Abstract
This study estimated Hg emission factors (EFs) and total Hg loading to the Jaguaribe Estuary, NE Brazil, from intensive shrimp farming, and compares this with other local anthropogenic activities. The EF reached 83.5 mg ha−1 cycle−1 (about 175 mg ha−1 year−1), resulting in an annual Hg load to the estuary of 0.35 kg. The calculated EF is comparable to Hg EFs from urban wastewaters (200 mg ha−1) and solid waste disposal (400 mg ha−1 year−1) from cities located in the estuary’s basin. However, due to the smaller area of aquaculture (2,010 ha), total annual loads are much lower than from these other sources (75 and 150 kg year−1, respectively). Since shrimp farming effluents are released directly into the estuary, the estimated high EF raises environmental concerns with this expanding industry, suggesting the inclusion of this element in ongoing environmental monitoring programs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Intensive shrimp farming has recently occupied extensive coastal areas worldwide, and its dependence on large inputs of artificial feed, fertilizers and other chemical additives, including acidity correctors and algacides, has triggered many studies to investigate the role of shrimp farm as pollutant sources to coastal environments. These inputs have prompted the calculation of EFs for nitrogen (N) and phosphorus (P) nutrients (Burford et al. 2003) as well as for cooper (Cu) (Lacerda et al. 2006a, b), in shrimp farm effluents.
Mercury (Hg) is present in aquaculture effluents due to its presence as a natural component in aquafeeds, particularly fish meal and as impurities in fertilizers and other chemicals used in the activity (Chou et al. 2002; Berntssen et al. 2004). Mercury is of high significance not only due to its ubiquitous presence in substances used in aquaculture but also to its toxicity toward phytoplankton and the shrimp (Barbieri et al. 2005).
Shrimp farming in NE Brazil has increased exponentially during the past 10 years from an annual production of about 7,000 tons, and less than 1,000 ha of pond area in 1998 to over 90,000 tons and about 15,000 ha of pond area in 2003 (ABCC 2005). This has resulted in an increase in nutrient emissions to estuaries in many areas, which formerly had no significant pollution sources (Figueiredo et al. 2005; Lacerda and Sena 2005). Since Hg may be present as an impurity in the feed and chemicals used in aquaculture, the rapid increase in intensive shrimp farming in NE Brazil may have also increased Hg emission to local estuaries. To our knowledge an EF for Hg due to intensive shrimp farming in northeastern Brazil has not been determined.
In this study we present the first estimate of Hg emission factor from intensive shrimp farming based on experimental data from a typical farm in Northeastern Brazil. The estimated EF is used to calculate the annual Hg emission and compare it with those from other anthropogenic sources located in the Jaguaribe estuary basin, based on statistics for the year 2009. Since published EFs for other elements (e.g. N, P and Cu) and the technological processes of intensive shrimp aquaculture are similar worldwide (Burford et al. 2003; Lacerda et al. 2006a, b), the estimated emission factor for Hg may be applied to the shrimp farming industry worldwide.
Materials and Methods
An emission factor (EF), i.e. the amount of a given element emitted per unit of production goods or production area or a natural process, allows fro the estimation of loading to the environment from a variety of natural and anthropogenic sources. An EF can generate a difficult measurable variable (element load) from an easily assessed parameter (e.g. area, amount of goods produced, inhabitants) and be applied to the global, regional and local level, to estimate pollutant emissions from natural and anthropogenic sources (Nriagu and Pacyna 1988).
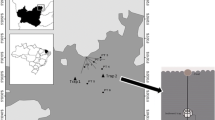
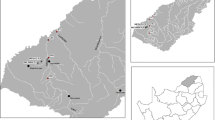
The emission factor was generated by analyzing Hg concentrations in aquafeeds and other chemical additives, in shrimp biomass and in inflow and outflow water and suspended particles of the largest shrimp farm of Ceará State NE Brazil, located at the Jaguaribe River estuary, latitude 4o23′ S and longitude 37o36′ W. Table 1 shows the major production parameters of this farm, which are also typical of intensive shrimp farming in Brazil and worldwide.
Samples for Hg determination were collected during one production cycle using pre-cleaned materials according to accepted protocols for Hg analysis (Marins et al. 2002). All glass- and plastic-ware used in sampling and preparation of samples were decontaminated by immersion for 2 days in 10% (v/v) Extran solution (MERCK), followed by immersion for 3 days in diluted HNO3 (10% v/v) before a final rinsing with Milli-Q water. Filters used for TSS and particulate Hg determinations were heated to 450°C for 12 h and transported enclosed in petri dishes prior to use. All chemical reagents used were of at least analytical grade. Water samples in the inflow canal (n = 6), inside two ponds (n = 8) and in the outflow canal (n = 6) were collected using pre-cleaned 1.5 L PET bottles and filtered through acid-cleaned cellulose acetate filter with a pore diameter of 0.45 μ for the collection of total suspended solids (TSS).
Two samples from each of 22 brands of aquafeeds (n = 44), 6 of fertilizers (n = 12) and 4 of lime (n = 8) used in the local shrimp farms were also analyzed. All these samples were oven-dried (60°C) to constant weight prior to digestion.
During the growth cycle from 10 to 24 shrimp depending on size but always totaling about 150 g, were sampled bi-weekly. Animals were separated in muscle tissue and exoskeleton and pooled into three composite samples from every collection and kept frozen till analysis, totaling 18 samples of each organism part. Prior to analysis biological samples were lyophilized.
Dissolved Hg was determined in filtered samples after oxidation with BrCl at room temperature. After oxidation, 1 N hydroxylamine solution was used to reduce the excess BrCl. This was followed by a reduction with 10% SnCl2 (Marins et al. 2002). Particulate Hg was obtained after partial digestion of the retained material on filters with 50% v/v aqua-regia at 80°C followed by reduction with SnCl2. For each batch, blank membrane samples were simultaneously analyzed. Water samples were analyzed in a PSA Millenium Merlin 10.025 Model cold vapor atomic fluorescence spectrophotometer (CVAFS). The analytical detection limit of the method was 0.24 ng L−1 for dissolved Hg and 1.0 ng g−1 for particulate Hg, based on the ratio between three standard deviations of blank reagents. In all cases, blank signals were lower than 0.5% of sample analysis. All samples were analyzed in duplicate. Differences between duplicates remained below 20%. For quality assurance of TSS analysis, a certified reference material (NRC PACS-2, Canada) was simultaneously analyzed to evaluate Hg determination accuracy. Such analysis showed a precision of 96%, as indicated by the relative standard deviation of three replicates, and presented an average Hg recovery of 103 ± 4%. Concentration values were not corrected for the recoveries found in the certified material. Aquafeed, fertilizer, lime and shrimp samples were digested in a manner similar to filters and also analyzed by cold vapor atomic fluorescence absorption spectrophotometer (CVAFS).
Results and Discussion
The average dissolved Hg concentrations were higher in input water (4.52 ng L−1) than in output water (0.09 ng L−1) (Table 2), suggesting the scavenging of Hg from the dissolved phase. Particulate Hg concentrations were; on the other hand, lower in input waters (0.72 ng L−1) than in output waters, which varied from 9.21 ng L−1, in the first 80% of the pond volume drained, to 179 ng L−1 in the 20% of the volume drained during the emptying of the pond. This confirms that at least part of the Hg scavenged from the dissolved phase adsorbs to suspended matter and may be deposited in pond bottom sediments.
Aquafeeds brands, fertilizers and lime used in the studied farm showed Hg concentrations ranging from 10.8 to 48.1; 0.1 to 9.7 and 5.0 to 90.3 ng g−1 d.w., respectively. The relatively large range in the observed concentrations is in agreement with the fact that Hg occurs as an impurity in these products. This results in a relatively large range of loading values, and contributes the most to the uncertainty associated with the estimated EF. Aquafeeds, due to the large amounts used, are the largest contributor of Hg to shrimp ponds (189 mg−1 ha−1 cycle−1), whereas lime addition results in the second most important source of Hg (41.2 mg−1 ha−1 cycle−1). Total Hg loading from the products added to ponds amounts to 231.2 mg ha−1 cycle−1.
Suspended particulate matter (TSS) was higher (80 to 164 mg L−1) in input waters than in pond waters (62 mg L−1). Extremely high TSS content was determined in bottom draining waters (1,675 mg L−1), a 19-fold increase relative to surface waters. This suggests that at the end of the cycle when the pond is emptied, water currents moving into the central draining canal may be strong enough to erode and transport at least the surface nefloid layer of bottom sediments.
Figueiredo et al. (2005) analyzed the TSS balance in another farm in the Jaguaribe estuary which uses a similar culturing approach, and found a 17-fold increase in bottom water TSS content relative to surface waters. These authors monitored the empting process at the end of the growing cycle and found that TSS-enriched water represents about 20% of the total pond volume.
Concentrations of Hg in shrimp biomass (16.2 ± 1.2 ng g−1 fresh weight) are far below the maximum permissible concentrations for Hg in seafood for human consumption adopted by Brazilian legislation. Since the sum of Hg export through waters and Hg export through biomass does not account for the total Hg added as chemicals and aquafeed, part of the Hg input is probably retained in sediments, as verified for other trace metals (Lacerda et al. 2006a, b). Considering this estimated deposition, Hg accumulation in pond bottom sediments would reach about 20 μg m−2 year−1, a value consistent with Hg deposition in sediments from coastal lagoons in non-industrialized areas (Marins et al. 2004).
The total export of Hg (i.e. 574.5 mg ha−1 cycle−1) through water is mostly (95%) associated with particulate matter (Table 2). From this total, 83.5 mg ha−1 cycle−1 constitute the anthropogenic excess contribution. This contribution is similarly to that reported for N, P and Cu in previous studies of element mass balances in intensive aquaculture of the Jaguaribe Estuary (Figueiredo et al. 2005; Lacerda et al. 2006a, b). Nutrient (N and P) and Cu exports from shrimp farming are ~60%, >98% and 65% for N, P and Cu, respectively mostly associated with particulates released at end of the draining of the pond (Burford et al. 2003; Figueiredo et al. 2005; Lacerda et al. 2006a, b).
Assuming that 2.1 production cycles per year is typical of this farm and of others in NE Brazil, the EF for Hg would reach 175 mgHg ha−1 year−1. Considering that 2,021 ha of ponds are operating around the estuary, the total loading of Hg from this source would reach about 0.35 kgHg year−1. Compared to other anthropogenic Hg sources to the Jaguaribe River estuary compiled by Lacerda and Sena (2005) (Table 3), where industrialization is non-existent, the Hg EF from shrimp farming is similar to that from urban waste waters (200 mgHg ha−1 year−1) and solid waste disposal (400 mgHg ha−1 year−1) from local towns and cities. As expected, however, total Hg load from shrimp farming compared to the other sources is much less significant (200–400 times lower) due to the small area occupied by shrimp farms relative to urban areas.
Evidence of environmental Hg contamination in areas receiving shrimp farm effluents are, however, still preliminary. Vaisman et al. (2005) measured higher Hg concentrations in mangrove oysters and creek sediments from the Jaguaribe estuary (2,021 ha of shrimp farm area) compared to oysters from the Pacotí estuary, an area harboring less than 10 ha of shrimp farms. These results are in agreement with a net export of particulate Hg from shrimp farm effluents to adjacent mangrove creeks.
The results presented here, although preliminary, show that intensive shrimp farming presents a measurable emission factor for Hg, originating mostly from Hg impurities present in aquafeeds, which include fish meal and oil in their formulation. Mercury emission is predominantly in the particulate form. The estimated EF is the first ever calculated for intensive shrimp farming. Since the technology and procedures used in these farms are used worldwide, the estimated EF may be applied to other production areas in the world.
The impact of intensive shrimp farm effluents on local coastal ecosystems may become more significant in the future relative to urban sources for Hg emissions, since the area dedicated to shrimp farming is increasing at a much faster rate (about 10% per year) than the growth of urban areas (about 0.2% per year) in the lower basin of the Jaguaribe River. This has already been noted (Lacerda et al. 2006a, b) for levels of N and P in other estuaries from the northeastern Brazilian coastline harboring significant shrimp farm areas.
References
ABCC (2005) Associação Brasileira dos Criadores de Camarão – Censo da produção do camarão marinho cultivado. Recife: ABCC. Available via: http://www.abcc.com.br. Accessed 25 Oct 2009
Barbieri E, Passos EA, Garcia CAB (2005) Use of metabolism to evaluate the sub lethal toxicity of mercury on Farfantepenaeus brasiliensis larvae (Latreille, 1817, Crustacea). J Shellfish Res 24:1229–1233
Berntssen MHG, Hylland K, Julshamn K, Lundebye AK, Waagbø R (2004) Maximum limits of organic and inorganic mercury in fish feed. Aquac Nutr 10:83–97
Burford MA, Costanzo SD, Dennison WC, Jackson CJ, Jone AB, McKinnon AD, Preston NP, Trott LA (2003) A synthesis of dominant ecological processes in intensive shrimp ponds and adjacent coastal environments in NE Australia. Mar Pollut Bull 46:1456–1469
Chou CL, Haya K, Paon LA, Burridge L, Moffatt JD (2002) Aquaculture-related trace metals in sediments and lobsters and relevance to environmental monitoring program ratings for near-filed effects. Mar Pollut Bull 44:1259–1268
Figueiredo MB, Araújo LFP, Gomes RB, Rosa MF, Paulino WD, Morais LFS (2005) Impactos ambientais do lançamento de efluentes da carcinicultura em águas interiores. Eng Sanit Amb 10:167–174
Lacerda LD, Sena DD (2005) Estimativas de cargas de nitrogênio, fósforo e metais pesados de interesse ambiental para as bacias inferiores do litoral do Estado do Ceará. Programa Zoneamento Ecológico e Econômico do Litoral do Ceará, SEMACE. Available via. www.semace.gov.ce/zee/produtos/EstimativasCargas.pdf. Accessed 20 Jun 2010. Accessed 20 Oct 2009
Lacerda LD, Paraquetti HHM, Rezende CE, Silva LFF, Silva Filho EV, Marins RV, Ribeiro MG (2002) Mercury concentrations in bulk atmospheric deposition over the coast of Rio de Janeiro, SE Brazil. J Brazilian Chem Soc 13:165–169
Lacerda LD, Santos JA, Madrid RM (2006a) Copper emission factor from intensive shrimp aquaculture. Mar Pollut Bull 52:1823–1826
Lacerda LD, Vaisman AG, Maia LP, Cunha E, Silva CAR (2006b) Relative importance of nitrogen and phosphorus emissions from shrimp farming and other anthropogenic sources for six estuaries along the NE Brazilian coast. Aquaculture 253:433–446
Marins RV, Paraquetti HH, Ayres GA (2002) Analytical alternative for the physical-chemical speciation of mercury in tropical coastal waters. Quím Nova 25:372–378
Marins RV, Paula Filho FJ, Maia SRR, Lacerda LD, Marques WS (2004) Distribuição de mercúrio total como indicador de poluição urbana e industrial na costa brasileira Quím Nova 27:763–770
Nriagu JO, Pacyna JM (1988) Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 333:134–139
Vaisman AG, Marins RV, Lacerda LD (2005) Characterization of the mangrove oyster Crassostraea rhizophora, as a biomonitor for mercury in tropical estuarine systems. Bull Environ Cont Toxicol 73:582–588
Acknowledgments
This study was supported by CNPq-INCT-TMCOcean Project (Proc. No. 573.601/2008-9) and FINEP/RECARCINE Initiative. The authors thank two anonymous reviewers for greatly improving an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lacerda, L.D., Soares, T.M., Costa, B.G.B. et al. Mercury Emission Factors from Intensive Shrimp Aquaculture and Their Relative Importance to the Jaguaribe River Estuary, NE Brazil. Bull Environ Contam Toxicol 87, 657–661 (2011). https://doi.org/10.1007/s00128-011-0399-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-011-0399-4