Abstract
Urolithiasis is one of the oldest diseases affecting humans, while plants are one of our oldest companions providing food, shelter, and medicine. In spite of substantial progress in understanding the pathophysiological mechanisms, treatment options are still limited, often expensive for common people in most parts of the world. As a result, there is a great interest in herbal remedies for the treatment of urinary stone disease as an alternative or adjunct therapy. Numerous in vivo and in vitro studies have been carried out to understand the efficacy of herbs in reducing stone formation. We adopted PRISMA guidelines and systematically reviewed PubMed/Medline for the literature, reporting results of various herbal products on in vivo models of nephrolithiasis/urolithiasis. The Medical Subject Heading Terms (Mesh term) “Urolithiasis” was used with Boolean operator “AND” and other related Mesh Unique terms to search all the available records (July 2019). A total of 163 original articles on in vivo experiments were retrieved from PubMed indexed with the (MeshTerm) “Urolithiasis” AND “Complementary Therapies/Alternative Medicine, “Urolithiasis” AND “Plant Extracts” and “Urolithiasis” AND “Traditional Medicine”. Most of the studies used ethylene glycol (EG) to induce hyperoxaluria and nephrolithiasis in rats. A variety of extraction methods including aqueous, alcoholic, hydro-alcoholic of various plant parts ranging from root bark to fruits and seeds, or a combination thereof, were utilized. All the investigations did not study all aspects of nephrolithiasis making it difficult to compare the efficacy of various treatments. Changes in the lithogenic factors and a reduction in calcium oxalate (CaOx) crystal deposition in the kidneys were, however, considered favorable outcomes of the various treatments. Less than 10% of the studies examined antioxidant and diuretic activities of the herbal treatments and concluded that their antiurolithic activities were a result of antioxidant, anti-inflammatory, and/or diuretic effects of the treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urolithiasis, the development of stones/crystals in the urinary tract, has been affecting humans since the dawn of history, as evident from the discovery of kidney and bladder stone in Egyptian mummies [1]. Estimated lifetime risk of urolithiasis around 2–5% in Asia, 8–15% in America and Europe, and about 20% in the Middle East [2]. It is estimated that in the United States, the population living in high-risk zones for nephrolithiasis will grow from 40% in 2000 to 56% by 2050, and to 70% by 2095 [3]. These projections highlight the need for finding ways to reduce the occurrence of this disease and less costly tests and treatments. In addition, the age of onset is decreasing and the annual incidence of urolithiasis is increasing, which can be linked to changes in climate, lifestyles, and diets [4]. The rate of recurrence is also increasing. After one year of the first episode the rate of urolithiasis recurrence is around 10–23%, within 5–10 years it is around 50% and 75% in 20 years. After every episode the recurrence interval decreases and the subsequent relapse rate increases [2, 5].
Kidney stones are composed of crystals and an organic matrix and are named for their main crystalline constituents. Calcium oxalate (CaOx) alone or in association with calcium phosphate (CaP) is the major component of 75–80% of the kidneys stones. Other types of stones include struvite, cystine, uric acid, and ammonium acid urate. [6, 7].
Herbal medicines are a part of the so-called traditional medicine (TM), often termed as “complementary”, “alternative” or “non-conventional” medicine [8]. It refers to local knowledge, belief systems and therapeutic practices that are used in developing countries of Africa, South-East Asia and/or the Western Pacific. Complementary and alternative medicine (CAM) is used when referring to developed countries of Europe, North America and/or Australia [9]. The evolution of different systems of traditional medicine emerged as a result of the traditional knowledge of using herbs for various disorders. The use of herbal medicine in the Indo-Pakistani sub-continent has persisted for centuries and, medicinal plants and their extracts are widely used in the traditional (Unani/Ayurvedic) system of medicine. Complementary and alternative medicine (CAM) is a multibillion-dollars industry globally [10] and has achieved an exponential growth in the last two decades in industrialized countries. Recent research is now showing a remarkable increase in support for, and usage of, therapeutic practices outside mainstream medicine [11]. Many plants have been traditionally used to treat kidney stones and have been shown to be effective [12]. According to the World Health Organization, plants provide an economical and affordable source of drugs for three-quarter of the world population [13] and their therapeutic use is increasing [12]. Within the European Community, annual sales of the herbal medicine are around 7 billion USD, while in the USA, the sale of herbal products has increased from $200 million in 1988 to > $3.3 billion in 1997 [14]. In spite of such a high dependency, little scientific research has been carried. This gap between the use of herbal remedies and their scientific basis exists mainly due to the lack of active interactions between the modern health professionals and traditional healers, a lack of modern testing technology, and a shortage of qualified scientists in the field of natural products pharmacology. However, pharmaceutical research centers, universities and even pharmaceutical companies are beginning to fill this gap by bringing teams together and focusing research on phytomedicine [15, 16]. A number of reviews have been published in the past few years on the herbal and traditional medicines for urolithiasis ([8, 17,18,19,20], summarizing the results of research articles and commenting on both the potential and challenges. We reviewed the literature related to the rat models of CaOx nephrolithiasis emphasizing the variety of approaches utilized to prepare and deliver the medicine, methods used to induce the disease, anti-urolithic activities of various treatments and their proposed mechanisms of action. There is a great variation in methods of drug preparation and delivery. Every part of the plant has been ranging from seeds, flowers, fruits, leaves, bark, even whole plants and mixture of various plants have been utilized.
Methodology
Literature search
Systematic review of literature was performed according to PRISMA guidelines [21] (Supplementary Figure; PRISMA flow diagram). All publications were retrieved from PubMed in July 2019, with Medical Subject Heading (Mesh) terms; a new and thoroughly revised version of lists of subject headings compiled by National Library of Medicine (NLM) for its bibliographies and cataloguing. The Mesh term “Urolithiasis” (MeshUnique ID: D014545) was used with Boolean operator “AND” and other related Mesh terms “Complementary Therapies/Alternative Medicine; Mesh Unique ID: D000529”, “Plant Extracts; Mesh Unique ID: D010936, “Traditional Medicine; Mesh Unique ID: D008519 were used to search all the records available up to date, i.e. “Urolithiasis” AND “Complementary Therapies/Complementary Therapies”, “Urolithiasis” AND “Plant Extracts”, “Urolithiasis” AND “Traditional Medicine”.
Inclusion criteria
In vivo original research articles, PubMed Indexed journals, indexed with Mesh Terms as stated above, studies on rats model of CaOx renal stones.
Exclusion criteria
In vitro studies, mechanistic in vivo studies without antiurolithic effect, studies on bladder stone disease, review articles, and studies in languages than English.
Results
A total of 234 articles were extracted using Mesh terms “Urolithiasis” AND “Complementary Therapies” Or “Alternative Medicine” in advance search of PubMed (Supplement Prisma Flow Chart) Based on inclusion and exclusion criteria, a total of 90 articles of animal origin were retrieved. A total of 249 articles were extracted using Mesh terms “Urolithiasis” AND “Plant Extract” in advance search of PubMed. After selecting animal studies, a total of 142 were retrieved. A total of 52 articles were extracted using Mesh terms “Urolithiasis” AND “Medicine, Traditional” in advance search of PubMed. After selecting animal studies, a total of 20 were retrieved.
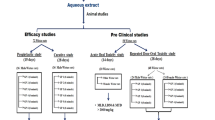
After combining all the articles and removing the duplicates, a total of 163 articles were left. After thoroughly screening and reading, another 27 articles were excluded due to unavailability of full text, while another 48 articles were excluded which were either review articles, written in languages other than English, studies involving stone induced by foreign body implantation, or studies focused on mechanisms of urolithiasis risk factors without any investigation of antiurolithic effect. A total of 88 publications were included in this systematic review. Data from all the eligible articles were independently extracted by two researchers and compiled in the form of tables and figures.
Tables 1, 2, 3, 4 show a systematic/chronological compilation of pharmacological studies conducted on aqueous (Table 1), hydroalcoholic (Table 2), alcoholic (Table 3), and other types of extract/formulation (Table 4) of medicinal plants. Each table provides information about the referenced study, year of study, common and scientific name of the plant, part of the plant used, type of extract, crystal inducing agent/model, and antiurolithic activity/mechanism. Reduced crystals deposition, improved renal morphology, reduced oxidative stress, change in urinary pH, decrease in lithogenic factors in urine such as calcium, oxalate, phosphate, increased urinary citrate, improved renal function, altered protein expression, and signs of diuresis were considered as signs of antiurolithic actions of the herbal extracts.
The majority of studies used 0.75–1% ethylene glycol (EG) in drinking alone [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37] or in combination of ammonium chloride [38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55] (Fig. 1). A small number of studies used other methods to induce CaOx nephrolithiasis. Several used EG along with zinc disk placed in the bladder of the rats for the development of a foreign body stone [56,57,58,59]. Others used a combination of gentamicin and calculi producing diet of 5% ammonium oxalate [60, 61], or intraperitoneal injection of sodium oxalate to the Wistar rats [62]. Introduction of CaOx pellet into the bladders of adult male Wistar rats [63], or 3% glycolate diet for 4 weeks [27], or daily intra-abdominal injection of glyoxylate (80 mg/kg) for 12 days [64], or insertion of a series of 15 knots of 5–0 chromic catgut into the urinary bladder [65] were some of the other methods employed. One study employed subcutaneous injection of gentamicin in addition to 5% ammonium oxalate in the rat diet [66]. Other studies involved a single dose of 200 mg glycolic acid given orally [67], implantation of a CaOx seed into the bladder of male Wistar rats [68], 3% glycolic acid mixed with food for 45 days [69], EG and 1 alpha(OH)D3 and 1 alpha-D3-induced calcium oxalate nephrolithiasis [70], high protein diet [71], and administration of glycollic acid [72].
As illustrated in Figs. 2 and 3 most of the studies utilized aqueous extracts [22,23,24,25,26,27,28,29,30,31,32, 35,36,37,38,39,40, 64, 67, 73,74,75,76,77,78,79], and leaves [29, 30, 33, 35, 38, 40, 44, 49, 66, 80, 81] were the most commonly utilized herbal preparation. Other parts used were fruits [25, 28, 35, 41, 82,83,84,85,86], roots [23, 26, 29, 37, 64, 87,88,89], seeds [32, 50, 90,91,92,93,94,95], rhizome [31, 53, 96,97,98], stem [22, 24, 41], flowers [39, 46], aerial part [23, 42, 45, 47, 76, 99, 100], in addition to other parts such as essential oil of seed [73] or root [73]. Some studies used whole plants [77,78,79, 101, 102], fronds [43], root bark [52, 103], bark [72], herbal tea [27], rose calyx [75], capitulum [104], pulp [105], herbal medication (Tutukon) [106], spores [107], polyphenolic compounds [108], diosmin: a flavanone glycoside [109], or poly-herbal formulations such as Cystone [110], polyherbal ayurvedic Gokshuradi [55], Mallorcan: a folk herbal extract [111], Wulingsan: a traditional Chinese herbal antilithic formula [112], Choreito [113], Kampou medicines like Takusha [114] and Takusva [70, 115], Zhulingtang (a traditional Chinese herbal formula) [116], Choreito (a herbal preparation), and urajirogashi (a herb) [113].
Other methods of extraction included hydroalcoholic [22,23,24,25, 27,28,29,30,31,32, 35, 36, 38,39,40, 64, 67, 73, 74, 76,77,78,79, 93], or alcoholic [29, 33, 47, 49, 60, 80, 83, 88, 90, 95, 99, 102, 105,106,107, 117], while some studies utilized formulations/constituents like infusions [104], decoctions [61, 69, 85, 118, 119], ultrahigh diluted homeopathic preparation [103], coconut water [120], polysaccharides [100], saponin-rich fraction [84], herbal powder [72, 81, 89, 92, 119], isolated constituents [28], pasteurized juice [91], juice [66, 86] or triterpenoids extracted from plant [121] (Fig. 2).
Calcium oxalate nephrolithiasis in rat models is associated with changes in urinary oxalate, citrate, pH, markers of oxidative stress, production of crystallization modulators and inflammatory molecules, crystalluria and CaOx crystal deposition in the kidneys [54]. All studies did not investigate all the above-mentioned changes associated with nephrolithiasis. But all of them examined crystal deposition in the kidneys and a reduction in crystal deposition was considered a successful outcome of the treatment (Fig. 4). Signs of improved renal structure and functions were reported by most of the studies. Changes in urinary excretion of calcium, oxalate, magnesium, and phosphate were also reported by most studies. Less than 20% of the studies reported changes in urinary pH, citrate, oxidative stress, altered protein expression, and diuresis.
Discussion
It is important for any drug development to understand the pathogenesis of a disease, in this case, kidney stones. Nephrolithiasis, formation of stones in the kidneys, is a complex, multifactorial, and multistep process resulting from physicochemical changes in the urinary environment, leading to crystal nucleation, growth aggregation, and retention at specific sites within the kidneys [2, 122, 123]. Interacting urinary ions and a variety of crystallization modulatory macromolecules are involved [124,125,126,127]. Most of the idiopathic CaOx stones develop over a foundation of biological apatite, called Randall’s plaque (RP). The plaque starts in the renal papillary interstitium, which grows outward to the papillary surface and becomes exposed to the pelvic urine once surface epithelium/urothelium disintegrates [128]. Some stones, however also form attached to tubular crystal deposits seen plugging the terminal collecting ducts [128, 129]. Thus kidney stones are crystal deposits in the kidneys, but all renal crystalline deposits are not kidney stones.
Several in vitro and in vivo models have been established to explore the pathogenesis of CaOx nephrolithiasis and to develop therapeutic agents and protocols for testing their efficacies [122, 130, 131]. In the in vitro crystallization experiments, calcium oxalate crystal nucleation, growth, and aggregation are investigated in the absence and presence of the crystallization modulators [132, 133]. These in vitro methods give an easy and quick estimate of crystallization modifying activity, preliminary screening for anti-urolithic activity and possible mode(s) of action. However, the biological system and the pathogenesis of urolithiasis is too complex and these in vitro results cannot be safely extrapolated for the therapeutic effect [131]. Therefore, in vivo animal models of CaOx nephrolithiasis to better understand the pathogenesis of nephrolithiasis and investigate the anti-urolithic actions and potential of various drugs have been developed [134].
Experimental nephrolithiasis is produced by the administration of hyperoxaluria inducing agents through diet, drinking water or injection [79, 122, 135]. These in vivo models have a great contribution in understanding of several human pathologies and will remain as a main tool for providing methods and designs to the researcher for the countless biochemical events, physiological processes, and testing of novel pharmaco-therapeutic agents [136]. Investigators have been utilizing these models of hyperoxaluric rats for decades despite obvious differences between rats and human kidneys. The rat kidneys are smaller weighing around 0.75–1.2 g, measuring 1.6 × 1 × 0.9 cm, and are unipapillate, while human kidneys weigh approx.. 170 g, measure 11 × 6 × 2 cm and are multi-papillary, however, despite the gross differences, the cortex–medulla ratio (2:1) of rat’s kidney is similar to human’s [137]. The majority of the studies of herbal use discussed here have utilized the rat model of nephrolithiasis by giving EG in their drinking water alone [33, 34] or with an addition of ammonium chloride [79, 138, 139]. EG, a precursor of oxalic acid, quickly absorbed from the GIT and metabolized to oxalic acid by hepatic enzymes. EG mostly affects the kidneys with considerable variability in sensitivity across the strains, species, and sexes. Rats are more sensitive than mice and male rats are more sensitive than females. EG (0.75–1%) alone gives variable results of CaOx deposition [140]. To achieve a uniformly high rate of renal crystal deposition and reducing the time required for crystal deposition, a pH reducing, hypercalciuric or nephrotoxic protocol such as ammonium chloride (AC) [140, 141], vitamin D3 [121], gentamicin [142] or a magnesium-deficient diet has been used in combination with EG. However, treatment with AC 1% or more cannot be extended beyond 4–5 days as rats become sick, lose body weight and drink less water [140]. Hydroxy-L-proline (HLP), a physiologic precursor of oxalate is also used for induction of hyperoxaluria in the rats by oral administration or by intraperitoneal injection [54, 143]. Other hyperoxaluric rat models are produced by inbreeding of hyperoxaluric progeny [144, 145], the administration of sodium oxalate [146], or glycolic acid [147]. In addition, several other models of nephrolithiasis have been developed such as transgenic mouse with selective knockout (KO) of osteopontin (OPN) [148] and Tamm–Horsefall protein (THP) [149], oxalate, sodium phosphate [150], and cysteine transporter [151]. KO mouse model and fly model of CaOx crystals have also been developed. Despite the genomic advantages of the mice model, its overall accuracy and consistency in relation to human kidney stone disease remains controversial among researchers [152]. However, the hyperoxaluric rat model of nephrolithiasis represents a well- established and relatively economic model for the study of urolithiasis [134]. When EG is given at a concentration of 0.75% or above in drinking water, rats developed hyperoxaluria, leading to crystalluria and CaOx crystal deposition in kidney’s tubules [122]. Frequency of crystal deposition in kidney range from 80 to 100% of the kidney’s area, depending upon the co-administered drug [121, 153] and it takes about 1–3 weeks to develop nephrolithiasis. The prominent features of hyperoxaluria-induced nephrolithiasis include development of oxidative stress in the kidneys, high water intake and polyuria, decreased urinary Ca++, Mg++, and citrate contents, lower urinary pH, increased phosphate excretion, renal hypertrophy, CaOx crystalluria, and loss of body weight [54, 79, 121]. Other signs of renal damage are increased urinary protein loss, lower creatinine clearance, and raised serum creatinine and blood urea nitrogen (BUN) [54, 154]. All rats, as well as other animal models of hyperoxaluria such as Drosophila [155, 156] and pig [157, 158], do not produce kidney stones similar to idiopathic CaOx stones of human. They produce intratubular deposits of CaOx crystals.
Both animal model and tissue culture studies have shown that high oxalate and CaOx as well as CaP crystals cause overproduction of reactive oxygen species (ROS) by the renal epithelial cells leading to renal injury [159, 160]. Signs of oxidative stress have also been reported from clinical studies [159, 161]. Reactive oxygen species also regulate the production of various crystallization modulators which are known to inhibit crystal nucleation, growth, and aggregation [162]. These crystallization modulators also participate in the cellular inflammatory cascade. In addition, the ROS may stimulate osteogenic changes in the renal epithelial [163], and/or vascular endothelial cells. Antioxidants are shown to reduce hyperoxaluria and crystal-associated injury [164]. Herbal treatments of hyperoxaluric rats appear to reduce the oxidative stress, thereby reducing renal epithelial injury, membrane-associated crystal nucleation, and crystal deposition within the renal tubules and kidneys.
A significant majority of studies reviewed here utilized the most well-recognized model of experimental CaOx nephrolithiasis, administration of ethylene glycol through diet. Eight different parts of the plants, including flowers, seeds, fruits, leaves, stems, roots, rhizomes, were used to prepare the herbal treatments. Leaves were the most common ingredient. Aqueous, alcoholic, and hydro-alcoholic solvents were employed to prepare the treatments. Common indicators of nephrolithiasis, such as renal CaOx crystal deposits, markers of oxidative stress, urinary calcium, citrate, oxalate, phosphate, and pH, in addition to improved renal structure and functions were assessed to determine the efficacy of herbal administration. Unfortunately, all studies did not investigate all of these aspects of nephrolithiasis. However, all the treatments resulted in reduction of CaOx crystal deposition in the kidneys. Majority of studies showed an improvement in both the structure and function of the kidneys following herbal treatments. Studies that determined oxidative stress found that the herbal treatments had antioxidant properties.
Based upon the available data, we can visualize the following sequence of events when hyperoxaluric rats are treated with the diverse herbal preparations. Under normal circumstances, renal epithelial cells produce sufficient ROS to regulate various metabolic processes including inhibition of crystallization by the production of needed macromolecular inhibitors. Crystals, if formed due to the increased intake of calcium and oxalate and the decreased water in the urine, stay small and un-aggregated. Cell membranes are intact and do not promote crystal adherence thereby reducing chances of their retention within the tubules. Crystals, if any, move freely with the urine and discharged during urination (Fig. 5a). The administration of hyperoxaluric agents such as EG alone or in combination with other agents, as discussed above, leads to the production of abnormal urine with high oxalate, low citrate and in the case with vitamin D, increased calcium. Epithelial cells respond by producing access ROS. Cells are injured and respond aberrantly, producing inactive molecular inhibitors or macromolecules that promote crystal nucleation instead of reducing it. Cell membranes promote crystal nucleation and aggregation even under lower supersaturation promoting crystal deposition within the tubules. Herbal preparations contain compounds which diminish the production of ROS or/and have antioxidants that neutralize the ROS, leading to a reduction in cell injury and direction towards normalization. As a result, there is a reduction in crystal deposition in the kidneys as has been reported by all the studies undertaken to date (Fig. 5b).
Diagrammatic representation of crystallization within the kidneys. The (A) side represents normal urinary conditions. Occasional changes in the urinary calcium (Ca), oxalate (Ox), citrate (Cit), conducive to crystallization lead to the production of reactive oxygen species (ROS) sufficient to generate crystallization inhibitors. Small crystals are formed which are urinated out. The (B) side denotes abnormal and lithogenic urinary conditions. Persistently high Ca and/or Ox, and low Cit causes overproduction of ROS, injuring the renal tubular epithelium. Cells die, release membrane vesicles promoting crystal nucleation and aggregation. Production of active inhibitors is affected thereby crystals grow unimpeded. Herbal therapies appear to act either on the production of ROS or as their neutralizers. These actions will reduce cell injury and influence the production of active/normal macromolecular inhibitors resulting in reduced crystallization, the formation of small un-aggregated crystals which should easily pass during urination
Conclusions and future direction
Idiopathic CaOx stones form by the deposition and growth of CaOx crystals on Randall’s plaques or the Randall’s plugs. Plaques are subepithelial deposits of CaP on renal papillae, originating deep inside the medullary/papillary interstitium. We do not yet fully understand the pathogenesis of plaques, which may involve both tubular and vascular inflammation. Plugs are crystal deposits within the terminal collecting ducts and form as the result of urinary supersaturation and crystal attachment to injured renal tubular epithelium. Most of the plugs are also made of CaP. Reactive oxygen species are likely involved in both the plaque and plug formation because they play a significant role in the production of crystallization modulators, tubular epithelial injury as well as inflammation. Tubular injury produces cellular debris that can promote heterogeneous nucleation of CaP and also provides sites for their adherence and retention within the tubules. Crystallization modulators affect crystal formation by promoting or inhibiting their formation and growth. Animal model studies show that herbal treatments lead to the production of antioxidants, which may be responsible for their positive role in stopping crystal formation/deposition in the animal kidneys and may reduce stone recurrence when given to stone patients.
Where do the recurrent stones form? Do the new stones form on the remnant of the plaque or plug left after stone has passed? Or do they form on the new plaques and new plugs? Whatever the case, herbal treatment with antioxidant properties can decrease stone recurrence by reducing the crystallization, which shall reduce crystal deposition on the plaques and plugs. In addition, the treatments may reduce crystal formation and deposition in the terminal collecting ducts and the formation of new plugs by reducing epithelial injury. It is unclear whether herbal treatments would have any effect on the formation and deposition of CaP in the renal interstitium and the initiation of the Randall’s plaque. It is however possible that herbal treatment stops disintegration of the urothelium and exposure of the sub-urothelial CaP deposits on the papillary surface to the pelvic urine, thereby lessening the possibility of a new stone development.
Great progress has been made in developing and understanding of the mode of action of herbal extracts/preparations as a therapy for stone recurrence. But the range of methodologies used for inducing nephrolithiasis, and in preparing and delivering the extracts does not allow for the comparison between various treatments. In addition, many studies do not consider all aspects of nephrolithiasis. There is also a dearth of knowledge about the bioactive components of various herbal preparations, and their safety profile. What is the effect of extraction method on the bioactive compounds, their quality and quantity? Many of the herbal/alternative medicines are shown to be injurious to the kidneys [19, 20]. Future studies are needed to determine the active compounds present in the herbal preparations and whether those compounds reach the kidneys and urine, and are nontoxic to the kidneys. Researchers should agree upon a panel of features to be investigated to determine the efficacy of a treatment. The herbal treatment should induce changes in the urinary environment that reduce supersaturation, inhibit crystallization, and eventual crystal deposition in the kidneys. It should have antioxidant properties and be able to reduce the production of reactive oxygen species. It should be nontoxic and improve renal structure and functions.
References
Lopez M, Hoppe B (2008) History, epidemiology and regional diversities of urolithiasis. Pediatr Nephrol
Pak CY (1998) Kidney stones. Lancet 351(9118):1797–1801
Brikowski TH, Lotan Y, Pearle MS (2008) Climate-related increase in the prevalence of urolithiasis in the United States. Proc Natl Acad Sci U S A 105(28):9841–9846. https://doi.org/10.1073/pnas.0709652105
Pedro RN, Aslam AU, Bello JO, Bhatti KH, Philipraj J, Sissoko I, Vasconcellos GS, Trinchieri A, Buchholz N (2020) Nutrients, vitamins, probiotics and herbal products: an update of their role in urolithogenesis. Urolithiasis:1–17
Moe OW, Huang CL (2006) Hypercalciuria from acid load: renal mechanisms. J Nephrol 19(Suppl 9):S53-61
Lemann J Jr (1993) Composition of the diet and calcium kidney stones. N Engl J Med 328(12):880–882. https://doi.org/10.1056/NEJM199303253281212
Evan AP (2010) Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatr Nephrol 25(5):831–841. https://doi.org/10.1007/s00467-009-1116-y
Butterweck V, Khan SR (2009) Herbal medicines in the management of urolithiasis: alternative or complementary? Planta Med 75(10):1095–1103. https://doi.org/10.1055/s-0029-1185719
World Health O (2002) WHO Traditional Medicine Strategy 2002–2005. World Health Organization Geneva, Switzerland
Eastwood HL (2000) Complementary therapies: the appeal to general practitioners. Med J Aust 173(2):95–98
Tovey PA, Broom AF, Chatwin J, Ahmad S, Hafeez M (2005) Use of traditional, complementary and allopathic medicines in Pakistan by cancer patients. Rural Remote Health 5(4):447
Hollenberg D, Zakus D, Cook T, Xu XW (2008) Re-positioning the role of traditional, complementary and alternative medicine as essential health knowledge in global health: do they still have a role to play? World Health Popul 10(4):62–75
Gilani AH, Rahman AU (2005) Trends in ethnopharmocology. J Ethnopharmacol 100(1–2):43–49. https://doi.org/10.1016/j.jep.2005.06.001
Mahady GB (2001) Global harmonization of herbal health claims. J Nutr 131(3s):1120S-1123S
Bashir S, Memon R, Gilani AH (2011) Antispasmodic and Antidiarrheal Activities of Valeriana hardwickii Wall. Rhizome Are Putatively Mediated through Calcium Channel Blockade. Evidence-based complementary and alternative medicine: eCAM 2011:304960. doi:https://doi.org/10.1155/2011/304960
Gilani A, Molla N, Rahman AU, Shah BH (1992) Phytotherapy - the role of natural products in modern medicine. J Pharm Med 2:111–118
Kasote DM, Jagtap SD, Thapa D, Khyade MS, Russell WR (2017) Herbal remedies for urinary stones used in India and China: A review. J Ethnopharmacol 203:55–68. https://doi.org/10.1016/j.jep.2017.03.038
Miyaoka R, Monga M (2009) Use of traditional Chinese medicine in the management of urinary stone disease. Int Braz J Urol 35(4):396–405. https://doi.org/10.1590/s1677-55382009000400002
Colson CR, De Broe ME (2005) Kidney injury from alternative medicines. Advances in chronic kidney disease 12(3):261–275. https://doi.org/10.1016/j.ackd.2005.03.006
Brown AC (2017) Kidney toxicity related to herbs and dietary supplements: Online table of case reports. Part 3 of 5 series. Food Chem Toxicol 107 (Pt A):502–519. doi:https://doi.org/10.1016/j.fct.2016.07.024
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62(10):e1–e34
Azaryan E, Malekaneh M, Shemshadi Nejad M, Haghighi F (2017) Therapeutic Effects of Aqueous Extracts of Cerasus Avium Stem on Ethylene Glycol- Induced Kidney Calculi in Rats. Urol J 14(4):4024–4029
Saeidi J, Bozorgi H, Zendehdel A, Mehrzad J (2012) Therapeutic effects of aqueous extracts of Petroselinum sativum on ethylene glycol-induced kidney calculi in rats. Urol J 9(1):361–366
Manjula K, Rajendran K, Eevera T, Kumaran S (2012) Effect of Costus igneus stem extract on calcium oxalate urolithiasis in albino rats. Urol Res 40(5):499–510. https://doi.org/10.1007/s00240-012-0462-6
Gadge NB, Jalalpure SS (2012) Curative treatment with extracts of Bombax ceiba fruit reduces risk of calcium oxalate urolithiasis in rats. Pharm Biol 50(3):310–317. https://doi.org/10.3109/13880209.2011.604332
Aggarwal A, Singla SK, Gandhi M, Tandon C (2012) Preventive and curative effects of Achyranthes aspera Linn. extract in experimentally induced nephrolithiasis. Indian J Exp Biol 50 (3):201–208
Woottisin S, Hossain RZ, Yachantha C, Sriboonlue P, Ogawa Y, Saito S (2011) Effects of Orthosiphon grandiflorus, Hibiscus sabdariffa and Phyllanthus amarus extracts on risk factors for urinary calcium oxalate stones in rats. J Urol 185(1):323–328. https://doi.org/10.1016/j.juro.2010.09.003
Vanachayangkul P, Chow N, Khan SR, Butterweck V (2011) Prevention of renal crystal deposition by an extract of Ammi visnaga L. and its constituents khellin and visnagin in hyperoxaluric rats. Urological research 39 (3):189–195. doi:https://doi.org/10.1007/s00240-010-0333-y
Geetha K, Manavalan R, Venkappayya D (2010) Control of urinary risk factors of stone formation by Salvadora persica in experimental hyperoxaluria. Methods Find Exp Clin Pharmacol 32(9):623–629. https://doi.org/10.1358/mf.2010.32.9.1549114
Moriyama MT, Suga K, Miyazawa K, Tanaka T, Higashioka M, Noda K, Oka M, Tanaka M, Suzuki K (2009) Inhibitions of urinary oxidative stress and renal calcium level by an extract of Quercus salicina Blume/Quercus stenophylla Makino in a rat calcium oxalate urolithiasis model. Int J Urol 16(4):397–401. https://doi.org/10.1111/j.1442-2042.2009.02268.x
Atmani F, Sadki C, Aziz M, Mimouni M, Hacht B (2009) Cynodon dactylon extract as a preventive and curative agent in experimentally induced nephrolithiasis. Urol Res 37(2):75–82. https://doi.org/10.1007/s00240-009-0174-8
Laroubi A, Touhami M, Farouk L, Zrara I, Aboufatima R, Benharref A, Chait A (2007) Prophylaxis effect of Trigonella foenum graecum L. seeds on renal stone formation in rats. Phytother Res 21 (10):921–925. doi:https://doi.org/10.1002/ptr.2190
Sikarwar I, Dey YN, Wanjari MM, Sharma A, Gaidhani SN, Jadhav AD (2017) Chenopodium album Linn. leaves prevent ethylene glycol-induced urolithiasis in rats. J Ethnopharmacol 195:275–282. https://doi.org/10.1016/j.jep.2016.11.031
Panigrahi PN, Dey S, Sahoo M, Dan A (2017) Antiurolithiatic and antioxidant efficacy of Musa paradisiaca pseudostem on ethylene glycol-induced nephrolithiasis in rat. Indian J Pharmacol 49(1):77–83. https://doi.org/10.4103/0253-7613.201026
Partovi N, Ebadzadeh MR, Fatemi SJ, Khaksari M (2018) Effect of fruit extract on renal stone formation and kidney injury in rats. Nat Prod Res 32(10):1180–1183. https://doi.org/10.1080/14786419.2017.1320790
Velu V, Das M, Raj NA, Dua K, Malipeddi H (2017) Evaluation of in vitro and in vivo anti-urolithiatic activity of silver nanoparticles containing aqueous leaf extract of Tragia involucrata. Drug Deliv Transl Res 7(3):439–449. https://doi.org/10.1007/s13346-017-0363-x
Li X, Wang W, Su Y, Yue Z, Bao J (2017) Inhibitory effect of an aqueous extract of Radix Paeoniae Alba on calcium oxalate nephrolithiasis in a rat model. Ren Fail 39(1):120–129. https://doi.org/10.1080/0886022X.2016.1254658
Saremi J, Kargar Jahromi H, Pourahmadi M (2018) Effect of Polygonum Aviculare L. on Nephrolithiasis Induced by Ethylene Glycol and Ammonium Chloride in Rats. Urol J 15 (3):79–82. doi:https://doi.org/10.22037/uj.v0i0.3815
Das P, Kumar K, Nambiraj A, Rajan R, Awasthi R, Dua K, M H, (2017) Potential therapeutic activity of Phlogacanthus thyrsiformis Hardow (Mabb) flower extract and its biofabricated silver nanoparticles against chemically induced urolithiasis in male Wistar rats. Int J Biol Macromol 103:621–629. https://doi.org/10.1016/j.ijbiomac.2017.05.096
Saremi J, Kargar-Jahroomi H, Poorahmadi M (2015) Effect of Malva Neglecta Wallr on Ethylene Glycol Induced Kidney Stones. Urol J 12(6):2387–2390
Kumar BN, Wadud A, Jahan N, Sofi G, Bano H, Makbul SA, Husain S (2016) Antilithiatic effect of Peucedanum grande C. B. Clarke in chemically induced urolithiasis in rats. J Ethnopharmacol 194:1122–1129. https://doi.org/10.1016/j.jep.2016.10.081
Xiang S, Zhou J, Li J, Wang Q, Zhang Q, Zhao Z, Zhang L, Chen Z, Wang S (2015) Antilithic effects of extracts from different polarity fractions of Desmodium styracifolium on experimentally induced urolithiasis in rats. Urolithiasis 43(5):433–439. https://doi.org/10.1007/s00240-015-0795-z
Ahmed A, Wadud A, Jahan N, Bilal A, Hajera S (2013) Efficacy of Adiantum capillus veneris Linn in chemically induced urolithiasis in rats. J Ethnopharmacol 146(1):411–416. https://doi.org/10.1016/j.jep.2013.01.011
Khalili M, Jalali MR, Mirzaei-Azandaryani M (2012) Effect of hydroalcoholic extract of Hypericum perforatum L. leaves on ethylene glycol-induced kidney calculi in rats. Urol J 9 (2):472–479
Ingale KG, Thakurdesai PA, Vyawahare NS (2012) Effect of Hygrophila spinosa in ethylene glycol induced nephrolithiasis in rats. Indian J Pharmacol 44(5):639–642. https://doi.org/10.4103/0253-7613.100402
Bayir Y, Halici Z, Keles MS, Colak S, Cakir A, Kaya Y, Akcay F (2011) Helichrysum plicatum DC. subsp. plicatum extract as a preventive agent in experimentally induced urolithiasis model. J Ethnopharmacol 138 (2):408–414. doi:https://doi.org/10.1016/j.jep.2011.09.026
Zhang H, Li N, Li K, Li P (2014) Protective effect of Urtica dioica methanol extract against experimentally induced urinary calculi in rats. Mol Med Rep 10(6):3157–3162. https://doi.org/10.3892/mmr.2014.2610
Yousefi Ghale-Salimi M, Eidi M, Ghaemi N, Khavari-Nejad RA (2018) Antiurolithiatic effect of the taraxasterol on ethylene glycol induced kidney calculi in male rats. Urolithiasis 46(5):419–428. https://doi.org/10.1007/s00240-017-1023-9
Das M, Malipeddi H (2016) Antiurolithiatic activity of ethanol leaf extract of Ipomoea eriocarpa against ethylene glycol-induced urolithiasis in male Wistar rats. Indian J Pharmacol 48(3):270–274. https://doi.org/10.4103/0253-7613.182886
Khan A, Khan SR, Gilani AH (2012) Studies on the in vitro and in vivo antiurolithic activity of Holarrhena antidysenterica. Urol Res 40(6):671–681. https://doi.org/10.1007/s00240-012-0483-1
Khan A, Bashir S, Khan SR, Gilani AH (2011) Antiurolithic activity of Origanum vulgare is mediated through multiple pathways. BMC Complement Altern Med 11:96. https://doi.org/10.1186/1472-6882-11-96
Bashir S, Gilani AH, Siddiqui AA, Pervez S, Khan SR, Sarfaraz NJ, Shah AJ (2010) Berberis vulgaris root bark extract prevents hyperoxaluria induced urolithiasis in rats. Phytother Res 24(8):1250–1255. https://doi.org/10.1002/ptr.3196
Bashir S, Gilani AH (2009) Antiurolithic effect of Bergenia ligulata rhizome: an explanation of the underlying mechanisms. J Ethnopharmacol 122(1):106–116. https://doi.org/10.1016/j.jep.2008.12.004
Khan SR, Glenton PA, Byer KJ (2006) Modeling of hyperoxaluric calcium oxalate nephrolithiasis: experimental induction of hyperoxaluria by hydroxy-L-proline. Kidney Int 70(5):914–923. https://doi.org/10.1038/sj.ki.5001699
Shirfule AL, Racharla V, Qadri S, Khandare AL (2013) Exploring antiurolithic effects of gokshuradi polyherbal ayurvedic formulation in ethylene-glycol-induced urolithic rats. Evidence-Based Complementary and Alternative Medicine 2013
Yuruk E, Tuken M, Sahin C, Kaptanagasi AO, Basak K, Aykan S, Muslumanoglu AY, Sarica K (2016) The protective effects of an herbal agent tutukon on ethylene glycol and zinc disk induced urolithiasis model in a rat model. Urolithiasis 44(6):501–507. https://doi.org/10.1007/s00240-016-0889-2
Vargas Solis R, Perez Gutierrez RM (2002) Diuretic and urolithiatic activities of the aqueous extract of the fruit of Randia echinocarpa on rats. J Ethnopharmacol 83(1–2):145–147
Vargas R, Perez RM, Perez S, Zavala MA, Perez C (1999) Antiurolithiatic activity of Raphanus sativus aqueous extract on rats. J Ethnopharmacol 68(1–3):335–338
Araújo Viel T, Diogo Domingos C, da Silva Monteiro AP, Riggio Lima-Landman MT, Lapa AJ, Souccar C (1999) Evaluation of the antiurolithiatic activity of the extract of Costus spiralis Roscoe in rats. J Ethnopharmacol 66(2):193–198
Sujatha D, Singh K, Vohra M, Kumar KV, Sunitha S (2015) Antilithiatic Activity of phlorotannin rich extract of Sarghassum Wightii on Calcium Oxalate Urolithiais - InVitro and In Vivo Evaluation. Int Braz J Urol 41(3):511–520. https://doi.org/10.1590/S1677-5538.IBJU.2014.0357
Mi J, Duan J, Zhang J, Lu J, Wang H, Wang Z (2012) Evaluation of antiurolithic effect and the possible mechanisms of Desmodium styracifolium and Pyrrosiae petiolosa in rats. Urol Res 40(2):151–161. https://doi.org/10.1007/s00240-011-0401-y
Orhan N, Onaran M, Sen I, Isik Gonul I, Aslan M (2015) Preventive treatment of calcium oxalate crystal deposition with immortal flowers. J Ethnopharmacol 163:60–67. https://doi.org/10.1016/j.jep.2015.01.009
Brancalion AP, Oliveira RB, Sousa JP, Groppo M, Berretta AA, Barros ME, Boim MA, Bastos JK (2012) Effect of hydroalcoholic extract from Copaifera langsdorffii leaves on urolithiasis induced in rats. Urol Res 40(5):475–481. https://doi.org/10.1007/s00240-011-0453-z
Ghalayini IF, Al-Ghazo MA, Harfeil MN (2011) Prophylaxis and therapeutic effects of raspberry (Rubus idaeus) on renal stone formation in Balb/c mice. Int Braz J Urol 37 (2):259–266; discussion 267. doi:https://doi.org/10.1590/s1677-55382011000200013
Portilla-de Buen E, Ramos L, Aguilar A, Ramos A, Garcia-Martinez D, Cardenas A, Rodriguez-Reynoso S, Leal C (2008) [Larrea tridentata. Effect on a non-metabolic model of urolithiasis in rats]. Rev Med Inst Mex Seguro Soc 46 (5):519–522
Doddola S, Pasupulati H, Koganti B, Prasad KV (2008) Evaluation of Sesbania grandiflora for antiurolithiatic and antioxidant properties. J Nat Med 62(3):300–307. https://doi.org/10.1007/s11418-008-0235-2
Al-Ghamdi SS, Al-Ghamdi AA, Shammah AA (2007) Inhibition of calcium oxalate nephrotoxicity with Cymbopogon schoenanthus (Al-Ethkher). Drug Metab Lett 1(4):241–244. https://doi.org/10.2174/187231207783221420
Barros ME, Lima R, Mercuri LP, Matos JR, Schor N, Boim MA (2006) Effect of extract of Phyllanthus niruri on crystal deposition in experimental urolithiasis. Urol Res 34(6):351–357. https://doi.org/10.1007/s00240-006-0065-1
Christina AJM, Priya Mole M, Moorthy P (2002) Studies on the antilithic effect of Rotula aquatica lour in male Wistar rats. Methods Find Exp Clin Pharmacol 24(6):357–359
Koide T, Yamaguchi S, Utsunomiya M, Yoshioka T, Sugiyama K (1995) The inhibitory effect of kampou extracts on in vitro calcium oxalate crystallization and in vivo stone formation in an animal model. Int J Urol 2(2):81–86
Grases F, Ramis M, Costa-Bauzá A, March JG (1995) Effect of Herniaria hirsuta and Agropyron repens on calcium oxalate urolithiasis risk in rats. J Ethnopharmacol 45(3):211–214
Varalakshmi P, Shamila Y, Latha E (1990) Effect of Crataeva nurvala in experimental urolithiasis. J Ethnopharmacol 28(3):313–321
Benhelima A, Kaid-Omar Z, Hemida H, Benmahdi T, Addou A (2016) Nephroprotective and Diuretic Effect of Nigella Sativa L Seeds Oil on Lithiasic Wistar Rats. Afr J Tradit Complement Altern Med 13(6):204–214. https://doi.org/10.21010/ajtcam.v13i6.30
Nishihata M, Kohjimoto Y, Hara I (2013) Effect of Kampo extracts on urinary stone formation: an experimental investigation. International journal of urology : official journal of the Japanese Urological Association 20(10):1032–1036. https://doi.org/10.1111/iju.12098
Laikangbam R, Damayanti Devi M (2012) Inhibition of calcium oxalate crystal deposition on kidneys of urolithiatic rats by Hibiscus sabdariffa L. extract. Urological research 40 (3):211–218. doi:https://doi.org/10.1007/s00240-011-0433-3
Soundararajan P, Mahesh R, Ramesh T, Begum VH (2006) Effect of Aerva lanata on calcium oxalate urolithiasis in rats. Indian J Exp Biol 44(12):981–986
Atmani F, Slimani Y, Mimouni M, Aziz M, Hacht B, Ziyyat A (2004) Effect of aqueous extract from Herniaria hirsuta L. on experimentally nephrolithiasic rats. J Ethnopharmacol 95 (1):87–93. doi:https://doi.org/10.1016/j.jep.2004.06.028
Atmani F, Farell G, Lieske JC (2004) Extract from Herniaria hirsuta coats calcium oxalate monohydrate crystals and blocks their adhesion to renal epithelial cells. J Urol 172(4 Pt 1):1510–1514. https://doi.org/10.1097/01.ju.0000131004.03795.c5
Atmani F, Slimani Y, Mimouni M, Hacht B (2003) Prophylaxis of calcium oxalate stones by Herniaria hirsuta on experimentally induced nephrolithiasis in rats. BJU Int 92(1):137–140. https://doi.org/10.1046/j.1464-410x.2003.04289.x
Christina AJ, Ashok K, Packialakshmi M, Tobin GC, Preethi J, Murugesh N (2005) Antilithiatic effect of Asparagus racemosus Willd on ethylene glycol-induced lithiasis in male albino Wistar rats. Methods Find Exp Clin Pharmacol 27(9):633–638. https://doi.org/10.1358/mf.2005.27.9.939338
Akanae W, Tsujihata M, Yoshioka I, Nonomura N, Okuyama A (2010) Orthosiphon grandiflorum has a protective effect in a calcium oxalate stone forming rat model. Urol Res 38(2):89–96. https://doi.org/10.1007/s00240-010-0265-6
Tayefi-Nasrabadi H, Sadigh-Eteghad S, Aghdam Z (2012) The effects of the hydroalcohol extract of Rosa canina L. fruit on experimentally nephrolithiasic Wistar rats. Phytother Res 26 (1):78–85. doi:https://doi.org/10.1002/ptr.3519
Mandavia DR, Patel MK, Patel JC, Anovadiya AP, Baxi SN, Tripathi CR (2013) Anti-urolithiatic effect of ethanolic extract of Pedalium murex linn. fruits on ethylene glycol-induced renal calculi. Urol J 10 (3):946–952
Patel PK, Patel MA, Vyas BA, Shah DR, Gandhi TR (2012) Antiurolithiatic activity of saponin rich fraction from the fruits of Solanum xanthocarpum Schrad. & Wendl. (Solanaceae) against ethylene glycol induced urolithiasis in rats. J Ethnopharmacol 144 (1):160–170. doi:https://doi.org/10.1016/j.jep.2012.08.043
Hosseinzadeh H, Khooei AR, Khashayarmanesh Z, Motamed-Shariaty V (2010) Antiurolithiatic activity of Pinus eldarica medw: fruits aqueous extract in rats. Urol J 7(4):232–237
Touhami M, Laroubi A, Elhabazi K, Loubna F, Zrara I, Eljahiri Y, Oussama A, Grases F, Chait A (2007) Lemon juice has protective activity in a rat urolithiasis model. BMC Urol 7:18. https://doi.org/10.1186/1471-2490-7-18
Divakar K, Pawar AT, Chandrasekhar SB, Dighe SB, Divakar G (2010) Protective effect of the hydro-alcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rats. Food Chem Toxicol 48(4):1013–1018. https://doi.org/10.1016/j.fct.2010.01.011
Xiang M, Zhang S, Lu J, Li L, Hou W, Xie M, Zeng Y (2011) Antilithic effects of extracts from Urtica dentata hand on calcium oxalate urinary stones in rats. J Huazhong Univ Sci Technolog Med Sci 31(5):673. https://doi.org/10.1007/s11596-011-0580-3
Christina AJM, Packia Lakshmi M, Nagarajan M, Kurian S (2002) Modulatory effect of Cyclea peltata Lam. on stone formation induced by ethylene glycol treatment in rats. Methods Find Exp Clin Pharmacol 24 (2):77–79
Hadjzadeh MA, Khoei A, Hadjzadeh Z, Parizady M (2007) Ethanolic extract of nigella sativa L seeds on ethylene glycol-induced kidney calculi in rats. Urol J 4(2):86–90
Ilbey YO, Ozbek E, Simsek A, Cekmen M, Somay A, Tasci AI (2009) Effects of pomegranate juice on hyperoxaluria-induced oxidative stress in the rat kidneys. Ren Fail 31(6):522–531. https://doi.org/10.1080/08860220902963871
Ahsan SK, Tariq M, Ageel AM, al-Yahya MA, Shah AH, (1989) Effect of Trigonella foenum-graecum and Ammi majus on calcium oxalate urolithiasis in rats. J Ethnopharmacol 26(3):249–254
Khan ZA, Assiri AM, Al-Afghani HM, Maghrabi TM (2001) Inhibition of oxalate nephrolithiasis with Ammi visnaga (AI-Khillah). Int Urol Nephrol 33(4):605–608
Saha S, Verma RJ (2015) Antinephrolithiatic and antioxidative efficacy of Dolichos biflorus seeds in a lithiasic rat model. Pharm Biol 53(1):16–30. https://doi.org/10.3109/13880209.2014.909501
Shah JG, Patel BG, Patel SB, Patel RK (2012) Antiurolithiatic and antioxidant activity of Hordeum vulgare seeds on ethylene glycol-induced urolithiasis in rats. Indian J Pharmacol 44(6):672–677. https://doi.org/10.4103/0253-7613.103237
Sharma I, Khan W, Parveen R, Alam MJ, Ahmad I, Ansari MH, Ahmad S (2017) Antiurolithiasis Activity of Bioactivity Guided Fraction of Bergenia ligulata against Ethylene Glycol Induced Renal Calculi in Rat. Biomed Res Int 2017:1969525. https://doi.org/10.1155/2017/1969525
Saha S, Shrivastav PS, Verma RJ (2014) Antioxidative mechanism involved in the preventive efficacy of Bergenia ciliata rhizomes against experimental nephrolithiasis in rats. Pharm Biol 52(6):712–722. https://doi.org/10.3109/13880209.2013.865242
Saha S, Verma RJ (2011) Bergenia ciliata extract prevents ethylene glycol induced histopathological changes in the kidney. Acta Pol Pharm 68(5):711–715
Bouanani S, Henchiri C, Migianu-Griffoni E, Aouf N, Lecouvey M (2010) Pharmacological and toxicological effects of Paronychia argentea in experimental calcium oxalate nephrolithiasis in rats. J Ethnopharmacol 129(1):38–45. https://doi.org/10.1016/j.jep.2010.01.056
Zhong YS, Yu CH, Ying HZ, Wang ZY, Cai HF (2012) Prophylactic effects of Orthosiphon stamineus Benth. extracts on experimental induction of calcium oxalate nephrolithiasis in rats. J Ethnopharmacol 144 (3):761–767. doi:https://doi.org/10.1016/j.jep.2012.09.052
Hiremath RD, Jalalpure SS (2016) Effect of hydro-alcoholic extract of Vernonia cinerea Less. against ethylene glycol-induced urolithiasis in rats. Indian J Pharmacol 48 (4):434–440. doi:https://doi.org/10.4103/0253-7613.186211
Nizami AN, Rahman MA, Ahmed NU, Islam MS (2012) Whole Leea macrophylla ethanolic extract normalizes kidney deposits and recovers renal impairments in an ethylene glycol-induced urolithiasis model of rats. Asian Pac J Trop Med 5(7):533–538. https://doi.org/10.1016/S1995-7645(12)60094-7
Jyothilakshmi V, Thellamudhu G, Kumar A, Khurana A, Nayak D, Kalaiselvi P (2013) Preliminary investigation on ultra high diluted B. vulgaris in experimental urolithiasis. Homeopathy 102 (3):172–178. doi:https://doi.org/10.1016/j.homp.2013.05.004
Onaran M, Orhan N, Farahvash A, Ekin HN, Kocabiyik M, Gonul II, Sen I, Aslan M (2016) Successful treatment of sodium oxalate induced urolithiasis with Helichrysum flowers. J Ethnopharmacol 186:322–328. https://doi.org/10.1016/j.jep.2016.04.003
Siddiqui WA, Shahzad M, Shabbir A, Ahmad A (2018) Evaluation of anti-urolithiatic and diuretic activities of watermelon (Citrullus lanatus) using in vivo and in vitro experiments. Biomed Pharmacother 97:1212–1221. https://doi.org/10.1016/j.biopha.2017.10.162
Sahin C, Sarikaya S, Basak K, Cetinel CA, Narter F, Eryildirim B, Saglam E, Sarica K (2015) Limitation of apoptotic changes and crystal deposition by Tutukon following hyperoxaluria-induced tubular cell injury in rat model. Urolithiasis 43(4):313–322. https://doi.org/10.1007/s00240-015-0777-1
Cho HJ, Bae WJ, Kim SJ, Hong SH, Lee JY, Hwang TK, Choi YJ, Hwang SY, Kim SW (2014) The inhibitory effect of an ethanol extract of the spores of Lygodium japonicum on ethylene glycol-induced kidney calculi in rats. Urolithiasis 42(4):309–315. https://doi.org/10.1007/s00240-014-0674-z
Youn SH, Kwon JH, Yin J, Tam LT, Ahn HS, Myung SC, Lee MW (2017) Anti-Inflammatory and Anti-Urolithiasis Effects of Polyphenolic Compounds from Quercus gilva Blume. Molecules 22 (7). doi:https://doi.org/10.3390/molecules22071121
Prabhu VV, Sathyamurthy D, Ramasamy A, Das S, Anuradha M, Pachiappan S (2016) Evaluation of protective effects of diosmin (a citrus flavonoid) in chemical-induced urolithiasis in experimental rats. Pharm Biol 54(9):1513–1521. https://doi.org/10.3109/13880209.2015.1107105
Bodakhe KS, Namdeo KP, Patra KC, Machwal L, Pareta SK (2013) A polyherbal formulation attenuates hyperoxaluria-induced oxidative stress and prevents subsequent deposition of calcium oxalate crystals and renal cell injury in rat kidneys. Chin J Nat Med 11(5):466–471. https://doi.org/10.1016/S1875-5364(13)60085-0
Grases F, Prieto RM, Gomila I, Sanchis P, Costa-Bauza A (2009) Phytotherapy and renal stones: the role of antioxidants. A pilot study in Wistar rats. Urological research 37 (1):35–40. doi:https://doi.org/10.1007/s00240-008-0165-1
Tsai CH, Chen YC, Chen LD, Pan TC, Ho CY, Lai MT, Tsai FJ, Chen WC (2008) A traditional Chinese herbal antilithic formula, Wulingsan, effectively prevents the renal deposition of calcium oxalate crystal in ethylene glycol-fed rats. Urol Res 36(1):17–24. https://doi.org/10.1007/s00240-007-0122-4
Ogawa Y, Morozumi M, Tanaka T, Yamaguchi K (1986) A comparison between effects of pyruvate and herb medicines in preventing experimental oxalate urolithiasis in rats. Hinyokika Kiyo 32(8):1127–1133
Yasui T, Fujita K, Sato M, Sugimoto M, Iguchi M, Nomura S, Kohri K (1999) The effect of takusha, a kampo medicine, on renal stone formation and osteopontin expression in a rat urolithiasis model. Urol Res 27(3):194–199
Yamaguchi S, Jihong L, Utsunomiya M, Yoshioka T, Okuyama A, Koide T, Sugiyama K (1995) The effect of takusha and kagosou on calcium oxalate renal stones in rats. Hinyokika Kiyo 41(6):427–431
Tsai CH, Pan TC, Lai MT, Lee SC, Chen ML, Jheng JR, Chen WC (2009) Prophylaxis of experimentally induced calcium oxalate nephrolithiasis in rats by Zhulingtang, a traditional Chinese herbal formula. Urol Int 82(4):464–471. https://doi.org/10.1159/000218539
Jagannath N, Chikkannasetty SS, Govindadas D, Devasankaraiah G (2012) Study of antiurolithiatic activity of Asparagus racemosus on albino rats. Indian J Pharmacol 44(5):576–579. https://doi.org/10.4103/0253-7613.100378
Liang Q, Li X, Zhou W, Su Y, He S, Cheng S, Lu J, Cao W, Yan Y, Pei X, Qi J, Xu G, Yue Z (2016) An Explanation of the Underlying Mechanisms for the In Vitro and In Vivo Antiurolithic Activity of Glechoma longituba. Oxid Med Cell Longev 2016:3134919. https://doi.org/10.1155/2016/3134919
Lin WC, Lai MT, Chen HY, Ho CY, Man KM, Shen JL, Lee YJ, Tsai FJ, Chen YH, Chen WC (2012) Protective effect of Flos carthami extract against ethylene glycol-induced urolithiasis in rats. Urol Res 40(6):655–661. https://doi.org/10.1007/s00240-012-0472-4
Gandhi M, Aggarwal M, Puri S, Singla SK (2013) Prophylactic effect of coconut water (Cocos nucifera L.) on ethylene glycol induced nephrocalcinosis in male wistar rat. Int Braz J Urol 39 (1):108–117. doi:https://doi.org/10.1590/S1677-5538.IBJU.2013.01.14
Hirayama H, Wang Z, Nishi K, Ogawa A, Ishimatu T, Ueda S, Kubo T, Nohara T (1993) Effect of Desmodium styracifolium-triterpenoid on calcium oxalate renal stones. Br J Urol 71(2):143–147. https://doi.org/10.1111/j.1464-410x.1993.tb15906.x
Khan SR (1997) Animal models of kidney stone formation: an analysis. World J Urol 15(4):236–243
Khan SR, Pearle MS, Robertson WG, Gambaro G, Canales BK, Doizi S, Traxer O, Tiselius HG (2016) Kidney stones Nature reviews Disease primers 2:16008. https://doi.org/10.1038/nrdp.2016.8
Robertson WG (2017) Do “inhibitors of crystallisation” play any role in the prevention of kidney stones? A critique Urolithiasis 45(1):43–56. https://doi.org/10.1007/s00240-016-0953-y
Robertson WG (2004) Kidney models of calcium oxalate stone formation. Nephron Physiology 98(2):p21-30. https://doi.org/10.1159/000080260
Rodgers AL (2017) Physicochemical mechanisms of stone formation. Urolithiasis 45(1):27–32. https://doi.org/10.1007/s00240-016-0942-1
Khan SR, Kok DJ (2004) Modulators of urinary stone formation. Front Biosci 9(14977559):1450–1482. https://doi.org/10.2741/1347
Khan SR, Canales BK (2015) Unified theory on the pathogenesis of Randall’s plaques and plugs. Urolithiasis 43(1):109–123. https://doi.org/10.1007/s00240-014-0705-9
Linnes MP, Krambeck AE, Cornell L, Williams JC Jr, Korinek M, Bergstralh EJ, Li X, Rule AD, McCollough CM, Vrtiska TJ, Lieske JC (2013) Phenotypic characterization of kidney stone formers by endoscopic and histological quantification of intrarenal calcification. Kidney Int 84(4):818–825. https://doi.org/10.1038/ki.2013.189
Verkoelen CF, van der Boom BG, Schroder FH, Romijn JC (1997) Cell cultures and nephrolithiasis. World J Urol 15(4):229–235
Kavanagh JP (2006) In vitro calcium oxalate crystallisation methods. Urol Res 34(2):139–145. https://doi.org/10.1007/s00240-005-0027-z
Hennequin C, Lalanne V, Daudon M, Lacour B, Drueke T (1993) A new approach to studying inhibitors of calcium oxalate crystal growth. Urol Res 21(2):101–108
Hess B, Meinhardt U, Zipperle L, Giovanoli R, Jaeger P (1995) Simultaneous measurements of calcium oxalate crystal nucleation and aggregation: impact of various modifiers. Urol Res 23(4):231–238
Khan A (2018) In vitro and in vivo models for the study of urolithiasis. Urologia 85(4):145–149. https://doi.org/10.1177/0391560317751578
Khan SR (2010) Nephrocalcinosis in animal models with and without stones. Urol Res 38(6):429–438. https://doi.org/10.1007/s00240-010-0303-4
Davis CM (2013) Animal Models of Drug Abuse: Place and Taste Conditioning. In: Animal Models for the Study of Human Disease. Elsevier Inc, pp 681–707
khan SR (2013) Animal Models of Calcium Oxalate Kidney Stone Formation. In: Conn PM (ed) Animal models for the study of human disease. Academic Press, pp 483–498
Thamilselvan S, Hackett RL, Khan SR (1997) Lipid peroxidation in ethylene glycol induced hyperoxaluria and calcium oxalate nephrolithiasis. J Urol 157(3):1059–1063
Thamilselvan S, Menon M (2005) Vitamin E therapy prevents hyperoxaluria-induced calcium oxalate crystal deposition in the kidney by improving renal tissue antioxidant status. BJU Int 96(1):117–126. https://doi.org/10.1111/j.1464-410X.2005.05579.x
Fan J, Glass MA, Chandhoke PS (1999) Impact of ammonium chloride administration on a rat ethylene glycol urolithiasis model. Scanning Microsc 13(2–3):299–306
Khan SR, Glenton PA (1995) Deposition of calcium phosphate and calcium oxalate crystals in the kidneys. J Urol 153(3 Pt 1):811–817
Hackett RL, Shevock PN, Khan SR (1990) Cell injury associated calcium oxalate crystalluria. J Urol 144(6):1535–1538
Tawashi R, Cousineau M, Sharkawi M (1980) Calcium oxalate crystal formation in the kidneys of rats injected with 4-hydroxy-L-proline. Urol Res 8(2):121–127. https://doi.org/10.1007/BF00271440
Bushinsky DA, Favus MJ (1988) Mechanism of hypercalciuria in genetic hypercalciuric rats. Inherited defect in intestinal calcium transport. The Journal of clinical investigation 82 (5):1585–1591. doi:https://doi.org/10.1172/jci113770
Bushinsky DA (1999) Genetic hypercalciuric stone-forming rats. Curr Opin Nephrol Hypertens 8(4):479–488
Khan SR, Finlayson B, Hackett R (1982) Experimental calcium oxalate nephrolithiasis in the rat. Role of the renal papilla. The American journal of pathology 107 (1):59
Ogawa Y, Yamaguchi K, Morozumi M (1990) Effects of magnesium salts in preventing experimental oxalate urolithiasis in rats. The Journal of Urology 144(2 Pt 1):385–389
Wesson JA, Johnson RJ, Mazzali M, Beshensky AM, Stietz S, Giachelli C, Liaw L, Alpers CE, Couser WG, Kleinman JG (2003) Osteopontin is a critical inhibitor of calcium oxalate crystal formation and retention in renal tubules. J Am Soc Nephrol 14(1):139–147
Mo L, Huang H-Y, Zhu X-H, Shapiro E, Hasty DL, Wu X-R (2004) Tamm-Horsfall protein is a critical renal defense factor protecting against calcium oxalate crystal formation. Kidney Int 66(3):1159–1166
Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS (2006) Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 38(4):474–478. https://doi.org/10.1038/ng1762
Weinman EJ, Mohanlal V, Stoycheff N, Wang F, Steplock D, Shenolikar S, Cunningham R (2006) Longitudinal study of urinary excretion of phosphate, calcium, and uric acid in mutant NHERF-1 null mice. Am J Physiol Renal Physiol 290(4):F838-843. https://doi.org/10.1152/ajprenal.00374.2005
Tzou DT, Taguchi K, Chi T, Stoller ML (2016) Animal models of urinary stone disease. Int J Surg 36(Pt D):596–606. https://doi.org/10.1016/j.ijsu.2016.11.018
Okada Y, Kawamura J, Nonomura M, Kuo YJ, Yoshida O (1985) Experimental and clinical studies on calcium urolithiasis: (I) Animal model for calcium oxalate urolithiasis using ethylene glycol and 1-alpha (OH) D3. Hinyokika Kiyo 31(4):565–577
Ozturk H, Ozturk H, Yagmur Y, Buyukbayram H (2006) The effect of L-arginine methyl ester on indices of free radical involvement in a rat model of experimental nephrocalcinosis. Urol Res 34(5):305–314. https://doi.org/10.1007/s00240-006-0061-5
Miller J, Chi T, Kapahi P, Kahn AJ, Kim MS, Hirata T, Romero MF, Dow JA, Stoller ML (2013) Drosophila melanogaster as an emerging translational model of human nephrolithiasis. J Urol 190(5):1648–1656. https://doi.org/10.1016/j.juro.2013.03.010
Chen YH, Liu HP, Chen HY, Tsai FJ, Chang CH, Lee YJ, Lin WY, Chen WC (2011) Ethylene glycol induces calcium oxalate crystal deposition in Malpighian tubules: a Drosophila model for nephrolithiasis/urolithiasis. Kidney Int 80(4):369–377. https://doi.org/10.1038/ki.2011.80
Mandel NS, Henderson JD Jr, Hung LY, Wille DF, Wiessner JH (2004) A porcine model of calcium oxalate kidney stone disease. J Urol 171(3):1301–1303. https://doi.org/10.1097/01.ju.0000110101.41653.bb
Penniston KL, Patel SR, Schwahn DJ, Nakada SY (2017) Studies using a porcine model: what insights into human calcium oxalate stone formation mechanisms has this model facilitated? Urolithiasis 45(1):109–125. https://doi.org/10.1007/s00240-016-0947-9
Khan SR (2013) Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J Urol 189(3):803–811. https://doi.org/10.1016/j.juro.2012.05.078
Khan SR (2014) Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Translational andrology and urology 3(3):256–276. https://doi.org/10.3978/j.issn.2223-4683.2014.06.04
Khan SR (2005) Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res 33(5):349–357. https://doi.org/10.1007/s00240-005-0492-4
Khan SR, Joshi S, Wang W, Peck AB (2014) Regulation of macromolecular modulators of urinary stone formation by reactive oxygen species: transcriptional study in an animal model of hyperoxaluria. Am J Physiol Renal Physiol 306(11):F1285-1295. https://doi.org/10.1152/ajprenal.00057.2014
Joshi S, Clapp WL, Wang W (1852) Khan SR (2015) Osteogenic changes in kidneys of hyperoxaluric rats. Biochem Biophys Acta 9:2000–2012. https://doi.org/10.1016/j.bbadis.2015.06.020
Joshi S, Khan SR (2019) NADPH oxidase: a therapeutic target for hyperoxaluria-induced oxidative stress - an update. Future medicinal chemistry 11(23):2975–2978. https://doi.org/10.4155/fmc-2019-0275
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
The article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khan, A., Bashir, S. & Khan, S.R. Antiurolithic effects of medicinal plants: results of in vivo studies in rat models of calcium oxalate nephrolithiasis—a systematic review. Urolithiasis 49, 95–122 (2021). https://doi.org/10.1007/s00240-020-01236-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-020-01236-0