Abstract
Metals can have significant impacts on inhabitants of mangrove swamps as well as consumers of mangrove-associated fauna. Yet, for several Caribbean islands, assessments regarding the impact of metals on such ecosystems are particularly sparse. The present study investigated the distribution and potential impact of Cd, Cr, Cu, Ni, Pb and Zn in the Caroni Swamp, Trinidad and Tobago’s largest mangrove ecosystem. Surface sediments and mangrove oysters (Crassostrea rhizophorae) from 10 sites in the swamp were analysed for the 6 identified metals. The concentration ranges (in μg/g dry wt.) of metals in sediments from Caroni Swamp were: Zn (113.4–264.6), Cr (27–69.7), Ni (10.7–41.1) and Cu (11–40.7). Based on Canadian Sediment Quality Guidelines (CSQGs), metals in sediments posed a low to medium risk to aquatic life. The concentration ranges (in μg/g wet wt.) for metals in Crassostrea rhizophorae tissues were: Zn (123.2–660), Cu (4.2–12.3), Ni (0.1–5.5), Pb (0.1–0.9), Cr (0.2–0.3) and Cd (0.1–0.2). Multiple evaluations indicated that zinc posed a potential threat to the health of oyster consumers. Information from this study is vital for managing the Caroni Swamp, safeguarding the health of consumers of shellfish on this Caribbean island and serving as a useful baseline for future local and regional risk assessments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangrove swamps in small island developing states (SIDS) are critically important ecosystems as they are key biodiversity hotspots and are depended upon by local populations for goods and services. Naturally occurring inorganic contaminants such as metals may threaten mangrove swamps due to their persistence, ubiquity and their capability to exert deleterious effects to biota (Luoma and Rainbow 2008). Among the direct negative effects of metals on inhabitants of mangrove swamps are mutations (Klekowski et al. 1999), increased mortalities and decreased growth rates (De Wolf and Rashid 2008). Metal bioaccumulation in commercially harvested mangrove fauna may also indirectly pose a threat to the health of consumers. Quantitative assessments have indicated that inorganic arsenic levels in oysters from Taiwan’s coastal areas may have led to an increased cancer risk for long-term oyster consumers (Han et al. 1998, 2000).
The Caroni Swamp, the largest mangrove swamp on the island of Trinidad, is a wetland of international importance under the Ramsar Convention on Wetlands of International Importance especially as Waterfowl Habitat. The Caroni River Basin, in which the swamp is located, is drained by 19 major rivers and currently houses six industrial estates comprising of over 172 industries, approximately 33 % of the national population and multiple agricultural enterprises (Juman et al. 2002; Trinidad and Tobago 2008). Metal contamination has been reported for several rivers and sites in the river basin (Mohammed et al. 1996; Ramsingh 2009; Surujdeo-Maharaj 2010). The potential for metal contamination in the Caroni Swamp is high as it is subjected to fluvial inputs from rivers which drain the Caroni River Basin, runoff from the Uriah Butler Highway (one of the major north–south highways that borders the swamp on its east) and influence by maritime activities in the Gulf of Paria (a semi-enclosed body of water between the South American mainland and Trinidad). Although previous studies (Klekowski et al. 1999; Astudillo et al. 2002, 2005; Norville 2007) have provided snapshot views of metal contamination in specific areas of the swamp, a comprehensive assessment of the distribution and potential impacts of metal contaminants in the entire swamp was lacking.
Historically, the Caroni Swamp has been important to the livelihood of fishermen as the swamp supports several commercially important species; mangrove oysters (Crassostrea rhizophorae), mussels (Mytella guyanensis, Mytella falcata), clams (Phacoides pectinatus), conch (Melongena melongena) and shrimp (Penaeus spp.; Nathai-Gyan and Juman 2005). Although the majority of the swamp is a Prohibited Area under the Forests Act, chapter 66:01 (Trinidad and Tobago 1915), the Blue River and Felicity areas are still ranked as the most popular on the island for the commercial harvesting of mangrove oysters (Bullock and Moonesar 2003). Oysters harvested from the Caroni Swamp showed significantly higher levels of microbial contamination than those from other areas (Laloo et al. 2000). Although evidence from Rampersad et al. (1999) and Laloo et al. (2000) indicated that the bacteriological quality of locally harvested raw oysters pose a threat to the health of consumers, comprehensive assessments on the risk posed by chemical contaminants such as metals is unavailable. Among the Caribbean islands, information is also particularly sparse on the impact of pollutants in mangrove fauna on human health (Fernandez et al. 2007). Locally, comprehensive quantitative assessments regarding the risks posed by chemical contaminants such as metals to shellfish consumers have never been conducted. Therefore, the objectives of this study were to determine the levels of metals (cadmium, chromium, copper, lead, nickel, zinc) in the sediments and mangrove oysters (Crassostrea rhizophorae) of the Caroni Swamp, to determine whether metals in the sediments posed a threat to aquatic life and to determine whether metal levels in Crassostrea rhizophorae posed a risk for human consumption.
Materials and method
Site description
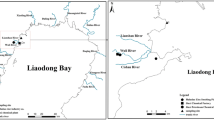
The Caroni Swamp is located on the western coast of Trinidad (Fig. 1) within the Caroni River Basin. The primary anthropogenic activities occurring in that river basin are domestic, agricultural and industrial. In its eastern region, three rivers (Caroni, Cunupia, Guaymare), which drain the Caroni River Basin, enter the swamp. The swamp is also bordered on its east by the Uriah Butler Highway and on its west by the Gulf of Paria. One site from an estuarine mangrove area in the Nariva Swamp was also included primarily for comparative purposes. The Nariva Swamp is the largest wetland in the country and is located on the eastern coast of Trinidad in the Nariva hydrometric area (Fig. 1). In contrast to the Caroni River Basin where anthropogenic activities are widespread, agriculture is the most prominent activity in the Nariva hydrometric area.
Map showing location of sampling sites in the Caroni Swamp, Trinidad (**Oysters and Sediments; *Sediments) [BR Blue River**, GOP Gulf of Paria**, CD Catfish Drain**, CR Caroni River*, EC Entrance Canal**, LL Large Lagoon**, NSD North/South Drain*, HS Herbaceous Swamp*, UMER Upper Madame Espagnol River**, MMER Mouth of the Madame Espagnol River**]
Sample collection
Sediment and oyster samples were collected in May (dry season) and December (wet season) 2009 from 10 sampling sites in Caroni Swamp and 1 sampling site in Nariva Swamp. Triplicate sediment samples were collected at each of the 11 sampling sites using either a stainless steel Petite Ponar or Ekman grab sampler (dependent on water depth). From each grab, two 100 g subsamples were taken, one each for metal and Organic Matter/Total Organic Carbon (OM/TOC) analysis. Fifty oysters (Crassostrea rhizophorae) of similar sizes, 1.6–2.6 (shell length) and 2.3–4.3 cm (shell height), were collected from the prop roots of a single red mangrove (Rhizophora mangle) tree and comprised a single sample. Triplicate oyster samples were collected at 7 sites (Fig. 1) over the same time period. All samples (sediments and oysters) were placed in clean Ziploc bags and transported on ice to the laboratory where they were stored at −20 °C (metal analyses) or 4 °C (OM/TOC analyses).
Chemical analyses
Fifty grams of each sediment sample was oven dried at 60 °C (to a constant weight), homogenized and sieved to a particle size <63 μm. Dried sediment (0.5 g) was pre-digested overnight with 10 mL of concentrated nitric acid (at room temperature) and then further digested on a heating block at 130–135 °C for 6 hours (Astudillo et al. 2005). Samples were cooled, diluted with 5 mL of deionised water and filtered into 25 mL volumetric flasks using No. 542 Whatman hardened ashless filter paper and the filtrate made up to 25 mL using deionised water (Astudillo et al. 2005). Twenty-five oysters (non-damaged and closed) from each sample were shucked, whole tissues removed and the composite sample homogenized. Five grams of each composite sample was placed into a boiling tube and oven dried at 105 °C overnight. Dried oyster samples also underwent the nitric acid digestion procedure described for the sediments (Astudillo et al. 2005). Sediment and oyster samples were analysed for chromium, nickel, copper, zinc, cadmium and lead on a Varian SpectrAA 880 flame atomic absorption spectrophotometer. Five external calibration standards for each metal were used to calibrate the FAAS and calibration curves with R 2 values > 0.9 were used. Method blanks were also simultaneously prepared and analysed with each batch of sediment and oyster samples. Validation of the methods for metals in sediments and oyster tissues were done by analysis of Estuarine Sediment (SRM 1646A) from the National Institute of Standards and Technology (NIST), USA and analysis of Lobster Hepatopancreas Reference Material for Trace Metals (TORT-2) from the National Research Council Canada (NRCC), respectively. Average percentage recoveries for SRM 1646A samples ranged from 41 to 107 % for all investigated metals (Cd 107 %, Cr 70 %, Cu 92 %, Ni 75 %, Pb 41 %, Zn 74 %). For TORT-2 samples, percentage recoveries ranged from 69 % to 119 % for all investigated metals (Cd 99 %, Cr 119 %, Cu 90 %, Ni 69 %, Pb 115 %, Zn 89 %). Since percentage recoveries for lead in sediments were very low (41 %), sediment lead level results were excluded. Correction factors (CFs) were applied to compensate for unacceptable percentage recoveries (i.e. those out of the 100 ± 5 % range). CFs were based on the percentage recovery of a specific metal and brought the recovery to 100 %. The organic matter content of sediment samples was determined by loss on ignition (LOI) following Commendatore and Esteves (2004). Organic matter content (%) from loss on ignition was calculated according to Sutherland (1998) and then transformed to total organic carbon content based on Gaspare et al. (2009). Moisture content determinations according to McDonald et al. (2006) were conducted for a sample of oyster tissues. Based on an average 17.87 % dry weight for oyster tissues, a conversion factor of 5.6 was used to convert metal concentrations reported on a wet weight basis to a dry weight basis.
Data analyses
Pearson correlation analyses were performed between sediment metal concentrations and OM/TOC content and used to determine whether sediment metal data needed to be corrected for this parameter. Univariate analyses (a two factor ANOVA test) was carried out on both sediment and oyster data to determine the effect of sites/seasons on metal concentrations. The a posteriori test used was a Fisher’s Least Significant Difference (LSD) test. Multivariate statistics (Principal Components Analysis—PCA) were also conducted on both the sediment and oyster data in order to assess whether spatial variability in metal levels existed in the Caroni Swamp.
Assessment of threat posed by metals in sediments to aquatic life
Due to the absence of local and regional sediment quality guidelines, the Canadian Sediment Quality Guidelines (CCME 2002) were utilized to assess the threat posed by metals in sediments to aquatic life. Metals levels were proposed to pose a (a) Low Risk if they were below the ISQG (Interim Sediment Quality Guideline), (b) Medium Risk if they were between the ISQG and the PEL (Probable Effect Level) and (c) High Risk if they exceeded the PEL.
Risk assessment of metals in Crassostrea rhizophorae to humans
Metal levels in Crassostrea rhizophorae were evaluated based on local (Trinidad and Tobago 1998) and international (USFDA 2007) guidelines for metals in seafood (Table 2). For essential metals, comparisons were made between metal levels in oyster tissues and the recommended dietary allowances (RDAs) and Tolerable Upper Intake Levels (ULs; Nutrition Board and I. o. M 2001). The Risk Quotient (RQ) was one of the quantitative methods used to assess the risk posed by metals in Crassostrea rhizophorae to human consumers. The Risk Quotient (RQ) according to Fung et al. (2004) was calculated as follows:
The assumptions made were (a) the tolerable daily intake of the relevant metal was equivalent to the tolerable upper intake level (UL) of the Nutrition Board and I. o. M (2001), (b) a single oyster cocktail contained 12 oysters’ total soft tissue (Laloo et al. 2000), each oyster total soft tissue weighed approximately 1.5 g (based on weights recorded for oysters in this study), (c) low consumption consumers had 1 oyster cocktail/day (18 g/day) and (d) high consumption consumers had 4 oyster cocktails/day (72 g/day). When determining risk quotients, the best case scenario (RQbcs), based on low consumption consumers, and the worst case scenarios (RQwcs), based on high consumption consumers, were calculated. The Target Hazard Quotient (THQ) was another quantitative method used to determine the non-carcinogenic risk to the public from consuming oysters contaminated with metals. The THQ, according to Han et al. (1998), was determined as follows:
The assumptions made were (a) exposure frequency (EFr) was 350 days/year, exposure duration (EDtot) was 30 years, (b) averaging time (ATn) was 365 days/year for 30 years (Han et al. 1998; Liu et al. 2006), (c) body weight (BWa) was 77 (male) and 70 kg (female; Gulliford et al. 2003) and (d) ingested metal was equal to absorbed metal with no loss of metal (Han et al. 1998). Oral reference doses (RfD) were based on USEPA (2012). The two seafood ingestion rates (SFI), as described for the RQ assumptions, were low consumption consumers (Typically General Consumers) and high consumption consumers (Maximum Exposed Individuals). Metal concentrations in the edible portion of seafood (MCS) were based on the findings of this study.
Results
Metal concentrations in sediments
Table 1 summarizes the mean concentrations (μg/g dry wt.) of Zn (113.4 - 264.6), Cr (27–69.7), Ni (10.7 - 41.1) and Cu (11–40.7) in sediments from the Caroni Swamp. The highest metal concentrations were found at the following sites: Herbaceous Swamp (HS)—highest Cu and Ni levels and high Zn levels, Upper Madame Espagnol River (UMER)—highest Zn levels, Mouth of the Madame Espagnol River (MMER)—high Cr and Ni levels and Gulf of Paria (GOP)—high Cr levels (Table 1). Average metal levels (μg/g dry wt.) in sediments of the Nariva Swamp site, Cu (8.5), Ni (12.9) and Zn (90.4), were significantly lower than those at various sites in the Caroni Swamp (Table 1). Overall, Cr, Cu and Ni levels in the sediments were significantly different between sites in the Caroni Swamp (ANOVA, p < 0.001). Nickel was the only metal for which there was a significant seasonal difference in sediment metal levels (ANOVA, p < 0.001) with higher Ni levels reported in the dry season. Zinc was also the only metal for which there was a significant interaction between the two factors of site and season (ANOVA, p = 0.007). Since correlations between sediment metal levels and TOC were not significant (Pearson’s Correlation, p > 0.05), sediment metal data was not corrected for this parameter. PCA on sediment metals in the dry season revealed that principal components 1 and 2 accounted for 78.5 % of the variation. Eigenvectors indicated that principal component 1 (PC1) was governed by decreasing concentrations of nickel (−0.637) and zinc (−0.485) while principal component 2 (PC2) was governed by decreasing concentrations of copper (−0.707) and increasing concentrations of Cr (0.696). PCA on sediment metal data in the wet season revealed that principal components 1 and 2 accounted for 76.01 % of the variation. Eigenvectors indicated that principal component 1 (PC1) was governed by increasing concentrations of nickel (0.634) and copper (0.583) while principal component 2 (PC2) was governed by increasing concentrations of zinc (0.918). The clustering of the majority of sites in the middle of the ordination plots (for both the dry and wet seasons) indicated that sediment metal levels at the majority of sites in the Caroni Swamp were similar (Fig. 2). This was corroborated by the a posteriori tests as for each metal there was a group of sites for which there were no significant differences (Fisher’s Least Significant Difference (LSD) Test, p > 0.05) in sediment metal levels. In both seasons, the relevant ordination plots (Fig. 2a, b) indicated that certain sites were dissimilar from the majority. The Herbaceous Swamp (HS) site (located in left quadrant of the plot for the dry season) was one site that reflected the highest sediment metal (Ni, Zn) contamination in the dry season in the Caroni Swamp (Fig. 2a). By contrast, the Nariva Swamp (N) and Caroni River (CR) sites (located in right quadrant of the plot for the dry season and the left quadrant of the plot for the wet season) reflected the lowest sediment metal (Ni, Cu, Zn, Cr) levels in both seasons (Figs. 2a, b). In the dry season, the Upper Madame Espagnol River (UMER) and Mouth of the Madame Espagnol River (MMER) sites reflected high sediment nickel and zinc levels (Fig. 2a) while in the wet season, these exact sites along with the Caroni River (CR) and Herbaceous Swamp (HS) sites (located in the upper quadrant) were distinct as they reflected high zinc levels (Fig. 2b).
Assessment of threat posed by metals in the sediments to aquatic life
Levels of none of the investigated metals at any of the sites exceeded the PEL (Table 1) and therefore sediment metals did not pose a high risk to aquatic life in the Caroni Swamp. Overall, sediment metal (Cu, Cr, Zn) levels were either below the ISQG or between the ISQG and the PEL (Table 1) and thereby posed only a low to medium risk to aquatic life. This implies that aquatic organisms are expected to rarely and in some cases to occasionally be exposed to adverse biological effects. Lead, copper, chromium and zinc simultaneously posed a medium risk to aquatic life at the EC, LL and HS sites in the dry season and at the NSD, UMER and HS sites in the wet season. The ERL and ERM (proposed by Long et al. 1995) which were used to assess the risks posed by nickel indicated that nickel levels posed a low to medium risk to aquatic life in the Caroni Swamp (Table 1).
Metal concentrations in oyster tissues
Table 2 summarizes the mean concentrations (μg/g wet wt.) of Zn (123.2 - 660.0), Cu (4.2 - 12.3), Ni (0.1 - 5.5), Pb (0.1 - 0.9), Cr (0.2 - 0.3) and Cd (0.1 - 0.2) in Crassostrea rhizophorae tissues from the Caroni Swamp. Crassostrea rhizophorae tissues at the Upper Madame Espagnol River (UMER) site reflected the highest levels of zinc in both seasons (382.7 - 660.0 μg/g wet wt.) while those at the Blue River (BR), Catfish Drain (CD) and Gulf of Paria (GOP) sites reflected the highest copper levels (10.6–12.3 μg/g wet wt.; Table 2). In contrast to the findings for sediments, metal concentrations in oyster tissues were significantly different among sites for nickel (ANOVA, p = 0.046) but this was not the case for cadmium, chromium or lead (ANOVA, p > 0.05). Chromium was the only metal for which there was a seasonal difference in oyster tissue metal levels (ANOVA, p = 0.015) with higher chromium levels being reported in the dry season. For both Zn (ANOVA, p < 0.001) and Cu (ANOVA, p = 0.014), there was a significant interaction between the two factors of site and season. PCA on Crassostrea rhizophorae metal levels in the dry season revealed that principal components 1 and 2 accounted for 67.7 % of the variation. Eigenvectors indicated that principal component 1 (PC1) was governed by decreasing concentrations of zinc (−0.708) while principal component 2 (PC2) was governed by increasing concentrations of chromium (0.665) and copper (0.697). PCA on Crassostrea rhizophorae metal levels in the wet season revealed that principal components 1 and 2 accounted for 56.5 % of the variation. Eigenvectors indicated that principal component 1 (PC1) was governed by decreasing concentrations of copper (−0.562) and nickel (−0.487) while principal component 2 (PC2) was governed by increasing concentrations of lead (0.729). The ordination plot based on metal levels in Crassostrea rhizophorae in the dry season indicated that the majority of sites were similar as evidenced by their clustering in the right quadrant of the plot (Fig. 3a). However, such was not the case in the wet season as there was no major clustering of sites (Fig. 3b). In the dry season, it was evident that the Upper Madame Espagnol River (UMER) site was dissimilar from the majority of sites as it reflected the highest levels of zinc in oyster tissues (660 μg/g wet wt.). In the wet season, the Catfish Drain (CD) and Blue River (BR) sites were located in the left quadrant of the ordination plot (Fig. 3b) and were characterised by high Cu and Ni levels.
Assessment of threat posed by metals to human health
Zinc was the only metal whose levels in oyster tissues exceeded established guideline levels (Table 2) and therefore was the only metal for which further risk assessment work was conducted. Levels of zinc in oyster tissues at all investigated sites in both seasons were between 3 to 13 times the maximum permitted level (50 μg/g wet wt.) proposed by Trinidad and Tobago (Table 2). Based on the ingestion rates of low consumption consumers (1 oyster cocktail/day), those individuals would be ingesting between 2.2 and 11.9 mg of zinc per cocktail depending on the site of harvest. Therefore, on a daily basis, zinc levels would have exceeded the recommended dietary allowance- RDA (women—8 mg/day, men—11 mg/day) for adults at certain sites (Upper Madame Espagnol River—UMER) but would not have exceeded the Tolerable Upper Intake Level—UL (40 mg/day). Based on the ingestion rates of high consumption consumers (4 oyster cocktails/day), those individuals would be ingesting between 8.9 and 47.5 mg of zinc per day and therefore the RDA at all sites would have been exceeded for those consumers. The only site at which the UL would have been exceeded was the Upper Madame Espagnol River (UMER) site. The best case risk quotient (RQbcs) for zinc based on ingestion rates for low consumption consumers (1 oyster cocktail/day) was between 0.055 and 0.297 and thus zinc would not have posed a threat to oyster consumers (Table 3). However, the worst case risk quotient (RQwcs) based on high consumption oyster consumers (4 oyster cocktails/day) was between 0.222 and 1.188 and would have posed a threat to oyster consumers (Table 3). THQs for zinc for TGCs were between 0.092 and 0.542 and were below the acceptable safe value of 1 (Table 4). THQs for MEIs ranged from 0.368 to 2.17 (Table 4) indicating that the acceptable safe value was exceeded at certain sites (MMER, UMER, BR and EC). Overall, THQ values for zinc suggested that this metal would have posed a human health threat to oyster consumers but this was also dependent on ingestion rate.
Discussion
Results of this study indicated that metal distribution in the sediments and oysters at the majority of sites in the Caroni Swamp were similar. However, a few sites distinctly revealed important information about metal contamination in the swamp. One such site, the Herbaceous Swamp (HS), reflected high levels of Cu, Ni and Zn in the sediments and was located mere metres from the Uriah Butler Highway (a major roadway on the island). Metals such as Cu and Zn are associated with motor vehicle roadways and typically originate from vehicle tailpipe emissions and brake/tyre wear (Sternbeck et al. 2002; Lough et al. 2004; Birmili et al. 2006). Therefore, metals at the HS site potentially originated from vehicular traffic on the adjacent roadway and entered the swamp via surface runoff. The Upper Madame Espagnol River (UMER) site, located downstream of the Cunupia River and Bejucal Canal, reflected high Zn levels in both the sediments and oysters while the Caroni River (CR) site, located in the lower course of the Caroni River, reflected high Zn levels in the sediments in the wet season. Metals at both sites may have originated from land-based activities in the Caroni River Basin and reached the swamp via fluvial transport. Specifically, zinc may have originated from improperly discarded tyres, runoff from galvanized metal roofs, domestic wastewater, sewage sludge, battery/smelter wastes, metal salvaging operations and runoff from roadways and agricultural areas (Mohammed et al. 1996; Wik and Dave 2009; Degaffe and Turner 2011). The Mouth of the Madame Espagnol River (MMER) site, located a few metres from the Gulf of Paria and subjected to intense boat activity by oyster collectors and fishermen, reflected high levels of Zn in both the sediments and oysters and high Ni and Cr levels in the sediments. Marine vessels contribute to metal contamination in aquatic environments via engine emission products and fuel leakages as metals are a component of fuels utilized by maritime vessels (Jüttner et al. 1995; Cooper and Gustafsson 2004). Metal contamination at the MMER site may have therefore been influenced by the operation of maritime vessels at the site or in the proximal Gulf of Paria.
Copper, nickel, chromium and zinc levels in the sediments of the Caroni Swamp were evaluated as being capable of posing a low to medium threat to aquatic organisms. It must be noted that although the ‘near total fraction’ of metals in the sediments were determined by this study, sediment-dwelling as well as other aquatic organisms are actually exposed to the bioavailable fraction of metals in the sediments. This must be taken into consideration when interpreting the risks posed by the ‘near total fraction’ of metals to aquatic organisms as risks may be overestimated. Nevertheless, metal contaminants in sediments affect benthic organisms by reducing their diversity and abundance, excluding or restricting certain species, leading to their decreased growth and increased juvenile mortality (Mayer-Pinto et al. 2010). Benthic organisms also provide several important ecosystem services and compromised health may impair their abilities for service provision (Covich et al. 1999). Benthos is also consumed by organisms (e.g. fishes) in higher trophic levels (Fugi et al. 2001) and threats to their health may ultimately have ripple effects on their predators. Once sediment-dwelling organisms in the Caroni Swamp are adversely affected by metals, marine organisms dependent on these resources may ultimately be negatively affected.
Although several metals (Zn, Cu, Ni, Cr, Pb, Cd) were detected in the tissues of Crassostrea rhizophorae in the Caroni Swamp, only zinc levels exceeded local maximum permitted levels. Furthermore, both the Risk Quotients (RQs) and Target Hazard Quotients (THQs) indicated that zinc would have posed a health risk to consumers depending on their ingestion rates. Due to limited resources, pooled oyster samples of similar shell lengths were analysed from each site. An assumption of this method was that the similar oyster size range would have either limited or eliminated any size effect on metal concentrations in oysters. Some previous studies (Rebelo et al. 2003; de Souza et al. 2011; Diaz Rizo et al. 2010) have also analysed pooled oyster samples of similar size ranges. However, size effect is a variable that may potentially influence oyster metal levels. Previous studies (Silva et al. 2001, 2003, 2006) have addressed this issue by analysing metal levels in individual oysters and then performing subsequent regression analyses (between metal levels in oysters and oyster tissue dry weight) to determine if size effect is a variable to be eliminated.
Risk assessments performed in this study may have over or underestimated risks since quantitative data on oyster consumption rates in Trinidad was lacking and a number of assumptions had to be made. One assumption that was made when assessing the risk posed by zinc in Crassostrea rhizophorae to humans was that the ‘ingested metal was equal to absorbed metal with no loss of metal’. While this assumption allows for an estimation of risk based on a ‘worst case scenario’, it also allows for an overestimation of risks. Previous research has indicated that a fraction of the total zinc concentrations in the soft tissues of oysters are actually bioavailable to humans. For Crassostrea gigas, Bragigand et al. (2004) estimated that between 50 % and 80 % of zinc was bioavailable while Amiard et al. (2008) indicated that 78–82 % of zinc was bioaccessible. Hence, the results of this risk assessment must be interpreted within the context that risks may have been overestimated. In future, attempts should be made to assess the fraction of metals in Crassostrea rhizophorae that is bioaccessible to humans. In spite of the limitations, information provided by risk assessments is critical to safeguarding the health of consumers. While consumers can consider oysters as a source of the essential metal zinc, they must be aware that if ingested in quantities above tolerable levels, some of the acute effects may include gastrointestinal distress while chronic effects may include impairment of immune and pancreatic functions and interference with metabolic cycles of copper and other essential elements (Nriagu 2007). Previous studies (Rampersad et al. 1999; Laloo et al. 2000) have indicated that high microbial loads in raw oysters pose a health risk to consumers in Trinidad and that oysters from the Caroni Swamp were significantly more contaminated than those from other areas. Collectively, previous and current work (this study) confirms that oyster consumers may be ingesting a literal ‘cocktail’ of biological and chemical contaminants.
One of the factors which influenced metal levels in both sediments and oyster tissues was season. In Trinidad, rainfall is the predominant factor which characterises seasons such that in the dry season rainfall is considerably less than in the wet season. This study indicated that nickel levels in the sediments and chromium levels in oyster tissues were significantly higher in the dry season at the majority of sites. Other local studies (Norville 2005, 2007) have also reported seasonal differences in metal levels in both sediments and oysters. Rainfall events lead to increased surface runoff, subsequent increased freshwater inputs into the Caroni Swamp as well as metal transfer from the terrestrial to estuarine/coastal environments. However, freshwater inputs may actually dilute metal concentrations in the aquatic phase of the Caroni Swamp leading to reduced sequestration in sediments as well as lower uptake by filter-feeding organisms such as oysters. Season also influences physiochemical parameters such as salinity and pH which will also influence bioavailability and metal uptake in biota (Luoma and Rainbow 2008). At some sites, the opposite pattern was exhibited whereby metal levels were higher in the wet season (e.g. zinc at the Caroni River site). In these cases, freshwater inputs may have been instrumental in transporting metals derived from land-based sources into the swamp. Therefore, fluctuating hydrological and physicochemical conditions may account for the influence of season on metal levels in sediments and biota.
In the context of other local studies conducted in the Caroni Swamp (Klekowski et al. 1999; Astudillo et al. 2005; Norville 2007), Cu, Cr, Ni and Zn levels in the sediments of this study were comparatively higher (Table 5). Similarly, levels of specific metals in Crassostrea rhizophorae of this study were also higher than those reported by previous studies (Norville 2007; Astudillo et al. 2002, 2005) for mangrove oysters from the Caroni Swamp (Table 6). Overall, this indicated that metal levels may have increased over time in the Caroni Swamp. Land-based sources within the Caroni river basin (the Uriah Butler Highway and fluvial inputs from the Caroni/Cunupia rivers) appeared to be one of the contributors to metal contamination in the Caroni Swamp. Once anthropogenic activities continue in the Caroni river basin and river entry into the swamp continues, it is likely that metal contamination will be an ongoing issue in the Caroni Swamp. In comparison to other sites in Trinidad and Tobago, sediment metal levels in the swamp were low as significantly higher metal levels (which exceeded the PEL of the CSQGs) were reported at several coastal sites (Singh 1989), in the Caroni River Basin (Mohammed et al. 1996; Ramsingh 2009) and in the Chaguaramas Peninsula (Mohammed 2005). Previous local studies also reported higher levels of Cr, Cu and Cd in Crassostrea rhizophorae from other locations in Trinidad (Table 6).
From a regional perspective, levels of Cr, Cu, Cd, Pb and Zn in Crassostrea rhizophorae tissues from this study were lower than those reported for mangrove oysters from the Dominican Republic and Brazil (Table 6). Levels of the majority of metals in Crassostrea rhizophorae tissues in this study were higher than those reported for oysters in Venezuela (Astudillo et al. 2002, 2005) and Cuba (Diaz Rizo et al. 2010). Of note is the fact that nickel levels in Crassostrea rhizophorae from this study were higher than those reported by all regional studies in Table 6. This must be taken in the context that these high nickel levels were reported for oysters at one site (Catfish Drain) in one season (wet) in the Caroni Swamp (Table 2) and thus this may have been an anomaly. Of note as well is the fact that zinc levels in mangrove oysters from this study were higher than those reported from some areas in Brazil (Cotegipe Channel, Macau, Curimatau and Potengi estuaries, Sambaqui Bay, Todos os Santos Bay) but lower than those reported from the Potengi estuary (Silva et al. 2001) and Sepetiba Bay, Brazil (Rebelo et al. 2003; Amaral et al. 2005) and the Dominican Republic (Sbriz et al. 1998). Overall, this indicates that the bioavailability of most metals to oysters in the Caroni Swamp is generally low when compared to other regional studies. However, zinc bioavailability to oysters is high but not as high as some of the most contaminated regional sites. Copper and zinc levels in mangrove sediments in the Caroni Swamp in this study were low in comparison to levels reported for Brazil (Table 6). Low sediment metal levels in the Caroni Swamp may be explained by the lack of major anthropogenic activities within the swamp. By contrast, the high sediment metal levels reported locally/regionally were in the proximity of metal salvaging operations, dump sites for battery/smelter wastes, active secondary smelters, municipal landfills, nickel mining sites and sites subjected to point source inputs of raw sewage/urban/industrial wastes. Of note is the fact that both chromium and nickel levels reported in the Caroni Swamp were the highest reported for mangrove sediments in both Trinidad as well as in Brazil. However, when compared to international studies, the levels of both metals were comparatively low as significantly higher levels were reported in Australia and Hong Kong (Lewis et al. 2011).
Conclusion
This study provided the first comprehensive assessment of the distribution and impact of metals in the sediments and mangrove oysters (Crassostrea rhizophorae) in the Caroni Swamp, Trinidad. It is also the first local study to quantitatively assess the risks posed by metal contaminants to oyster consumers. Metals presented a direct threat to inhabitants of the swamp and thus to ecosystem well-being. Evaluations based on the Canadian Sediment Quality Guidelines (CSQGs) indicated that metals posed a low to medium risk to aquatic life. Metals in the swamp were also shown to be capable of indirectly affecting human consumers of mangrove-associated oysters. Based on multiple evaluations, local/international guidelines for metals in seafood, recommended daily allowances (RDAs), tolerable intake levels (ULs), Risk Quotient (RQs) and Target Hazard Quotient (THQs), zinc was the only metal whose levels were capable of posing a human health threat to oyster consumers. Information from this study will be invaluable in the management of the Caroni Swamp Ramsar Site, it will be critical to safeguarding the health of consumers of shellfish and will serve as a useful baseline for future local and regional risk assessments.
References
Amaral, M. C. R. D., Rebelo, M. D. F., Torres, J. P. M., & Pfeiffer, W. C. (2005). Bioaccumulation and depuration of Zn and Cd in mangrove oysters (Crassostrea rhizophorae, Guilding, 1828) transplanted to and from a contaminated tropical coastal lagoon. Marine Environmental Research, 59(2005), 277–285.
Amiard, J.-C., Amiard-Triquet, C., Charbonnier, L., Mesnil, A., Rainbow, P. S., & Wang, W.-X. (2008). Bioaccessibility of essential and non-essential metals in commercial shellfish from Western Europe and Asia. Food and Chemical Toxicology, 46(6), 2010–2022.
Astudillo, L. R. D., Yen, I. C., Agard, J., Bekele, I., & Hubbard, R. (2002). Heavy metals in green mussel (Perna viridis) and oysters (Crassostrea sp.) from Trinidad and Venezuela. Archives of Environmental Contamination and Toxicology, 42(2002), 410–415.
Astudillo, L. R. D., Yen, I. C., & Bekele, I. (2005). Heavy metals in sediments, mussels and oysters from Trinidad and Venezuela. International Journal of Tropical Biology and Conservation, 53(Supplement 1), 41–53.
Birmili, W., Allen, A. G., Bary, F., & Harrison, R. M. (2006). Trace metal concentrations and water solubility in size-fractionated atmospheric particles and influence of road traffic. Environmental Science & Technology, 40(4), 1144–1153.
Bragigand, V., Berthet, B., Amiard, J. C., & Rainbow, P. S. (2004). Estimates of trace metal bioavailability to humans ingesting contaminated oysters. Food and Chemical Toxicology, 42(11), 1893–1902.
Bullock, C., & Moonesar, I. (2003). The use of roadside shellfish vendors interviews to guide the selection of shellfish harvesting areas for a study of the status of microbiological pollution of shellfish and shellfish growing waters in the Gulf of Paria and a brief insight into the roadside shellfish vending trade in Trinidad. Chaguaramas: Institute of Marine Affairs.
Canadian Council Ministers of Environment (CCME). (2002). Canadian Sediment Quality Guidelines for the protection of aquatic life: Summary tables. Winnipeg: Canadian Council Ministers of the Environment. http://ceqg-rcqe.ccme.ca/. Accessed 25 June 2013.
Commendatore, M. G., & Esteves, J. L. (2004). Natural and anthropogenic hydrocarbons insediments from the Chubet River (Patagonia, Argentina). Marine Pollution Bulletin, 48(2004), 910–918.
Cooper, D., & Gustafsson, T. (2004). Methodology for calculating emissions from ships. 1. Update of emission factors. Norrkoping, Sweden: Swedish Methodology for Environmental Data. http://westcoastcollaborative.org/files/sector-marine/SMED%20Methodology%20for%20 Calculating%20Emissions%20from%20Ships.pdf. Accessed July 5 2013.
Covich, A. P., Palmer, M. A., & Crowl, T. A. (1999). The role of benthic invertebrate species in freshwater ecosystems. BioScience, 49(2), 119–127.
de Souza, M. M., Windmoller, C. C., & Hatje, V. (2011). Shellfish from Todos os Santos Bay, Bahia, Brazil: treat or threat? Marine Pollution Bulletin, 62(10), 2254–2263.
De Wolf, H., & Rashid, R. (2008). Heavy metal accumulation in Littoraria scabra along polluted and pristine mangrove areas of Tanzania. Environmental Pollution, 152(3), 636–643.
Degaffe, F. S., & Turner, A. (2011). Leaching of zinc from tire wear particles under simulated estuarine conditions. Chemosphere, 85(5), 738–743.
Diaz Rizo, O., Olivares Reumont, S., Viguri Fuente, J., Diaz Arado, O., Lopez Pino, N., D’Alessandro Rodriguez, K., et al. (2010). Copper, zinc and lead enrichments in sediments from Guacanayabo Gulf, Cuba, and its bioaccumulation in oysters, Crassostrea rhizophorae. Bulletin of Environmental Contamination and Toxicology, 84(1), 136–140.
Farias, C. O., Hamacher, C., Wagener, A. D. L. R., Campos, R. C. D., & Godoy, J. M. (2007). Trace metal contamination in mangrove sediments, Guanabara Bay, Rio de Janeiro, Brazil. Journal of the Brazilian Chemical Society, 18, 1194–1206.
Fernandez, A., Singh, A., & Jaffé, R. (2007). A literature review on trace metals and organic compounds of anthropogenic origin in the Wider Caribbean Region. Marine Pollution Bulletin, 54(11), 1681–1691.
Fugi, R., Agostinho, A. A., & Hahn, N. S. (2001). Trophic morphology of five benthic-feeding fish species of a tropical floodplain. Revista Brasileira de Biologia, 61, 27–33.
Fung, C. N., Lam, J. C. W., Zheng, G. J., Connell, D. W., Monirith, I., Tanabe, S., et al. (2004). Mussel-based monitoring of trace metal and organic contaminants along the east coast of China using Perna viridis and Mytilus edulis. Environmental Pollution, 127(2), 203–216.
Gaspare, L., Machiwa, J. F., Mdachi, S. J. M., Streck, G., & Brack, W. (2009). Polycyclic aromatic hydrocarbon (PAH) contamination of surface sediments and oysters from the inter-tidal areas of Dar es Salaam, Tanzania. Environmental Pollution, 157(1), 24–34.
Gulliford, M. C., Mahabir, D., & Rocke, B. (2003). Food insecurity, food choices, and body mass index in adults: nutrition transition in Trinidad and Tobago. International Journal of Epidemiology, 32(4), 508–516.
Han, B. C., Jeng, W. L., Chen, R. Y., Fang, G. T., Hung, T. C., & Tseng, R. J. (1998). Estimation of target hazard quotients and potential health risks for metals by consumption of seafood in Taiwan. Archives of Environmental Contamination and Toxicology, 35(4), 711–720.
Han, B.-C., Jeng, W.-L., Hung, T.-C., Ling, Y.-C., Shieh, M.-J., & Chien, L.-C. (2000). Estimation of metal and organochlorine pesticide exposures and potential health threat by consumption of oysters in Taiwan. Environmental Pollution, 109(1), 147–156.
Harris, R. R., & Santos, M. C. F. (2000). Heavy metal contamination and physiological variability in the Brazilian mangrove crabs Ucides cordatus and Callinectes danae (Crustacea: Decapoda). Marine Biology, 137(4), 691–703.
Juman, R., Bacon, P., & Gerald, L. (2002). Environmental modifications and impacts on the Caroni River basin, Trinidad. In B. Kjerfve, W. J. Wiebe, H. H. Kremer, W. Salomons, J. I. M. Crossland, N. Morcom, et al. (Eds.), Caribbean Basins, LOICZ (Land-Ocean Interaction in the Coastal Zone) Global Change Assessment and Synthesis of River Catchment/Island-Coastal Sea Interaction and Human Dimensions; with a desktop study of Oceania Basins (Vol. LOICZ Reports and Studies # 27). Texel: LOIZ.
Jüttner, F., Backhaus, D., Matthias, U., Essers, U., Greiner, R., & Mahr, B. (1995). Emissions of two- and four-stroke outboard engines—II. Impact on water quality. Water Research, 29(8), 1983–1987.
Kehrig, H. A., Pinto, F. N., Moreira, I., & Malm, O. (2003). Heavy metals and methylmercury in a tropical coastal estuary and a mangrove in Brazil. Organic Geochemistry, 34(5), 661–669.
Klekowski, E. J., Temple, S. A., Siung-Chang, A. M., & Kumarsingh, K. (1999). An association of mangrove mutation, scarlet ibis, and mercury contamination in Trinidad, West Indies. Environmental Pollution, 105(1999), 185–189.
Laloo, S., Rampersad, F. S., Borde, A. L., Maharaj, K., Sookhai, L., Teelucksingh, J. D., et al. (2000). Bacteriological quality of raw oysters in Trinidad and the attitudes, knowledge and perceptions of the public about its consumption. International Journal of Food Microbiology, 54(2000), 99–107.
Lewis, M., Pryor, R., & Wilking, L. (2011). Fate and effects of anthropogenic chemicals in mangrove ecosystems: a review. Environmental Pollution, 159(10), 2328–2346.
Liu, C.-W., Liang, C.-P., Huang, F. M., & Hsueh, Y.-M. (2006). Assessing the human health risks from exposure of inorganic arsenic through oyster (Crassostrea gigas) consumption in Taiwan. Science of the Total Environment, 361(1–3), 57–66.
Long, E. R., MacDonald, D. D., Smith, S. L., & Calder, F. D. (1995). Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environmental Management, 19(1), 81–97.
Lough, G. C., Schauer, J. J., Park, J.-S., Shafer, M. M., DeMinter, J. T., & Weinstein, J. P. (2004). Emissions of metals associated with motor vehicle roadways. Environmental Science & Technology, 39(3), 826–836.
Luoma, S. N., & Rainbow, P. S. (2008). Metal contamination in aquatic environments: science and lateral management. New York: Cambridge University Press.
Machado, W., Moscatelli, M., Rezende, L. G., & Lacerda, L. D. (2002a). Mercury, zinc, and copper accumulation in mangrove sediments surrounding a large landfill in southeast Brazil. Environmental Pollution, 120(2), 455–461.
Machado, W., Silva-Filho, E. V., Oliveira, R. R., & Lacerda, L. D. (2002b). Trace metal retention in mangrove ecosystems in Guanabara Bay, SE Brazil. Marine Pollution Bulletin, 44(11), 1277–1280.
Mayer-Pinto, M., Underwood, A. J., Tolhurst, T., & Coleman, R. A. (2010). Effects of metals on aquatic assemblages: what do we really know? Journal of Experimental Marine Biology and Ecology, 391(1–2), 1–9. doi:10.1016/j.jembe.2010.06.013.
McDonald, S. J., Frank, D. S., Ramirez, J. A., Wang, B., & Brooks, J. M. (2006). Ancillary methods of the National Status and Trends Program: Update 2000–2006. Silver Springs, MD: NOAA. http://coastalscience.noaa.gov/documents/ancillarymethodsnsandt.pdf. Accessed 7th April 2009.
Mohammed, A. (2005). Investigation of heavy metals and butyltin in Chaguaramas, Trinidad. St. Augustine: MPhil Thesis, University of the West Indies.
Mohammed, T. I., Chang-Yen, I., & Bekele, I. (1996). Lead pollution in East Trinidad resulting from lead recycling and smelting activities. Environmental Geochemistry and Health, 18, 123–128.
Nathai-Gyan, N., & Juman, R. (2005). Information sheet on Ramsar wetlands (RIS). http://www.ramsar.wetlands.org/Database/SearchforRamsarsites/tabid/765/ Default.aspx. Accessed 26th June 2013.
Norville, W. (2005). Spatial distribution of heavy metals in sediments from the Gulf of Paria, Trinidad. International Journal of Tropical Biology and Conservation, 53(Supplement 1), 33–40.
Norville, W. (2007). Environmental monitoring of the Gulf of Paria: assessment of biota quality in the Gulf of Paria: heavy metals component. Chaguaramas: Institute of Marine Affairs.
Norville, W., & Banjoo, D. (2011). Water and sediment quality in a tropical swamp used for agricultural and oil refining activities. Journal of Environmental Science and Health, Part A, 46(2), 149–156.
Nriagu, J. (2007). Zinc toxicity in humans. In E.-i.-C. J. O. Nriagu (Ed.), Encyclopedia of environmental health (pp. 801–807). Burlington: Elsevier.
Food and Nutrition Board, I. o. M. (2001). Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: The National Academies Press. http://www.nap.edu/catalog.php?record_id=10026. Accessed 20 January 2012.
Rampersad, F. S., Laloo, S., Borde, A. L., Maharaj, K., Sookhai, L., Teelucksingh, J., et al. (1999). Microbial quality of oysters sold in Western Trinidad and potential health risk to consumers. Epidemiology and Infection, 123(2), 241–250.
Ramsingh, D. C. (2009). Identification and quantification of trace metals in the Caroni Arena Watershed, Trinidad; a chemical and spatial approach. St. Augustine: MPhil Thesis, University of the West Indies.
Rebelo, M. D. F., Amaral, M. C. R. D., & Pfeiffer, W. C. (2003). High Zn and Cd accumulation in the oyster Crassostrea rhizophorae, and its relevance as a sentinel species. Marine Pollution Bulletin, 46(2003), 1341–1358.
Sbriz, L., Aquino, M. R., Alberto de Rodriguez, N. M., Fowler, S. W., & Sericano, J. L. (1998). Levels of chlorinated hydrocarbons and trace metals in bivalves and nearshore sediments from the Dominican Republic. Marine Pollution Bulletin, 36(12), 971–979.
Silva, C. A. R., Lacerda, L. D., & Rezende, C. E. (1990). Metals reservoir in a red mangrove forest. Biotropica, 22(4), 339–345.
Silva, M. R., Lamotte, M., Donard, O. F. X., Soriano-Sierra, E. J., & Robert, M. (1996). Metal contamination in surface sediments of mangroves, lagoons and Southern Bay in Florianopolis Island. Environmental Technology, 17(10), 1035–1046.
Silva, C. A. R., Rainbow, P. S., Smith, B. D., & Santos, Z. L. (2001). Biomonitoring of trace metal contamination in the Potengi Estuary, Natal (Brazil), using the oyster Crassostrea rhizophorae, a local food source. Water Research, 35(17), 4072–4078.
Silva, C. A. R., Rainbow, P. S., & Smith, B. D. (2003). Biomonitoring of trace metal contamination in mangrove-lined Brazilian coastal systems using the oyster Crassostrea rhizophorae: comparative study of regions affected by oil, salt pond and shrimp farming activities. Hydrobiologia, 501(2003), 199–206.
Silva, C. A. R. E., Smith, B. D., & Rainbow, P. S. (2006). Comparative biomonitors of coastal trace metal contamination in tropical South America (N. Brazil). Marine Environmental Research, 61(2006), 439–455.
Singh, J. G. (1989). A study of heavy metals and hydrocarbons in fish crabs and mussels found in Trinidad. St. Augustine: PhD Thesis, University of the West Indies.
Sternbeck, J., Sjödin, A. Å., & Andréasson, K. (2002). Metal emissions from road traffic and the influence of resuspension–results from two tunnel studies. Atmospheric Environment, 36(30), 4735–4744.
Surujdeo-Maharaj, S. (2010). Heavy metals in rivers in Trinidad and Tobago. St. Augustine: PhD Thesis. The University of the West Indies.
Sutherland, R. (1998). Loss on ignition estimates of organic matter and relationships to organic carbon in fluvial bed sediments. Hydrobiologia, 389, 153–167.
Trinidad and Tobago. (1915). Forests Act Chapter 66:01. (pp. 21). Trinidad and Tobago. http://rgd.legalaffairs.gov.tt/Laws2/Alphabetical_List/lawspdfs/66.01.pdf. Accessed May 21 2011.
Trinidad and Tobago. (1998). Fish and fishery products regulations (LN220/1998). Trinidad and Tobago. http://rgd.legalaffairs.gov.tt/Laws2/Alphabetical_List/lawspdfs/30.01.pdf. Accessed May 21 2011.
Trinidad and Tobago. (2008). Business Establishments Register Port of Spain. Trinidad: Central Statistical Office.
United States Environmental Protection Agency (USEPA) (2012) Regional Screening Level (RSL) Summary Table. United States Environmental Protection Agency (USEPA). http://www.epa.gov/reg3hwmd/risk/human/rb-concentration_table/Generic_Tables /docs/master_sl_table_01run_MAY2013.pdf. Accessed 21 June 2013.
United States Food and Drugs Administration (USFDA) (2007) National Shellfish Sanitation Program: Guide for the control of molluscan shellfish. Center for Food Safety and Applied Nutrition, U. S. Food and Drug Administration, Washington, DC. http://www.fda.gov/downloads/ Food/GuidanceRegulation/FederalState FoodPrograms/UCM241512.pdf. Accessed 21 June 2013.
Wallner-Kersanach, M., Theede, H., Eversberg, U., & Lobo, S. (2000). Accumulation and elimination of trace metals in a transplantation experiment with Crassostrea rhizophorae. Archives of Environmental Contamination and Toxicology, 38(2000), 40–45.
Wik, A., & Dave, G. (2009). Occurrence and effects of tire wear particles in the environment - A critical review and an initial risk assessment. Environmental Pollution, 157(1), 1–11.
Acknowledgements
This work was funded by a Campus Research Grant (CRP.5.NOV09.4) from the University of the West Indies, St. Augustine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanhai, L.D.K., Gobin, J.F., Beckles, D.M. et al. Metals in sediments and mangrove oysters (Crassostrea rhizophorae) from the Caroni Swamp, Trinidad. Environ Monit Assess 186, 1961–1976 (2014). https://doi.org/10.1007/s10661-013-3510-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-013-3510-y