Abstract
Cadmium (Cd), copper (Cu), mercury (Hg), lead (Pb), and zinc (Zn) were assessed in the edible tissues of Crassrotrea corteziensis oysters collected during the rainy and dry seasons in 27 sites from 8 coastal lagoons of the southeast Gulf of California. In addition, C. palmula oysters were sampled at 9 sites from the same mangrove roots where C. corteziensis oysters were collected. Metal analyses were performed by flame atomic absorption spectrophotometry (Cd, Cu, and Zn), graphite furnace (Pb), and cold vapor detection (Hg). The obtained mean levels were (µg g−1 dry weight) as follows: Cd 6.05 ± 2.77, Cu 60.0 ± 33.4, Hg 0.38 ± 0.17, Pb 1.11 ± 0.63, and Zn 777 ± 528 µg g−1. For all metals except Hg, the concentrations were greater during dry season than during rainy seasons. The high levels, particularly that for Cd, were related to upwelling along the eastern Gulf of California. High Hg levels in the rainy season were associated with the transport of materials from the watershed to the lagoon. Shrimp farming, agriculture, and other sources were considered as potential sources to explain the differences in metal bioavailability in the 8 lagoons. The mean concentrations of Cd (Santa María-La Reforma lagoon), Cu [San Ignacio–Navachiste–El Macapule (SINM), Urías (URI), and Altata-Ensenada del Pabellón lagoons], and zinc (Zn) (URI, Santa María–Ohuira–Topolobampo, El Colorado, and SINM lagoons) during the dry season were greater than the maximum permissible limits. C. palmula collected in 8 sites where they were present simultaneously with C. corteziensis had consistently greater metal levels than C. corteziensis, but correlation analyses showed a high and significant (P < 0.05) correlation between metal concentrations in both species. The correlation equations obtained are useful where the same species is not distributed and is necessary to compare results from distinct regions.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Trace metals occur naturally in aquatic systems and are leached from soil and rocks into water at low concentrations. They typically do not result in any serious or deleterious effects on human health; however, anthropogenic activities promote a rapid increase in environmental metal pollution. Therefore, the monitoring of metal availability in organisms from these environments is an important topic of concern. Bivalve organisms are in the second trophic level of aquatic ecosystems and have long been known to accumulate both essential and nonessential trace elements. Several studies have reported the potential of using bivalve organisms, particularly mussels and oysters, as biomonitors or biomarkers for monitoring metal contamination in aquatic systems (Rainbow and Phillips 1993; Kanthai et al. 2014).
Mangroves and their associated aquatic habitats are ecosystems that provide numerous and important ecological services. In these ecosystems, a great diversity of biota is present that are significant from a commercial and ecological viewpoint. Recent interest has been placed on the ecological and socioeconomic importance of mangroves for the adjacent populations in terms of food resources, employment, and generation of income (Bayen 2012). The impact of anthropogenic chemicals on tropical and subtropical habitats has been of limited interest until the last 15 years despite several routes of contaminant exposure. At least 53 species of mangrove habitat-associated biota, with bivalves as the main group (Lewis et al. 2011), have been reported in studies of contaminant bioaccumulation.
Three species of wild oysters are found along the Pacific coast of Mexico: Crassostrea iridescens Hanley 1854, C. corteziensis Hertlein 1951, and C. palmula Carpenter 1857. Although C. iridescens is associated with rocky substrata and inhabits subtidal areas in open coasts, C. palmula and C. corteziensis reside within coastal lagoons (Páez-Osuna et al. 2002) and are mostly associated with mangrove roots. Therefore, C. corteziensis and C. palmula were selected to be the main cosmopolitan species associated with mangrove roots in the southeast (SE) Gulf of California. In this study, the contents of cadmium (Cd), copper (Cu), mercury (Hg), lead (Pb), and zinc (Zn) in the soft tissue of mangrove oysters from eight coastal lagoons in the SE Gulf of California were analyzed to evaluate the possible risk to human health on consumption of these organisms and understand the influence of the main anthropogenic activities in each watershed. In addition, we investigated the spatial and temporal variation (dry and rainy periods) of trace metals in oysters. Also, we compared the accumulated trace metals between the two oyster species that occur in the mangrove roots, i.e., C. corteziensis and C. palmula.
Materials and Methods
Study Site and Sampling
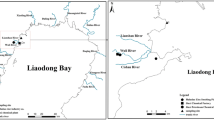
The SE Gulf of California region includes a coastline of approximately 750 km with 12 lagoon complexes. It is centrally located to the north of a agricultural area (approximately 900,000 ha of intensive agriculture), has a moderate population (approximately 3.1 million inhabitants), and has recently incorporated shrimp aquaculture (approximately 50,000 ha) (Table 1). The region is characterized by two well-defined periods of rainfall, the dry season and the rainy season, which are defined, respectively, by monthly average air temperatures between 22 and 25 and 27 and 30 °C (CNA 2005). The pluvial precipitation in Sinaloa has a gradient that increase of north to south and from the coast to the mountain. On the coastal plain from El Colorado (EC) and Santa María–Ohuira–Topolobampo (SMOT) lagoon systems to Ceuta lagoon (CEU) it ranges from 125 to 400 mm, and from Urías lagoon (URI) to north of Nayarit state it ranges from 600 to 800 mm (Páez-Osuna et al. 2007). At the control site (CS; Estero del Rey, Nayarit) it is 1,100 mm (INEGI 2010).
Four mangrove species occur in the region: white mangrove (Laguncularia racemosa), black mangrove (Avicennia germinans), buttonwood mangrove (Conocarpus erectus), and red mangrove (Rhizophorae mangle); the latter is more often associated with the presence of oysters in the roots. The estimated area of mangroves in the region is approximately 249,702 ha (Páez-Osuna et al. 2003). Oysters were sampled directly by hand from mangrove roots in 7 coastal lagoons of Sinaloa state: EC, SMOT, San Ignacio–Navachiste–El Macapule (SINM), Santa María-La Reforma (SMLR), Altata-Ensenada del Pabellón (AEP), CEU, and URI (Fig. 1). Each lagoon is influenced by several anthropogenic sources, which are listed in Table 1. In addition, a CS was selected in Estero de Rey, San Blas, Nayarit, Mexico, because this area and the associated drainage basin have low anthropogenic pressure. Oysters were sampled from the roots of R. mangle mangrove trees in October 2008 (days 2, 9, 10, 16, 17, 23, and 28) at the end of the rainy season and in April (days 16, 17, 23, 24, and 30) and May 2009 (8 and 16) during the dry season. At each sampling site, salinity (±0.2 g/L; refractometer Shimadzu 2000C) and temperature (±0.1 °C) were recorded. In addition, the coordinates of each site were recorded using a handheld global positioning system (GPS). Each sample consisted of 25 wild oysters C. corteziensis of similar size (40- to 60-mm length); specimens were collected in duplicate (i.e., a total of 50 oysters) from 27 sampling sites along the 8 coastal lagoons (Table 2). The range of sizes was selected considering the availability of oysters for each season and corresponds to individuals of commercial size that have reached maturity (Chávez-Villalba et al. 2005). All oysters were collected from an interval depth of 20–60 cm with respect to mean high water. This strategy was adopted because most metals do not show significant differences in concentrations (Osuna-López et al. 1990). In addition, C. palmula oysters were sampled at eight sites (SINM8, SINM10, SMLR11, SMLR12, SMLR16, AEP17, CEU21, and URI24) from the same mangrove roots where C. corteziensis oysters were collected; similarly 25 individuals of similar size (40- to 60-mm length) were selected in duplicates (i.e., a total of 50 oysters). Just after sampling, live oysters were transported to the laboratory and subsequently maintained in lagoon water from the mouth of each lagoon for 36 h to defecate their gut contents (Szefer et al. 2006).
Metal Analyses
Once the oyster shells were cleaned, they were measured (mm) and weighed [total tissue (g)]. The soft tissue in the oysters was extracted, homogenized, freeze-dried for 72 h (–49 °C and 133 × 10−3 mbar), weighed, and then ground in an automatic agate mortar. The condition index (CI) was determined by the following equation (Walne and Mann 1975): CI = soft tissue dry weight (g) × 1,000/shell dry weight (g). The glassware and other plastic utensils were previously washed according to Osuna-Martínez et al. (2010). The samples were digested using a microwave system (Model MARS-X, CEM Corporation, USA) in one step, in which 5 mL of concentrated HNO3 (70 % J. T. Baker for trace-metal analysis) was added to 0.25 g of tissue samples, and the mixture was heated to 100 °C for 5 min, to 120 °C for a further 5 min, and then to 140 °C for 10 min. For every 13 samples, a blank run was inserted during the digestion step. The digested samples, blanks, and reference material were diluted with Milli-Q water to a final volume of 20 mL. Blanks and standard reference material (mussel tissue 2976 NIST, EVISA) were digested (1 set each of 28 samples) with the same procedure. These samples were analyzed by atomic absorption spectrometry (Varian SpectrAA 220, Varian Inc, Palo Alto, California) with flame (Cd, Cu, and Zn), graphite furnace (Pb) (Varian GTA110), and cold vapor detection (Hg) (Varian VGA76). The analytical results of the certified mussel material value were in satisfactory agreement, and the recoveries varied from 103.6 to 117.6 %. Depending of the level of concentration and the element, the coefficient of variation fluctuated from 1.5 to 7.1 %. The limits of detection were 0.06, 3.6, 0.06, 0.44, and 10.3 µg g−1 for Cd, Cu, Hg, Pb, and Zn, respectively.
Statistical Analysis
Normality tests (Kolmogorov–Smirnov) were performed on the biometric data of oysters, salinity, temperature, and the concentrations of metals. Comparisons of the average concentrations of metals between the lagoons and between seasons were performed using multiple comparison of means. The analyses were performed using parametric or nonparametric tests depending on the results of the normality tests. Correlation analysis was performed for the metal concentrations and other variables (temperature, salinity, and CI) using GraphPad Prism 5 (GraphPad Software, San Diego, California, USA). Finally, Pearson correlation analysis was performed to obtain the linear equations that were related to the content of the metals between both species (C. corteziensis and C. palmula). For all statistical results, a level of significance of P < 0.05 was considered.
Results and Discussion
Physicochemical Parameters of Lagoon Water
During each sampling, the salinity and temperature of the waters in each site where the oysters were collected were recorded. According to the number of sampling sites, we calculated an average for each season and for each lagoon. The mean salinities for dry and rainy season were as follows: 35.0 and 31.5 for EC, 32.0 and 28.9 for SMOT, 36.5 and 31.5 for SINM, 38.0 and 36.7 for SMLR, 35.0 and 33.1 for AEP, 31.5 and 32.8 for CEU, 39.5 and 32.8 for URI, and 39.1 and 32.6 g/L for CS, respectively. The mean temperatures for the dry and rainy seasons were as follows: 28.5 and 32.5 °C for EC, 26.5 and 32.0 °C for SMOT, 26.3 and 30.6 °C for SINM, 27.6 and 28.3 °C for SMLR, 27.8 and 29.6 °C for AEP, 29.0 and 30.8 °C for CEU, 30.2 and 31.3 °C for URI, and 33.5 and 31.1 °C for CS, respectively. The lower salinity and temperature corresponded to the SMOT (rainy season) and SINM lagoons (dry season) respectively, and the greater to URI (dry season) and SMOT (rainy season) lagoons, respectively.
Biometric Parameters of Oysters
The size, expressed as length and weight (total and soft, respectively), and the CI of each pool of oysters (duplicate samples) are listed in Table 2. During the rainy season, the average size of C. corteziensis was 45.3 ± 2.6 mm, and the average weights of the total and soft tissue were 13.5 ± 3.2 and 1.7 ± 0.3 g, respectively. Regarding CI, the values were comparable; they were slightly greater during the dry season (25.5 ± 7.1) than during the rainy season (24.7 ± 3.9). The means were not significantly (P < 0.05) different. The CI can serve to establish the quality of the product for commercial purposes and to characterize the apparent health of organisms for physiological purposes (Lucas and Beninger 1985). Hypothetically, high CI values indicate a better health status than low CI values. In C. corteziensis, the highest CI values were consistently recorded at the SMOT6, SINM10, and CEU23 sites and the CS in the two seasons, which indicates a physiologically better status of these oysters.
Metal Concentrations in C. corteziensis and their Sources
The order of magnitude of metal concentrations in the soft tissues of C. corteziensis was Zn > Cu > Cd > Pb > Hg for both the rainy and dry season samples (Fig. 2; Table 3). A correlation matrix clearly showed the existence of several significant (P < 0.05) correlations: Hg had the highest number of correlations with Pb (r = 0.298), temperature (r = 0.265), Zn (−0.474), CI (r = −0.584), and salinity (r = −0.296). CI had a negative correlation with Cu (r = −0.242), Hg (r = −0.584), and Pb (r = −0.464). Cd was correlated with Cu (r = 0.312), Zn (r = −0.375), and temperature (r = −0.417). It is important indicate that water salinities and temperatures vary continuously due to the tidal cycles; however, such variations have a characteristic pattern and contrast between the dry and rainy season (particularly for temperature). The negative behavior of Cd with temperature is indirectly related to upwelling events; these events occur in the SE Gulf of California during the winter and spring when the water temperatures are relatively low (Staines-Urías et al. 2009). In addition, the negative correlation of Hg with salinity is indirectly related to the rainy season when lagoon waters decrease in salinity and simultaneously transport Hg-enriched materials by way of runoff from the watershed. Aside from the species and level of exposure, the body size of oysters is one of the most important factors that affect metal accumulation; to avoid this influence, we collected specimens of similar sizes (Table 2). However, the soft tissue weight was sometimes slightly greater in samples from the dry season when the CI value was greater. This pattern has previously been observed in other species such as C. rhizophorae (Rebelo et al. 2005) and C. gigas (Osuna-Martínez et al. 2010).
Globally, mean concentrations were greater during the dry season for all metals except Hg (Fig. 2). However, the differences in most cases were not significant (P > 0.05). This consistent pattern may be explained by several factors including seasonal differences in food supply (associated with an increase in productivity), changes in the runoff of particulate metals to the lagoons in periods of high precipitation, and variations related to the reproductive cycle (Páez-Osuna et al. 1995). Greater Hg concentrations during the rainy season could be related to the transport of materials from the watershed to the lagoons during runoff processes. Greater Hg concentrations during the rainy season have been recorded in other bivalves from different regions, e.g., in the Moroccan Atlantic coast in Mytilus galloprovincialis and C. gigas (Maanan 2008) and in the Setiu lagoon, Malaysia, in C. iredalei (Affizah et al. 2009). During precipitation periods, a first effect of dilution and transport of metals to the sea occur and lower concentrations of most of metals should be found. However, for Hg (which forms organic complexes as methylmercury), the formation of organic molecules could explain better the retention within the lagoon systems even during the rainy season.
Greater levels of Cd in soft tissue of C. corteziensis were found during the dry season. A similar result was found by García-Rico et al. (2010) in the same species along the Sonora coast. This pattern could be related to the presence of an upwelling regime during the dry season. The Gulf of California is characterized by upwelling events along its eastern coast, which typically occur from February to April (Staines-Urías et al. 2009); this trend has also been recorded in the mussel M. californianus from the northeast (NE) Pacific Ocean (Lares et al. 2002). It is interesting to note that the lagoons where we found more increased Cd concentrations in soft tissue of C. corteziensis, the SMLR and SINM lagoons (Fig. 2), are those with two mouths (Fig. 1); thus, they have a greater exchange of water with the Gulf of California. Moreover, the influence of upwelling events appears to be more evident in Cd than in other metals, such as Cu and Zn, due to the relative magnitude of fluxes from other sources than upwelling, which are likely lower for Cd and not for Cu and Zn. Cd is a good example of nutrient behavior in the ocean (a depletion of an element in surface waters and an enrichment at depth) in which the metal is associated with the soft parts of living and dead biological material. Such enrichment is more evident in the North Pacific, and it has been shown that most nitrates and phosphates have a near linear correlation (Millero 2006).
However, Zn and Cu are examples of essential metals, which may be the main reason for their high concentrations in soft tissue. The high absorption of these metals by oysters is biologically balanced with a high use of such compounds in metabolic processes. Zn and Cu act as natural cofactors in the enzyme reactions of cells, which therefore require oysters to ingest high concentrations of these metals in their diets (Azlisham et al. 2009). The tendency of greater levels of both metals in C. corteziensis from lagoons, such as the SINM and AEP lagoons, may be due to the use of Cu- and Zn-based products (fungicides and fertilizers) in agriculture. High levels of Cu and Zn were also found in the URI lagoon (Fig. 2) where agricultural activity is absent. The URI lagoon is one of the smallest lagoons (Table 1) and is characterized as the most urbanized system; there is a large ship fleet in the area, and the high levels of Cu could be related to the Cu-based antifouling paints used in the shipping industry. Another important source is sewage because Cu and Zn take part in the formulation of commercial detergents, shampoos, and other personal care products.
In addition, seafood industry, shipping industry, and one thermoelectric power plant are present in the area. The highest concentrations of Zn were found at sites URI24 and SMOT6; both sites were the closest to the two thermoelectric plants. It is well known that metals, such as Cu, Zn, and Hg, are emitted from thermoelectric power stations to the atmosphere and mainly expand close to the source (Ruíz-Fernández et al. 2009).
The highest Pb levels were found in the lagoons from the central zone of Sinaloa (AEP, SMLR) and in the URI lagoon, where most of the state population resides (Table 1). The major transport routes of Pb for the coastal ecosystem is runoff from urban settlements into nearby watersheds where it is transported by combined sewage systems, agricultural effluents, rural runoff, and direct atmospheric deposition (Soto-Jiménez and Flegal 2008). The atmospheric deposition involves a source of Pb that is transported from vehicle emissions, which mainly comes from the cities of Culiacan and Mazatlan, as well as several roads that surround the study area, which is consistent with the number of vehicles in this northwest region. According to the isotopic ratios of the Pb found in environmental samples from AEP and URI lagoons, Pb has an anthropogenic origin, primarily from the use of leaded gasoline between the 1940s and 1997 (Soto-Jiménez et al. 2008).
The CS was selected by considering a less-urbanized and less-populated area compared with the lagoons from Sinaloa state (Fig. 1; Table 1). Cd and Cu levels were clearly lower in the CS than in the other sites. Nevertheless, Hg, Pb, and Zn concentrations were greater in the CS than in the various sites that were studied in the coastal lagoons from Sinaloa. The relatively high Pb concentration in the CS appears to be a result of the natural background values of the alluvial soils and rocks of the Pacific coastal plain (Soto-Jiménez et al. 2008).
Lyle-Fritch et al. (2006) documented the use and application rates of chemicals and biological products for shrimp farming in the SE Gulf of California. A total of 106 different types of products were identified, and an average of 42 products was applied at each farm. The most commonly used products were feed additives, liming materials, inorganic fertilizers, and antibiotics; the common components of feed additives and fungicides include copper (CuSO4) and zinc (ZnSO4, ZnO). Lacerda et al. (2006) estimated an emission factor of 168 g/ha/cycle for Cu in shrimp farms from NE Brazil. By assuming such loading and considering 2.3 cycles/year (Lyle-Fritch et al. 2006) in lagoons such as the SINM, SMLR, and AEP lagoons, where thousands of hectares are dedicated to shrimp farming (Table 1), it is possible to estimate annual loads of 2558, 2985, and 2995 g Cu/year, respectively, which are often discharged directly into the lagoons. An additional metal that has recently been considered in shrimp farming is Hg. This element is present in aquaculture effluents due to its presence as a natural component in aquaculture feed, particularly fishmeal, and as an impurity in fertilizers and other chemicals. Lacerda et al. (2011) estimated an emission factor of 83.5 mg/ha/cycle for Hg in shrimp farms from NE Brazil. Assuming such levels here, we estimated an annual load of 1272, 1483, and 1488 g Hg/y for the SINM, SMLR, and AEP lagoons, respectively.
Once present in lagoon waters, trace metals are biotically and abiotically distributed in the different reservoirs. The abiotic fraction can be dissolved and linked to distinct materials in the water column (e.g., iron and manganese oxides, humic and fulvic acids) or deposited in sediments. Similarly, living microorganisms (e.g., microalgae, bacteria, fungus) has an important role on the metal distribution in the coastal lagoons. Finally, these biogeochemical carriers influence on the concentrations of each metal available for the biota including filter-feeding animals.
Generally, concentrations in both the water and surface sediments tend to decrease as the distance increases from specific sources. Costa et al. (2013) examined the Hg distribution in a mangrove creek affected by intensive shrimp farming using Ward quadratic distance cluster analysis and showed the effects of shrimp-farming effluents on the Hg distribution pattern. In the SE Gulf of California lagoons, according to the surface area dedicated to shrimp farming (Table 1), we expect that the SINM, SMLR, and AEP lagoons could be the most affected by metals associated with shrimp farming. However, the morphology, topography, depth, and number of mouths and their size determine the hydrodynamics and the potential availability of trace metals. Another point relevant is the resuspension of contaminated sediments, which are a source of metals to biota. In various coastal lagoons of SE Gulf of California, a predominance of shallow areas is characteristic; thus, the occurrence of phenomena that induces resuspension may increase the transference of metals to oysters.
Metal Concentrations in C. corteziensis Over Time
In general, Cd, Cu, and Zn levels found in C. corteziensis (sampled from 2008 to 2009) are comparable with previously recorded levels. In the case of Hg, the concentrations found in this study were greater than those previously documented (Table 4). Nevertheless, there are lagoons, such as the AEP and URI lagoons, that have been studied in greater detail when we compare their historical concentrations: The metal bioavailability appears to be different between lagoons. In the AEP system (Páez-Osuna et al. 2002; Ruelas-Inzunza and Páez-Osuna 2008; Frías-Espericueta et al. 2009), the decrease in mean levels of Pb, Cu, and Zn was noticeable, whereas in the URI system (Páez-Osuna and Marmolejo-Rivas 1990; Páez-Osuna et al. 1988, 2002; Osuna-López et al. 1990; Ruelas-Inzunza and Páez-Osuna 2000; Soto-Jiménez et al. 2008; Frías-Espericueta et al. 2005; Jara-Marini et al. 2008), there was an increasing trend in concentrations for the same metals. In the case of Pb, despite an increased number of vehicles in the cities of Culiacan and Mazatlan and increased drainage from the watershed of both lagoons, the use of Pb in gasoline as an antiknock agent has decreased. The decrease in Cu and Zn concentrations in the AEP system could be related to their lower use in agriculture operations, whereas their slightly increased concentrations in the URI lagoon could be attributed to the recent expansion of shrimp farming.
Comparison of Metal Levels Among the Different Mangrove Oyster Species
Table 4 lists the data obtained in this study and those reported in two mangrove species of the genus Crassostrea (C. rhizophorae and C. corteziensis) from elsewhere in the world. The concentrations in C. corteziensis from the coastal lagoons of the Gulf of California can be considered increased for Hg and Cd and intermediate for Cu, Pb, and Zn, thus indicating moderate or increased bioavailability. Thus, lagoons such as the URI lagoon for Zn, the AEP lagoon for Pb, the CEU lagoon for Hg, the SINM lagoon for Cu and Cd, and the SMLR lagoon for Cd could be interpreted as being metal-contaminated. However, such comparisons should be made with caution because they are somewhat general due to the complexity of considering the influence of size, reproductive stage, and physiological condition on metal concentrations in oysters (Páez-Osuna et al. 1995; Alfonso et al. 2013).
Metal Intake and Human Consumption of Oysters
Global mean concentrations of these metals and their mean concentrations for C. corteziensis in each coastal lagoon are listed in Table 3. The nonessential metal (Cd, Hg, and Pb) concentrations were lower than the maximum permissible limits (MPLs) that have been previously established (WHO 1982) (Fig. 2; Table 3). Nevertheless, global mean concentrations of Cu and Zn, which are considered as essential metals, in various lagoons (SINM, URI, and AEP for Cu and URI, SMOT, EC, and SINM for Zn) were greater than the MPL established by the WHO (1982) and Mexican legislation (Norma Official Mexicana NOM-242 2009), whose values are in agreement with each other. As expected, these greater levels were found in the lagoon systems of the central and north portion of the state of Sinaloa, which is characterized by greater levels of agricultural activity and shrimp farming as well as a greater population (Table 1). According to the WHO (1996), the upper limit of the safe range of population mean intakes of Zn for adults (age range 18–60 years) has accordingly been set at 45 mg Zn/d for men and 35 mg Zn/d for women. Similarly, for Cu it has been set at 12 mg Cu/d for men and 10 mg Cu/d for women. Concerning the maximum mean level of Zn and Cu registered in the soft tissue of C. corteziensis from coastal lagoons of the SE Gulf of California, the required amount to reach 35–45 mg/day for Zn and 10–12 mg/day for Cu would be approximately 100–129 and 444–533 g wet weight of oysters (soft tissue), respectively. In the case of Zn, the typical consumption ration of oysters in restaurants is equivalent to the wet weight estimated to reach the upper limit of the safe range. However, the apparent exposure may not reflect a consistent intake of this magnitude because individuals who consume oysters usually consume them occasionally. In contrast to the general population, the subpopulation of fishermen (composed of artisanal fishermen and communities associated) has a different consumption rate of seafood, including oysters, and so they are evidently more exposed to metals than the rest of the population.
Comparative Bioavailability of Trace Metals Using C. corteziensis and C. palmula
According to their distribution patterns, the two oyster species, C. corteziensis and C. palmula, can be found together at mangrove roots in these coastal lagoons. In some cases, as they were collected from the same roots in various sites (Table 2), it was evident that both species were exposed to the same water conditions (temperature, salinity, pH, and available food); consequently, they would also be exposed to the same levels of metals. The range of concentrations found was for Cd 0.85–7.8 and 0.91–14.1 µg/g, for Cu 23.5–125.0 and 28.2–128.1 µg/g, for Hg 0.18–0.62 and 0.20–0.78 µg/g, for Pb 0.26–2.81 and 0.15–1.56 µg/g, and for Zn 110–2755 and 410–2961 µg/g in C. corteziensis and C. palmula, respectively. With the exception of Pb, the soft tissue of C. palmula contained consistently greater metal concentrations than that of C. corteziensis, but the correlation analyses showed significant (P < 0.05) and high correlation coefficients between the metal levels of both species as shown in Fig. 3. These results demonstrate that closely related species from the same site can accumulate significantly different concentrations of the same component as shown in different organisms from temperate regions. The mussels Mytilus edulis and M. trossulus from the same site in Newfoundland (Canada) accumulated different metal concentrations (Lobel et al. 1990); similarly, the barnacles Balanus amphitrite and B. uliginosus (or B. kondakovi) inhabiting adjacent areas in Fujian province (China) also had different levels of metals (Rainbow and Phillips 1993).
It is clear that it is necessary to biomonitor species that are cosmopolitan in their geographical distribution to compare the metal bioavailabilities over large geographical distances or between published studies from different parts of the world. However, in most tropical and subtropical regions, the same species is not distributed throughout the entire zone of interest covered by a biomonitoring program. In such cases, the determination of the levels of metals (or other pollutants) that are accumulated in different species may or may not be related by a simple relationship equation. If biomonitoring data from multiple species are available, useful interpretations can be expanded. If the same species is not found at all sites of interest, protocols that include adequate equations of intercomparison could be useful to compare data obtained from different species.
Conclusions
The results of the present study noted several concerns regarding relatively increased Cd concentration in oysters from the SMLR lagoon during the dry season, moderate Cu availability in the SINM, URI, and AEP lagoons during the dry season, high Zn availability for the SMOT, EC, and SINM lagoons during the dry season, and high Zn availability for the URI lagoon during both seasons. These results are of great significance in measuring recent and future impacts on the study area; development is projected to increase in the short term to mark the completion of the construction of the road that connects this SE Gulf of California region with the Atlantic Ocean. This work also provides useful information (equations) on the intercomparison of metal data (Cd, Cu, Hg, Pb, and Zn) of the two main mangrove oyster species (C. corteziensis and C. palmula) that occur in the coastal environments of the eastern Pacific from the Gulf of California to northern Peru. It is important in future studies to verify such equations at other sites and experimentally.
References
Affizah N, Vedamanikam VJ, Shazilli NAM (2009) Concentration of arsenic and mercury in the oyster (Crassostrea iredalei) from Setiu lagoon, Terengganu. Toxicol Environ Chem 91:259–265
Alfonso JA, Handt H, Mora A, Vásquez Y, Azocar J, Marcano E (2013) Temporal distribution of heavy metal concentrations in oysters Crassostrea rhizophorae from the central Venezuela coast. Mar Pollut Bull 73:394–398
Azlisham M, Vedamanikam VJ, Shazilli NAM (2009) Concentrations of cadmium, manganese, copper, zinc, and lead in the tissues of the oyster (Crassostrea iredalei) obtained from Setiu Lagoon, Terengganu, Malaysia. Toxicol Environ Chem 91:251–258
Bayen S (2012) Occurrence, bioavailability and toxic effects of trace metals and organic contaminants in mangrove ecosystems: a review. Environ Int 48:84–101
Chávez-Villalba J, López-Tapia M, Mazón-Suástegui J, Robles-Mungaray M (2005) Growth of the oyster Crassostrea corteziensis (Hertlein 1951) in Sonora, Mexico. Aquac Res 36:1337–1344
Comisión Nacional del Agua Servicio Meteorológico Nacional (2005) Datos estadísticos climáticos del observatorio meteorológico de Mazatlán, Sinaloa, México [in Spanish]. CNA
Costa BGB, Soares TM, Torres RF, Lacerda LD (2013) Mercury distribution in a mangrove tidal creek affected by intensive shrimp farming. Bull Environ Contam Toxicol 90:537–541
Frías-Espericueta MG, Osuna-López JI, Flores-Reyes S, López-López G, Izaguirre-Fierro G (2005) Heavy metals in the oyster Crassostrea corteziensis from Urías lagoon, Mazatlan, Mexico, associated with different anthropogenic discharges. Bull Environ Contam Toxicol 74:996–1002
Frías-Espericueta MG, Osuna-López JI, Bañuelos-Vargas I, López-López G, Muy-Rangel MD, Izaguirre-Fierro G et al (2009) Cadmium, copper, lead and zinc contents of the mangrove oyster, Crassostrea corteziensis, of seven coastal lagoons of NW Mexico. Bull Environ Contam Toxicol 83:595–599
García-Rico L, Tejeda-Valenzuela L, Burgos-Hernández A (2010) Seasonal variations in the concentrations of metals in Crassostrea corteziensis from Sonora, Mexico. Bull Environ Contam Toxicol 85:209–213
Instituto Nacional de Geografía y Estadística (2010) Available at: http://www.inegi.org.mx/. Accessed: November 6, 2014
Jara-Marini ME, Soto-Jiménez MF, Páez-Osuna F (2008) Trace metal accumulation patterns in a mangrove lagoon ecosystem, Mazatlan Harbour, SE Gulf of California. J Environ Sci Health A 43:1–11
Kanthai LD, Gobin JF, Beckles DM, Lauckner B, Mohammed A (2014) Metals in sediments and mangrove oysters (Crassostrea rhizophorae) from the Caroni Swamp, Trinidad. Environ Monit Assess 186:1961–1976
Lacerda LD, Santos JA, Madrid RM (2006) Copper emission factors from intensive shrimp aquaculture. Mar Pollut Bull 52:1823–1826
Lacerda LD, Soares TM, Costa BGB, Godoy MDP (2011) Mercury emission factors from intensive shrimp aquaculture and their relative importance to the Jaguaribe River estuary, NE Brazil. Bull Environ Contam Toxicol 87:657–661
Lares ML, Flores-Muñoz G, Lara-Lara R (2002) Temporal variability of bioavailable Cd, Hg, Zn, Mn and Al in an upwelling regime. Environ Pollut 120:595–608
Lewis M, Pryor R, Wilking L (2011) Fate and effects of anthropogenic chemicals in mangrove ecosystems: a review. Environ Pollut 159:2328–2346
Lobel PB, Belkhode SP, Jackson SE, Longerich HP (1990) Recent taxonomic discoveries concerning the mussel Mytilus: implications for biomonitoring. Arch Environ Contam Toxicol 19:508–512
Lucas A, Beninger P (1985) The use of physiological condition indices in marine bivalve aquaculture. Aquaculture 44:187–200
Lyle-Fritch LP, Romero-Beltrán E, Páez-Osuna F (2006) Survey on use of the chemical and biological products for shrimp farming in Sinaloa (NW Mexico). Aquac Eng 35:135–146
Maanan M (2008) Heavy metal concentrations in marine molluscs from the Moroccan coastal region. Environ Pollut 153:176–183
Millero F (2006) Chemical oceanography. Taylor & Francis, Boca Raton
Norma Oficial Mexicana 242 (NOM-242-SSA1) (2009) Productos y servicios. Productos de la pesca frescos, refrigerados, congelados y procesados. Especificaciones sanitarias y métodos de prueba [in Spanish]
Osuna-López JI, Zazueta-Padilla HM, Rodríguez-Higuera A, Páez-Osuna F (1990) Trace metal concentration in mangrove oyster (Crassostea corteziensis) from tropical lagoon environments, Mexico. Mar Pollut Bull 21:486–488
Osuna-Martínez CC, Páez-Osuna F, Alonso-Rodríguez R (2010) Mercury in cultured oysters (Crassostrea gigas Thunberg 1793 and C. corteziensis Hertlein 1951) from four coastal lagoons of the SE Gulf of California, Mexico. Bull Environ Contam Toxicol 85:339–343
Páez-Osuna F, Marmolejo-Rivas C (1990) Trace metals in tropical coastal lagoon bivalves, Crassostrea corteziensis. Bull Environ Contam Toxicol 45:538–544
Páez-Osuna F, Izaguirre-Fierro G, Godoy-Meza RI, González-Fernández F, Osuna-López JI (1988) Metales pesados en cuatro especies de organismos filtradores de la región costera de Mazatlán: técnicas de extracción y niveles de concentración [in Spanish]. Contam Amb 4:37–41
Páez-Osuna F, Frías-Espericueta MG, Osuna-López JI (1995) Trace metal concentrations in relation to season and gonadal maturation in the oyster Crassostrea iridescens. Mar Environ Res 40:19–31
Páez-Osuna F, Ruíz-Fernández AC, Botello AV, Ponce-Vélez G, Osuna-López JI, Frías-Espericueta MG et al (2002) Concentrations of selected trace metals (Cu, Pb, Zn), organochlorines (PCBs, HCB) and total PAHs in mangrove oysters from the Pacific Coast of Mexico: an overview. Mar Pollut Bull 44:1296–1313
Páez-Osuna F, Gracia-Gasca A, Flores-Verdugo F, Lyle-Fritch ML, Alonso-Rodríguez R, Roque A et al (2003) Shrimp aquaculture development and the environment in the Gulf of California ecoregion. Mar Pollut Bull 46:806–815
Páez-Osuna F, Ramírez-Reséndiz G, Ruiz-Fernández AC, Soto-Martínez MF (2007) La Contaminación por Nitrógeno y Fósforo en Sinaloa: Flujos, Fuentes, Efectos y Opciones de manejo. In: Páez-Osuna F (ed) La Serie las Lagunas Costeras de Sinaloa. UNAM, El Colegio de Sinaloa
Rainbow PS, Phillips DJS (1993) Cosmopolitan biomonitors of trace metals. Mar Pollut Bull 26:593–601
Rebelo MF, Amaral MCR, Pfeiffer WC (2005) Oyster condition index in Crassostrea rhizophorae (Guilding 1828) from a heavy-metal polluted coastal lagoon. Braz J Biol 65:345–351
Ruelas-Inzunza JR, Páez-Osuna F (2000) Comparative bioavailability of trace metals using three filter-feeder organisms in a subtropical coastal environment (Southeast Gulf of California). Environ Pollut 107:437–444
Ruelas-Inzunza JR, Páez-Osuna F (2008) Trophic distribution of Cd, Pb, and Zn in a food web from Altata-Ensenada del Pabellón subtropical lagoon, SE Gulf of California. Arch Environ Contam Toxicol 54:584–596
Ruíz-Fernández AC, Frignani M, Hillaire-Marcel C, Ghaleb B, Arvizu MD, Raygoza-Viera JR et al (2009) Trace metals (Cd, Cu, Hg, and Pb) accumulation recorded in the intertidal mudflat sediments of three coastal lagoons of the Gulf of California, Mexico. Estuar Coast 32:551–560
Silva CAR, Rainbow PS, Smith BD, Santos ZI (2001) Biomonitoring of trace metal contamination in the Potengi estuary, Natal (Brasil), using the oyster Crassostrea rhizophorae a local food source. Water Res 17:4072–4078
Soto-Jiménez MF, Flegal AR (2008) Origin of lead in the Gulf of California ecoregion using stable isotope analysis. J Geochem Explor 101:209–217
Soto-Jiménez MF, Páez-Osuna F, Scelfo G, Hibdon S, Franks R, Aggarawl J et al (2008) Lead pollution in subtropical ecosystems on the SE Gulf of California Coast: a study of concentrations and isotopic composition. Mar Environ Res 66:451–458
Staines-Urías F, Douglas RG, Gorsline DS (2009) Oceanographic variability in the southern Gulf of California over the past 400 years: evidence from faunal and isotopic records from planktonic foraminifera. Palaeog Palaeoclim Palaeoecol 284:337–354
Szefer P, Fowler SW, Ikuta K, Páez-Osuna F, Ali AA, Kim B (2006) A comparative assessment of heavy metal accumulation in soft parts and byssus from subartic, temperate, subtropical and tropical marine environments. Environ Pollut 139:70–78
Walne PR, Mann R (1975) Growth and biochemical composition in Ostrea edulis and Crassostrea gigas. Proceedings of the 9th European Marine Biology Symposium, pp 587–607
World Health Organization (1982) Toxicological evaluation of certain food additives and contaminants. Joint FAO/WHO expert committee on food additives. WHO food additives. WHO, Geneva, pp 28–35
World Health Organization (1996) Trace elements in human nutrition and health. WHO, Geneva, p 178
Acknowledgments
We thank H. Bojórquez Leyva for assistance in the laboratory, M.C. Ramírez Jáuregui for bibliographic support, and G. Ramírez-Reséndiz and C. Suárez Gutiérrez for the preparation of figures. This work was supported by the Universidad Nacional Autonóma de México (Grant Nos. PAPIIT IN210609 and PAPIIT IN208813-2).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Páez-Osuna, F., Osuna-Martínez, C.C. Bioavailability of Cadmium, Copper, Mercury, Lead, and Zinc in Subtropical Coastal Lagoons from the Southeast Gulf of California Using Mangrove Oysters (Crassostrea corteziensis and Crassostrea palmula). Arch Environ Contam Toxicol 68, 305–316 (2015). https://doi.org/10.1007/s00244-014-0118-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-014-0118-3