Abstract
The aim of this study was to test the effect of l-arginine methyl ester (l-Arg) on indices of free radical involvement in a rat model of experimental nephrocalcinosis. Twenty-eight Sprague–Dawley rats were randomized into four groups of seven. The first group (G1), the sham-control group received pure distilled drinking water. The second group (G2) received drinking water containing 0.7% ethylene glycol (EG) in distilled water for 3 weeks. The third group (G3) received drinking water containing 0.7% EG in distilled water for 3 weeks and l-Arg was administered for 3 weeks. The fourth group (G4) received drinking water containing 0.7% EG in distilled water for 3 weeks and l-NAME was administered for 3 weeks. Urine and aortic blood was collected to determine some parameters. The kidneys were also removed for histological examination. The increase in blood urea nitrogen, serum creatinine, K+, Mg2+ and uric acid were mild in group 3 compared with the groups 2 and 4. The urinary concentrations of Na+, K+, Mg2+ and uric acid were noticed to be similar among the groups. However, Ca2+ and oxalate excretion were significantly higher in groups 2, 3 and 4 than in group 1. The mean values of SOD, CAT and GSH-Px values were significantly increased in group 3 when compared to groups 2 and 4. Presence of aggregated urinary crystals was clearer in experimental groups compared to group 1. The tubular dilatation, epithelial degeneration and lymphocytic infiltration were significantly found in groups 2 and 4. Mild tissue damage was observed in l-Arg-pretreated rats. Under polarized light microscope intense crystals in the cortex and medulla were observed in the kidney of group 2 and 4 and moderate crystals were noticed in group 3. In conclusion, l-Arg supplementation may decrease free radicals and tubulary membrane injury in nephrocalcinosis due to infiltrating leukocytes and decreased antioxidant enzyme activities in rats fed with EG diet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Earlier studies of experimental nephrocalcinosis in rat models demonstrated that increased urinary excretion of oxalate and deposition of calcium oxalate crystals in the renal tubules was associated with renal epithelial injury [1, 2] and that renal injury was a risk factor for nephrocalcinosis [2–4]. In addition, an exposure to oxalate was associated with lipid peroxidation in both cell culture and animal studies [5–7]. Lipid peroxides in kidney tissue and urine increased after 15, 30 and 60 days in rats supplied with 0.75% ethylene glycol (EG) and renal cell damage was associated with lipid peroxide production and the damage appeared to be caused primarily by hyperoxaluria [6]. Lipid peroxidation is one of the results of toxicity mediated by oxygen free radicals and represents oxidative tissue damage by superoxide, hydroxyl radicals and hydrogen peroxide, which results in structural alteration to membranes and the functional impairment of the cellular component [7]. The cell is endowed with several antioxidant systems to limit the extent of lipid peroxidation; these include the enzyme catalase, superoxide dismutase (SOD) and glutathione peroxidase [7, 8].
Nitric oxide (NO) is an important signaling molecule for vascular homeostasis by the regulation of blood vessel diameter, platelet aggregation, leukocyte adhesion, and smooth muscle proliferation [9]. In addition, NO antagonizes the vasoconstrictive effect of angiotensin II on the afferent arteriole and helps regulate renal blood flow, glomerular filtration rate and sodium homeostasis [10]. It has been clearly shown that in renal ischemic conditions, the production of NO is increased. Endothelial release of NO causes a local relaxation in vessel walls and as a result of renal blood flow improvement, ischemic tissue damage could be well limited. Thus, NO may also serve as a potent antioxidant due to this specific vasodilator effect [10–12]. Thus, the aim of this study was to test the effect of l-arginine methyl ester (l-Arg) on indices of free radical involvement in a rat model of experimental nephrocalcinosis.
Materials and methods
The study was performed with 28 male prepubertal (35 days old) Wistar-albino rats weighing 150–160 g. All rats were housed in a temperature- and light-controlled room with ad libitum access to water and rat chow. For surgery, the rats were anesthetized with intraperitoneal sodium pentobarbital (120 mg/100 g body weight).
Twenty-eight Wistar rats were randomized into four groups of seven. The first group (G1), the sham-control group received pure distilled drinking water orally. The second group (G2), the untreated group, received drinking water containing 0.7% EG in distilled water for 3 weeks orally. The third group (G3) received drinking water containing 0.7% EG in distilled water for 3 weeks orally and l-Arg (150 mg/kg/day dissolved 0.5 ml in distilled water; Sigma Chemical Co., St. Louis, USA) was intraperitoneally administered for 3 weeks. The fourth group (G4) received drinking water containing 0.7% EG in distilled water for 3 weeks orally and NG-nitro-l-arginine methyl ester (L-NAME) (30 mg/kg/day dissolved 0.5 ml in distilled water; Sigma Chemical Co., St. Louis, USA) was intraperitoneally administered for 3 weeks.
The day before the end of the treatment, the animals were placed in metabolic cage and a 24 h urine sample collected from each. Urine samples were centrifuged at 2,000g for 10 min to remove debris. Immediately thereafter, all the rats were anesthetized with diethyl ether and aortic blood was collected to determine the same biochemical parameters. Biochemical determinations included urinary and plasma concentrations of sodium, potassium (photometry, Pegasus II, Tecnow, Brazil), calcium (Vitros 750 XRC autoanalyzer system), uric acid, magnesium, urea and creatinine (Labtest Diagnostics, Brazil). The urinary oxalate level was measured using the oxalate oxidase enzymatic method with a commercial oxalate assay kit (Sigma Chemical Co., St. Louis, MO, USA) [13].
Urinary crystal study
From all the groups, urine sample was collected after 24 h and a drop of which allowed to spread over a clean glass slide and visualized under light microscope.
Histopathological study
The kidneys were removed. The extracted the right kidneys were divided into two pieces in each rat. One of the pieces was immediately fixed using 10% buffered formalin and routinely processed. Then, they were stained with hematoxylin–eosin (H&E) to examine any pathological changes and calcium oxalate crystals under light microscope.
Enzymatic assays
The other piece was washed in ice-cold 0.9% saline solution, weighed and stored at − 70°C. Homogenates of the tissues were prepared as 1.0 g/10 ml in 250 mM sucrose, 1 mM EDTA, 1 mM-dl-dithiothreitol and 15 mM Tris HCl (pH 7.4), using an all-glass Potter Elvehjem homogenizer (Selecta, Barcelona, Spain). Each homogenate was centrifuged for 20 min at 800g. The resulting supernatant fraction was used to determine enzyme activities. The protein concentrations of the supernatant were determined by the method described by Bradford [14].
SOD activity was measured using the xanthine-oxidase-cytochrome c method as described by McCord and Fridovich [15]. The final concentrations in the cuvettes were 50 mM potassium phosphate (pH 7.8), 0.1 mM EDTA, 10 mM cytochrome c, 50 mM xanthine, 50 or 2 mM cyanide, 1 U catalase and tissue sample (0.05–0.1 mg). The reaction was initiated by the addition of 1 U xanthine-oxidase. The inhibition of xanthine-oxidase was followed spectrophotometrically at 550 nm. One unit of SOD activity is defined as the amount of enzyme that gave 50% inhibition of the control rate of cytochrome c reduction.
CAT activity was assayed according to the method of Beers and Sizer [16]. The final concentrations in the cuvettes were 500 mM potassium phosphate (pH 7), 100 mM H2O2 and tissue sample (0.05–0.1 mg). The decrease in the absorbance at 240 nm after the addition of the substrate was followed spectrophotometrically.
GSH-Px activity was assayed with a coupled enzyme system in which oxidized glutathione (GSSG) reduction was coupled to NADPH oxidation by glutathione reductase [17]. The assay mixture contained 50 mM potassium phosphate (pH 7.5), 1 mM EDTA, 1 mM NaN3, 1 mM reduced glutathione (GSH), 0.2 mM NADPH, 1 U glutathione reductase and tissue sample (0.05–0.2 mg). After 5 min preincubation (20–25 °C), the reaction was initiated by the addition of 0.25 mM H2O2. The decrease in the absorbance at 340 nm was followed spectrophotometrically.
In addition, the number of infiltrating polymorphonuclear leukocyte (PNL) per kidney tissue was assessed by counting neutrophils manually at a ×400 (Olympus Eyepiece Micrometer®) magnification in ten portal tracts per slide (n = 10 in each group).
Statistical analysis
Data were entered and analyzed on an IBM compatible personal computer using SPSS version 9.0. All values were expressed as medians and ranges. Multiple non-parametric comparative analyses were done by Kruskal–Wallis test. Differences were analyzed by the Mann–Whitney U test. P values of less than 0.05 were considered significant.
Results
Table 1 presents the general variables. There were no differences in the 24-h volume of water ingested and urinary volume among the groups. The blood urea nitrogen (BUN), serum creatinine, K+, Mg2+ and uric acid values were significantly higher in groups 2 and 4 than in group 1; however, the increase in these values were mild in group 3 compared with group 1. The plasma concentrations of Na+, Ca2+, oxalate and pH were noticed to be similar among the groups.
Urinary Na+, K+, uric acid and were similar among the groups (Table 1), but the concentration of protein was increased in experimental groups compared to group 1. However, Ca2+ and oxalate excretion were significantly higher in groups 2, 3 and 4 than in group 1. In addition, the increase in the concentration of urinary protein was moderate in group 3 compared with groups 2 and 4. The urinary excretion of the inhibitors such as magnesium and citrate was unaffected by l-Arg and l-NAME treatment (Table 1).
The values of SOD, CAT and GSH-Px measurements for the different groups are given in Table 2. The values were significantly decreased in 2, 3 and 4 groups in comparison with the group 1. However, these values were significantly increased in group 2 when compared to groups 2 and 4.
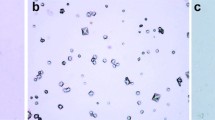
Presence of aggregated urinary crystals under light photomicrography is shown in Fig. 1 as (a) group1: Sham-control, (b) group 2: EG/Untreated, (c) group 3: EG/L-Arg, and (d) group 4: EG/L-NAME. Crystal aggregation was clearer in experimental groups compared to group 1.
The histological sections of urolithic rat kidney are presented in Fig. 2. In group 1 rats, normal architecture of glomeruli and proximal convoluted tubule was noticed (Fig. 2a). The tubular epithelial degeneration and lymphocytic infiltration were significantly found in groups 2 and 4 (Fig. 2b). Mild tissue damage was observed in l-Arg-pretreated rats (Fig 2c). A positive sign of tissue injury such as interstitial, tubular dilatation and mononuclear inflammatory cells was observed in l-NAME pretreated rats (Fig. 2d).
Histopathological observation of kidney tissue. a Group 1: showing normal architecture of glomeruli and proximal convoluted tubule (H&E, 40×), b group 2: EG 0.75% + untreated: oxalate rat showing tubular dilatation (






Cross-section of the kidney of control and experimental rats were viewed under polarized light microscope. A few crystals were noticed in group 1 rats’ kidney (Fig. 3a); however, intense crystals in the cortex and medulla were observed) in the kidney from groups 2 (Fig. 3b) and 4 (Fig. 3d) and moderate crystals was noticed in group 2 (Fig. 3c). A histological examination exhibited less medullar calcium oxalate crystals in group 3, whereas calcium crystals in the cortex tissue were found to be similar in all the experimental groups.
Cross-section of the kidney of control and experimental rats as viewed under polarized light microscope of (a) a Sham-control rat kidney (magnification, 100×), b a kidney from a rat that received EG 0.75% only where numerous crystals in the cortex and medulla can be seen (magnification, 100×), and c a kidney from a rat that received EG 0.75% and l-Arg (magnification, 100×), and d a kidney from a rat that received EG 0.75% and l-NAME (magnification, 100×)
The count of PNL in control group was less than one in renal parenchyma. Whereas, it was found as 16 ± 3, 6 ± 1.9 and 33 ± 5 in the experimental groups 2, 3 and 4, respectively. The number of polymorphonuclear leukocyte (PNL) was significantly decreased in group 3 when compared to groups 2 and 4 (P < 0.0001, P < 0.0001).
Discussion
A relationship between deposition of calcium oxalate crystals in the renal tubules and epithelial injury has been demonstrated in experimental urolithiasis [1–8]. An increased level of oxalate in the tissues has been reported to cause lipid peroxidation [7, 8]. In a cell line study, Scheid et al. [5] suggested that free radicals may be induced after adding oxalate to cultures of LLC-PK1 cells. Thamilselvan et al. [6] found that lipid peroxidation occurred in kidney tissue and urine samples of male rats treated with 0.75% EG during all periods. Huang et al. [18] experimental nephrolithiasis study in a rat model demonstrated that the production of reactive oxygen species in arterial blood samples increased significantly on day 7 after a 0.75% EG treatment. However, reactive oxygen species returned to a normal baseline level on days 21 and 42, although hyperoxaluria, deposition of calcium oxalate in the renal tubules and enzymuria were still increased during these periods. Lipid peroxidation is one of the results of toxicity mediated by oxygen free radicals. Lipid peroxidation represents oxidative tissue damage by hydrogen peroxide (H2O2), superoxide (\( {\text{O}}^{ - }_{{\text{2}}} \)) and hydroxyl radicals (OH-), resulting in structural alteration to membranes with release of cell and organelle contents, loss of essential fatty acids with formation of cytosolic aldehyde and peroxide products [19, 20]. The cell is endowed with several antioxidant systems to limit the extent of lipid peroxidation; these include the enzyme CAT, SOD) and GSH-Px [21]. The development of tissue injury probably depends on the balance of the generation of reactive oxygen species and the tissue antioxidant defense mechanism [22]. The present study supports earlier studies suggestions that 0.75% EG when supplied to rats for 21 days can induce free radical damage to the renal epithelial cells along with hyperoxaluria and deposition of calcium oxalate in the tubules. In our study, after 3 weeks of EG supply, the endogenous scavenger SOD, which catalyses the dismutation of the highly reactive superoxide anion to H2O2 [23], was significantly decreased in the renal tissues studied. Inhibition of GSH-Px as seen in our study, which disposes of cellular H2O2 by utilizing GSH as the co-factor, might be due to the depletion of GSH-Px along with the high degree of peroxides being formed [24]. Catalase, which also detoxifies H2O2 was significantly found to be low in the tissues of stone-forming animals.
Nitric oxide, known as an endothelium-derived relaxing factor, is formed from the terminal guanidino nitrogen atom of l-arginine by NO synthase [25]. NO binds to the haem moiety of guanylate cyclase and increases its activity by 400-fold, catalyzing the conversion of guanosine triphosphate to cyclic guanosine monophosphate (cGMP). Elevation of cGMP relaxes smooth muscle—in blood vessels, the including genitourinary tract—inhibits platelet aggregation and adhesion, and blocks the adhesion of white cells to the blood vessel wall [26–28]. Currently, inhibitors of NOS are substrate analogues such as N-monomethyl-l-arginine (L-NMMA) or N-nitro-l-arginine methyl ester (l-NAME) [27–29]. Supplementation of nitric oxide in the form of nitric oxide donors can, thus, be used to correct the nitric oxide deficiency encountered in many disease states. In the setting of ischemic acute renal failure, the administration of l-arginine had a beneficial effect on GFR and RPF, decreased \( {\text{O}}^{ - }_{{\text{2}}} \) production, diminished up-regulation of soluble guanylate cyclase, and prevented up-regulation of inducible NO synthase [30]. A significant decrease in SOD, Catalase and glutathione peroxidase activity in EG-treated rats as also noticed in our study, could be attributed to the interminable increase in calcium levels that is known to increase the \( {\text{O}}^{ - }_{{\text{2}}} \) and H2O2 production [31]. The inactivation of NO by \( {\text{O}}^{ - }_{{\text{2}}} \) creates NO deficiency. Oxidative stress can promote the production of vasoconstrictor molecules [32]. l-Arg administration restored the enzyme activities to that of controls. In addition, it is possible that activation of human neutrophils in vivo could decrease surrounding NO levels by reacting with the superoxide anion synthesized. Decreased NO by reaction with \( {\text{O}}^{ - }_{{\text{2}}} \) could potentially cause vasoconstriction, platelet aggregation and adhesion, and peroxynitrite formation [33]. Under the light of our current knowledge and previous information, we made a diagram to show the putative pathways of free radical production, crystal nucleation and renal tubular cell injury, along with the possible points of involvement of l-Arg, l-NAME and enzymes (Fig. 4).
A diagram showing the putative pathways of free radical production, crystal nucleation and renal tubular cell injury, along with the possible points of involvement of l-Arg, l-NAME and enzymes. ROS reactive oxygen species, \( {\text{O}}^{ - }_{{\text{2}}} \) superoxide, H 2 O 2 hydrogen peroxide, OH - hydroxyl radicals, NO nitric oxide, NOS nitric oxide synthase, L-NAME N-nitro-l-arginine methyl ester)
Oxalate is secreted along the entire length of the proximal tubule, which may be the first part to be damaged when the oxalate concentration becomes high enough to have a toxic effect in the kidney, and damage to the distal tubule occurs only when the concentration becomes higher [34]. In addition, Huang et al. [18] suggested that lipid peroxidation correlated significantly with proximal tubule damage and urinary Ca and oxalate levels. Previous studies pointed that the inner medullary collecting duct contains the highest capacity for NO synthesis of all nephron segments [35] and expresses all three isoforms of NOS [35–38]. From these findings, we may explain why histological examination exhibited less medullar calcium oxalate crystals in l-Arg group.
Blockade of the enzymatic production of NO lead to vasoconstriction and in this case, intracellular calcium may increase due to elevated pressure on the endothelium. In a recent study, exogenous NO, or stimulation of NO production in endothelial cells, resulted in the activation of plasma membrane Ca2+-ATPase and a decrease in basal [Ca2+]i. This effect was abolished by l-NAME [39]. NO donors have the capacity to control the intracellular rise in calcium levels [40]. So, l-Arg supplementation inhibits the synthesis of oxalate and increases the bioavailability of NO to sequester calcium through the cGMP pathway.
Several hypertensive animal models showed increased activity of nicotine adenine dinucleotide phosphate (NADPH) oxidase, which is the chief source of \( {\text{O}}^{ - }_{{\text{2}}} \) in the vessel wall and kidneys [41]. Superoxide is formed upon one-electron reduction of oxygen mediated by enzymes such as NADPH oxidase or xanthine oxidase or from the respiratory chain. In the arginine supplemented group, inhibition of \( {\text{O}}^{ - }_{{\text{2}}} \) radical formation may be due to the correction of the NAD+/NADH ratio [42].
Proteinuria is an important manifestation of renal disease and is always associated with increased glomerular injury. Increased protein excretions in urolithic rats as well as in stone formers have already been reported [43]. Diabetic rats given l-Arg had significantly lower excretion of protein and cyclic guanosine monophosphate than diabetic rats not receiving l-Arg [44]. In our study, in group 3, proteinuria was noticed less than other experimental groups.
As a conclusion, l-Arg supplementation may decrease free radicals and tubulary membrane injury in nephrocalcinosis due to infiltrating leukocytes and decreased antioxidant enzyme activities in rats fed with EG diet.
References
Finlayson B (1978) Kidney Int 13:344–360
Huang HS, Ma MC, Chen CF, Chen J (2003) Urology 62:1123–1425
Finlayson B, Khan SR, Hackett RL (1984) Scan Electr Microsc 3:1419–1425
Khan SR, Hackett RL (1994) (eds) Renal epithelial injury: a risk factor in urolithiasis. Plenum, New York
Scheid C, Koul H, Hill WA, Luber-Narod J, Jonassen J, Honeyman T, Kennington L, Kohli R, Hodapp J, Ayvazian P, Menon M (1996) J Urol 155:1112–1116
Thamilselvan S, Hackett RL, Khan SR (1997) J Urol 157:1059–1063
Thamilselvan S., Byer K. J., Hackett R. L. Khan SR (2000) J Urol 164:224–229
Thamilselvan S, Khan SR, Menon M (2003) Urol Res 31:3–9
Shimizu S, Ishii M, Miyasaka Y, Wajima T, Negoro T, Hagiwara T, Kiuchi Y (2005) Int J Biochem Cell Biol 37:864–875
Waz WR, Van Liew JB, Feid LG (1998) Pediatr Nephrol 12:26–29
Mashiach E, Sela S, Winaver J, Kristal B (1998) Nephron 80:458–467
Jefayri MK, Grace PA, Mathie RT (2000) BJU Int 85:1007–1013
Rajagopal G (1984) Ind J Exp Biol 22:391–392
Bradford MM (1976) Anal Biochem 72:248–254
McCord JM, Fridovich I (1969) J Biol Chem 244:6049–6055
Beers RF Jr, Sizer IW (1952) J Biol Chem 195:133–140
Lawrence A, Burk RF (1976) Biochem Biophys Res Commun 71:952–958
Huang HS, Chen CF, Chien CT, Chen J (2000) BJU Int 85:1143–1149
Comporti M (1985) Lab Invest 53:599–623
Burton KP, Morris AC, Massey KD, Buja LM, Hager HK (1990) J Mol Cell Cardiol 22:1035–1047
Janssen YM, Van Houten B, Borm PJ, Mossman BT (1993) Lab Invest 69:261–274
Ernster L, Nordenbrand K (1967) (eds) Microsomal lipid peroxidation. Methods in enzymology. Academic, New York
Husain K, Somani SM (1998) J Appl Toxicol 18:421–429
Ross D (1988) Pharamacol Ther 37:231–243
Moncada S, Palmer RMJ, Higgs EA (1991) Pharmacol Rev 43:109–142
Russwurm M, Koesling D (2002) Mol Cell Biochem 230:159–164
Vallance P (2003) Fundam Clin Pharmacol 17:1–10
Ozturk H, Dokucu AI, Otcu S, Gezici A, Ketani A, Yildiz FR, Ozdemir E, Yucesan S (2001) BJU Int 88:93–99
Moncada S, Palmer RMJ, Higgs AE (1991) Pharmacol Rev 43:109–142
Klahr S, Morrissey J (2004) Semin Nephrol 24:389–394
Kramp RA, Fourmanoir P, Ladriere L, Joly E, Gerbaux C, El Hajjam A, Caron N (2000) Am J Physiol Renal Physiol 278:F561–F569
Modlinger PS, Wilcox CS, Aslam S (2004) Semin Nephrol 24:354–365
McBride AG, Brown GC (1997) FEBS Lett 417:231–234
Greger R, Lang F, Oberleithner H, Deetjen P (1978) Pflugers Arch 374:243–238
Wu F, Park F, Cowley AW Jr, Mattson DL (1999) Am J Physiol Renal Physiol 276:F874–F881
Ahn KY, Mohaupt MG, Madsen KM, Kone BC (1994) Am J Physiol Renal Fluid Electrolyte Physiol 267:F748–F757
Roczniak A, Zimpelmann J, Burns KD (1998) Am J Physiol Renal Physiol 275:F46–F54
Terada Y, Tomita K, Nonoguchi H, Marumo F (1992) J Clin Invest 90:659–665
Chen J, Wang Y, Nakajima T, Iwasawa K, Hikiji H, Sunamoto M, Choi DK,Yoshida Y, Sakaki Y, Toyo-Oka T (2000) J Biol Chem 275:28739–28749
Meszaros LG, Minarovic I, Zahradnikova A (1996) FEBS 380:49–52
Modlinger PS, Wilcox CS, Aslam S (2004) Semin Nephrol 24:354–365
Fujii S, Zhang L, Igarashi J, Kosaka H (2003) Hypertension 42:1014–1020
Grover PK, Resnick MI (1995) J Urol 153:1716–1721
Klahr S, Morrissey J (2004) Semin Nephrol 24:389–394
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ozturk, H., Ozturk, H., Yagmur, Y. et al. The effect of l-arginine methyl ester on indices of free radical involvement in a rat model of experimental nephrocalcinosis. Urol Res 34, 305–314 (2006). https://doi.org/10.1007/s00240-006-0061-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-006-0061-5