Abstract
The effects of aqueous and ethanolic extracts of Costus igneus (stem) and isolated compounds lupeol and stigmasterol on calcium oxalate urolithiasis have been studied in male albino Wistar rats. Ethylene glycol feeding resulted in hyperoxaluria as well as increased renal excretion of calcium and oxalate. The increased deposition of stone-forming constituents in the urine, serum, and kidney homogenate of urolithic rats was significantly (p < 0.05) lowered by treatment using aqueous and ethanolic extracts of C. igneus (stem), and isolated compounds lupeol and stigmasterol. The calcium oxalate crystal deposition in the kidney was significantly greater in ethylene glycol-induced urolithic rats. After administration of aqueous and ethanolic extract of C. igneus, the deposition of calcium and oxalate was significantly lowered. Treatment with lupeol and stigmasterol significantly reduced the deposition of calcium and oxalate in the kidney, and also in the blood serum; the lipid profile serum total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL) and high-density lipoprotein (HDL) levels at 50 and 100 mg/kg were significantly (p < 0.05) lowered in urolithiatic rats. From this study, we conclude that both the treatments with aqueous and ethanolic extract of C. igneus (stem) and isolated compounds lupeol and stigmasterol had an inhibitory effect on calcium oxalate urinary stone. Lupeol and stigmasterol were identified from the stem of C. igneus by high-performance thin layer chromatography technique. The isolated compounds were confirmed by Fourier transform infrared (FTIR) and 13C NMR spectra.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kidney stone disease is a common disorder estimated to occur in approximately 12% of the population, with a recurrence rate of 70–81% in males, and 47–60% in females [1]. The majority of stones, up to 80%, are composed mainly of calcium oxalate (CaOx) [2]. Urinary stones are characterized by its high recurrence rate if patients are not treated appropriately. Among the treatments used are surgical removal, percutaneous techniques based on laparoscopic and extracorporeal shock wave lithotripsy (ESWL), and drug treatment [3]. Besides, these treatments cause undesirable side effects such as hemorrhage, hypertension, tubular necrosis, and subsequent fibrosis of the kidney leading to cell injury and recurrence of renal stone formation [4]. The kidney-stone-forming patients are prone to its recurrence even after its surgical removal [5]. From the above facts it is clear that there is a need to study herbal plants for the treatment of urinary stones. Herbal medicines are in great demand in the developed world for primary health care because of their efficacy, safety, lesser side effects, and better compatibility with human body [6–9].
Costus igneus, also known as Fiery Costus, Spiral Flag or Insulin Plant, belongs to the Costaceae family are rich in protein (18%), iron (40 mg), and antioxidant components such as ascorbic acid, β-carotene, α-tocopherol, glutathione, phenols, flavonoids, steroids, alkaloids, and terpenoids, and is traditionally used in India to control diabetes [10, 11]. Administration of the aqueous extract of Costus spiralis to rats with experimentally induced urolithiasis has been found to reduce the growth of urinary stones [12]. In addition, the blood glucose levels of alloxan-induced diabetic rats were controlled after the administration of ethanolic extracts of C. igneus leaves [13]. However, it has been cautioned that the constant use of Costus pictus increases the LDL to HDL cholesterol ratio due to higher levels (24.51% in leaves, 28.30% in the stem and 25.26% in the rhizome) of hexadecanoic acid found in diethyl ether extractions [14]. In the present study, the effects of aqueous and the ethanolic extracts of stem of C. igneus and isolated compounds lupeol and stigmasterol in experimentally induced calcium oxalate urolithiatic rats are reported for the first time. Lupeol and stigmasterol were identified and isolated by HPTLC techniques and column chromatography. The isolated compounds were confirmed by Fourier transform infrared (FTIR) and 13C NMR spectra. This study incorporates a multidisciplinary approach for the growth of calcium oxalate urinary crystal grown in vivo to facilitate the development of prevention and dissolution strategies aimed at managing urinary stone growth.
Materials and methods
Collection of plant materials
The fresh C. igneus (Costaceae family) stem was collected from the Periyar Maniammai University nursery in the month of April (2010). The plant was identified, confirmed, and authenticated by Rapinat herbarium, St. Joseph College, Tiruchirapalli, Tamil Nadu, India. The voucher of these plants was deposited at the herbarium of the Periyar Maniammai University, Vallam, Thanjavur. All the chemicals and reagents were certified analytical grade purchased from Himedia (Mumbai, India).
Preparation of extracts
The stem of C. igneus was air-dried at room temperature (37°C) for 2 weeks, after which it was ground to a uniform powder of 40 mesh size. The ethanol extracts were prepared by soaking 100 g of the dried, powdered stems in 1 L of ethanol using a soxhlet extractor continuously for 10 h [15]. The aqueous extracts were prepared by soaking 100 g each of the dried, powdered plant stems in 1 L of aqueous (double distilled water) using a soxhlet extractor continuously for 10 h at temperature of 60–70°C [16]. The extract was filtered through Whatman filter paper No. 42 (125 mm) to remove all unextractable matter, including cellular materials and other constitutions that are insoluble in the extraction solvent. The entire extracts were concentrated to dryness using a rotary evaporator under reduced pressure. The final dried samples were stored in labeled sterile bottles and kept at −20°C.
Animals
Thirty-six male young adult albino Wistar rats, 9 months, weighing approximately 200 g were used. All animal experiments and maintenance were carried out according to the ethical guidelines suggested by the Institutional Animal Ethics Committee (Approval number: CPC SEA/265). Animals were housed in polypropylene cages under controlled conditions of 12 h light/dark cycle at 27 ± 2°C. All the rats received standard pellet diet (Amrut rat feed, pune) and water ad libitum.
Stone induction
A kidney stone was induced by 0.75% of ethylene glycol in drinking water for 28 days ad libitum. At the end of the 28th day, the animals were used for the biochemical and histopathological studies [17–19].
Acute toxicity (AOT) studies
The acute oral toxicity studies was carried out as per the guidelines set by Organization for Economic Co-operation and Development (OECD) (AOT-423) received from Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). Acute toxicity studies observed that animals tolerated a maximum dose of 1,000 mg/kg b.w. for aqueous and ethanolic extracts and a maximum dose 500 mg/kg b.w. for isolated compounds lupeol and stigmasterol with no noticeable behavioral changes in all groups. Therefore, 1/10th of the maximum tolerated dose 100 mg/kg b.w. for aqueous and ethanolic extracts, and 50 and 100 mg/kg b.w. for lupeol and stigmasterol were chosen for further experimental studies.
Experimental design
For studying the effect of C. igneus, and isolated compounds lupeol and stigmasterol on calcium oxalate stones, the 36 rats were divided into nine groups comprising four animals per each group. Each group underwent a different treatment protocol for 28 days. Group I: normal, ad libitum access to regular food and drinking water and administered 6 μl of distilled water per 1 g of body weight by gavage (oral administration). Groups II, III, IV, V, VI, VII, VIII, and IX ad libitum access to regular food and ad libitum access to drinking water containing 0.75% (v/v) ethylene glycol in order to promote hyperoxaluria and CaOx crystals deposition in the kidney for 28 days. Group III administered 100 mg/kg aqueous extract of stem of C. igneus from the 1st to 28th day. Group IV administered 100 mg/kg ethanolic extract of stem of C. igneus from 1st to 28th day. Group V and VI administered 50 and 100 mg/kg of Lupeol from 1st to 28th day. Group VII and VIII administrated 50 and 100 mg/kg of stigmasterol from 1st to 28th day. Group IX administered 50 mg/kg standard drug gallium nitrate from 1st to 28th day by oral administration [6].
Assessment of antiurolithiatic activity
Serum analysis
After the 28th day experimental period, the rats were anaesthetized and blood was collected from the retro-orbital region, centrifuged at 10,000 rpm for 10 min, and the serum collected and analyzed for level of calcium, urea, phosphate, uric acid, and creatinine are expressed as milligram per deciliter [18, 20]. Biochemical estimation of the lipid profile serum total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) were determined by enzymatic assay methods using analytical kits (Biolabo SA., Maizy, France).
Assessment of urinary parameters
The rats were hydrated with distilled water (5 ml/animal), housed in metabolic cages and urine sample was collected under acidified conditions with 1.0 ml of 6.0 N hydrochloric acid for 24 h, on days 0, 7, 14, and at the end of 28 days. The urine samples were centrifuged at 2,500 rpm (REMI, R24) for 5 min and the supernatant was estimated for calcium, oxalate, phosphate, uric acid, and creatinine are expressed as milligrams/decilitre.
Kidney homogenate analysis
At the end of the experimental period, the rats were killed by cervical dislocation, the abdomen opened, and both kidneys removed. The kidneys were carefully removed, washed in ice-cold 0.15 M KCl. Left kidney from each animal was put in 10% formalin and used for histological studies. The right kidney was sliced and homogenized in 10% HCl. The homogenate was centrifuged at 2,500 rpm for 3 min and the supernatant was used for the estimation of calcium, oxalate, creatinine, and phosphate are expressed as milligrams/gram of dry kidney [21].
Histopathological examination
For microscopic evaluation, the tissue piece taken from the kidney of the rats was fixed by 10% neutral phosphate buffered formalin solution and subsequently embedded in paraffin. The sections (5 μm thick) were stained by haematoxylin and eosin to study the histopathological changes and calcium oxalate crystal deposition [22].
Isolation and identification of active compounds
Sample preparation
All the chemicals, including solvents, were of analytical grade from E. Merck, India. The HPTLC plates Si 60F254(20 cm × 10 cm) were purchased from E. Merck (India). Standards of lupeol (97% purity), stigmasterol (99% purity) were purchased from Sigma (New Delhi, India). 100 mg/ml of ethanolic extracts of stem of C. igneus was taken for analysis. The extracts were filtered and vacuum-dried at 45°C. The dried extracts were separately redissolved in 1 ml of ethanol, and sample of varying concentration (1–3 μl) for lupeol and (5–30 μl) for stigmasterol were spotted for quantification. 1 mg of standard 1 (lupeol) and standard 2 (stigmasterol) were prepared in 1 ml of chloroform, and different amounts (5,000–10,000 ng) of lupeol and stigmasterol (1,000–6,000 ng) were loaded onto a TLC plate to get the calibration curve [23].
Thin layer chromatography
A Camag HPTLC system equipped with an automatic TLC sampler ATS4, TLC scanner 3, and integrated software Win CATS version 3, was used for the analysis. Samples were washed on a pre-coated silica gel HPTLC plates Si 60F254 (20 cm × 10 cm) plate of 200 μm layer thickness, for quantification of lupeol and stigmasterol in stem of C. igneus. The samples and standards were applied on the plate as 8-mm-wide bands with a constant application rate of 150 Nl s−1, with an automatic TLC sampler (ATS4) under a flow of N2 gas, 15 mm from the bottom, 15 mm from the side, and the space between two spots was 6 mm in the plate.
Detection and estimation of lupeol and stigmasterol
The linear ascending development was carried out in a Camag twin through chamber (20 cm × 10 cm), which was pre-saturated with a 25 ml mobile phase, with n-hexane: ethyl acetate (80:20v/v) for lupeol, toluene: acetone: acetic acid (8.9: 0.9:0.2 v/v/v) for stigmasterol for 30 min, at room temperature (25 ± 2°C) and 50 ± 5% relative humidity. The length of the chromatogram run was up to 90 mm. Subsequent to the development; the TLC plate was dried in a current of air, with the help of air dryer, in a wooden chamber with adequate ventilation. The dried plate was dipped into freshly prepared Anisaldehyde–sulphuric acid reagent and subsequently in Liebermann–Burchard reagent. Quantitative estimation of the plate was performed in the absorption–reflection mode at 538 nm, using a slit width 6.00 × 0.45 mm, with data resolution 100 μm/step and scanning speed 20 mm/sec. The source of radiation utilized was a tungsten lamp emitting continuous visible spectra of 366 nm. Each was carried out in triplicate.
Calibration curve and linearity
The calibration were performed by analysis of working standard solutions of lupeol (5,000 to 1,0000 ng for C. igneus), stigmasterol (1,000 to 6,000 ng for C. igneus) were spotted on pre-coated TLC plate, using semiautomatic spotter under nitrogen stream. The TLC plates were developed, dried by hot air and photometrically analyzed as described earlier. The calibration curves were prepared by plotting peak area versus concentration (ng/spot) corresponding to each spot.
Isolation of lupeol and stigmasterol by column chromatography
The condensed ethanol extract of stem powder (1 kg) of C. igneus was subjected to column chromatography over TLC grade silica gel. Elution of the column first with petroleum ether, increasing amount of ethyl acetate in petroleum ether, and finally with methanol, yielded a number of fractions. The preparation of solvent systems used to obtain lupeol (4,252 mg/898 g) and stigmasterol (4,278 mg/224 g) were petroleum ether–ethyl acetate (90:10) from fraction 5 and 6. The compounds were detected on TLC plates by spraying with Liebermann–Burchard reagent and heated at 100°C for 10 min.
Statistical analysis
The results were expressed as mean ± SEM for four rats of each group. Statistical analysis was carried out using one way ANOVA followed by the Tukey–Kramer test. p < 0.05 was considered statistically significant.
Results
Biochemical analysis of samples
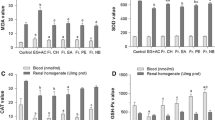
Serum analysis showed that calcium, creatinine, urea, uric acid, and phosphate levels were significantly (p < 0.05) higher in Group II, III, IV,V, VI, VII, VIII, and IX compared to Group I (Table 1). These data indicate marked renal damage in the ethylene glycol-treated albino rats. The data also showed that urea, uric acid, calcium, phosphate, and creatinine levels were significantly retained near normal level in albino rats treated with aqueous and ethanolic extracts of stem of C. igneus (Group III and IV) compared to rats treated with ethylene glycol alone (Group II, positive control). Administration of lupeol Group V (50 mg/kg) and Group VI (100 mg/kg) and stigmasterol Group VII (50 mg/kg) and Group VIII (100 mg/kg) showed significant decrease in the levels of serum urea, uric acid, calcium, phosphate, and creatinine level (p < 0.05) as compared to urolithiatic rats Group II. Similarly, gallium nitrate Group IX (50 mg/kg) showed significant decrease in the levels of serum urea, uric acid, calcium, phosphate, and creatinine level (p < 0.05) as compared to urolithiatic rats Group II.
The lipid profile in control and experimental rats are depicted in Table 2. The ethylene glycol-induced urolithic rats (Group II), there was a significant (p < 0.05) increase in serum TC, TG, LDL and significant (p < 0.05) decrease in HDL cholesterol in serum compared with normal control (Group I). Administration of aqueous and ethanolic extracts of stem of C. igneus (Group III and IV) and isolated compounds lupeol Group V (50 mg/kg), Group VI (100 mg/kg) and stigmasterol Group VII (50 mg/kg), Group VIII (100 mg/kg) for 28th day has significantly (p < 0.05) lowered the high values to normal levels of TC, TG, LDL, and significantly (p < 0.05) increased the HDL cholesterol. This indicates that C. igneus and isolated compounds lupeol and stigmasterol had favorable effects on lipid metabolism of urolithiatic rats.
Urine analysis showed that calcium, oxalate, phosphate, creatinine, and uric acid levels were significantly (p < 0.05) higher in Groups II, III, IV, V, VI, VII, VIII, and IX compared to Group I (Table 3). These data indicate renal damage in the ethylene glycol-treated albino rats. The data also showed that calcium, oxalate, creatinine, phosphate, and uric acid levels were significantly (p < 0.05) lower in rats treated with aqueous and ethanolic extracts of stem of C. igneus (Group III and IV) compared to rats treated with ethylene glycol alone on days 14 and 28 (Group II, positive control). At the 0th day there was no difference among the Groups I–IX in urine calcium, oxalate, creatinine, phosphate, and uric acid levels. Administration of lupeol Group V (50 mg/kg) and Group VI (100 mg/kg) and stigmasterol Group VII (50 mg/kg) and Group VIII (100 mg/kg) showed significant decrease in the levels of urine calcium, oxalate, phosphate, uric acid, and creatinine level (p < 0.05) as compared to urolithiatic rats Group II on days 14 (p < 0.05) and 28 (p < 0.05). Similarly, gallium nitrate Group IX (50 mg/kg) showed significant decrease in the levels of urine calcium, oxalate, phosphate, uric acid, and creatinine level (p < 0.05) as compared to urolithiatic rats Group II. These results of the urinary excretion data clearly support that C. igneus and isolated compounds lupeol and stigmasterol can reduce supersaturating of urine with calculogenic ions.
Kidney analyses were assessed for calcium, creatinine, oxalate, phosphate levels. Ethylene glycol-treated alone (Group II) resulted in increased kidney calcium, oxalate level compared to the negative control rats (Group I), while the administration of aqueous and ethanolic extract of stem of C. igneus significantly (p < 0.05) reduced this calcium and oxalate accumulation (Groups III and IV) are depicted in (Table 4). On treatment with lupeol Group V (50 mg/kg) and Group VI (100 mg/kg) and stigmasterol Group VII (50 mg/kg) and Group VIII (100 mg/kg) for 28th day produced a significant decrease in the calcium and oxalate deposition when compared to their urolithiatic rats Group II. On administration of gallium nitrate (Group IX) 50 mg/kg for 28 days the calcium and oxalate deposition significant decrease. These results indicate that the efficiency of C. igneus and isolated compounds lupeol and stigmasterol in preventing the formation also in dissolving pre-formed calcium oxalate calculi in the kidney.
Histological examination
Examination of kidney paraffin sections showed that Group II rats (ethylene glycol alone, positive control) had the greatest amount of calcium oxalate deposition compared to Group III and IV albino rats (aqueous and ethanolic extract of stem of C. igneus) (Fig. 1). Ethylene glycol-induced albino rats (Group II) had major calcium deposits on the surface of the kidney section, while no such deposits were observed in the negative control albino rats (Group I). Calcium oxalate deposits were lower in albino rats treated with extracts of stem of C. igneus (Groups III and IV) compared to rats treated with ethylene glycol alone (Group 2, positive control). On treatment with lupeol Group V (50 mg/kg) and Group VI (100 mg/kg), and stigmasterol Group VII (50 mg/kg) and Group VIII (100 mg/kg) for 28th day have shown reduction in the calcium and oxalate deposition in the kidney when compared to their urolithiatic rats Group II. On administration of gallium nitrate Group IX (50 mg/kg) for 28 days the calcium and oxalate deposition on the surface of the kidney section was reduced compared to their urolithiatic rats Group II.
Histopathology (×100) of crystalline formation on the surface of rat kidney. a Tissue from negative control rats, b tissue from rats treated with 0.75% ethylene glycol (EG) (positive control), c tissue from rats treated with EG and aqueous extract of stem of Costus igneus (treated group), d tissue from rats treated with EG and ethanolic extract of stem of C. igneus (treated group), e tissue from rats treated with EG and 50 mg/kg lupeol (treated group), f tissue from rats treated with EG and 100 mg/kg lupeol (treated group), g tissue from rats treated with EG and 50 mg/kg stigmasterol (treated group), h tissue from rats treated with EG and 100 mg/kg stigmasterol (treated group), i tissue from rats treated with EG and Gallium nitrate standard drug (treated group). Arrows indicates the presence of calcium oxalate crystals deposition
Quantitative determination of lupeol and stigmasterol in extracts of C. igneus by HPTLC technique
HPTLC fingerprint patterns have been therefore evolved for extracts of C. igneus. Lupeol standard was quantitated accurately using silica gel F254 HPTLC pre-coated plates with the mobile phase for n-hexane:ethyl acetate (80:20v/v),the Rf value for Lupeol was about 0.55. The chromatographs of lupeol and ethanol extract of C. igneus are shown in Fig. 2. The Rf value of lupeol was matched with the Rf value of C. igneus extract was about 0.55. Stigmasterol standard was quantitated accurately using silica gel F254 HPTLC pre-coated plates with the mobile phase toluene:acetone:acetic acid (8.9:0.9:0.2 v/v/v), the Rf value was about 0.58. The chromatographs of stigmasterol and ethanol acetate of C. igneus are shown in Fig. 3. The Rf value of stigmasterol was matched with the Rf value of extract was about 0.58. The amount of lupeol (0.473 mg/100 mg) and stigmasterol (1.913 mg/100 mg) in stem of C. igneus were quantified from the HPTLC plate.
Calibration curve and linearity
The calibration curve was prepared by plotting peak area versus concentration (ng/spot) corresponding to each spot. The regression equation and correlation curves for lupeol in C. igneu were, regression via height y = 149.076 + 32.745x and r = 0.99794 sdv = 0.72, regression via area y = 213.109 + 1731.406x and r = 0.99914 sdv = 0.72. Stigmasterol in C. igneus were, regression via height y = 116.129 + 0.052x and r = 0.99956 sdv = 1.78, regression via area y = 1732.776 + 2.151x and r = 0.99999 sdv = 0.08.
Structural elucidation of isolated compounds
Lupeol melting point 213°C which corresponds to the molecular formulae C30H50O, IR: (KBr) v mas: 3431.79 cm−1 (hydrogen bonded OH stretch), 2,941.78 and 2,357.89 cm−1 (C–H stretch in CH2 and CH3), 2,103.72 cm−1 (C≡C stretch), 1,641.94 cm−1 (C=C symmetric stretch), 1,563.48 (C=C asymmetric stretch), 1,418.25 cm−1 (C–H deformation in CH2 and CH3), 1,365.93 cm−1 (C–H stretch), 1,036.39 cm−1 (C–O stretch of secondary alcohol), 887.86 cm−1 (=C–H bending exocyclic CH2). In the 13C NMR spectrum of lupeol showed δC: δ 37.17 (C-1), δ 20.9 (C-2), δ 79.0 (C-3), δ 38.0 (C-4), δ 55.2 (C-5), δ 18.01 (C-6), δ 27.9 (C-7), δ 38.87 (C-8), δ 50.4 (C-9), δ 34.2 (C-10), δ 19.31 (C-11), δ 20.9 (C-12), δ 35.5 (C-13), δ 40.01 (C-14), δ 25.1 (C-15), δ 29.8 (C-16), δ 40.8 (C-17), δ 48.2 (C-18), δ 48 (C-19), δ 151.01 (C-20), δ 27.4 (C-21), δ 38.7 (C-22), δ 25.1 (C-23), δ 15.3 (C-24), δ 15.98 (C-25), δ 15.98 (C-26), δ 14.5 (C-27), δ 16.13 (C-28), δ 109.34 (C-29) and δ 18.32 (C-30) (Fig. 4).
Stigmasterol melting point 165°C which corresponds to the molecular formulae C29H48O. IR: 3345.5 (br, OH), 2945.9 (C–H str. in CH3 and CH2), 1649.8 (C=C str.), 1452.6 C–H deformation in gem dimethyl), 1055.8 (C–O str. of secondary alcohol). In the 13C NMR spectrum of stigmasterol showed δC: δ 140.75 (C-5), δ 138 (C-6), δ 129 (C-20), δ 121 (C-21), δ 77.45 (C-3), δ 56.8 (C-14), δ 55.9 (C-17), δ 50.1 (C-9), δ 42.3 (C-20), δ 40.5 (C-12), δ 39.6 (C-13), δ 37.2 (C-4), δ 36.5 (C-1), δ 36.5 (C-10), δ 31.9 (C-8), δ 31.6 (C-22), δ 31.6 (C-7), δ 28.9 (C-16), δ 28.9 (C-25), δ 25.4 (C-16), δ 24.3 (C-15), δ 21.2 (C-28), δ 21.1 (C-11,26), δ 21.0 (C-27), δ 19.4 (C-19), δ 18.9 (C-21), δ 12.2 (C-18), δ 12.05 (C-29) (Fig. 5).
Discussion
Kidney stone formation or nephrolithiasis is a complex process that is a consequence of an imbalance between promoters and inhibitors in the kidney. Human kidney stones are usually composed of calcium oxalate (CaOx) [24]. Several studies have examined the effect of the lemon juice [25], Melia azedarach, Tribulus terrestris, Moringa oleifera, Raphanus sativus, Phyllanthus niruri, Homonia riparia, Crataeva nurvala, Coleus aromaticus, Cyclea pertata, Trigonella-foenum graecem, Ammi majus, Ammania baccifera. Costus spiralis on experimentally induced urolithiasis in albino rats [26]. Many in vivo models have been developed to investigate the mechanisms involved in the formation of urinary stones, and to ascertain the effect of various therapeutic agents on the development and progression of the disease [27]. Rats are the most frequently used animals in models of CaOx deposition in the kidneys, a process that mimics the etiology of kidney stone formation in humans. Rat models of CaOx urolithiasis induced by either ethylene glycol alone or in combination with other drug like ammonium chloride, are often used to study the pathogenesis of kidney crystal deposition [28]. In the present study rats were treated with 0.75% ethylene glycol for 28 days. All positive control rats (Group II) developed CaOx depositions during that time.
In this study, aqueous and ethanolic extract of stem of C. igneus and isolated compounds lupeol and stigmasterol had an inhibitory effect on CaOx calculus formation in the kidney of rats. The ethanolic extract also decreased the number of CaOx calculi in the treated group (Group IV), and therefore, demonstrates a therapeutic effect, on the disruption of CaOx calculi formed in the kidney due to ethylene glycol consumption. Administration of lupeol (Groups V and VI) and stigmasterol (Groups VII and VIII) showed significant decrease in the levels of urine and serum calcium, oxalate, phosphate, uric acid and creatinine level (p < 0.05) as compared to urolithiatic rats (Group II) and also could be great significance in diminution of calcium oxalate crystal formation in the kidney. A pentacyclic triterpenoid compound lupeol and a steroid compound stigmasterol were identified and isolated by HPTLC techniques. Previous study has reported that lupeol, a triterpene compound, has been isolated from C. nurvala showed antioxaluric and anticalciuric effects in rats against hydroxyproline-induced hyperoxaluria [29]. A number of lupeol derivatives has been synthesized and studied in rats for their antioxaluric and anticalciuric effect against hyperoxaluria [30]. The earlier investigators isolated lupeol from the methanol extract of stem bark of Grewia titiaefolia and evaluated the cytotoxic properties on in vitro cell lines [31]. The standard drug gallium nitrate has been used to prevent loss of calcium from hypercalcemia, osteopenia, and bone destruction due to metastasis from hyperparathyroidism [32]. Lupeol and lupeol linoleate are effective in alleviating the renal abnormalities in hyperoxaluric rats by regulating oxidative stress and protecting the membrane integrity [33]. All structures of lupeol and stigmasterol were confirmed by comparison with spectral analysis data reported in literature [34–36].
The interesting findings of our study was the observation of high concentration of total cholesterol, triglycerides and LDL levels in serum of ethylene glycol-induced urolithic rats (Group II). Administration of aqueous and ethanolic extract of stem of C. igneus (Group III and IV) and isolated compounds lupeol (Groups V and VI) and stigmasterol (Groups VII and VIII) significantly reduced the cholesterol and triglycerides levels in serum, whereas both the lupeol and stigmasterol show significantly reduction in LDL levels and improvement in HDL levels. The serum lipid profile is generally considered as a reflection of the tissue metabolism and the permeability of cell membrane to various ions, which in turn depends on lipid composition [37]. It has been reported that hyperlipidemia is secondary cause to nephrolithiasis, and it can aggravate the primary renal disorder [38]. Hyperlipidemia has an adverse effect on glomerular function on normal and experimental animals [39]. Serum total cholesterol was raised in the stone-forming rats [40].
In the present study, higher concentration of calcium and oxalate was observed in ethylene glycol-induced urolithic rats. Hypercalcuria might be a factor favoring the nucleation and precipitation of calcium oxalate of apatite (calcium phosphate) from urine and subsequent crystal growth [22]. Treatment with aqueous and ethanolic extracts, and lupeol and stigmasterol reduced the level of calcium and oxalate excretion in ethylene glycol-induced urolithic rats. Uric acid is known to promote calcium oxalate crystal. The predominance of uric acid crystals in calcium oxalate stones and the observation that uric acid binding proteins are capable of binding to calcium oxalate and modulate its crystallization also suggests its primary role in stone formation [41]. In the present study, higher concentration of urinary uric acid was observed in ethylene glycol-induced urolithic rats. The ethanolic extract of C. igneus restored the uric acid level to normal thus reducing the risk of stone formation. Microscopic examination of kidney sections derived from ethylene glycol-induced urolithic rats showed polymorphic irregular crystals deposits inside the tubules which cause more calcium and oxalate deposits was observed. Ethylene glycol-induced urolithic rats treated with aqueous and ethanolic extracts of stem of C. igneus had increased the solubility of CaOx crystal deposits and restored the normal renal tubules. This inhibitory effect may be due to the presence of a triterpenes compound lupeol, a steroid compound stigmasterol has been identified by HPTLC in the present study and antioxidant components, such as ascorbic acid, β-carotene, α-tocopherol, glutathione, phenols, flavonoids, alkaloids [10]. Treatment with ethanolic extract of stem of C. igneus (100 mg/kg), and lupeol and stigmasterol (50 and 100 mg/kg) caused a significant reduction in kidney homogenate: calcium, oxalate, creatinine, and phosphate level, and also reduction in urinary excretion of calcium, oxalate, uric acid, and phosphate level in ethylene glycol-induced albino rats.
Conclusion
The aqueous and ethanolic stem extracts of C. igneus having the isolated compounds lupeol and stigmasterol were found significantly reduced the elevated level of calcium oxalate ions in treated albino Wistar rats. The histopathological findings also confirm the effect of the C. igneus on animals treated with extract principally having lupeol and stigmasterol compounds. All these observation provided the basis for the conclusion that C. igneus stems extract could inhibit the stone (CaOx crystals) formation induced by ethylene glycol in albino Wistar rats.
References
Soundararajan P, Mahesh R, Ramesh T, Hazeena Begum V (2006) Effect of Aerva lanata on calcium oxalate urolithiasis in rats. Indian J Exp Biol 44:981–986
Daudon M, Bader CA, Jungers P (1993) Urinary calculi: review of classification methods and correlations with etiology. Scanning Microsc 7(3):1081–1106
Atmani F, Slimani Y, Mimouni M, Hacht B (2003) Prophylaxis of calcium oxalate stones by Herniaria hirsuta on experimentally induced nephrolithiasis in rats. BJU Int 92:137–140
Goldfarb DS, Coe FL (1999) Prevention of recurrent nephrolithiasis. Am Fam Physician 60(8):2269–2276
Abraham PA, Smith CL (1984) Medical evaluation and management of calcium nephrolithiasis. Med Clin North Am 68(2):281–299
Khan NI, Shinge JS, Naikwade NS (2010) Antilithiatic effect of Helianthus annuus Linn. Leaf extract in ethylene glycol and ammonium chloride induced nephrolithiasis. Int J Pharm Pharm Sci 2:180–184
Aggarwal S, Tandon CD, Forouzanveh M, Simgla SK, Kiran R, Jethi RK (2000) Role of biomolecules from human renal stone matrix on COM crystal growth. Mol Cell Biochem 210:109–119
Atmani F, Farell G, Lieske JC (2004) Extract from Herniaria hirsuta coats calcium oxalate monohydrate crystals and blocks their adhesion to renal epithelial cells. J Urol 172:1510–1514
Bensatal A, Ouahrani MR (2008) Inhibition of crystallization of calcium oxalate by the extraction of Tamarix gallica L. Urol Res 36:283–287
Devi VD, Urooj A (2010) Nutrient profile and antioxidant components of Costus speciosus Sm. and Costus igneus Nak. Indian J Nat Prod Resour 1(1):116–118
Devi VD, Urooj A (2008) Hypoglycemic potential of Morus indica L and Costus igneus Nak: a preliminary study. Indian J Exp Biol 46(8):614–616
Viel TA, Cristina D, Silva Monterio AP, Lima-Landman MTR, Lapa AJ, Souccar C (1999) Evaluation of the antiurolithiatic activity of the extract of Costus spiralis Roscoe in rats. J Ethnopharmacol 66:193–198
Bhat V, Naveen A, Akshay K, Sikarwar Mukesh S, Patil MB (2010) Antidiabetic activity of insulin plant (Costus igneus) leaf extract in diabetic rats. J Pharm Res 3(3):608–611
Jose B, Reddy LJ (2010) Analysis of the essential oils of the stems, leaves and rhizomes of the medicinal plant Costus pictus from Southern India. Int J Pharm Pharm Sci 2(2):100–101
Hadjzadeh MAR, Khoei A, Hadjzadeh Z, Parizady M (2007) Ethanolic extract of Nigella sativa L seeds on ethylene glycol induced kidney calculi in rats. Urol J 4:86–90
Vimal S, Joshi Bharat B, Parekh Mihir J, Joshi Ashok DBV (2005) Herbal extract of Tribulus terrestris and Bergenia ligulat inhibit growth of calcium oxalate monohydrate crystals in vitro. J Cryst Growth 275:1403–1408
Huang HS, Ma MC, Chen J, Chen CF (2002) Changes in the oxidant-antioxidant balance in the kidney of rats with nephrolithiasis induced by ethylene glycol. J Urol 167:2584–2593
Selvam P, Kalaiselvi P, Govindaraj A, Murugan VB, Sathishkumar AS (2001) Effect of A. lanata leaf extract and Vediuppu chunnam on the urinary risk factors of calcium oxalate urolithiasis during experimental hyperoxaluria. Pharmacol Res 43:89–93
Karadi RV, Gadge NB, Alagawadi KR, Savadi RV (2006) Effect of Moringa oleifera Lam. Root wood on the ethylene glycol induced urolithiasis in rats. J Ethnopharmacol 105:306–311
Anand R, Patnaik GK, Kulshrestha DK, Dhawan BN (1994) Activity of certain fractions of Tribulus terrestris fruits against experimentally induced urolithiasis in rats. Indian J Exp Biol 32(1):548–552
Varalakshmi P, Shamila Y, Latha E (1990) Effect of Crataeva nurvala in experimental urolithiasis. J. Ethnopharm 20:313–321
Dodoala S, Diviti R, Koganti B, Prasad KVSEG (2010) Effect of ethanolic extract of Phyla nodiflora (Linn.) greene against calculi producing diet induced urolithiasis. Indian J Nat Prod Resour 1(3):314–321
Badami S, Gupta MK, Ramaswamy S, Rai SR, Nanjaian M, Bendell DJ, Subban R, Bhojaraj S (2004) Determination of betulin in Grewia titiaefolia by HPTLC. J Sep Sci 27:129–131
Daudon M, Jungers P (2001) Epidemiologie de la lithiase urinaire. Eurobiologiste 253:5–15
Touhami M, Laroubi A, Elhabazi K, Loubna F, Zrara I, Eljahiri Y, Oussama A, Grases F, Chait A (2007) Lemon juice has protective activity in a rat urolithiasis model. BMC Urol 7:18
Prasad KVSRG, Sujatha D, Bharathi K (2007) Herbals drug in urolithiasis: a review. Pharma Rev 1(1):175–178
Atmani F, Slimani Y, Minouni M, Aziz M, Hacht B, Ziyyat A (2004) Effect of aqueous extract from Herniaria hirsute L. on experimentally nephrolithiasic rats. J Ethnopharmacol 95:87–93
Khan SR, Glenton P (1995) Deposition of calcium phosphate and calcium oxalate crystals in the kidneys. J Urol 153:811–817
Anand R, Patnaik GK, Kulshreshtha DK (1994) Antiurolithiatic activity of lupeol, the active constituents isolated from Crataeva nurvala. Phytother Res 8:417–421
Vidya L, Varalakshmi P (2000) Control of urinary risk factors of stones by betulin and Lupeol in experimental hyperoxaluria. Fitoterapia 75:533–543
Badami S, Vijayan R, Mathew N, Chandrashekhar R, Godavarthi A, Dhanaraj SA, Suresh B (2003) In vitro cytotoxic properties of Grewia titiaefolia bark and lupeol. Indian J Pharmacol 35:250–251
Bernstein LR (1998) Mechanism of therapeutic activity for gallium. Pharmacol Rev 50:665–682
Sudhahar V, Kandaswamy Veena C, Varalakshmi P (2008) Antiurolithic effect of lupeol and lupeol linoleate in experimental hyperoxaluria. J Nat Prod 71(9):1509–1512
Jain PS, Bari SB (2010) Isolation of lupeol, stigmasterol and campesterol from petroleum ether extract of woody stem of Wrightia tinctoria. Asian J Plant Sci 9(3):163–167
Kamboj A, Saluja AK (2011) Isolation of stigmasterol and β-sitosterol from petroleum ether extract of aerial parts of Ageratum conyzoides (Asteraceae). Int J Pharm Pharm Sci 3(1):94–96
Hague E, Isham N, Gupta DD, Hossain M, Sneeekhar HU (2008) Triterpenoids from the stem bark of Crataeva nurvala. Dhaka Univ J Pharm Sci 7(1):71–74
Scheuer H, Gwinner W, Hohbach J, Grone EF, Brandes RP, Malle E (2000) Oxidant stress in hyperlipidemia-induced renal damage. Am J Physiol 278:F63–F74
Sumathi R, Jayanthi S, Kalpanadevi V, Varalakshmi P (1993) Effect of DL alpha-lipoic acid on tissue lipid peroxidation and antioxidant systems in normal and glycollate treated rats. Pharmacology 27:309–318
Sumathi R, Jayanthi S, Varalakshmi P (1995) Impaired lipid metabolism in calcium oxalate stone forming rats and DL-alpha-Lipoic acid supplementation. Nutr Res 15:59–70
Varalakshmi G, Shanmuga sundaram KR, Venugopal A (1977) Blood lipids in renal stone disorder. Indian J Med Res 66:840–846
Grover PK, Ryall RL, Marshall VR (1990) Effect of urate on calcium oxalate crystallization in human urine, evidence for a prominent role of hyperuricosuria in urolithiasis. Clin Sci 79:9
Acknowledgments
The authors acknowledge DBT, New Delhi (BT/PR10018/NNT/28/2007) for providing financial support and M.K acknowledges DBT for Junior Research Fellowship, Dr. S. John Britto, Director, Rapinat herbarium, St. Joseph College, Tiruchirapalli, Tamil Nadu for identifying the plants. Authors also thank Dr. N. Ramachandran, Vice-Chancellor of Periyar Maniammai University for continued support for this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manjula, K., Rajendran, K., Eevera, T. et al. Effect of Costus igneus stem extract on calcium oxalate urolithiasis in albino rats. Urol Res 40, 499–510 (2012). https://doi.org/10.1007/s00240-012-0462-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-012-0462-6