1990) Effect of oxygenation conditions on submerged cultures of Phanerochaete chrysosporium. Appl Microbiol Biotechnol 34:131–137
Fan KW, Chen F, Jones EBG, Vrijmoed LLP (2001) Eicosapentaenoic and docosahexaenoic acids production by and okara-utilizing potential of thraustochytrids. J Ind Microbiol Biotechnol 27:199–202
Ganuza E, Anderson AJ, Ratledge C (2008) High-cell-density cultivation of Schizochytrium sp. in an ammonium/pH-auxostat fed-batch system. Biotechnol Lett 30:1559–1564
Gill I, Valivety G (1997) Polyunsaturated fatty acids, part 1: occurrence, biological activities and applications. Trends Biotechnol 15:401–409
Horrocks LA, Yeo YK (1999) Health benefits of docohexaenoic acid (DHA). Pharmacol Res 40:211–225
Jakobsen AN, Aasen IM, Josefsen KD, Strom AR (2008) Accumulation of docosahexaenoic acid-rich lipid in thraustochytrid Aurantiochytrium sp. strain T66: effects of N and P starvation and O2 limitation. Appl Microbiol Biotechnol 80:297–306
Jiang Y, Fan KW, Wong RT, Chen F (2004) Fatty acid composition and squalene content of the marine microalga Schizochytrium mangrovei. J Agric Food Chem 52:1196–1200
Ji XJ, Huang H, Du J, Zhu JG, Ren LJ, Hu N, Li S (2009) Enhanced 2, 3-butanediol production by Klebsiella oxytoca using a two-stage agitation speed control strategy. Bioresour Technol 100:3410–3414
Liang Y, Sarkany N, Cui Y, Yesuf J, Trushenski J, Blackburn JW (2010) Use of sweet sorghum juice for lipid production by Schizochytrium limacinum SR21. Bioresour Technol 101:3623–3627
Lippmeier JC, Crawford KS, Owen CB, Rivas AA, Metz JG, Apt KE (2009) Characterization of both polyunsaturated fatty acid biosynthetic pathways in Schizochytrium sp. Lipids 44:621–630
Metz JG, Roessler P, Facciotti D, Levering C, Dittrich F, Lassner M, Valentine R, Lardizabal K, Domergue F, Yamada A, Yazawa K, Knauf V, Browse J (2001) Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science 293:290–293
Morita N, Nishida T, Tanaka M, Yano Y, Okuyama H (2005) Enhancement of polyunsaturated fatty acid production by cerulenin treatment in polyunsaturated fatty acid-producing bacteria. Biotechnol Lett 27:389–393
Morita E, Kumon Y, Nakahara T, Kagiwada S, Noguchi T (2006) Docosahexaenoic acid production and lipid-body formation in Schizochytrium limacinum SR21. Mar Biotechnol 8:319–327
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nakahara T, Yokochi T, Higashihara T, Tanaka S, Yaguchi T, Honda D (1996) Production of docosahexaenoic and docosapentaenoic acids by Schizochytrium sp. isolated from Yap Islands. J Am Oil Chem Soc 73:1421–1426
Ratledge C (2004) Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie 86:807–815
Ren LJ, Huang H, Xiao AH, Lian M, Ji XJ (2009) Enhanced docosahexaenoic acid production by reinforcing acetyl-CoA and NADPH supply in Schizochytrium sp. HX-308. Bioproc Biosyst Eng 32:837–843
Rosa SM, Soria MA, Vélez CG, Galvagno MA (2010) Improvement of a two-stage fermentation process for docosahexaenoic acid production by Aurantiochytrium limacinum SR21 applying statistical experimental designs and data analysis. Bioresour Technol 101:2367–2374
Sijtsma L, de Swaaf ME (2004) Biotechnological production and applications of the ω-3 polyunsaturated fatty acid docosahexaenoic acid. Appl Microbiol Biotechnol 64:146–153
Unagul P, Assantachai C, Phadungruengluij S, Suphantharika M, Tanticharoen M, Verduyn C (2007) Coconut water as a medium additive for the production of docosahexaenoic acid (C22:6 n3) by Schizochytrium mangrovei Sk-02. Bioresour Technol 98:281–287
Ward OP, Singh A (2005) Omega-3/6 fatty acids: alternative sources of production. Process Biochem 40:3627–3652
Wu ST, Yu ST, Lin LP (2005) Effect of culture conditions on docosahexaenoic acid production by Schizochytrium sp. S31. Process Biochem 40:3103–3108
Yamanè T, Shimizu S (1984) Fed-batch techniques in microbial processes. In: Fiechter A (ed) Advances in biochemical engineering/biotechnology. Springer, Berlin, pp 147–194
Yaguchi T, Tanaka S, Yokochi T, Nakahara T, Higashihara T (1997) Production of high yields of docosahexaenoic acid by Schizochytrium sp. strain SR21. J Am Oil Chem Soc 74:1431–1434
Acknowledgments
This work was financially supported by the Key Program of National Natural Science Foundation of China (no. 20936002), the National Basic Research Program of China (nos. 2007CB707805 and 2009CB724700), the Scientific Research Project for Post-graduate in Jiangsu Province (no. CX07s_032z), the Fifth of Six Projects Sponsoring Talent Summits of Jiangsu Province (2008), and the College Industrialization Project of Jiangsu Province (2009). We also wish to thank both managerial and technical staff of Jiangsu TianKai Biotechnology Co., Ltd. (Nanjing, P. R. China) for providing the manufacturing facilities and technical assistance. We also thank Dr. Mingjie Jin from Michigan State University and Mr. Samuel St-Pierre for their language assistance with this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, LJ., Ji, XJ., Huang, H. et al. Development of a stepwise aeration control strategy for efficient docosahexaenoic acid production by Schizochytrium sp.. Appl Microbiol Biotechnol 87, 1649–1656 (2010). https://doi.org/10.1007/s00253-010-2639-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2639-7