Abstract
This study examines seasonal variations in the concentrations of Cd, Cu, Pb, and Hg in experimentally cultured Crassostrea corteziensis, an oyster species known to have high resistance to physical and chemical stressors. The highest levels of Cd (4.92 mg/kg), Cu (3.45 mg/kg), and Pb (0.67 mg/kg) were detected in oyster samples collected during the summer, while Hg concentrations were similar (0.03 to 0.04 mg/kg) throughout all seasons. Results indicate that except for Cd, Crassostrea corteziensis accumulates metals to levels below those recommended by the US. FDA and the Mexican government. For Cd, its concentration correlates more strongly with the temperature of the oyster’s environment rather than to the oyster growth cycle.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In marine environments, oysters have the ability to accumulate metals in high concentrations. As a result, they have been widely used to assess environmental contamination in different ecosystems (Cherkasov et al. 2006). Moreover, oysters are valued as food, and have and important commercial value. Some species, such as Crassostrea gigas and Crassostrea virginica are cultivated in specific areas. A number of regulatory programs addressing the concentration levels of metals in various ecosystems have been legislated to protect the environment and human health (Chávez-Villalba et al. 2005; Samain et al. 2007). However, these regulations and monitoring programs have not significantly decreased the levels of metal contamination, and high mortality rates still afflect the oyster communities in several countries (Chávez-Villalba et al. 2005; Samain et al. 2007).
Since 1998, the Crassostrea gigas oyster industry located in the Gulf of California has been afflicted with high mortality rates (Chávez-Villalba et al. 2005). The same phenomenon has been reported for oyster industries along the coasts of the US (Cheney et al. 2000) and France (Samain et al. 2007). These reports indicated that some of the factors that affect oyster mortality are temperature, techniques associated with aquaculture practices, genetic characteristics, pathogens and viruses, and pollutants.
Metals are major water pollutant and cause detrimental effects such as respiratory or osmoregulatory dysfunctions in aquatic organisms. They are known to be sulflydryl-reactive and their toxicity is believed to be due to their strong binding capacities to sensitive groups such as thiols and histidyls, resulting in the deterioration of biologically important molecules (Samain et al. 2007).
According to Chávez-Villalba et al. (2005), a species such as Crassostrea corteziensis in the Sonoran coast, is a good alternative for the oyster industry due to its resistance to physical and chemical stressors. Crassostrea corteziensis acclimates easily, and is more resistant to high temperatures than Crassostrea gigas. However, data on the toxicity of the metals found in cultivate Crassostrea corteziensis is scarce, if not available. Therefore, to improve the culture conditions of oyster species such as Crassostrea corteziensis in the coastal and estuarine areas, we investigated the concentration of metals in cultivated oyster Crassostrea corteziensis.
Materials and Methods
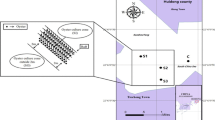
Our experiments on the cultured oyster Crassostrea corteziensis were performed in Bacochibampo Bay, which is the central part of the Sonora, México between 27° 53′ N latitude and 110° 56′ W longitude (Fig. 1). The Bay is more than 10 m deep and connects to the Gulf of California via a 2.5 km wide mouth. The climate of the region is dry and hot with rainy summers. The Crassostrea corteziensis were sampled in November (Winter 2004), March (Spring 2005), June (Summer 2005), and October (Fall 2005). During each sampling period, 30 oysters were collected; in the winter, the size of the oysters that were selected was 6–7 cm; in the spring, their size was 8–9 cm; in the summer, 7–10.5 cm; and in the fall, 7–9 cm. The oysters were rinsed with sea water, packed in polyethylene plastic bags and transported on ice to the laboratory within 12 h of collection. At the laboratory, the 30 oysters from each sampling period were cleaned with distilled water. They were not depurated so that the soft tissue included the digestive gland and sediment particles. Following washing, the oysters were shucked with a knife, and the entire organisms were removed from their shells, homogenized in a food processor for 30 s at low speed, and stored frozen until further analyses.
From each of the 30 oysters within a sampling period, 3.0 g of oyster homogenate was weighed in a microwave digestion lineal vessel (MARSX, CEM Corp., Matthews, NC) and combined with 5 mL HNO3 (50%, v/v). The vessels were digested for 5 min at 90°C, 10 min at 120°C, and 15 min at 140°C, following which 3.0 mL H2O2 (30%, v/v) was slowly added to the vessels. The vessels were returned to the microwave oven, and a second digestion was performed for 5 min at 100°C, 10 min at 140°C, and 15 min at 160°C. After the microwave digestion, the samples were cooled, vented, and diluted with HPLC grade water. This digestion procedure was carried out twice, one for Cd, Pb, and Cu determination and the other for Hg determination. For the Cd, Cu, and Pb measurement, each of the digested samples was diluted to a final volume of 50 mL. Cu was determined by atomic absorption spectrometry and Cd and Pb by furnace atomic absorption spectrometry using the Varian model SpectrAA-20 (Varian, Victoria, Australia), along with a graphite furnace (GTA-96). For the Hg measurement, 0.5 mL of K2Cr2O7 (1%, w/v) and 4.0 mL of HNO3 (50%, v/v) were added to the digested samples. Afterwards, each sample was diluted with HPLC grade water to a final volume of 50 mL. Hg was determined after reduction with SnCl2 (20%, w/v) in HCl (20%, v/v) by the cold vapor technique. The wavelength and slit were set at (in nm): Cd (228.8, 0.5), Cu (324.8, 0.5), Pb (283, 0.5), Hg (253.7, 0.5). Argon (purity > 99.99%) was used as the inert gas in the graphite furnace and hydride vapour generation.
HPLC water was used for the preparation of reagents and standards. All chemicals were trace metal grade. Calibration standard solutions were prepared from commercial standard solutions (High-Purity Standards, Charleston, SC). All glassware was treated with 20% (v/v) HNO3, and then rinsed with double-distilled water. For quality control purposes, reagent blanks and triplicates samples were analyzed during the experiment and the results were averaged. The Standard Reference Material, SBR-1566b, Oyster Tissue from the National Institute of Standards and Technology (NIST, Gaithersburg, MD) was analyzed for quality assurance. The recovery rates were: Cd (99%), Cu (102%), Pb (94%), Hg (102%). The variation coefficient was below 7%. Detection limits (mg/kg) were determined by the blanks method: Cd (0.006), Cu (0.02), Pb (0.003), Hg (0.002). t-test were conducted at a significance level of p < 0.05 to compare the mean metal concentrations in oyster from among the different seasons.
Results and Discussion
The concentrations of Cd, Cu, Pb, and Hg found in oysters from each season of the study are shown in Table 1. The most abundant were Cd and Cu, followed by Pb and then Hg. It has been found that variations in the concentration of the metals are related to the seasonal cycle of oyster reproduction and growth (Cherkasov et al. 2006). In this study, the concentration of Cd showed the highest seasonal variation; it ranged from 0.90 to 4.92 mg/kg.
The variations in the concentrations of metals may also be linked to mean annual temperature patterns since it has been reported that the amount of metals in oyster is positively correlated with seasonal variation (Cherkasov et al. 2006). In this study, the lowest levels of Cd (0.90 mg/kg) were detected during the winter; the Cd levels increased in oysters gathered in the spring (p < 0.05), peaked in those selected in the summer (4.92 mg/kg) (p < 0.05), and fell again in oysters collected in the fall (p < 0.05).
For Cu and Pb, the lowest levels (1.72 and 0.39 mg/kg) were detected in oysters gathered during the fall and the highest levels (p < 0.05) in those from the summer (3.45 and 0.67 mg/kg). Meanwhile, Hg concentrations were similar (0.03 to 0.04 mg/kg) for all seasons. Similar trends in Cd concentrations were observed in Crassostrea rhizophorae from the Sepetiba Bay, Brasil (Rebelo et al. 2003), in Crassostrea corteziensis from the coastal lagoons of Culiacán and Mazatlán, México (Frías-Espericueta et al. 2008). As for seasonal variations in Pb and Cu concentrations, similar patterns were also reported for the Crassostrea corteziensis from Culiacán and from Mazatlán, México (Soto-Jiménez et al. 2001). The seasonal fluctuations are related to an increase in effluents along the coasts and to an increase in oyster feeding rate due to an increase in temperature of the seawater in the summer (Soto-Jiménez et al. 2001; Rebelo et al. 2003).
There are a number of studies that measure the amount of metals found in oysters. However, there are none that put these numbers into a larger context; studies that investigate changes in the concentrations of metals in Crassostrea corteziensis for commercial purpose over a certain time period are scarce. Cd concentrations were found to be higher in Crassostrea corteziensis than those in Crassostrea gigas (García-Rico et al. 2001) where levels of Cd ranged from 0.40 to 1.43 mg/kg. Pb and Hg levels (0.40 to 0.60 and 0.02 to 0.06 mg/kg) were similar in both species, but Cu concentrations were lower than those found (1.40 to 7.87 mg/kg) in a previous report. These differences may be related to the feeding behaviors of the oysters, Crassostrea corteziensis has a higher particle-sorting ability throughout the year than Crassostrea gigas (Chávez-Villalba et al. 2005). Cd levels were shown to be related to the sulfur content and hydrothermal activity of the area (Ruelas-Inzunza et al. 2003), both of which promote the transfer of Cd from the sediment to the seawater, where the Cd is then taken up by oysters.
Seasonal variations in metal concentrations have also been shown to be associated with the seasonal cycle of oyster reproduction and growth (Rebelo et al. 2003). In the first evaluation of growth and survival of Crassostrea corteziensis along the Sonora Coasts, the cultivated oysters reached a length of 7.1 ± 0.19 cm over 13 months and showed the lowest and highest growth rates during the winter and summer, respectively (Chávez-Villalba et al. 2005). In the present study, the highest oyster growth occurred during the summer (8.7 ± 0.78 cm of length over 14 months), the same season in which we detected the highest levels of Cd.
Oysters are exposed to different levels of Cd in their habitats and accumulate Cd in their soft tissues to concentrations greater than that in their surroundings by an order of magnitude (Cherkasov et al. 2006). Through bioconcentration, particulate matter and phytoplankton are the main sources of Cd in oysters. The bioconcentration factor (BCF, defined as the ratio between the metal concentration in the organism and that in the particulate matter) provides information about how enriched an organism is in its metal content, with respect to its surrounding environment. In this study, we calculated the BCF using a previously reported metal concentration in particulate matter (Ochoa-Valenzuela et al. 2009). The order from the highest values to lowest of the mean BCF values of the various metals in Crassostrea corteziensis was Cd (2.5) > Cu (1.75) > Hg (0.62) > Pb (0.06). The highest BCF values for Cd, Pb, and Cu were observed in the summer (3.81, 0.07, and 2.17) those for Hg were observed in the winter (0.91). Except for Cd, our BCF values for the metals are in agreement with those measured in the mangrove root Crassostrea corteziensis by Jara-Marini et al. (2008). Our mean Cd values were 5 times higher than those observed by Jara-Marini et al. (2008); this difference may be related to variations in growth conditions.
There are no reports relating metal concentrations in the Crassostrea corteziensis cultivated in Sonoran Coasts to human and environmental health standards. When metals concentrations determined in this study were compared to the levels set by Mexican regulations, we found that, except for that of Cd, all our metal concentrations were lower. The Cd concentrations we measured were 6–8 times higher than those allowed by the Mexican government (0.5 mg/kg) (Secretaria de Salud 1995), but lower or similar to those dictated by US. FDA regulation (4 mg/kg) (FDA 2001). Since in and around the Gulf of California, Cd levels in organisms are related to high Cd concentrations in phytoplankton and sediment due natural phenomenons as the hydrothermal systems (Ruelas-Inzunza et al. 2003), high levels of Cd in oysters indicated that culture conditions need to be improved. Moreover, to protect the public health, more studies are needed to evaluate the Crassostrea corteziensis’s Cd exposure and Cd bioavailability. Their Cd daily intake needs to be monitored and limited to 0.07 mg (for a body weight of 70 kg).
The levels of accumulation of Cu, Pb and Hg in the oyster Crassostrea corteziensis was below the levels recommended by the Mexican government, and the levels of Cd was below that the level recommended by the US. FDA. The high levels of Cd in cultivated oysters were found to be related to the seasonal temperatures of the oysters’ surrounding environment more than the oyster growth cycle. However, to improve aquaculture practices to better protect public and ecosystem health, more studies on Crassostrea corteziensis are needed to determine the other chemical and physiological factors that may affect its Cd accumulation, and to gather more information about conditions that optimize its growth.
References
Chávez-Villalba J, López-Tapia M, Mazón-Suástegui J, Robles-Mungaray M (2005) Growth of the oyster Crassostrea corteziensis (Hertlein, 1951) in Sonora, Mexico. Aquaculture Res 36:1337–1344
Cheney DP, Macdonald BF, Elston RA (2000) Summer mortality of pacific oysters, Crassostrea gigas (Thunberg): initial findings on multiple environmental stressors in Puget Sound, Washington, 1998. J Shellfish Res 19:353–359
Cherkasov AS, Biswas PK, Ridings DM, Ringwood AH, Sokolova IM (2006) Effects of acclimation temperature and cadmium exposure on cellular energy budgets in the marine mollusk Crassostrea virginica: linking cellular and mitochondrial responses. J Exp Biol 209:1274–1284
FDA (2001) Environmental chemical contaminants and pesticides. Fish and Fisheries Products, Hazards and Controls Guidance, 3rd edn. Washington, DC
Frías-Espericueta MG, Osuna-López JI, Voltolina D, López-López G, Izaguirre-Fierro G, Muy-Rangel MD (2008) The metal content of bivalve molluscs of a coastal lagoon of NW Mexico. Bull Environ Contam Toxicol 80:90–92
García-Rico L, Ruiz RER, Jiménez JV (2001) Determination of total metals in cultivated oysters (Crassostrea gigas) from the Northwest coast of Mexico by microwave digestion and atomic absorption spectrometry. AOAC Int 84:1909–1913
Jara-Marini ME, Soto-Jiménez MF, Páez-Osuna F (2008) Trace metals accumulation patterns in a mangrove lagoon ecosystem, Mazatlán harbor, southeast Gulf of California. J Environ Sci Heal Part A 43:995–1005
Ochoa-Valenzuela LE, Gómez-Alvarez A, García-Rico L, Villalba-Atondo AI (2009) Distribution of Heavy Metals in Surface Sediments of the Bacochibampo Bay, Sonora, Mexico. Chem Spec Bio 21:211–218
Rebelo MF, Rebouças do Amaral MC, Pfeiffer WC (2003) High Zn and Cd accumulation in the oyster Crassostrea rhizophorae, and its relevance as a sentinel species. Mar Pollut Bull 46:1341–1358
Ruelas-Inzunza J, Soto LA, Páez-Osuna F (2003) Heavy-metal accumulation in the hydrothermal vent clam Vesicomya gigas from Guaymas basin, Gulf of California. Deep-Sea Res I 50:757–761
Samain JF, Dégremont L, Soletchnik P, Haure J, Bédier E, Ropert M, Moal J, Huvet A, Bacca H, Van Wormhoudt A, Delaporte M, Costil K, Pouvreau S, Lambert C, Boulo V, Soudant P, Nicolas JL, Le Roux F, Renault T, Gagnaire B, Geret F, Boutet I, Burgeot T, Boudry P et al (2007) Genetically based resistance to summer mortality in the Pacific oyster (Crassostrea gigas) and its relationship with physiological, immunological characteristics and infection processes. Aquaculture 268:227–243
Secretaria de Salud NOM-031-SSA1-1993 (1995) Bienes y Servicios. Productos de la Pesca. Moluscos bivalvos frescos-refrigerados y congelados. Especificaciones Sanitarias., México, DF. Secretaría de Salud
Soto-Jiménez M, Páez-Osuna F, Morales-Hernández F (2001) Selected trace metals in oysters (Crassostrea iridescens) and sediments from the discharge zone of the submarine sewage outfall in Mazatlán Bay (southeast Gulf of California): chemical fractions and bioaccumulation factors. Environ Pollut 114:357–370
Acknowledgments
This work was supported by funds provided by Consejo Nacional de Ciencia y Tecnología (CONACyT). The authors thank Dr. Jorge Chávez-Villalba for providing the oysters and to Perlas del Mar de Cortez for providing its culture facilities for this experiment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García-Rico, L., Tejeda-Valenzuela, L. & Burgos-Hernández, A. Seasonal Variations in the Concentrations of Metals in Crassostrea corteziensis from Sonora, México. Bull Environ Contam Toxicol 85, 209–213 (2010). https://doi.org/10.1007/s00128-010-0055-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-010-0055-4