Abstract
This study assessed the contamination extent and potential ecological and human health impacts for chromium (Cr), manganese (Mn), nickel (Ni), copper (Cu), zinc (Zn), cadmium (Cd), and lead (Pb) in sediments and indigenous benthic organisms along the coastal area of Huludao, China. We analyzed a total of eight species: two benthic fish species, two bivalves, two snails, and two decapod crustaceans. Cu, Zn, and Cd levels in sediment exceeded the Chinese marine sediment quality criteria. The geoaccumulation index was highest for Cd followed in a decreasing order by Zn, Pb, Cu, Ni, and Cr. Metal levels were highest in the four mollusk species. The oyster and veined rapa whelk had the highest bioaccumulation factors, indicating that these two species would be well suited for monitoring the metal pollution in this area. Our comparison of estimated daily intake values for human consumption of the seafood species to the Food and Agricultural Organization-recommended daily dietary allowances indicate potential health risks from the intake of Cd from all shellfish other than our crab species and Zn intake from oyster consumption. An analysis of target hazard quotients identified noncarcinogenic health risks from Cd (in all shellfish analyzed except for our crab species), Cu, and Zn (in oysters and veined rapa whelks). Moreover, an analysis of cancer risk from Pb ingestion detected an increased risk for consumption of all shellfish except for the crab species. Health risks seem especially pronounced for the consumption of oysters and the veined rapa whelks; a seafood advisory may be warranted for these mollusks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Metals occur in aquatic ecosystems as a result of both natural processes and anthropogenic activities. Weathering of rocks and volcanic eruptions can result in the accumulation of metals in aquatic environments. More commonly, locally increased levels are a consequence of human activities such as the burning of fossil fuels, smelting of metal ores, metal processing, insecticide and fertilizer applications, urban runoff, and the discharge of domestic effluents (Fishbein 1981). Once released into aquatic ecosystems, almost all of this metal load becomes associated with suspended particulate matter and sediments (Hare et al. 2003). Consequently, benthic organisms associated with the sediment ingest relatively high metal levels compared with pelagic organisms. For example, for some deposit-feeders, the ingestion of sediments can account for almost 100 % of their accumulation for certain metal species (e.g., methylmercury) (Wang and Fisher 1999). This metal bioaccumulation can cause a suite of adverse effects including changes in ion regulation, oxidative stress, and DNA damage, and these cellular-level effects can have effects at (and even beyond) the individual and population levels (Valavanidis et al. 2006). Because some metals are toxic, persistent, and bioaccumulative (Allan 1997; Diaz et al. 2006), metal pollution has been identified as a threat to wildlife habitats and ecosystems (Pan and Wang 2012), and can pose a health risk to the general population by way of consumption of contaminated seafood.
The present study assessed metal pollution and associated impacts and risks for Liaodong Bay. This estuary is located in the northeast part of the Bohai Sea in northeast China, and the main freshwater input comes from the Daliao, Liao, Shuangtaizi, Daling, Luan, and Daqing rivers. The area surrounding Liaodong Bay, including the economically important coastal city of Huludao, has seen rapid economic growth. The area is also home to the Huludao Zinc Smelting Plant, which is located southeast of the city and situated directly on Liaodong Bay. These factors have led to high inputs of pollutants into the bay and thus to its ecological degradation, especially in its coastal areas (Zhang et al. 2006). Although an earlier study reported that the mean metal levels in the sediments of the Liaodong Bay [46.4, 22.5, 19.4, 71.7, and 31.8 mg/kg dry weight (dw) for chromium (Cr), nickel (Ni), copper (Cu), zinc (Zn) and lead (Pb)], respectively) were close to background values (Hu et al. 2013), the Jinzhou bay (part of the Liaodong Bay), adjacent to the Huludao city, was heavily polluted. The average concentrations of As, Cd, Cu, and Pb recorded in surface sediments of the Jinzhou Bay were several times to nearly 500 times greater than the upper limit of the background values (Gao et al. 2014). Similarly, highly increased metal levels have been reported for the oyster Crassostrea gigas in Bohai Bay with concentrations of 11.6, 1337, 356, 2.5, and 1.8 mg/kg dw for Cd, Zn, Cu, Pb, and Ni, respectively (Liang et al. 2004). Although these results indicate that metal pollution is an issue here, the severity of the bioaccumulation in species other than oysters is unclear. It is also not clear what the metal levels are in the commonly consumed seafood species in this region, other than in oysters, and what the associated health risks are (Wang et al. 2010).
This study quantified metal levels in sediment, six benthic organisms and two fish species (goby and tongue sole) from the coast of Huludao City, Liaoning, P. R. China. Ecological risks associated with increased metal levels in sediment were evaluated. In addition, the health risk from eating seafood contaminated with Cd, Cu, Cr, manganese (Mn), Ni, Pb, and Zn was estimated. Estimates of daily intake (EDIs), the noncarcinogenic target hazard quotient (THQ), and target carcinogenic risk (TR) were used to estimate the daily intake and potential risks for two categories of consumers (adults and children) (Copat et al. 2012; USEPA 1989, 2000).
Materials and Methods
Study Area
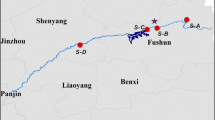
This study focused on the northwestern part of Liaodong Bay (known as Jinzhou Bay) in the Bohai Sea (Fig. 1). The Huludao economic zone (Longgang and Lianshan districts) borders Jinzhou Bay and has several factories including the Huludao Zinc Smelting Plant, the Jinxi Chemical Factory, and the Jinxi Petroleum Chemical Factory (Fig. 1). The Huludao Zinc-Smelting Plant, a smelting plant built in 1937, produces 330,000 tons of Zn every year (Zheng et al. 2007b). Its atmospheric releases and wastewater discharges have deposited large quantities of metals in nearby terrestrial and marine ecosystems (Zheng et al. 2007a). Less attention has been paid to the potential of metal pollution from the Jinxi Chemical Factory and the Jinxi Petroleum Chemical Factory, though their effluents could also be sources of metal pollution in this region.
Sampling and Pretreatment
All of the samples were collected in July 2014 along the coastal area of Huludao city. Sampling of sediment and three benthic species was performed at a site directly on the bay (40°47′13″N, 120°58′36″E; see Fig. 1), situated 7.8 km away from the Huludao Zinc-Smelting Plant and 12.5 km away from Jinxi Chemical Factory and Jinxi Petroleum Chemical Factory. Three benthic species (the oyster C. gigas, the Asian periwinkle Littorina brevicula, and the clam Neotrapezium liratum) were collected manually in the sediment or removed from rocks. Five species of commonly consumed seafood (the veined rapa whelk Rapana venosa, the crab Charybdis spp., the shrimp Oratosquilla oratoria, the goby Synechogobius hasta, and the red tongue sole Cynoglossus joyneri) were purchased from local fishermen who had collected these specimens in the open sea within approximately 18 km from the coast. The organisms were brought to the laboratory alive. To decrease the effects of size variability, developmental (reproductive) stages, and sex on metal levels, five to six shellfish were pooled to form a compound sample. In the laboratory, the mollusks were allowed to purge their gut contents in artificial seawater for 3 days before they were killed for analysis. Due to inherent difficulties for sample collection in the field, the sample size for certain species was limited (n = four to eight dependent on number of organisms collected). All specimens were rinsed thoroughly with deionized water, wrapped in aluminum foil, and frozen at −20 °C until later processing. All species were identified to the lowest possible taxonomic level. The details of individual species are listed in Table 1.
After thawing on ice, muscle and liver tissues of the fish (N = 6) were dissected and weighted before oven-drying. For crabs (N = 6), only the muscle tissue inside the claws was used. The dorsal muscles of shrimps (N = 6) and the soft tissues of oysters (N = 8) and clams (N = 4) were obtained using an acid-rinsed steam-cleaned stainless steel knife. For veined rapa whelks (N = 4) and Asian periwinkles (N = 6), whole soft tissues were used. For some of the species, especially for those smaller animals, several individuals were combined into a compound sample (see Table 1).
All samples [wet weight (ww) approximately 1–2 g] were dried in an oven at 60 °C for 48 h to obtain a constant weight. The dry samples were digested using 5.0 or 10.0 mL of concentrated nitric acid on a hotplate (Numerical Stainless Steel Electric Heating Board, China) at 110 °C for 1 h. The digested samples were then diluted by bringing the sample volumes up to a final volume of 25 or 50 mL with deionized water. Acid blank samples were prepared for each batch of samples in the digestion process. The accuracy of metal determinations was validated by concurrent analysis of two certified reference materials (GBW10024-scallop tissue and GBW07309-sediment) from the National Institute of Metrology, China. During the analysis, calibrated standards and spiked samples with known concentrations of metals were analyzed after every 15 samples to ensure accurate performance of the instrument. In addition, all of the analyses were performed in triplicate, and the SDs were within ±5 % of the mean. Recoveries for all of the analyzed metals in the standard reference materials ranged from 87 to 103 % of the certified values.
Sediment samples (N = 3) were collected using a sediment grab and brought to the laboratory where they were stored in a freezer at 4 °C until further processing. The sediment was oven-dried at 60 °C for 24 h and then ground with a ceramic mortar and pestle. Approximately 1 g of each sample was extracted using 10 mL of nitric acid (70 %) on a hotplate at 140 °C for 3 h. After that, each sediment/acid mixture was transferred to a 50-mL plastic centrifuge tube and centrifuged at 1780 × g for 10 min. The supernatant was further diluted with deionized water to a final volume of 50 mL before metal analysis.
Chemicals and Reagents
All glassware was soaked with 10 % v/v nitric acid (HNO3) for 24 h and then rinsed three times with deionized water before use. Chromatographic-grade nitric acid (Aladdin, China) was used for tissue digestion. Other chemicals of analytical purity or better were obtained from Sinopharm Chemical Reagent Corporation (SCRC Company, China). All of the reagents were prepared using deionized water (18.2 MΩ/cm).
Metal Determination
Concentrations of Cu, Cd, and Zn were determined by flame atomic absorption spectroscopy (AAS) (PE Analyst 100, Perkin-Elmer, USA). Levels of Cr, Mn, Ni, and Pb were determined using inductively coupled plasma mass spectrometry (ICP-MS) (NexIon 300, Perkin-Elmer). All analyses were performed in triplicate using the external calibration method. Mixed-metal standards were freshly prepared from 1000 mg/L of certified mixed-metal stock solution (National Testing Center of Nonferrous Metals and Electronic Materials Analysis, China) to establish the standard curves.
Risk Assessment
The geoaccumulation index (Igeo) was used to evaluate metal pollution and sediment quality (Müller 1979):
where Cn is the measured sediment concentration of the metal of interest (n), and Bn is the geochemical background concentration (values in mg/kg dw: 49 for Cr, 36.1 for Ni, 25.3 for Cu, 71.2 for Zn, 0.14 for Cd, and 20.5 for Pb) (Feng et al. 2011) of the same metal. According to Müller (1981), metal pollution was categorized into seven classes ranging from 0 (Igeo <0, unpolluted) to 6 (Igeo >5, extremely polluted).
The potential ecological risk index (RI) was used in this study to assess the ecological risks of the toxic metals (Hakanson 1980). This index combines biological toxicology, environmental chemistry, and ecology. RI is determined as follows:
where Ti represents the toxic-response factor for a given metal i (Cd = 30, Ni = Cu = Pb = 5, Cr = 2, and Zn = 1) (Hakanson 1980); C 0 is the regional background value of a metal in the sediment (the values are the same as the Bn values); Ci represents the actual concentration in metal i in the sediment; and n is the number of metals analyzed.
The bioaccumulation factor (BAF) was calculated according to the following formula (Chen and Chen 1999):
where Corg is the metal concentration in the organism (mg/kg dw), and Csed is the metal concentration in the sediment (mg/kg dw).
Risk Assessment for Public Health
We first calculated the estimated daily intake (EDI) of metals from seafood using the following equation (Copat et al. 2012):
where FIR is the food-ingestion rate (55 g/person/d for adults and 17.4 g/person/d for children) (Zheng et al. 2007a); C is the metal concentration (mg/kg ww); and BW is the average body weight (60 kg for adults and 32.7 kg for children) (Fu et al. 2013).
Health risks were also assessed using the target hazard quotient (THQ) and target carcinogens risk (TR) approaches. THQ relates the exposure to a reference dose and was determined using the standard assumption for an integrated USEPA risk analysis. The TR value estimates the probability of an individual to develop cancer during their lifetime (USEPA 1989). THQ and TR were calculated using Eqs. (5) and (6), respectively:
where EF is the exposure frequency (365 days/year); ED is the exposure duration (adults, 70 years; children, 10 years); FIR is the food meal size (g/person/day); C is the metal concentration in seafood (mg/kg, ww); RfDo is the oral reference dose [values in mg/kg/d: 4.0 × 10−2 for Cu, 2.4 × 10−2 for Mn, 2.0 × 10−2 for Ni, 1.0 × 10−3 for Cd, 4.0 × 10−3 for Pb, and 0.3 for Zn (USEPA 2010; Nadal et al. 2008); for Cr we used the RfDo value for Cr(III), which is 1.5 mg/kg/day]; BW is the body weight (kg); AT is the number of days over which the exposure is averaged [365 days/year × ED for noncarcinogenic effects, and 25,500 days (70 years × 365 days/year) for carcinogenic effects]; and CSF is the oral carcinogenic slope factor, which is 8.5 × 10−3 (mg/kg/day)−1 for Pb (USEPA 2010).
The total target hazard quotient (TTHQ) was used to estimate the total noncarcinogenic health hazards caused by exposure to multiple metals (USEPA 2007). The TTHQ is estimated by Eq. (7):
where THQi is the THQ value of element i.
Statistical Analysis
Student t test was used to test for differences between the two tissues (liver and muscle) for each of the two fish. One-way analysis of variance (ANOVA) was used to test for differences in metal levels among species. When the overall ANOVA was significant (P < 0.05), Duncan test was used to determine which species differed from each other (at the P = 0.05 level) for that metal. Data were checked for normality (Kolmogorov–Smirnov test) and homogeneity of variances (Levene’s F test). Logarithmic and square-root transformation of some of the data improved the normality and homogeneity of variances and met the assumptions for ANOVA. If the transformations did not achieve homoscedasticity and normality requirements, nonparametric tests, i.e., Kruskal–Wallis followed by Mann–Whitney U test, were used. All statistical analyses were performed using SPSS Statistics (version 17.0; SPSS, Chicago, Illinois, USA).
Results and Discussion
Sediment Metal Levels
Chinese marine sediment-quality standards (MSQs) (GB18668-2002) have three levels (MSQ1 = low contamination, MSQ2 = moderate contamination, and MSQ3 = high contamination) for five metals: Cr, Cu, Cd, Zn, and Pb (CSBTS 2002). Metal levels in sediments in the present study were greater than their standards except for Cr (21 mg/kg) (Table 2). Average concentrations of Cu (71 mg/kg) and Pb (66 mg/kg) were between MSQ1 and MSQ2 values, whereas Zn values (mean 564 mg/kg) were between MSQ2 and MSQ3 values (Table 2). Relative to the standards, the highest values were observed for Cd (19 mg/kg), the values of which were approximately four-fold greater than the MSQ-3 value (i.e., 5 mg/kg). No MSQ values are available for Mn and Ni. The average Mn value (606 mg/kg) for our sampling site was approximately similar to levels measured near Hong Kong (524 mg/kg) (Zhou et al. 2007) and Potter Cove (Antarctica) (695 mg/kg) (Vodopivez et al. 2015), whereas the average Ni level (16 mg/kg) was lower than those reported for other sites in the region (Hu et al. 2013; Gao and Chen 2012).
The severity of the sediment metal pollution was also assessed using Igeo values excluding Mn (according to Müller 1979). The Igeo values were negative for Cr (−1.85) and Ni (−1.75) indicating again the absence of contamination for these two metals (Table 2). The Igeo values for Cu ranged from −0.20 to 1.60 indicating a relatively modest Cu contamination. The Igeo values for Zn (2.40), Cd (6.51), and Pb (1.09) all exceeded 1 implying that the sediment was more severely polluted by these metals. Igeo values were particularly high (>5) for Cd showing that the study area was severely polluted by Cd with sediment levels being >100-fold above the background value.
As explained previously, the ecological risk index provides an assessment for the overall ecological risk caused by all of the contaminants analyzed. We excluded Mn from this analysis (according to Hakanson 1980). The combined RI value for Cr, Ni, Cu, Zn, Cd, and Pb was 4162. The fact that this value is well above 600 is indicative of a high ecological risk (Hakanson 1980). Taken together, these assessments and indices show that the sediment collected at this region was extremely polluted by metals and that this pollution resulted in a high ecological risk.
The coastal area of Liaodong Bay is surrounded by heavy-industry factories; among them is the Huludao Zinc Smelting Plant, the largest Zn smelter plant in Asia. When we compare metal concentrations in sediments of the Huludao region with those in other parts of Liaodong Bay as well as the Bohai Bay and Hong Kong areas, it is clear that metal pollution of the Huludao coastal region is generally more serious than that in those other regions (Hu et al. 2013; Gao and Chen 2012; Zhou et al. 2007). Our results showed that mean levels of Zn, Cd, and Pb at the Huludao coast exceeded those at all of the other sites. Much greater levels of Pb, Cd, Zn, and Cu—with values as high as, respectively, 1551, 1463, 19,789, and 1072 mg/kg—have been reported for sediments of Cishan River (Fig. 1) next to the Huludao Zinc Smelting Plant (Zheng et al. 2008). This indicates that the Huludao Zinc Smelting Plant is likely to be a major contributor to the observed metal pollution in the coastal area. Furthermore, these studies showed that sediments from both the marine and freshwater aquatic ecosystems in this region were heavily polluted and that assessments of the ecological risk of the contaminated marine sediment in the study area are warranted.
Metal Levels in Shellfish
Among the species studied, metal levels were generally highest in the mollusks (Table 3). Among these, the highest levels of Mn and Ni were found in the Asian periwinkle. The highest levels of Cu, Zn, and Cd were found in the oyster. Cu levels in the oyster and the veined rapa whelk were in the range of 269–632 mg/kg, which is significantly greater than in the other species, and differed significantly between these two species. The latter was consistent with previous studies (Beliaeff et al. 1998). Cr levels (1.0–4.0 mg/kg) in these organisms were relatively low, which is in line with a previous study (Wang et al. 2005). Meanwhile, Pb levels were relatively low in the selected shellfish (means 0.08–5.9 mg/kg). The fact that these levels were much lower than the average sediment Pb level of 65.6 mg/kg showed that Pb bioaccumulation was relatively low with a BAF <1. This same pattern was reported previously for mollusks in Pb-contaminated sediment (Wang et al. 2005). The observed metal levels were generally greater than those in organisms from other contaminated regions, especially for Zn and Cd (Liang et al. 2004; Thiyagarajan et al. 2012; Cui et al. 2011; Pan and Wang 2012). In nature, Zn and Cd coexist in Zn ore (Vallee 1959). The high levels of both Zn and Cd in the shellfish therefore suggest that the effluents from the Huludao Zinc Smelting Plant may be a major source of metal contamination.
In general, BAFs for Cr, Mn, Ni, and Pb were <1, whereas BAFs for Cu, Zn, and Cd were close to or >1 depending on species (Fig. 2). In addition, the BAFs were consistent with the relative degree of sediment enrichment of metals. BAF values were especially high (and exceeded 30) for Zn and Cd in oysters. Clear evidence of bioaccumulation was also found for the veined rapa whelk. It is well known that mollusks tend to accumulate metals from their surrounding environment (Pan and Wang 2012). Consequently, they are frequently employed in biomonitoring programs such as the Mussel Watch Program in the United States (Kimbrough et al. 2008). Both the present study and previous ones show that oysters and veined rapa whelks would be very well suited for monitoring Cu, Cd, and Zn pollution in the Bohai Sea and other coastal areas in this region (de Astudillo et al. 2002).
BAFs calculated as the metal level in benthic animals divided by the metal concentration in the sediment for benthos and sediment collected on the Huludao coastal area western Liaodong Bay. A BAF value >1 (dashed line) is indicative of bioaccumulation. BAF values for Cr, Mn, Ni and Pb were all <1 (data not shown)
Bioaccumulation was much lower in the crustacean species. For the shrimp species, the BAF was <1 for Cu and Zn and was approximately 1 for Cd. For the crab genus Charybdis, the BAFs of all of the measured metals were <1. Although concentrations of Cu and Zn were much greater than those of the other metals (Table 3), this may reflect the fact that Cu and Zn are essential elements. Decapod crustaceans require these elements for hemocyanin and a variety of enzymes, and levels of these metals are generally highly regulated in decapod crustaceans (Bryan 1968). Interestingly, Cd levels were significantly greater in our shrimp species than in our crab species. However, we do not know the reason for this observation.
Metal Levels in Fish
Overall, metals levels in the two fish species (S. hasta and C. joyneri) were much lower than those in other species (Table 3) despite the these two fish species having their benthic lifestyle in common with that of the shellfish. The observed metal levels were similar to those in S. hasta collected off the coast of India (Thiyagarajan et al. 2012) and in a different Cynoglossus species (C. arel) inhabiting China’s Yellow River delta (Cui et al. 2011). Metal levels were general greater in livers than they were in muscle tissue, and this was especially pronounced for Cu, Zn, and Cd (Table 3). For fish, metal uptake is generally followed by redistribution to internal organs such as the liver, which typically leads to internal organs having greater levels of metals than that observed in muscle tissue (Szebedinszky et al. 2001).
Risk Assessment
Our risk assessment for human health risks associated with the consumption of seafood from Liaodong Bay followed the approach established by the USEPA (1989, 2000) and developed for minimizing the risk of both cancer and noncancer end points resulting from the consumption of seafood. We determined the EDI on the basis of the metals levels in seafood and the amount of seafood ingested by individuals (Table 4). For Cd, EDI values exceeded the recommended daily dietary allowance for all of the shellfish other than the crab in both children and adults. The highest EDI value, with an EDI that was >100 times the reference dose, was obtained for adults consuming oysters. The very high level of Zn in our oysters also translated into EDI values for this metal greatly exceeding those for children and adults consuming oysters. In contrast, EDI values of Cr, Mn, Ni, Cu, and Pb in our study were lower than recommended daily dietary allowances for all of the shellfish species. For our finfish species, EDI values were lower than reference doses for both adults and children suggesting that it is safe to consume the two fish species. The EDI approach thus points out that the risks are especially pronounced for the consumption of oysters because a single contaminated oyster may contain more Cd and Zn than the recommended daily dietary allowance.
THQ values were <1 for Cr, Mn, Ni, and Pb in all of the species sampled (Table 5) indicating that the intake for these metals derived from the lifetime consumption of these species would not result in significant deleterious effects. In contrast, THQ values for Cd were >1 for the consumption of most species (all except the two fish and the crab) for children as well as adults. For Zn and Cu, THQ values exceeded 1 for both adults and children consuming oysters and veined rapa whelks. When the THQ for all of the analyzed metals was combined into TTHQ values, it was clear that only the consumption of the two fish and the crab species can be considered safe; TTHQ values ranged from approximately 2.79–152.9 and were again highest for the oyster and veined rapa whelk (Table 5).
Because Pb is the metal that poses generally the highest threat to human health (Agency for Toxic Substances and Disease Registry (ATSDR) 2013) and has been categorized as a human carcinogen (Vieira et al. 2011), we also calculated the TR derived from the intake of Pb. These TR values ranged from 1.18 × 10−8 to 1.08 × 10−5 among the different seafood species (Table 5). The carcinogenic risk was greater for adults than for children as a consequence of the adults’ greater food intake and longer life-stage length (and thus longer exposure time). Although Pb levels in our seafood species were relatively low, an added cancer risk in the 10−6 to 10−5 range should nevertheless not be ignored because a 10−5 value still translates into 10 additional cancers for a population of 1 million. Furthermore, Pb causes a variety of other health effects such as neurotoxicity and nephrotoxity (Vieira et al. 2011). The developing nervous system of children is especially sensitive to Pb exposure (Menezes et al. 2012). For adults, chronic exposure by way of the consumption of Pb-contaminated food has been associated with an increased incidence of a number of diseases including cardiovascular diseases and Alzheimer’s disease (Bakulski et al. 2014).
Conclusions
In summary, our data indicated that sediment along the Huludao coast of Liaodong Bay is highly polluted by Cd, Zn, and Pb and to a lesser extent by Cu. These metals were bioavailable as evident from increased metal levels in shellfish species. Metal levels varied among species, and Cd levels were highly increased in all five shellfish species. The oyster C. gigas and the veined rapa whelk R. venosa accumulated high levels of Cu, Zn, and Cd. Although the whelk accumulated metals, more extensive study is warranted to determine whether metal body burdens generally correspond to the spatial and temporal pattern of metal contamination. Our findings also show that ecological effects are likely from the high Cu, Zn, and Cd levels. Moreover, human health risks from the consumption of seafood, especially from eating oysters and whelks from this region, appear to be substantial. We recommend further study of this area’s metal pollution and the associated ecological effects to determine whether or not there is need for seafood-consumption advisories. Future work would benefit from larger sample sizes and speciation analysis of metals both in the abiotic media and in the concerned species. Information on the speciation of metals in the environment helps to better understand their bioavailability, whereas information on the metal’s specific form and compartmentalization in the organisms would be useful for assessing potential ecological and human risks (Bragigand et al. 2004; Rainbow and Smith 2010).
References
Allan R (1997) Introduction: mining and metals in the environment. J Geochem Explor 58:95–100
Bakulski KM et al (2014) Lead exposure, B vitamins, and plasma homocysteine in men 55 years of age and older: the VA Normative Aging Study. Environ Health Perspect 122:1066–1074
Beliaeff B, O’Connor TP, Claisse D (1998) Comparison of chemical concentrations in mussels and oysters from the United States and France. Environ Monit Assess 49:87–95
Bragigand V, Berthet B, Amiard JC, Rainbow PS (2004) Estimates of trace metal bioavailability to humans ingesting contaminated oysters. Food Chem Toxicol 42:1893–1902
Bryan GW (1968) Concentrations of zinc and copper in the tissues of decapod crustaceans. J Mar Biol Assoc UK 48:303
Chen MH, Chen CY (1999) Bioaccumulation of sediment-bound heavy metals in grey mullet, Liza macrolepis. Mar Pollut Bull 39:239–244
Copat C, Bella F, Castaing M, Fallico R, Sciacca S, Ferrante M (2012) Heavy metals concentrations in fish from Sicily (Mediterranean Sea) and evaluation of possible health risks to consumers. Bull Environ Contam Toxicol 88:78–83
Cui BS, Zhang QJ, Zhang KJ, Liu XH, Zhang HG (2011) Analyzing trophic transfer of heavy metals for food webs in the newly-formed wetlands of the Yellow River Delta, China. Environ Pollut 159:1297–1306
de Astudillo LR, Yen IC, Agard J, Bekele I, Hubbard R (2002) Heavy metals in green mussel (Perna viridis) and oysters (Crassostrea sp.) from Trinidad and Venezuela. Arch Environ Contam Toxicol 42:410–415
Diaz S, Martin-Gonzalez A, Gutierrez JC (2006) Evaluation of heavy metal acute toxicity and bioaccumulation in soil ciliated protozoa. Environ Int 32:711–717
Feng HA et al (2011) Metal contamination in sediments of the western Bohai Bay and adjacent estuaries, China. J Environ Manag 92:1185–1197
Fishbein L (1981) Sources, transport and alterations of metal compounds—an overview. 1. Arsenic, beryllium, cadmium, chromium, and nickel. Environ Health Perspect 40:43–64
Fu JJ et al (2013) Influence of e-waste dismantling and its regulations: temporal trend, spatial distribution of heavy metals in rice grains, and its potential health risk. Environ Sci Technol 47:7437–7445
Gao XL, Chen CTA (2012) Heavy metal pollution status in surface sediments of the coastal Bohai Bay. Water Res 46:1901–1911
Gao X, Zhou F, Chen C-TA (2014) Pollution status of the Bohai Sea: an overview of the environmental quality assessment related trace metals. Environ Int 62:12–30
Hakanson L (1980) An ecological risk index for aquatic pollution control—a sedimentological approach. Water Res 14:975–1001
Hare L, Tessier A, Borgmann U (2003) Metal sources for freshwater invertebrates: pertinence for risk assessment. Hum Ecol Risk Assess 9:779–793
Hu BQ, Li J, Zhao JT, Yang J, Bai FL, Dou YG (2013) Heavy metal in surface sediments of the Liaodong Bay, Bohai Sea: distribution, contamination, and sources. Environ Monit Assess 185:5071–5083
Joint FAO/WHO Expert Committee on Food Additives (1982) Evaluation of certain food additives and contaminants. Twenty-sixth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO technical report series no. 683. WHO, Geneva
Joint FAO/WHO Expert Committee on Food Additives (1989) Evaluation of certain food additives and contaminants. Twenty-sixth report of the joint FAO/WHO Expert Committee on Food Additives. WHO technical report series no. 776. WHO, Geneva
Joint FAO/WHO Expert Committee on Food Additives (2000) Evaluation of certain food additives and contaminants. Twenty-sixth report of the joint FAO/WHO Expert Committee on Food Additives. WHO technical report series no. 896. WHO, Geneva
Kimbrough KL, Lauenstein G, Christensen J, Apeti D (2008) An assessment of two decades of contaminant monitoring in the nation’s coastal zone. NOAA/National Centers for Coastal Ocean Science, Silver Spring
Liang LN, He B, Jiang GB, Chen DY, Yao ZW (2004) Evaluation of mollusks as biomonitors to investigate heavy metal contaminations along the Chinese Bohai Sea. Sci Total Environ 324:105–113
Menezes JA, Viana GFD, Paes CR (2012) Determinants of lead exposure in children on the outskirts of Salvador, Brazil. Environ Monit Assess 184:2593–2603
Müller G (1979) Schwermetalle in den Sedimenten des Rheins-Veränderungen seit. Umschau 79:778–783
Müller G (1981) Die Schwermetallbelastung der Sedimente des Neckars und seiner Nebenflusse: Eine Bestandsaufnahme. Chemiker Zeitung 105:157–164
Nadal M, Ferre-Huguet N, Marti-Cid R, Schuhmacher M, Domingo JL (2008) Exposure to metals through the consumption of fish and seafood by the population living near the Ebro River in Catalonia, Spain: Health risks. Hum Ecol Risk Assess 14:780–795
National Research Council (1989) National Research Council recommended dietary allowances, 10th edn. National Academy of Sciences, Washington, DC, pp 241–243
Pan K, Wang WX (2012) Trace metal contamination in estuarine and coastal environments in China. Sci Total Environ 421:3–16
Rainbow PS, Smith BD (2010) Trophic transfer of trace metals: subcellular compartmentalisation in bivalve prey and comparative assimilation efficiencies of two invertebrate predators. J Exp Mar Biol Ecol 390:143–148
Szebedinszky C, McGeer JC, McDonald DG, Wood CM (2001) Effects of chronic Cd exposure via the diet or water on internal organ-specific distribution and subsequent gill Cd uptake kinetics in juvenile rainbow trout (Oncorhynchus mykiss). Environ Toxicol Chem 20:597–607
Thiyagarajan D, Dhaneesh KV, Kumar TTA, Kumaresan S, Balasubramanian T (2012) Metals in fish along the southeast coast of India. Bull Environ Contam Toxicol 88:582–588
United States Environmental Protection Agency (USEPA) (1989) Risk assessment guidance for superfund. Volume I. Human health evaluation manual (Part A), Interim final. EPA 540/1–89/002. United States Environmental Protection Agency, Washington, DC
United States Environmental Protection Agency (USEPA) (2000) Guidance for assessing chemical contamination data for use in fish advisories. Volume II. Risk assessment and fish consumption limits. EPA/823-B94-004. United States Environmental Protection Agency, Washington, DC
United States Environmental Protection Agency (USEPA) (2007) Concepts, methods and data sources for cumulative health risk assessment of multiple chemicals, exposures and effects: A resource document; EPA/600/R-06/013F. National Center for Environmental Assessment, Office of Research and Development, United States Environmental Protection Agency, Cincinnati
United States Environmental Protection Agency (USEPA) (2010) Risk-based concentration table. http://www.epa.gov/reg3hwmd/risk/human/index.htm
Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M (2006) Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf 64:178–189
Vallee BL (1959) Biochemistry, physiology and pathology of zinc. Physiol Rev 39:443–490
Vieira C, Morais S, Ramos S, Delerue-Matos C, Oliveira MBPP (2011) Mercury, cadmium, lead and arsenic levels in three pelagic fish species from the Atlantic Ocean: intra- and inter-specific variability and human health risks for consumption. Food Chem Toxicol 49:923–932
Vodopivez C, Curtosi A, Villaamil E, Smichowski P, Pelletier E, Mac Cormack WP (2015) Heavy metals in sediments and soft tissues of the Antarctic clam Laternula elliptica: more evidence as a possible biomonitor of coastal marine pollution at high latitudes? Sci Total Environ 502:375–384
Wang WX, Fisher NS (1999) Assimilation efficiencies of chemical contaminants in aquatic invertebrates: a synthesis. Environ Toxicol Chem 18:2034–2045
Wang YW, Liang L, Shi JB, Jiang GB (2005) Study on the contamination of heavy metals and their correlations in mollusks collected from coastal sites along the Chinese Bohai Sea. Environ Int 31:1103–1113
Wang J, Chen S, Xia T (2010) Environmental risk assessment of heavy metals in Bohai Sea, North China. Procedia Environ Sci 2:1632–1642
WHO (1996) Guidelines for drinking water quality, vol 2, 2nd edn. World Health Organization, Geneva
Zhang ZH, Zhu MY, Wang ZL, Wang J (2006) Monitoring and managing pollution load in Bohai Sea, PR China. Ocean Coast Manag 49:706–716
Zheng N, Wang QC, Zhang XW, Zheng DM, Zhang ZS, Zhang SQ (2007a) Population health risk due to dietary intake of heavy metals in the industrial area of Huludao city, China. Sci Total Environ 387:96–104
Zheng N, Wang QC, Zheng DM (2007b) Health risk of Hg, Pb, Cd, Zn, and Cu to the inhabitants around Huludao zinc plant in china via consumption of vegetables. Sci Total Environ 383:81–89
Zheng N, Wang QC, Liang ZZ, Zheng DM (2008) Characterization of heavy metal concentrations in the sediments of three freshwater rivers in Huludao City, Northeast China. Environ Pollut 154:135–142
Zhou F, Guo HC, Hao ZJ (2007) Spatial distribution of heavy metals in Hong Kong’s marine sediments and their human impacts: a GIS-based chemometric approach. Mar Pollut Bull 54:1372–1384
Acknowledgments
The authors thank Professor Peiwen Liang from the South China Sea Fishery Research Institute and Fangcan Chen from the Pearl River Fisheries Research Institute, Chinese Academy of Fishery Sciences, for their help with species identification. This work was financially supported by National Natural Science Foundation of China Grant (NSFC Nos. 31270549 and 41303087) and the One-Hundred Talent program of the Chinese Academy of Sciences to L. Xie.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, M., Klerks, P.L., Wu, X. et al. Metal Concentrations in Sediment And Biota of the Huludao Coast in Liaodong Bay and Associated Human and Ecological Health Risks. Arch Environ Contam Toxicol 71, 87–96 (2016). https://doi.org/10.1007/s00244-016-0274-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-016-0274-8