Abstract
The effect of seed phytate content (regular and low) on the composition (protein and mineral content), protein quality [in vitro protein digestibility-corrected amino acid score (IVPDCAAS)], iron bioavailability, and functionality (solubility, oil/water holding capacity, foaming capacity and stability, and emulsion stability) of pea flours and extracted protein isolates was investigated. There was 37–45% less phytate in the flours of the low-phytate varieties compared to the regular varieties and approximately 39% less for the isolates. Upon extraction of protein, phytate increased over threefold, but for the mineral ions, this was selective in that Fe2+ ions increased more than threefold, while Ca2+ content halved. The phytate content did not influence the IVPDCAAS of the flours or isolates. The functional properties of the isolates and flours were largely similar between the low and regular phytate varieties. For each variety, iron was more bioavailable in the flours (10.5–22.0 ng ferritin/mg protein) than in the isolates (2.9–16.5 ng/mg). The low-phytate flours (20.6 ng/mg) had overall higher iron bioavailability than the regular phytate pea flours (10.7 ng/mg). For the isolates, this trend was not significant, possibly due to high intra-variety variation and the limited number of samples; however, the mean iron bioavailability value of the three low-phytate isolates was three times greater than that of the two regular phytate isolates. In conclusion, protein isolates extracted from low-phytate varieties did not show deleterious or positive impacts on the functional characteristics or protein quality; more evidence is required for iron bioavailability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The current trend of utilizing plant, rather than animal, sources of protein for human consumption was predicted 4 decades ago by Cheryan [1], among other academic researchers, whose reasoning included the energy intensiveness of animal production and limited land availability, as contributing to this shift. Pulses, such as lentils, chickpeas, beans, peas, and faba beans, are emerging as effective plant protein alternatives to soy. The increasing appeal of pulses to farmers, the food industry, researchers, and consumers is founded in their universal presence in global agriculture, ability to fix atmospheric nitrogen, eco-friendliness, nutritional profile, health promoting bioactive components, versatility, and affordability. Pulses can be utilized as whole seeds, whole flour, and pulse-derived ingredients, including protein extract (> 80% protein), starch-rich flour, protein-rich flour, and fiber fraction [2]. The most valuable component for food applications is protein. Besides the nutritional value it provides, a protein’s functionality (solubility, emulsifying, foaming, water and fat binding, rheological properties, etc.) determines its usefulness as a food ingredient [3].
Over the decades, there has been a paradigm shift from focusing on nutrient content to nutrient bioavailability [4]. In the context of protein, components that are co-extracted with protein from the food matrix can influence the protein bioavailability, depending on the nature of interactions and their propensity to destruction during food digestion. The interactions between the protein and other components may occur inherently in the pulse matrix prior to processing or they may be induced by food processing. Anti-nutrient effects due to compounds, such as phenolics, condensed tannins, lectins, protease inhibitors, and phytic acid, are inherent in pulses [2]. Phytic acid, a reservoir for phosphorus, is known to adversely affect protein digestibility and the bioavailability of minerals which are vital for metabolism in humans [4, 5]. This is especially important for bioavailability of iron, as iron deficiency is prevalent worldwide and has the highest disease burden of all nutritional inadequacies [6]. Notwithstanding, phytate also can have positive effects, such as reducing the risk of some forms of cancer and cardiovascular disease [5]. As reported by Shi et al. [7], phytate, the salt form of phytic acid, is approximately 8–10 mg/g dry matter in whole peas.
Various strategies have been employed to reduce the negative effects of phytate on protein and minerals. For example, exogenous phytase has been applied during the production of pea protein isolate to reduce or eliminate the phytate content in the product [8]. The enzymatic partial removal of the phosphate groups from phytate hinders its chelating ability, thus potentiating an increase in mineral bioavailability. However, enzyme application adds time and costs to the extraction process. Alternatively, a solution for reducing phytate content in pulses has been sought via plant breeding. Breeders have developed low-phytate pea varieties with the objective of improving the bioavailability of (micro)nutrients, specifically protein and iron, as well as other minerals. Warkentin et al. [9] developed low-phytate pea varieties from CDC Bronco, a regular phytate pea variety.
Substantial documentation of the low-phytate pea varieties has been reported, including the influence of iron, phytate, carotenoid, and polyphenol concentration on iron bioavailability [10,11,12]. However, these studies exclusively focused on pea flour; protein isolates extracted from low-phytate pea varieties were not examined. The research reported here investigated the in vitro digestibility of protein and bioavailability of iron in pea protein isolates produced from low and regular phytate varieties. Flours of low and regular phytate varieties were also examined as a comparison to the protein isolates. Since phytate is inherently associated with protein in the pulse matrix, concentrating protein from flour also leads to a concentration effect of phytate in the protein extract [8]. Therefore, we hypothesized that an increase in bioavailability of iron and protein would occur in protein extracts generated from low-phytate pea varieties. A functionality comparison of the flours and protein isolates from low and regular phytate peas was also conducted due to the importance of these properties for using the flours and isolates as food ingredients, and the novel nature of the low-phytate varieties. We hypothesized that within the flours or isolates, the samples from low-phytate peas would have similar functional properties as ones from the regular varieties. This was a small, well-defined study with a limited number of samples; however, findings from this work could lead to further development of novel pea protein ingredients with unique nutritional aspects for certain market segments, such as supplements for athletes or infants.
Materials and methods
Materials
For this study, two biological replicates of five varieties were utilized. The biological replicates were grown in different locations in Saskatchewan (Canada); Kamsack (KAM, in 2019) and Rosthern (ROS, in 2020). Two of the five varieties, CDC Amarillo, and CDC Bronco, were regular phytate varieties and the other three, 4802-8-87Y-L, 4802-8-1Y-L, and 4803-4-78G-L were low-phytate (LP) varieties herein and henceforth referred to as LP-1, LP-2, and LP-3, respectively. LP varieties were previously developed from CDC Bronco as the parent variety [9]. CDC Amarillo was included for comparison, as it is widely grown in Canada and often used as a check variety. The peas were milled to flours using a household WonderMill (Grainmaster, UK).
Extraction of protein
The pea flours were defatted in hexane (1:3 w/v) with stirring for 10 min, and thereafter, the suspension was vacuum filtered using Whatman No. 1 filter paper (Whatman International Ltd., Maidstone, UK). The defatting process was repeated twice. The defatted flours were air-dried overnight under a fume hood to evaporate residual hexane. Protein was isolated from the defatted pea flours using an alkaline extraction with subsequent isoelectric precipitation method [13]. Pea flour was suspended in distilled and deionized water (ddH2O) in a 1:10 (w/v) ratio and the pH of the suspension was adjusted to 9.5 using 1 M NaOH, before incubation with stirring at room temperature for 1 h. The suspension was centrifuged (4500 × g, 4 °C, 15 min) (Sorvall RC-6 Plus centrifuge, Thermo Scientific, Ashville, NC, USA) and the supernatant was decanted with the aid of glass wool filtration to remove any insoluble particles. Isoelectric precipitation of the solubilized protein was achieved by adjusting the pH of the supernatant to 4.5 using 1 M HCl. After centrifugation, the extracted protein was collected in pellet form and washed twice with ultra-pure water. The extracted protein was freeze-dried and stored at 4 °C until use.
Composition of the flour and extracted pea protein
Protein and moisture content
The protein content of flours and isolates was determined by measuring total nitrogen using the micro-Kjeldahl digestion and distillation unit (Labconco Corp., Kansas City, MO) following the AOAC official method (960.52; [14]). A protein factor of 6.25 was used to convert nitrogen content into protein content (%N × 6.25). Moisture content was measured following the AOAC official method (925.10; [14]).
Phytate content
Phytate content was determined based on measurement of phosphorus released when phytate is hydrolyzed by phytase and alkaline phosphatase (K-PHYT 05/19; Megazyme International, Ireland). The phytate was extracted from pea flour or isolate by suspending 1.0 g or 0.5 g, respectively, in 20 mL of 0.66 M HCl and incubated with stirring for 16 h. A 1 mL aliquot of the suspension was centrifuged (15,871 × g, room temperature, 10 min; Eppendorf centrifuge 5424, Eppendorf, Hamburg, Germany), after which 0.5 mL of the supernatant was neutralized by an equal amount of 0.75 M NaOH solution. A 50 µL aliquot of the neutralized sample was analyzed for free and total phosphorus in varying amounts of distilled water and buffer solutions as outlined in the kit. Phytase and alkaline phosphatase enzymes were applied sequentially for total phosphorus determination. The enzymatic reactions were terminated by addition of 0.30 mL of trichloroacetic acid (50% w/v) before centrifugation as described earlier. An ascorbic acid (10% w/v)/1 M sulphuric acid solution was mixed with a 5% (w/v) ammonium molybdate solution in a 5:1 ratio to produce the color reagent for quantification of phosphorus. To 1 mL of sample, 0.5 mL of the color reagent was added followed by vortexing, then incubation at 40 °C for 1 h. After cooling for 10 min, the mixture was vortexed, and 1 mL was pipetted into a 1.5 mL semi-micro-cuvette and the absorbance was read at 655 nm using a spectrophotometer (Genesys™ 10S UV–Vis spectrophotometer, Thermo Fisher Scientific, Waltham, MA). A standard curve was prepared from standard phosphorus solutions that were treated for colorimetric quantification, as was done for the samples.
Mineral ion content
Specifically, Fe2+, Zn2+, Ca2+, and P were quantified using the inductively coupled plasma-atomic emission spectrometry (ICP-AES). Each sample (500 mg) was pre-digested with 3 mL of a concentrated ultra-pure nitric acid and perchloric acid mixture (60:40 v/v) for 16 h at room temperature. Samples were then placed in a digestion block (Martin Machine, Ivesdale, IL, USA) and heated incrementally over 4 h to a temperature of 120 °C with refluxing. After incubating at 120 °C for 2 h, 2 mL of concentrated ultra-pure nitric acid was subsequently added to each sample before raising the digestion block temperature to 145 °C for an additional 2 h. The temperature of the digestion block was then raised to 190 °C and maintained for at least 10 min before samples could cool at room temperature. Digested samples were re-suspended in 20 mL of ultra-pure water prior to analysis using ICP-AES (Thermo iCAP 6500 Series, Thermo Scientific, Cambridge, United Kingdom) with quality control standards (High Purity Standards, Charleston, SC, USA) following every ten samples. Yttrium purchased from High Purity Standards (10M67-1) was used as an internal standard. All samples were digested and measured with 0.5 μg/mL of Yttrium (final concentration) to ensure batch-to-batch accuracy and to correct for matrix inference during digestion.
Functional properties
Solubility
The solubility of the pea flours and isolates differing in phytate content were determined at pH 7.0 [3]. A solution of 200 mg flour or isolate in 20 mL of Milli-Q water was prepared and stirred for 1 h while maintaining the pH at 7.0. Thereafter, the solution was left to stand for 10 min to induce sedimentation of insoluble particles. Eight grams of the solution was centrifuged (Sorvall ST 8, Thermo Fisher Scientific, Waltham, MA) at 4180 × g at room temperature for 10 min. The supernatant was analyzed for protein content as previously described and protein solubility was determined by expressing the protein content of the supernatant as a percentage of the flour or protein extract.
Water and oil holding capacity
The water holding capacity (WHC) was determined by measuring the quantity of water absorbed per gram of thoroughly wetted flour or protein extract [3]. To this end, 0.5 g of sample was weighed into a tube, and then, 5 mL of water was added followed by vortexing for 10 s at 5 min intervals for a total period of 30 min. Thereafter, the sample was centrifuged (Sorvall ST 8) at 1000 × g for 15 min and the supernatant was discarded as unabsorbed water. WHC calculation:
WHC = (weight of wet sample—weight of initial sample)/weight of initial sample.
The same procedure was followed for oil holding capacity (OHC) with the use of canola oil in place of water.
Foaming capacity and stability
The foaming capacity (FC) and foaming stability (FS) were determined using a single test [3]. An aliquot of pea flour or isolate was suspended in water to make a 1% w/w suspension whose pH was adjusted to 7 before stirring overnight at room temperature. The pH was readjusted to 7 just before analysis, which involved measuring 15 mL of the suspension into a narrow 400 mL beaker and homogenizing (Polytron PT2100, Kinematica AG, Lucerne, Switzerland) at 11,000 rpm for 2 min with the blade part of the homogenizer probe submerged in the suspension. The formed foam and any remaining liquid were transferred immediately into a 50 mL graduated cylinder and the volume of foam was measured at the starting time (t = 0) and finishing time (t = 30 min) of incubation on a vibration free surface at room temperature. The foaming capacity and stability were calculated as follows:
%FC = (volume of foam at t = 0/initial volume of starting solution (15 mL)) × 100%
%FS = (volume of foam at t = 30/volume of foam at t = 0) × 100%.
Emulsion stability
Emulsion stability (ES) was determined using the same suspensions prepared for the foaming capacity and stability test at pH 7 [3]. A 5 mL aliquot was measured into a 50 mL plastic centrifuge tube followed by 5 g of canola oil. With the probe at the oil–water interface, the contents of the tube were homogenized (as described for the foaming test) to produce an emulsion. The emulsion was transferred immediately into a wide mouth 10 mL graduated cylinder and incubated on a vibration free surface at room temperature for 30 min during which the emulsion exuded an aqueous layer (Vaq). The ES was calculated as follows:
%ES = ((Vaq before emulsification (5 mL)—Vaq at t = 30)/ Vaq before emulsification) × 100%.
Nutritional evaluation
Amino acid composition and score
Samples were analyzed for amino acid composition at the Protein Quality Research Laboratory at the University of Manitoba (Winnipeg, MB), according to established methods. The tryptophan content was quantified following method 13,904 of the ISO (International Organization for Standardization, 2016) as described in detail by Nosworthy et al. [15]. Methionine and cysteine were quantified following method 985.28 [14]. The concentrations of the remaining 15 amino acids were determined following the AOAC Official Method 982.30. Analyses were conducted without repetitions on each sample from each location (KAM 2019 and ROS 2020); therefore, no statistical analysis was done.
The amino acid score for each essential amino acid was calculated as the ratio of the content of the essential amino acid of the protein under investigation to that of the reference protein based on FAO/WHO guidelines [16]. The essential amino acid with the lowest amino acid score was identified as the limiting essential amino acid. In mg amino acid/g protein, the reference protein is as follows: histidine, 19; isoleucine, 28; leucine, 66; lysine, 58; methionine + cysteine, 25; phenylalanine + tyrosine, 63; threonine, 34; tryptophan, 11; valine, 35 (FAO, 1991).
In vitro protein digestibility and in vitro protein digestibility-corrected amino acid score
The in vitro protein digestibility (IVPD) of flour and isolate samples was determined using the pH drop method utilizing a multienzyme solution [17]. The multienzyme solution was prepared using 31 mg of chymotrypsin (bovine pancreas ≥ 40 units/mg protein), 16 mg of trypsin (porcine pancreas 13,000–20,000 BAEE units/mg protein), and 13 mg of protease (Streptomyces griseus ≥ 15 units/mg solid) dissolved in 10 mL of MilliQ water. The solution was adjusted to pH 8.0 with 0.1 M NaOH and 0.1 M HCl before incubating in a water bath at 37 °C for 1 h. Sample solutions consisting of 62.5 ± 0.5 mg flour or protein extract in 10 mL MilliQ water were incubated in a water bath at 37 °C for 1 h after which pH was adjusted to 8.0. The sample solutions were left to stabilize for 10 min before adding 1 mL of the multienzyme solution. The initial pH was recorded, and subsequent pH changes were recorded every 1 min for 10 min. The in vitro protein digestibility was calculated as
where ΔpH10min refers to the change in pH from initial 8.0 to the end of the 10 min.
The score of the limiting essential amino acid was multiplied with the IVPD to obtain the in vitro protein digestibility-corrected amino acid score (IVPDCAAS).
Bioavailability of iron
An established in vitro digestion/Caco-2 cell culture model was used to assess the iron bioavailability of the flours and protein isolates. The Caco-2 bioassay was performed according to the most recent methods described in detail by Glahn [18]. The bioassay works according to the following principle: in response to increases in cellular iron concentrations, Caco-2 cells produce more ferritin protein; therefore, iron bioavailability was determined as the increase in Caco-2 cell ferritin production expressed as a ratio to total Caco-2 cell protein (ng ferritin per mg of total cell protein) after exposure to a digested sample. Ferritin was measured by enzyme-linked immunoassay (Eagle Biosciences, Amherst, NH, Product number FRR31-K01) and total cell protein concentrations were quantified using the Bio-Rad DC™ protein assay kit (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Statistical analyses
All the analyses in this study were conducted in triplicate for each of the growing locations, KAM 2019 and ROS 2020, with the exception of amino acid composition. For statistical analyses, samples from these two locations were considered as replications and, therefore, results from the replicates were averaged and presented as mean ± standard deviation (n = 2). The means within the flours or isolates were compared as a function of variety using a one-way analysis of variance and a Tukey test to determine statistically significant differences (P < 0.05). For iron bioavailability, ANOVA with Fisher’s least significant difference for variety and location was used.
Results and discussion
Composition of flours and protein isolates
Protein content
The protein content of the pea flours ranged between 25 and 26% with no clear distinction between flours generated from regular and low-phytate peas, as shown in Table 1. These results indicate that the protein content of the flours was not related to the level of phytate in the pea varieties. In a study on the agronomic performance of low-phytate peas, Lindsay et al. [19] reported the protein content in low-phytate and regular varieties to be similar, with an overall mean of 26.5%. For 12 regular phytate pea varieties grown at four different locations over 4 years, Nosworthy et al. [20] reported that variety genetics accounted for 39% of the variability in the protein content (20.8–25.6%), whereas year accounted for only 4% of the variability. However, the authors also found that the two-way interactions of year × location and year × genotype were significant for protein content. A similar or lower range in protein content (23.4–26.1%) than the current study was reported by Maharjan et al. [21] for nine pea genotypes grown at two different locations; however, the differences between the varieties were still significant and there was a significant genotype × environment effect. In a review by Hall et al. [22], the upper level of protein found in peas was 31%.
Protein extracts from the different pea flours in the present study were characterized by a protein content ranging from 85 to 90%. The protein content of the isolates was similar, as indicated by the absence of significant differences (Table 1). Therefore, neither the phytate levels of the pea varieties from which the isolates were extracted, nor differences in the varieties in a particular phytate level category (regular or low) influenced the protein content of the isolates. Regardless of the similarity in protein content of the isolates, the extraction efficiency ranged from 52 to 57%, with no overall distinction between peas from the regular and low-phytate varieties (data not shown). The protein content of isolates extracted from different pea varieties using alkaline extraction and isoelectric precipitation, as in the current study, ranged between 90.8% and 94.7% [23]. Lam et al. [24] reported negligible differences in both the protein content (89.7–92.5%) and extraction efficiency (70.3–73.5%) of pea protein isolates as a function of variety.
Phytate content
As expected, the phytate content was higher in the flours of the regular phytate varieties (7.1 and 7.7 mg phytate/g) than the low-phytate pea varieties (4.2–4.5 mg phytate/g), as shown in Table 1. There was up to a 45% reduction in phytate content in the low-phytate pea varieties as compared to the regular ones. A similar result (of approximately 50% reduction) was noted in a related study on pea by Liu et al. [11]. Flours from CDC Bronco and LP-1 (varieties equal to the current study; grown in 2017) were analyzed for phytate content in an in vivo study, utilizing the Caco2 assay, wherein the implications of a diet containing low-phytate pea variety flours on iron bioavailability were investigated [12]. The phytate content was 5.82 ± 0.01 mg/g for CDC Bronco and 3.84 ± 0.05 mg/g for LP-1 flour. These values are lower than those reported in the current study and this can be attributed to the presoaking and cooking treatment of the peas before producing the flours in the study by Warkentin et al. [12], unlike the flours from raw peas used in the current study. Hídvégi and Lásztity [25] reported a phytate content range of 7.2–12.3 mg/g in pea flour from one variety grown over 2 different years. In the current study, phytate content was similar among the varieties within each group (i.e., the regular phytate content group as well as within the low-phytate group). Similar to these results, Maharjan et al. [21] reported the phytate content of nine varieties (4 Kaspa type, 4 dun type, 1 white) to not be significantly affected by genotype. However, the authors did find a significant difference in the phytate content as a function of growing location. Shi et al. [7] found no difference in the phytic acid content of whole yellow and green pea (9–10 mg/g), but noted a significant increase in phytic acid to 12 mg/g after the seeds had been split, as the dehulling process concentrated the phytate in the flour.
In the present study, the phytate content of the protein isolates was higher than that of the flours (Table 1). Importantly, the isolates extracted from the flours of the regular varieties had a higher phytate content (24.8–25.5 mg/g) than those extracted from the low-phytate varieties (14.4–15.7 mg/g). Since phytate is inherently associated with protein in the pulse matrix, extracting the protein leads to a concentration effect on phytate [8]. Therefore, it is logical that the phytate concentration was higher in the protein isolates than in the flours. In fact, the concentration increased by a factor of around 3.5 for the low-phytate varieties, and a factor of 3.2 and 3.6 for CDC Bronco and CDC Amarillo, respectively. This matched the increase in protein in the isolates, which was concentrated by 3.3–3.5-fold from the flour. Carnovale et al. [26] reported the ratio of protein to phytic acid to be the same in an air-classified protein fraction as the initial faba bean flour. The interactions between phytate and protein are governed by pH, being electrostatic at low pH, because protein will have a net positive charge, while phytic acid has a negative one [1]. Cheryan and Rackis [1] hypothesized that at pH > 6, protein–cation–phytic acid interactions, which increasingly become soluble with increase in pH (up to 10), are formed with the minerals being multivalent cations. A soy protein isolate precipitated from a protein extract (isolated at pH 7) at pH 4.5 exhibited the highest quantity of phytate in comparison to precipitation at higher pHs [27].
Mineral ion content
The mineral ion content of the flours and protein isolates is shown in Table 2. The concentrations of the ions in the flours decreased in the order P, Ca2+, Fe2+, and Zn2+. No significant differences were observed in the concentration of any of the four mineral ions across all the varieties, and therefore, there was no overall trend in the mineral content of the flours based on phytate content. On average, the flours contained 49 ppm Fe2+, 38 ppm Zn2+, 739 ppm Ca2+, and 3678 ppm P. While the low-phytate and regular phytate varieties contained similar levels of total P, the low-phytate varieties had a much higher proportion as free P (as shown by the phytate quantification analysis, results not shown). This would reflect less P stored in the form of phytate. Of the four minerals, the highest variability (largest relative range) across the varieties was for Ca2+ content.
The mineral ion content results of the protein isolates were interesting because they revealed that the protein extraction process, which led to the concentration of protein (Sect. 3.1.1) and phytate (Sect. 3.1.2) in the isolate, is selective in regard to mineral ions. The concentration of Fe2+ increased markedly in the isolates by a factor of 4–4.8. This may be due to the presence of protein–iron, phytate–iron, or protein–phytate–iron complexes that would enable the iron to stay with the protein phase and not be discarded during the extraction procedure. The concentration of P was also much higher in the isolates than in the flours and increased by 3.6–3.8-fold in the regular varieties and 2.4–2.6 in the low-phytate varieties; however, the CDC Bronco isolate had similar P levels as both LP-1 and LP-2. There were no significant differences in the Zn2+ content of the isolates, which had marginally higher levels than the flours. In contrast, the concentration of Ca2+ in the isolates was on average less than half that of the flours. Similar to the flours, the Ca2+ content had the highest variability in the isolates.
Functionality
Solubility
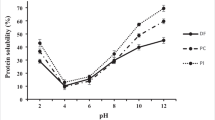
The solubility of the protein in the flours and isolates obtained from the different pea varieties is shown in Table 3. Among the flours, CDC Amarillo had the highest protein solubility at almost 89% followed by CDC Bronco; however, the solubility of protein for CDC Bronco was not statistically different from that of LP-3. The solubility of protein in the flours of the low-phytate varieties ranged between 57 and 64%, and decreased in the order LP-3, LP-2, and LP-1, although the variation was not statistically significant. Overall, the regular varieties had higher solubility than the low-phytate ones with mean values of 79.7% and 60.6%. Phytate has been reported to form insoluble complexes with protein; however, this is highly dependent on the pH of the medium, with the most detrimental effects being at pHs where the protein has a net positive charge [1, 28, 29]. Furthermore, the solubility of phytic acid (in faba bean) at pH 7 has been reported to be approximately 85% [26]. The protein composition (legumin–vicilin ratio, secondary structure, etc.) of the varieties in this study has not yet been studied and may provide useful information related to solubility. Dai et al. [28] found that the phytate content of 100 barley varieties correlated to not only total protein content but also the content of specific protein fractions.
The protein isolates had higher solubility than flours for the low-phytate varieties only, whereas for the regular varieties, the protein isolate solubility was lower than the respective flours. For the protein isolates, the solubility of protein was overall higher for the low-phytate (mean value of 68.9%) versus regular varieties (mean value of 63.4%); however, most differences were minimal. Nonetheless, LP-1 (71.3%) and LP-2 (68.0%) had significantly higher protein solubility than CDC Amarillo (61.1%) with all other differences being not significant. Hídvégi and Lásztity [25] reported no change in the solubility of different protein preparations (soy concentrate and isolate; vital wheat gluten) with the addition of exogenous phytate. Similarly, Taherian et al. [29] reported minimal differences in the pH 7 solubility of pea protein isolates produced by acid extraction–ultrafiltration/diafiltration containing different amounts of phytate, whereas, at other pHs, the isolates with lower phytate levels had better solubility. This corresponds to the work of Ali et al. [30] where phytate content in soy protein isolates only influenced protein solubility at pHs < 4.5.
Pea protein isolate extracted from CDC Leroy flour exhibited a solubility of 61.4% [31], while that of five different lines of pea ranged between 64.2% and 79.9% [23]. Results from a study by Shevkani et al. [23] on the structural and functional characterization of pulse protein isolates indicated a probable relationship between protein solubility and the secondary structure components alpha-helices, beta-sheets, and anti-parallel beta-sheets. A high solubility implies more hydrophilic (protein–solvent) interactions, while lower solubility highlights more abundant hydrophobic interactions among the proteins.
Water and oil holding capacity
The WHC of flour from the regular pea varieties was 1.2 g/g and was significantly, but minimally, lower than that of the flour from the low-phytate varieties (1.3–1.4 g/g), as shown in Table 3. The findings on flour from regular pea varieties by Setia et al. [32] were 1.1–1.2 g/g, being consistent with results from the current study. In the current study, the WHC of the protein isolates was 1.8–2.3 g/g, with no distinct variation between the regular and the low-phytate varieties (Table 3). Furthermore, LP-3 had the highest WHC of the 5 varieties and LP-1 had the lowest. In comparison with the flour samples, the WHC values of the protein isolates were higher by 28–75%. In a study on the functional properties of protein isolates from different pea varieties by Stone et al. [3], the WHC values ranged between 2.4 g/g and 2.6 g/g. The WHC of protein isolate from five different lines of pea ranged between 3.9 and 4.8 g/g [23], being approximately double that in the current study. This can be attributed to differences in pH of the protein isolate, namely, Shevkani et al. [23] neutralized the protein isolate (pH 7.0) unlike the isolates in the current study, which were not neutralized (pH 4.5).
In the current study, the OHC of the regular phytate flours was 0.9 g/g, whereas that of the low-phytate flours was significantly, but minimally, higher, ranging between 1.0 g/g and 1.1 g/g. These values are lower than the 1.5 g/g reported for the flours of regular pea by Setia et al. [32]. The OHC of all the protein isolates in the current study was in the range of 1.4–1.6 g/g and no clear trend based on variety phytate level was exhibited (Table 3). As observed for the WHC, the OHC of the protein isolates was higher (by 27–67%) than that of the flours. An OHC of 1.2 g/g for a pea protein isolate was reported by Fernández-Quintela et al. [33], and 3.5–3.8 g/g was reported for protein isolates of three pea varieties by Stone et al. [3]. The protein isolates from five different lines of a pea variety exhibited OHC in the range 5.5–7.2 g/g [23], which is 4–4.5 times the OHC of protein isolates in the current study. This may be explained by the difference in pH of the protein isolates as mentioned earlier for WHC. Clearly, the influence of pH on WHC is different from OHC, being more pronounced for the latter.
Foaming capacity and stability
The foaming capacity (FC) of the flours ranged between 56 and 69% and the foaming stability (FS) was between 65 and 78%, as shown in Table 3. Only LP-1 and CDC Amarillo exhibited significant differences, and hence, an influence of variety but not the level of phytate was observed. For the FS, there were no significant differences among all the flours; thus, neither the variety nor the level of phytate influenced this parameter. Higher FC (115% and 108%) and lower FS (21% for both) values were reported for regular pea and faba bean flours by Setia et al. [32]. In the current study, the protein isolates had markedly higher FC (values of 116%-137%) than their corresponding flours. The LP-1 isolate exhibited the lowest FC value, which was significantly lower than CDC Bronco and CDC Amarillo, but statistically similar to the rest of the isolates. For the FS of the isolates (values of 67–76%), the results were comparable to those of the corresponding flours. The only notable difference in the FS of the isolates was LP-1 had a significantly lower FS compared with the rest of the samples.
The FC and FS of protein isolates from different pea lines were in the range of 87–132% and 94–96%, respectively, and were similar to kidney bean protein isolates in the same study [23]. While the FC of protein isolates in that study is similar to that of the protein isolates in the current study, the FS of the protein isolates in the study by Shevkani et al. [23] is higher by about 20%. This may be related to method used, where in the aforementioned study, the protein solutions were foamed directly in a graduated cylinder versus being poured into a cylinder after the foam was formed and therefore disturbing the foam structure. The FC is reported to be related to the solubility of the protein [3]. However, this relationship is not apparent from the results of the current study.
Emulsion stability
The emulsion stability (ES) results of the flours and the protein isolates are shown in Table 3. The values for the flours had a larger range than the other functional properties studied. Of the low-phytate variety flours, LP-1 exhibited a high ES of 70%, which was more than double the ES values for LP-2 and LP-3 (23%–28%). The reason for this is not clear. The flours from the regular pea varieties had comparable ES values in the range of 60–63%. For the protein isolates in the current study, ES was higher (90%-93%) than for the flours. Moreover, neither type of variety nor level of phytate influenced these values, as they were all statistically similar. Since the considerably low ES of the LP-2 and LP-3 flours did not carry over to the respective protein isolates, it is hypothesized that non-protein and non-phytate related compounds were responsible for the flours having minimal ES. The carbohydrate fraction of the flours may have important differences for ES; however, investigating that hypothesis was beyond the scope of the current study; total starch and water-soluble carbohydrate content has been reported to vary based on pea genotype [21]. Stone et al. [3] reported significant differences in the ES of three pea protein isolates extracted from different varieties; however, the magnitude of difference was only approximately 3%. Lam et al. [34] also reported minimal differences between the ES (95.1–96.1%) of pea protein isolates derived from five different varieties.
Protein quality
In vitro protein digestibility
The protein quality, starting with the in vitro protein digestibility (IVPD), of the flours and protein isolates is shown in Table 4. There was limited variation in the IVPD of the flours (79–81%), although that of LP-2 was significantly higher than the rest of the flours. No influence of the level of phytate was exhibited. In contrast, Chitra et al. [35] reported a negative correlation between phytic acid content and IVPD for the flours of five different legume species. Previous results from a study by our group showed an IVPD of 78% for CDC Amarillo [32], which is consistent with the results of the current study. Nosworthy et al. [20] reported no significant effect of genotype (× 12), environment (× 4), or location (× 4) for the IVPD of pea flours, with values of approximately 77%.
Although the isolates also had small variation in the IVPD (86–88%), a significant difference between the low and regular phytate categories was apparent with LP-1, -2, and -3 all having higher IVPD than CDC Bronco and CDC Amarillo. No significant differences were found among the varieties within each of these categories (Table 4). The protein isolates had 8.4–10.6% higher IVPD values than the respective flours from which they were extracted. In a study by de la Rosa‐Millán et al. [36] on faba bean, chickpea, lentil, and white bean, a similar finding of higher in vitro protein digestibility for the isolates than flours was reported; however, the increase (48.5–63.0%) was much larger than what was found in the current study. The low IVPD of the pulse flours (50–62%) in the aforementioned study was attributed to proteolytic enzyme inhibitors, and the higher starch and insoluble dietary fiber contents found in the flours as compared to the isolates [36].
The results from this study indicate that phytate, which was more concentrated in the protein isolates than in the flours (Table 1), plays a non-significant role, if any, toward IVPD, which was higher for the protein isolates. However, it may not be the content of phytate but the protein-phytate interactions that would play a role in the IVPD. The protein–phytate interactions may differ in the flours compared to protein isolates due to, among other factors, changes in pH during the protein extraction process. The higher in vitro digestibility of the protein in the protein isolates compared to flour suggests a possible role of other components in the flour, which are absent in the isolates, notably those which have an ability to bind protein [36]. Another possibility is that protease inhibitors, commonly present in pulses, may have contributed to a decrease in the in vitro protein digestibility of the flours as they have been reported to be partially lost or degraded in the alkaline extraction-isoelectric precipitation pea protein isolation process [37].
Amino acid score
Reported in Table 4 are the limiting essential amino acids (AAs) and scores for the flours and isolates. These were derived from the AA composition (Supplementary Table 1). The AA scores (Supplementary Table 2) were calculated based on the reference values of the essential amino acids given by FAO/WHO. All flours had tryptophan as the first limiting AA, with minor differences in scores among the varieties. CDC Amarillo had the highest score of 0.85, followed by CDC Bronco and LP-3 at 0.82 for both. LP-1 had the lowest AA score (0.79) followed closely by LP-2 (0.80). There was no trend in the AA scores based on the phytate content of the varieties. Others have also reported tryptophan to be the limiting AA in peas, with scores ranging from 0.79 to 0.92 [20, 32].
For two of the five varieties, LP-1 and LP-2, protein extraction shifted the limiting AA from tryptophan to methionine + cysteine, whereas for the other three varieties, tryptophan remained the limiting AA in the protein isolates. All isolates had lower AA scores than their respective flours. For three types of legumes, including pea, Shi et al. [38] reported protein isolates to have lower AA scores than the flours that they were extracted from and also that tryptophan was limiting in pea flour, whereas, for isolates, it was methionine + cysteine. The alkaline extraction–isoelectric precipitation process is known to result in the loss of albumins, which contain a higher proportion of essential amino acids, especially those containing sulfur, than the globulin fraction [39, 40]. The lowest score in the protein isolates was 0.57 for LP-3. CDC Bronco had the second lowest score (0.61) of the isolates. Similar to the flours, CDC Amarillo had the highest score (0.75). Phytate content was not an influencing factor on protein isolate amino acid score.
In vitro protein digestibility-corrected amino acid score
After correction of the amino acid score with the IVPD, the in vitro protein digestibility-corrected amino acid score (IVPDCAAS) values were obtained (Table 4). The IVPDCAAS of the flours and isolates followed the same trend as the AA score values. For each variety, the flour had a higher IVPDCAAS than the protein isolate. Furthermore, for IVPDCAAS, there was no overall impact from the phytate content. In both the flours and isolates, CDC Amarillo had the best overall protein quality (i.e., highest IVPDCAAS), whereas the lowest IVPDCAAS was for LP-1 among the flours and LP-3 among the isolates. Nosworthy et al. [20] reported differences in the IVPDCAAS of pea flours to be attributed to genotype (responsible for 19% of variation), year (17% of variation), and location (9% of variation).
Bioavailability of iron
The bioavailability values of iron in the flours and protein isolates are shown in Table 5. For the flours, the bioavailability values were overall higher in the low-phytate varieties (17.9–22.0 ng ferritin/mg protein) than in the regular varieties (10.5–10.9 ng ferritin/mg protein); however, the value of LP-1 was not significantly different from that of CDC Bronco or CDC Amarillo. In contrast, LP-2 and LP-3 had double the iron bioavailability of the regular varieties. As expected, the results in Tables 1 and 5 show an inverse relationship between phytate content and iron bioavailability for both flour and isolates. These results are supported by the previous studies in the literature for flour. For example, Bangar et al. [10] found a negative correlation between pea phytate content and iron bioavailability utilizing a similar in vitro digestion Caco-2 cell culture bioassay. Liu et al. [11] reported the iron bioavailability values of low-phytate pea varieties to be 1.4 to 2 times higher than that of regular varieties, including CDC Bronco. Higher iron bioavailability in low versus regular phytate peas has also been shown in vivo for CDC Bronco and LP-1 flours fed to Gallus gallus for 42 days [12]. Diets containing flour from the LP-1 variety resulted in a higher total body hemoglobin iron, a biomarker of iron bioavailability [12]. Moreover, Petry et al. [41] demonstrated an increase in bioavailability of iron in vivo after feeding healthy women porridges made from low-phytate bean. In addition to phytate content, the phytate:iron content molar ratio and polyphenol content [11], condensed tannin [42], carotenoid content, and the cotyledon cell wall [10] may be important factors concerning iron bioavailability. Moore et al. [43] reported an increase in iron bioavailability from 7.7 to 23.1 ng ferritin/mg protein when phytase was added to the in vitro digestion of cooked peas which contained 11.18 mg/g phytic acid before phytase treatment.
In the current study, the bioavailability of iron in the protein isolates was less than that of the corresponding flour and ranged from 2.9 to 16.5 ng ferritin/mg protein, with all values being statistically similar. This could be explained by the enhanced concentration of phytate in the isolates as compared to the flours (Table 1), which can bind Fe2+ among other divalent cations and form insoluble complexes. Degrading the phytate, via phytase, to trace amounts has been shown to improve the in vivo iron absorption of a pea protein isolate product by approximately 50% [44]. Soy protein itself, specifically conglycinin, is known to suppress iron absorption [45]; however, the major proteins in pea, legumin, vicilin, convicilin, and the albumin fraction are not known to inhibit iron bioavailability. Liu et al. [11] showed that dehulling a low-phytate non-pigmented seed coat pea variety resulted in an improvement in the iron bioavailability. The seed coat contains phenolic compounds that can impair iron absorption. As whole seeds were used in this study, it is hypothesized that the phenolics would be concentrated in the protein isolate and, therefore, could also be a contributing factor for the lower iron bioavailability in the protein isolates as compared to the flours. This is supported by the work of Shi et al. [38] who reported that the total phenolics content and condensed tannins content was higher in pea protein isolate as compared to the flour when using a similar protein extraction procedure as the current study. And Campion et al. [46] found that the presence of tannins negated the benefits of breeding low phytate, lectin-free, common bean in terms of iron bioavailability. From the scope of this study, it is not known the extent of how much Fe2+ in the isolates is being bound by phytate, phenolics, or if other matrix effects are taking place to inhibit the bioavailability.
There were no significant differences in the iron bioavailability values between the five isolates; however, it is important to note the high standard deviations of the iron bioavailability values, the limited number of samples, and the overall differences in the values when grouping the low and regular varieties. The mean iron bioavailability value of the isolates from the three low-phytate varieties was 3.5 times higher than the mean value of the two regular varieties. The high standard deviations were not a result of the bioassay itself, but of the different values obtained for the biological replicates. The varieties were grown in different years and locations. To examine this further an ANOVA with Fisher’s least significant difference post hoc test was performed for variety and location (Supplementary Table 3). The effect of location was significant for the isolates, but not the flours. For the iron bioavailability of the isolates, the mean value of the ROS-2020 location was 12.2 ng ferritin/mg protein, whereas the mean value of the KAM-2019 location was 5.8 ng ferritin/mg protein. The effect of variety was also significant with LP-3 having higher iron bioavailability than CDC Bronco and CDC Amarillo. This initial study on the comparison of protein isolation from low and regular phytate varieties included only five varieties and two biological replicates which limits the statistical power of the analysis. It is prudent that more studies be conducted with a larger number of samples and the effect of growing environment taken into consideration.
Conclusions
In this study, the efficacy of using low-phytate pea varieties to reduce the effect of phytate on pea protein nutrition was explored for flour and protein isolate samples. There was approximately 40% less phytate in the flours and isolates compared to the regular phytate varieties. As expected, differences in the functional properties of either the flours or isolates were due to individual variety variation and only minimal effects of low versus regular phytate variety grouping were found, indicating that low-phytate flours and isolates can be used equivalently in food ingredient applications. The protein extraction process concentrated phytate and iron by over threefold in the isolates. The IVPD of the protein isolates was improved by less than 2% when extracting from low versus regular phytate flours and overall protein quality (IVPDCAAS) showed no improvements. The iron bioavailability results were mixed, as the flours from low-phytate varieties had higher values than the regular phytate flours, but the protein isolates showed no significant differences due to high variation within each variety replicate and the limited number of samples in the study. Despite this, the overall trend was improved iron bioavailability in the protein isolates from low-phytate varieties. This indicates the need for further studies, with a higher number of variety replications, to determine if there is an advantage in using low-phytate varieties for producing pea protein isolates in terms of iron bioavailability.
References
Cheryan M, Rackis JJ (1980) Phytic acid interactions in food systems. C R C Crit Rev Food Sci Nutr 13:297–335. https://doi.org/10.1080/10408398009527293
Patterson CA, Curran J, Der T (2017) Effect of processing on antinutrient compounds in pulses. Cereal Chem 94:2–10. https://doi.org/10.1094/CCHEM-05-16-0144-FI
Stone AK, Karalash A, Tyler RT et al (2015) Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res Int. https://doi.org/10.1016/j.foodres.2014.11.017
Gharibzahedi SMT, Jafari SM (2017) The importance of minerals in human nutrition: bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci Technol 62:119–132. https://doi.org/10.1016/J.TIFS.2017.02.017
Gemede HF (2014) Potential health benefits and adverse effects associated with phytate in foods. Glob J Med Res 27:45–55
Wang M, Gao H, Wang J et al (2022) Global burden and inequality of iron deficiency: findings from the Global Burden of Disease datasets 1990–2017. Nutr J 21:1–10. https://doi.org/10.1186/S12937-022-00771-3/FIGURES/4
Shi L, Arntfield SD, Nickerson M (2018) Changes in levels of phytic acid, lectins and oxalates during soaking and cooking of Canadian pulses. Food Res Int 107:660–668. https://doi.org/10.1016/j.foodres.2018.02.056
Fredrikson M, Biot P, Alminger ML et al (2001) Production process for high-quality pea-protein isolate with low content of oligosaccharides and phytate. J Agric Food Chem 49:1208–1212. https://doi.org/10.1021/jf000708x
Warkentin TD, Delgerjav O, Arganosa G et al (2012) Development and characterization of low-phytate pea. Crop Sci 52:74–78. https://doi.org/10.2135/cropsci2011.05.0285
Bangar P, Glahn RP, Liu Y et al (2017) Iron bioavailability in field pea seeds: Correlations with iron, phytate, and carotenoids. Crop Sci 57:891–902. https://doi.org/10.2135/CROPSCI2016.08.0682
Liu X, Glahn RP, Arganosa GC, Warkentin TD (2015) Iron Bioavailability in Low Phytate Pea. Crop Sci 55:320–330. https://doi.org/10.2135/cropsci2014.06.0412
Warkentin T, Kolba N, Tako E (2020) Low phytate peas (Pisum sativum L.) improve iron status, gut microbiome, and brush border membrane functionality in vivo (gallus gallus). Nutrients 12:1–18. https://doi.org/10.3390/nu12092563
Boye JI, Aksay S, Roufik S et al (2010) Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res Int 43:537–546. https://doi.org/10.1016/J.FOODRES.2009.07.021
AOAC (2005) Official methods of analysis, 16th ed. Association of official analytical chemists, Washington D.C.
Nosworthy MG, Medina G, Franczyk AJ et al (2018) Effect of processing on the in vitro and in vivo protein quality of beans (Phaseolus vulgaris and Vicia Faba). Nutrients 10:671. https://doi.org/10.3390/nu10060671
FAO (1991) Protein quality evaluation: report of the joint FAO/WHO expert consultation. Italy, Rome
Tinus T, Damour M, Van Riel V, Sopade PA (2012) Particle size-starch-protein digestibility relationships in cowpea (Vigna unguiculata). J Food Eng 113:254–264. https://doi.org/10.1016/j.jfoodeng.2012.05.041
Glahn RP (2022) The Caco-2 cell bioassay for measurement of food iron bioavailability. J Vis Exp. https://doi.org/10.3791/63859
Lindsay DL, Jha AB, Arganosa G et al (2021) Agronomic performance in low phytic acid field peas. Plants 10:1589. https://doi.org/10.3390/plants10081589
Nosworthy MG, Huang S, Franczyk A et al (2021) Effect of genotype, year, and location on the proximate composition and in vitro protein quality of select pea cultivars. ACS Food Sci Technol 1:1670–1676. https://doi.org/10.1021/ACSFOODSCITECH.1C00186
Maharjan P, Penny J, Partington DL, Panozzo JF (2019) Genotype and environment effects on the chemical composition and rheological properties of field peas. J Sci Food Agric 99:5409–5416. https://doi.org/10.1002/jsfa.9801
Hall C, Hillen C, Robinson JG (2017) Composition, nutritional value, and health benefits of pulses. Cereal Chem 94:11–31
Shevkani K, Singh N, Kaur A, Rana JC (2015) Structural and functional characterization of kidney bean and field pea protein isolates: a comparative study. Food Hydrocoll 43:679–689. https://doi.org/10.1016/j.foodhyd.2014.07.024
Lam ACY, Can Karaca A, Tyler RT, Nickerson MT (2018) Pea protein isolates: Structure, extraction, and functionality. Food Rev Int 34:126–147
Hídvégi M, Lásztity R (2002) Phytic acid content of cereals and legumes and interaction with proteins. Period Polytech Chem Eng 46:59–64
Carnovale E, Lugaro E, Lombardi-Boccia G (1988) Phytic acid in faba bean and pea: effect on protein availability. Cereal Chem 65:114–117
de Rham O, Jost T (1979) Phytate-protein interactions in soybean extracts and low-phytate soy protein products. J Food Sci 44:596–600. https://doi.org/10.1111/j.1365-2621.1979.tb03844.x
Dai F, Wang J, Zhang S et al (2007) Genotypic and environmental variation in phytic acid content and its relation to protein content and malt quality in barley. Food Chem 105:606–611. https://doi.org/10.1016/j.foodchem.2007.04.019
Taherian AR, Mondor M, Labranche J et al (2011) Comparative study of functional properties of commercial and membrane processed yellow pea protein isolates. Food Res Int 44:2505–2514. https://doi.org/10.1016/j.foodres.2011.01.030
Ali F, Ippersiel D, Lamarche F, Mondor M (2010) Characterization of low-phytate soy protein isolates produced by membrane technologies. Innov Food Sci Emerg Technol 11:162–168. https://doi.org/10.1016/j.ifset.2009.08.004
Karaca AC, Low N, Nickerson M (2011) Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res Int 44:2742–2750. https://doi.org/10.1016/j.foodres.2011.06.012
Setia R, Dai Z, Nickerson MT et al (2019) Impacts of short-term germination on the chemical compositions, technological characteristics and nutritional quality of yellow pea and faba bean flours. Food Res Int 122:263–272. https://doi.org/10.1016/J.FOODRES.2019.04.021
Fernández-Quintela A, Macarulla MT, Del Barrio AS, Martínez JA (1997) Composition and functional properties of protein isolates obtained from commercial legumes grown in northern Spain. Plant Foods Hum Nutr 51:331–341. https://doi.org/10.1023/A:1007936930354
Lam ACY, Warkentin TD, Tyler RT, Nickerson MT (2017) Physicochemical and functional properties of protein isolates obtained from several pea cultivars. Cereal Chem 94:89–97. https://doi.org/10.1094/CCHEM-04-16-0097-FI
Chitra U, Vimala V, Singh U, Geervani P (1995) Variability in phytic acid content and protein digestibility of grain legumes. Plant Foods Hum Nutr 47:163–172
de la Rosa-Millán J, Orona-Padilla JL, Flores-Moreno VM, Serna-Saldívar SO (2018) Physicochemical, functional andATR-FTIR molecular analysis of protein extracts derived from starchy pulses. Int J Food Sci Technol 53:1414–1424. https://doi.org/10.1111/ijfs.13719
Pedrosa MM, Varela A, Domínguez-Timón F, Tovar CA, Moreno HM, Borderías AJ, Díaz MT (2020) Comparison of bioactive compounds content and techno-functional properties of pea and bean flours and their protein isolates. Plant Foods Hum Nutr 75:642–650. https://doi.org/10.1007/s11130-020-00866-4
Shi D, House JD, Wanasundara JPD, Nickerson MT (2022) Comparative evaluation of the nutritional value of faba bean flours and protein isolates with major legumes in the market. Cereal Chem 99:1013–1029. https://doi.org/10.1002/cche.10575
Kiosseoglou V, Paraskevopoulou A, Poojary MM (2021) Functional and physicochemical properties of pulse proteins. Pulse Foods Process Qual Nutraceutical Appl. https://doi.org/10.1016/B978-0-12-818184-3.00006-4
Liu LH, Hung TV, Bennett L (2008) Extraction and characterization of chickpea (Cicer arietinum) albumin and globulin. J Food Sci. https://doi.org/10.1111/J.1750-3841.2008.00773.X
Petry N, Egli I, Campion B, Nielsen E, Hurrell R (2013) Genetic reduction of phytate in common bean (Phaseolus vulgaris L.) seeds increases iron absorption in young women. J Nutr 143:1219–1224. https://doi.org/10.3945/jn.113.175067
Luo YW, Xie WH, Min-Xu LFX (2012) Effects of phytase and polyphenol oxidase treatments on in vitro iron bioavailability in faba bean (Vicia faba L.). CYTA: J Food 10:165–171. https://doi.org/10.1080/19476337.2011.631222
Moore KL, Rodríguez-Ramiro I, Jones ER, Jones EJ, Rodríguez-Celma J, Halsey K, Domoney C, Shewry PR, Fairweather-Tait S, Balk J (2018) The stage of seed development influences iron bioavailability in pea (Pisum sativum L.). Sci Reports. https://doi.org/10.1038/s41598-018-25130-3
Davidsson L, Dimitriou T, Walczyk T, Hurrell RF (2001) Iron absorption from experimental infant formulas based on pea (Pisum sativum)-protein isolate: the effect of phytic acid and ascorbic acid. Br J Nutr 85:59–63. https://doi.org/10.1079/BJN2000232
Lynch SR, Dassenko SA, Cook JD, Juillerat MA, Hurrell RF (1994) Inhibitory effect of a soybean-protein–related moiety on iron absorption in humans. Am J Clin Nutr 60:567–572. https://doi.org/10.1093/AJCN/60.4.567
Campion B, Glahn RP, Tava A, Perrone D, Doria E, Sparvoli F, Cecotti R, Dani V, Nielsen E (2013) Genetic reduction of antinutrients in common bean (Phaseolus vulgaris L.) seed, increases nutrients and in vitro iron bioavailability without depressing main agronomic traits. F Crop Res 141:27–37. https://doi.org/10.1016/j.fcr.2012.10.015
Funding
Funding to support this research was provided by the Saskatchewan Agriculture Development Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Compliance with ethics Requirements
This article does not contain any studies with animals or human participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chigwedere, C.M., Stone, A., Konieczny, D. et al. Examination of the functional properties, protein quality, and iron bioavailability of low-phytate pea protein ingredients. Eur Food Res Technol 249, 1517–1529 (2023). https://doi.org/10.1007/s00217-023-04232-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-023-04232-x