Abstract
This research examined the physicochemical, functional, and nutritional aspects of pea (PPI) and faba bean protein isolates (FBPI) modified using trypsin at varying levels of hydrolysis. Enzyme hydrolysis decreased the protein content for both pulse protein isolates and increased the lipid content for pea. The surface charge became more negative, and the surface hydrophobicity was enhanced. The surface and interfacial tension, in general, decreased upon hydrolysis. The oil holding capacity for both pulses increased while the water holding capacity decreased. The foaming capacity of FBPI was significantly lowered by hydrolysis, while in the case of PPI, it increased at pH 4.5 and was unaffected at 7.0. The foaming stability increased for both pulses at pH 4.5 but was reduced at pH 7.0. At pH 4.5, hydrolysis enhanced the emulsion activity index of FBPI, while it decreased the index of PPI. The indices increased for both pulses at pH 7.0. The emulsion stability index was generally reduced for FBPI but improved for PPI after hydrolysis. All samples were limiting in tryptophan and the sulfur-containing amino acids, and in vitro protein digestibility decreased after hydrolysis for both pulses. As the results of these changes, overall protein quality improved for FBPI but not PPI. The degree of protein hydrolysis was an important factor in determining the functional and nutritional attributes of modified pulse proteins along with pH and pulse type.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pulse ingredients are typically sold as whole or split seeds, and flour. The flour is further processed into enriched flours, starch fraction, protein concentrates, isolates or peptide fractions prepared through either dry or wet fractionation/isolation processes. Depending on the process, ingredients can give nutritional and functional profiles that can then be tailored to specific applications, such as beverages, infant formulas, baked goods, meat analogs/texturized vegetable proteins, expanded snacks and nutritional supplement products. Further processing can be performed to modify the ingredient fractions to obtain desired properties, such as improved digestibility, protein quality and novel functional attributes. For this processing, current trend is to employ “clean-label” processing. One such clean-label process for protein modifications involves enzymatic treatments. For example, proteinase enzymes (e.g., trypsin) can be used under controlled conditions to cleave sites along the protein structure to alter its conformation, surface properties and molecular size and generate a mix of amino acids and peptides of diverse lengths that modifies a protein’s properties. Enzymatic hydrolysis is one of the safest and most reliable means of protein modification. The enzymatic hydrolysis conditions are mild and can prevent loss of free amino acids which may occur during chemical hydrolysis, while the formation of by-product is also minimal due to enzyme specificity [1, 2]. Typically, proteinases are used to obtain more-controlled partial hydrolysis (i.e., not totally random hydrolysis) of the protein structure (e.g., 5–20% degrees of hydrolysis (DH)) to elicit a positive response in their functional attributes [3,4,5]. However, the release of small peptides at high DH can also induce bitterness in the product.
Various research in the field of protein modification indicates a positive and promising future for proteins modified enzymatically, but there is still a research gap pertaining to the interactions between various enzyme and substrate combinations since there are numerous variables to consider (e.g., enzyme: substrate (E/S) ratio, optimum working pH, and temperature). In addition, plant proteins from different sources will behave differently when interacting with specific enzymes. Many plant proteins, such as faba bean proteins, are still underutilized in Western countries, and very little is known about their modification. Despite being richer in protein content than most pulses, the presence of anti-nutritional factors (ANF) and various health concerns associated with faba bean consumption (such as favism) have led to the underutilization of the respective protein products [6]. Pea protein, though nutritionally rich, is also not utilized as much (as soy) due to the lower protein digestibility, presence of ANF and reduced levels of sulfur-containing amino acids [7]. Plant proteins in general also show lower functionality as compared to those from animal sources due to their inherent structural constraints [8].
Thus, this research was aimed at improving the functional and nutritional properties of pea (PPI) and faba bean protein isolates (FBPI) using trypsin at different DH (5%, 10%, 15%, and 20%). Trypsin was chosen as it is one of the most studied and highly specific serine-type endopeptidases which is easy to use and control. The reason for choosing these levels of hydrolysis was to draw conclusions on the changes in the aforementioned properties for both pulse proteins at regular intervals of hydrolysis up to 20%. Trypsin hydrolysis has been reported to introduce bitterness in pea protein hydrolysates at a DH of ~ 18% [9]. Therefore, further hydrolysis (beyond 20%) would likely contribute adversely to the sensory properties of the protein ingredients. The effects on the protein structure, surface properties, functionality and protein quality were examined. The knowledge gathered from this work would be helpful for the food sector in developing a commercial pulse protein ingredient with desired functionality and protein quality.
Materials and methods
Materials
FBPI and PPI were kindly donated by AGT Food and Ingredients (Saskatoon, SK, Canada). All chemicals used in this study including the enzyme (lyophilized trypsin powder from porcine pancreas; product no. T6567; specific activity: 10,000 BAEE units per mg protein) were purchased from Sigma Aldrich (Oakville, ON, Canada) and were of reagent grade. The water used for all analyses was obtained using the Milli-Q™ filtration system (Millipore Corporation, MA, USA).
Production of protein hydrolysates
FBPI and PPI solutions at 1% (w/w) were prepared by dissolving 50 g of protein isolate into 5 kg of 10 mM sodium phosphate buffer at pH 7.8. The solutions were stirred overnight at 4 °C and brought to 25 °C the next day. The protein solutions were then readjusted to pH 7.8 using 1 N HCl or NaOH, and stirred for another 1 h. Trypsin was added to each protein solution at an E/S ratio of 1:5000, and a 10-L fermenter (BioFlo III Batch/Continuous Fermenter; New Brunswick Scientific, Hamburg, Germany) was used for the following reactions. For faba bean protein solutions, enzymatic reactions were carried out at 25 °C for 11, 22, 45, and 78 min to reach the DH of 5%, 10%, 15%, and 20%, respectively. The enzyme hydrolysis for pea protein solutions was carried out for 12 and 31 min to reach DH of 5% and 10%, respectively. The DH of 15% and 20% for pea protein was not achieved, presumably due to the high trypsin inhibitor activity in the material (~ 5.5 TIU/mg for pea and ~ 4.0 TIU/mg for faba bean; data not shown). All enzymatic conditions were selected based on preliminary tests (data not shown). To deactivate the enzyme, 10 mL of 4 N HCl was added to each protein solution which lowered the pH down to ~ 5.6, followed by heating at 85 °C for 30 min. For the heat-treated control isolate samples, the protein solutions were prepared similarly as described above, except that no trypsin was added. All protein solutions (enzyme treated and heat-treated control) were brought to room temperature (21–23 °C), and the pH of each solution was adjusted to its corresponding isoelectric point (pI) (ranged between pH 4.0–4.5) as determined using a Zetasizer (Nano-ZS90; Malvern Instruments, Westborough, MA, USA). Centrifugation (Avanti JE; Beckman Coulter, ON, Canada) at 7100 × g at 4 °C for 20 min was applied to each solution to collect the protein precipitate. The protein pellets were frozen at − 18 °C and freeze-dried (FreeZone® 6; Labconco, Kansas City, MO, USA). The freeze-dried samples were ground with a coffee grinder into fine powders. The original FBPI and PPI were used as untreated controls in this study.
Total acid hydrolysis and determination of degrees of hydrolysis
To determine the levels of protein hydrolysis by trypsin, the 2, 4, 6-trinitrobenzenesulfonicacid (TNBS) method [10] was used after deactivating trypsin and cooled to room temperature (21–23 °C) using a UV–VIS spectrophotometer (Genesys 20; Thermo Fischer Scientific Inc., Waltham, MA, USA) at 340 nm. To calculate the DH, the absorption of TNBS reacted mixture for total hydrolysis and unhydrolyzed samples was also determined according to the method described in Avramenko et al. [11]. The DH (%) was calculated based on the ratio of the difference between the absorbance of the hydrolyzed sample and unhydrolyzed sample, and the absorbance of 1 g equivalent of total acid hydrolysis of the isolate.
Proximate composition
The crude protein, ash, lipid, and moisture were determined using AOAC methods 997.09 (%N × 6.25), 923.03, 920.85 and 925.10, respectively [12].
Capillary gel electrophoresis
The capillary gel electrophoresis (CGE) for protein size analysis was performed based on the method described in Das et al. [13]. A capillary electrophoresis system (P/ACE MDQ plus; AB Sciex LLC, Framingham, MA, USA) enabled with a UV detector and the 32Karat 10.3 software was used for data collection and analysis. The preparation of CGE samples and instrument setup were established as per the application guide (RUO-IDV-05–6934-A) for the SDS-MW analysis kit (390953).
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
The samples of FBPI and PPI were dissolved in a buffer (100 mM Tris pH 9.0 and 1% SDS) with a 1:10 ratio (w:v, protein isolates: buffer) for 30 min at room temperature in a mixer, followed by clarification at 15,000 × g for 10 min. The supernatant is mixed with the same amount of SDS-PAGE dye, and the same amount of mixtures was individually loaded onto 8–16% Mini-PROTEAN® TGX™ Precast Protein Gels (Bio-Rad 4561105) for separation at 200 V for 30 min. The gels were visualized with the application of Coomassie R-250 stain.
Fourier transform infrared spectroscopy
The Renishaw inVia™ Reflex Raman Microscope (Renishaw, Gloucestershire, UK) was used to record the infrared spectra of each sample using an IlluminatIR II Fourier transform infrared spectroscopy (FTIR) microscope accessory (Smith’s Detection, Danbury, CT, USA) having a 36 × ATR objective lens within the wavelength range of 4000–650 cm−1 with 4 cm−1 resolution. After loading the sample on a glass slide, it was placed under the ATR lens, and an average of 518 spectra was collected. The data analysis was done using the WiRE 3.3 software (Renishaw) after the second derivation of the amide I peak (1600–1700 cm−1) and the identification of the buried peaks corresponding to secondary proteins. The peaks were then fitted inside the original amide I curve (also known as curve fitting) using Gaussian fit, and the percentage of area conforming to each secondary protein structure was obtained.
Surface properties
Surface charge
The surface charge (SC) of all samples at pH 7 and 4.5 was measured based on the method of Avramenko et al. [11] using a Zetasizer Nano-ZS90 instrument (Malvern Instruments, Westborough, MA, USA). The protein solutions (0.05%, w/w, protein basis) were stirred for 1 h at room temperature (21–23 °C) before adjusting the pH and were stirred for another 1 h prior to the analysis.
Surface hydrophobicity
The surface hydrophobicity (SH) for all samples was measured at pH 7.0 and pH 4.5 using the fluorescent probe 8-anilino-1-naphthalenesulfonic acid (ANS) based on the method of Kato and Nakai [14] as described by Stone et al. [15]. Protein solutions were prepared similarly as previously described for SC except that the protein concentration was decreased to 0.025% (w/w). The measurements were done using a FluoroMax-4 spectrofluorometer (Horiba Jobin Yvon Inc, Edison, NJ, USA) with excitation and emission wavelengths of 390 and 470 nm, respectively.
Surface and interfacial tension
The surface (ST) and interfacial tension (IT) of all samples were determined based on the method of Can Karaca et al. [16] at pH 7.0 as described by Wang et al. [17]. The ST and IT between the proteins and air, and between the proteins and canola oil, respectively, were measured using a Force Tensiometer-K20 and a Wilhelmy plate (Krüss, Hamburg, Germany). For ST, a 1% (w/w, protein basis) protein solution was prepared, while for IT, a 0.25% (w/w, protein basis) protein solution was prepared. The control measurements were done by measuring between water (without protein) and air/oil.
Functional properties
Solubility
The solubility of the protein samples was measured in situ during the hydrolysis procedure using the Coomassie dye (Coomassie Plus Reagent; Thermo Fisher Scientific Inc., Waltham, MA, USA) according to a modified method as described by Achouri et al. [18]. Briefly, for the hydrolysates and heat-treated isolates, 1 mL of each solution was taken from each batch exactly at the time when the desired DH was achieved (after heating to 85 °C; no HCl addition). The solutions were in the pH range of 7.2 to 7.8 depending on the level of hydrolysis. Solutions were centrifuged (Centrifuge 5418 R; Eppendorf Corporate, Hamburg, Germany) at 12,100 × g for 10 min. Then 50 µL of the supernatant was diluted with 450 µL of sodium phosphate buffer. For the untreated samples, 20 mg protein was dissolved in 20 mL water at pH 7, followed by the same centrifugation process. The supernatant protein content was determined according to Bradford [19] using a UV–VIS spectrophotometer (Genesys 20; Thermo Fisher Scientific Inc., Waltham, MA, USA) at 595 nm. The total protein (soluble and insoluble) was determined by solubilizing 20 mg of isolate in 20 mL of 0.5 N NaOH followed with the same procedure above. The solubility was calculated as the percentage ratio of protein in the supernatant to the total protein in the initial batch solution.
Water and oil holding capacity
The water (WHC) and oil holding capacity (OHC) of all samples were determined using a modified method of Yin et al. [20]. To determine the WHC, 0.5 g of protein was mixed with 10 mL water and vortexed for 30 s every 5-min interval for 30 min. The solution was left untouched for 30 min at room temperature (21–23 °C) and vortexed again for 10 s every 5 min for 30 min. The vortexed solution was centrifuged (Thermo Electron LED GmbH 37520 Osterode; Thermo Fisher Scientific Inc., Waltham, MA, USA) at 5000 × g for 15 min. The supernatant was drained, and the pellet was weighted. The WHC was reported as g water/g protein and calculated as below:
A similar procedure was used to determine OHC, where the water was replaced with canola oil. The OHC was reported as g oil/g protein.
Foaming capacity and stability
The foaming capacity (FC) and stability (FS) of all samples at pH 7.0 and 4.5 were measured based on the method of Wilde and Clark [21] as described in detail by Konieczny et al. [5] using an IKA® T10 basic ULTRA-TURRAX® Homogenizer (IKA Werke GmbH & Co. KG, Staufen im Breisgau, Germany). The FC and FS were calculated using the following equations:
Emulsifying activity and stability index
The emulsifying activity (EAI) and stability index (ESI) of all samples at pH 7.0 and 4.5 were determined using the methods of Pearce and Kinsella [22] as described in detail by Konieczny et al. [5] using an IKA® T10 basic ULTRA-TURRAX® Homogenizer (IKA Werke GmbH & Co. KG, Staufen im Breisgau, Germany). The EAI and ESI were calculated using the following equations:
where T = 2.303, \({A}_{0}\) is the absorbance at 500 nm immediately after emulsion formation (at time 0 min), the dilution factor is 151, C is the weight of the protein per unit volume of aqueous phase before emulsion formation (g/mL), \(\phi\) is the oil volume fraction of the emulsion, \(\Delta t\) is the change in time from 0 to 10 min, and \(\Delta A\) is the change in absorbance (\({A}_{0}-{A}_{10}\)).
Protein quality
Amino acid determination
The amino acid composition of each sample was determined using an AccQ-Tag Ultra C18, 1.7 µm column (Waters Corporation, Milford, MA, USA) on a Shimadzu UPLC. All the amino acids, except for tryptophan, methionine, and cysteine, were determined using 20-h hydrolysis with 6 N HCl according to AOAC method 982.30 [12]. Methionine and cysteine were determined according to AOAC method 985.28 [12] with performic acid oxidation followed by the 6 N HCl hydrolysis. Tryptophan was conducted through alkaline hydrolysis using 10 N NaOH, as described in AOAC method 988.15 [12].
Amino acid score
The amino acid score was calculated as the ratio of the amino acid content (mg) in 1 g of the target protein to that in the reference protein. The reference protein amino acid composition (mg/g) protein for children from 2 to 5 years old was recommended by FAO/WHO [23]: Histidine, 19; isoleucine, 28; leucine, 66; lysine, 58; methionine + cysteine, 25; phenylalanine + tyrosine, 63; threonine, 34; tryptophan, 11; and valine, 35. The amino acid score is the lowest score among the essential amino acids.
In vitro protein digestibility
The in vitro protein digestibility (IVPD) was determined using a multi-enzyme pH drop method according to Tinus et al. [24]. Briefly each NBPI and PPI sample (equivalent of 62.5 ± 0.5 mg protein) was mixed with 10 mL of water preheated to 37 °C. The mixture was stirred for 1 h at 37 °C. The pH of the solution was adjusted to 8.0 ± 0.05 with 0.1 M NaOH and HCl before adding 1 mL of a multi-enzyme solution. The enzyme solution was prepared freshly with 31 mg chymotrypsin (bovine pancreas ≥ 40 units/mg protein), 16 mg trypsin (porcine pancreas 13,000–20,000 BAEE units/mg protein) and 13 mg protease (Streptomyces griseus ≥ 15 units/mg solid) with 10 mL water and kept at 37 °C (pH 8.0 ± 0.05. The pH of the mixture was recorded after 10 min (∆pH10min). The IVPD was calculated using the following equation:
where the \(\Delta pH_{10 min}\) represents the change in pH from time 0 to 10 min.
In vitro protein digestibility corrected amino acid score
The in vitro protein digestibility corrected amino acid score (IVPDCAAS) was calculated by multiplying the amino acid score and IVPD values.
Statistical analysis
The enzyme hydrolysis was performed in triplicate batches for each pulse. All measurements were made three times on each triplicate batch. Data represent the mean ± one standard deviation (n = 3). The statistical analysis was done using IBM SPSS Statistics (IBM Corp., Armonk, NY, USA). A one-way analysis of variance (ANOVA) was performed on each pulse group separately to identify differences between untreated, heat-treated, and enzymatically modified FBPI and PPI. A Pearson correlation (r, two-tailed) was also performed to determine the relationship between the protein properties. The degree of significance used was p < 0.05.
Results and discussion
Proximate composition
The proximate composition of the faba bean and pea protein samples is presented in Table 1 on a dry weight basis. Samples analyzed include the untreated, heat-treated (85 °C, 30 min; 0% DH, no enzyme), and hydrolyzed protein isolates of both pulses. There were no significant differences between the protein contents of the untreated and heat-treated isolates for both pulses (~ 92% for faba bean; ~ 84% for pea). Upon hydrolysis, the protein levels decreased to ~ 85% and ~ 75% for faba bean and pea, respectively, regardless of the DH. Approximately 70% of the initial sample weight was retained after the hydrolysis procedure and therefore the reduction in protein content after hydrolysis is hypothesized to be due to the loss of albumin (water-soluble) in the supernatant. In the case of heat-treated isolates, since there was no breaking down of proteins via hydrolysis, limited loss of proteins in the form of albumin took place. Goertzen et al. [4] also reported a decrease in protein levels with the hydrolysis of chickpea protein isolates using pepsin, trypsin, and papain. García Arteaga et al. [25] reported a slight decrease in protein levels in PPI after 120 min of trypsin treatment. There was no significant change with DH for the lipid content in FBPI (0.1%), whereas, for PPI, a significant increase was observed with 5% and 10% DH (3.4–3.6%) relative to the controls (1.1%). The increased lipid content observed was likely due to the hydrolyzing activities of trypsin that cleaved protein-lipid complexes [26], thereby releasing lipids into the solution which were later quantified by the Goldfisch method. A similar trend was not seen for faba bean, possibly due to the extremely low lipid levels in the starting material. The ash content for the untreated FBPI and PPI were 3.8% and 3.9%, respectively. The values declined slightly for the heat-treated isolates and hydrolysates; however, no trend based on DH was found. Konieczny et al. [5] reported a similar effect on hydrolyzed pea protein-enriched flour. It was hypothesized that the decline was because of the loss of minerals post-hydrolysis by heat treatment and centrifugation.
Capillary and sodium dodecyl sulfate–polyacrylamide gel electrophoresis
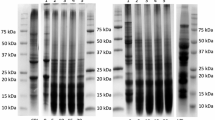
The CGE stacks for the untreated, heat-treated, and hydrolyzed FBPI and PPI are represented in Fig. 1. It was observed that the UV absorbance signal intensity of the peaks for the untreated FBPI and PPI are much higher than the heat-treated (0% DH) isolates, indicating that more proteins were extracted from the same sample weight of the untreated isolate than the heat-treated isolates for both pulses. A similar reduction in overall band intensities was observed in the heat-treated (0% DH) isolates compared to the untreated protein isolate controls in the SDS-PAGE analysis (Fig. S1). This reduction in overall extractable protein was more pronounced in the PPI samples than in FBPI. The CGE profiles of untreated and treated (heat and enzymatically) samples for both pulses were analyzed for protein size distribution, which are presented in Fig. 2 and Table S1. For pea protein, the majority of proteins above the 30 kDa range were readily hydrolyzed in the 5% DH sample, with a corresponding increase in the proportion of proteins and peptides in the < 15 and 15 to 30 kDa range. At the 10% DH stage, further hydrolysis of the medium molecular weight proteins in the range of 15 to 30 kDa was observed, with a corresponding increase in the proportion of smaller peptides below 15 kDa. Similar to PPI, the FBPI showed an initial hydrolysis of larger proteins above the 30 kDa range at the 5% DH stage. However, the proportion of medium molecular weight proteins in the range of 15 to 30 kDa range was slightly higher even at the 20% DH FBPI sample when compared to the 10% DH PPI sample. This reveals the presence of a subset of FBPI proteins in the medium molecular weight range that is resistant to complete hydrolysis by trypsin. The SDS-PAGE analysis of faba bean and pea protein samples is shown in Fig. 3. Overall, PPI and FBPI were susceptible to tryptic hydrolysis as most high and medium molecular weight bands were progressively degraded in the 5% DH samples and completely hydrolyzed at 10% DH. Larger proteins were almost completely broken down at higher DH (15%–20%) in the case of faba bean, with the exception of a few persistent bands between the 25 and 37 kDa range, correlating with the observations noted above in the CGE size distribution profile analysis.
Capillary gel electrophoresis of a heat-treated 0% DH FBPI control and 5 to 20% DH samples, b untreated FBPI control, c heat-treated 0% DH PPI control and 5 to 10% DH samples and d untreated PPI control. Red lines indicate the 15 and 30 kDa molecular weight range determined by an external molecular with standard calibration curve
Fourier transform infrared spectroscopy
The FTIR analysis via Gaussian spectral deconvolution of amide I peak for the untreated, heat-treated, and hydrolyzed FBPI and PPI was performed. Percent relative spectral weights of the secondary structures are presented in Table 2. It was observed that peak heights and positions shifted upon treatment for both pulse proteins, which indicates that the secondary structures of the proteins changed as hydrolysis progressed (Fig. S2). For faba bean, hydrolysis increased the β-sheet content, which ranged between ~ 26%-38%, compared to the untreated isolate (~ 21%), but decreased the α-helix content as the concentration dropped from ~ 37% in the untreated isolate to ~ 26–30% in the hydrolysates. This is supported by findings reported by Carbonaro et al. [27] wherein β-sheets formed adverse complexes in legumes due to proteolysis. Concentrations for the side chains of amino acid A2 remained unchanged, while A1 decreased initially upon hydrolysis, from ~ 11% for the untreated control to ~ 8% at 5% DH, but then increased to ~ 12% till 15% DH before decreasing again to ~ 8% at 20% DH.
The relative weight percentages of α-helices, β-sheets, and β-turns for the untreated PPI were ~ 34%, 28%, and 222%, respectively, which were similar to the findings reported by Shevkani et al. [28] where the respective proportions for pea were approximately 28%, 30%, and 22%. The side chains of amino acid A1 remained unchanged after treatment (~ 10%-13%). Heat treatment had a major effect on the α-helix content as it decreased from ~ 34% in the untreated control to ~ 27% in the heat-treated isolate. Thermal treatment of proteins leads to their denaturation and aggregation caused by the breaking of bonds that hold the α-helices and β-sheets together [29]. However, no significant reduction was observed for the content of β-sheet (~ 24%-28%). This was expected as β-conformations are thermally more stable than α-helices [30]. The content of β-turns increased from ~ 22% to ~ 34% after heating. For the hydrolyzed samples, a trend similar to that of faba bean proteins was observed with β-sheets increasing from ~ 28% (untreated control) to ~ 35% (10% DH). The content of α-helices increased initially upon hydrolysis (~ 32% at 5% DH) compared to the heated sample (~ 27%) and later decreased with further hydrolysis (~ 26% at 10% DH). Hydrolysis did not affect the level of β-turns (~ 21%-24%) or the side chains of amino acid A2.
Surface properties
Surface charge
The SC (zeta potential) of the untreated, heat-treated, and hydrolyzed FBPI and PPI are presented in Table 3. At pH 7, all protein samples carried net negative charges. The untreated FBPI carried a charge of − 22.3 mV and became more negative after the heat treatment and hydrolysis. The heat-treated isolated carried a charge of − 36.6 mV, while the hydrolysates carried charges ranging from − 36.7 to − 40.4 mV. In the case of PPI, a similar trend was observed where the untreated control carried a charge of − 22.5 mV, while the heat-treated and hydrolyzed samples carried charges of approximately − 36 and − 40 mV, respectively. This could be due to the partial unfolding of proteins after hydrolysis, resulting in the exposure of ionizable amino and carboxyl groups [31]. Similar trends were reported by Eckert et al. [32] wherein faba bean and pea protein extracts and hydrolysates had negative charges at a neutral pH. At pH 4.5, the charge possessed by each sample was significantly less negative than those at pH 7.0. In the case of faba bean, the untreated isolate had a net positive charge at pH 4.5 (4.0 mV), and it became negative after the heat treatment (− 3.6 mV). This became more negative with hydrolysis; the FBPI hydrolysates carried charges ranging from − 5.4 to − 6.4 mV. Pea protein samples also showed a similar trend at pH 4.5 where the untreated and heat-treated isolates had positive charges (2.8 and 1.3 mV, respectively), while their hydrolysates possessed net negative charges (− 4.2 and − 7.6 mV at 5% and 10% DH, respectively). Since pulse proteins generally have pIs of pH 4.5 [32], above this pH, deprotonation of amines occurs, leaving a net negative charge on them. Also, hydrolysis causes the unravelling of proteins, resulting in the exposure of hydrophilic amino acid groups which leads to a higher negative charge on the hydrolysates [5].
Surface hydrophobicity
The SH for the untreated, heat-treated and hydrolyzed FBPI and PPI at pH 7.0 is given in Table 3. The SH at pH 4.5 could not be calculated due to the absence of ANS binding sites on the proteins as a result of protein–protein aggregation. At pH 7.0, the untreated and heat-treated FBPI had relatively similar SH, however, the untreated isolate was slightly more hydrophobic than the heat-treated sample (99.1 and 93.0 arbitrary units (A.U.), respectively). Enzyme hydrolysis increased the SH by opening the protein structure and exposing the buried hydrophobic sites. The values for the hydrolysates were ~ 141–148 A.U. at 5–10% DH and ~ 176 A.U. at 15–20% DH. For PPI, the untreated isolate (91.8 A.U.) and the hydrolyzed samples (~ 142 A.U.) had a significantly lower SH value than the heat-treated isolate (171.7 A.U.). It was expected that the heat-treated isolate would exhibit significantly higher SH due to protein denaturation and the increase in the amount of exposed hydrophobic patches. Peng et al. [34] reported an increase in SH post heat treatment (95 °C for 30 min) for pea protein at pH 7.0. Opposing the findings for faba bean, the pea protein hydrolysates showed significantly lower hydrophobicity than the heat-treated isolate. This could be due to the enzymatic breakdown of hydrophobic regions that results in the reburying of hydrophobic patches via hydrophobic interactions and the reduced surface area of those patches due to protein–protein aggregation as a result of heating [33, 35]. In general, both FBPI and PPI showed improvement in SH after hydrolysis. In the case of faba bean, the increase was more pronounced at higher DH (15%–20%), and for both pulse proteins, at lower DH (5%–10%), the values were similar (> 140 A.U.).
Surface and interfacial tension
ST exists when there is an air–water interaction, which also determines the foaming properties of the solution, while IT occurs at the oil–water interface and generally defines the emulsifying properties of the protein solution. Proteins act as surfactants, thereby, reducing ST and IT by adsorbing and unfolding at the water–air or water–oil interface, respectively. The ST and IT for the untreated, heat-treated and hydrolyzed FBPI and PPI at pH 7.0 are presented in Table 3. The ST and IT was not performed at pH 4.5 due to the pulse proteins’ excessive aggregation and precipitation at this pH, which prevents obtaining meaningful data from the measurements. Both pulse protein solutions lowered ST and IT relative to water (~ 72 and ~ 22 mN/m, respectively). The ST values for the untreated pulse protein isolates were similar at ~ 49 mN/m. Heat treatment of the isolates increased ST to > 50 mN/m. This may be due to the denaturation of the proteins after heating at 85 °C for 30 min, which makes them more difficult to align at the interface. Enzyme hydrolysis gradually reduced ST to < 50 mN/m and eventually lower (~ 47 mN/m at 10% and 20% DH for PPI and FBPI, respectively) than that of the respective untreated isolate. The IT was also reduced by enzyme hydrolysis for both pulses (~ 10–11 mN/m); however, heating did not increase the IT like for ST but instead maintained the same level of surface activity as the untreated isolates (~ 13 mN/m). The values were not significantly different among the hydrolysates for either pulse. Protein hydrolysis decreases ST and IT as proteins break down into low molecular weight aggregates with greater rates of diffusion to the surface/interface, which in the meantime are also more flexible and readily adsorbed to form viscoelastic cohesive films, effectively reducing the tension [36].
Functionality
Solubility
The protein solubility was analyzed in situ prior to centrifugation in the hydrolysate production process (pH 7.2–7.8) (Table 4). An increasing trend in solubility was observed for both pulses after hydrolysis. For FBPI, the solubility of the untreated and heated isolates was 10.2% and 40.4%, respectively, and it increased with DH for the hydrolysates and reached 69.0% at 20% DH. In the case of PPI, the untreated and heat-treated isolates had solubility of 21.7% and 57.2%, respectively, and 5% and 10% DH hydrolysates showed values of ~ 73%. Similar findings were reported by Wu et al. [37] wherein papain increased the solubility of soy protein isolate with increases in the length of hydrolysis. Eckert et al. [32] also reported enhanced solubility of FBPI by pepsin hydrolysis. The reason for the increased solubility is possibly due to the breaking of hydrogen bonds between aggregates in commercially spray-dried products during heating. This leads to reduced particle size and enhanced solubility of the hydrolysates. This is supported by the data shown by CGE analysis (Table S1). Smaller peptides interact and form hydrogen bonds with water as more hydrophilic sites become available after hydrolysis [35].
Water holding capacity
The WHC values for the untreated and heated isolates and their hydrolysates for both pulses are shown in Table 4. The WHC for faba bean was the highest for the untreated isolate (4.03 g/g). Among the hydrolysates, the 5% DH sample showed the highest WHC (3.09 g/g), which was significantly higher than the heat-treated isolate (2.46 g/g), while the 10%–20% DH samples had similar values (2.32–2.68 g/g) to the heat-treated sample. For pea protein samples, there was no significant difference in WHC between the untreated and heat-treated isolates (~ 3.2 g/g), while the hydrolyzed samples showed significantly lower WHC (~ 2.5 g/g). A similar finding was reported by Periago et al. [38] where hydrolyzed pea protein flour showed lower WHC as compared to the unhydrolyzed flour. Hydrolysis of proteins can lead to increased or decreased exposure of buried hydrophilic or hydrophobic amino acid moieties, depending on the structure, conformation, and level of aggregation. For the untreated isolate, since there was no heat or enzymatic treatment followed by centrifugation, there was no loss of hydrophilic peptides which would otherwise be available to hold water. It is hypothesized that, after hydrolysis, peptides break down and get lost in the supernatant after centrifuging, resulting in reduced WHC. Among the faba bean protein hydrolysates, the 5% DH sample showed the highest WHC, likely due to the lower loss of such protein fragments in the supernatant post centrifugation as compared to higher DH samples. Similar findings were reported by Yin et al. [20] where the authors attributed the decreases in WHC as a result of reduced hydrophilic pockets and increases in insoluble protein aggregates. In our study, the thermal inactivation of the enzyme played a major role in globulin aggregation, which is not as soluble in water. A study by Goertzen et al. [4] also reported lower WHC following trypsin hydrolysis of chickpea protein isolates as compared to hydrolysis by other proteases. The authors hypothesized that the lower WHC may be due to the compact protein structures (probably due to isoelectric extraction) that prohibited water accessibility.
Oil holding capacity
The OHC values for the untreated and heated isolates and their hydrolysates for both pulses are shown in Table 4. In the case of faba bean proteins, OHC was the lowest for the untreated and heat-treated isolates (~ 1.7 g/g), while hydrolyzed samples showed higher values (~ 2.0–2.5 g/g). The OHC was the highest in 5%–10% DH samples (~ 2.5 g/g) while 15%–20% DH samples showed lower values at ~ 2.0 g/g. In the case of the pea protein samples, the heat-treated isolate had the highest OHC (~ 2.3 g/g) followed by the hydrolysates, which had similar values (~ 1.8–2.0 g/g) at 5%-10%, whereas the unheated isolate showed the lowest OHC (~ 1.5 g/g). Overall, both faba bean and pea protein hydrolysates showed higher OHC as compared to their respective untreated controls. Similar trends have been reported by Konieczny et al. [5], Wani et al. [39], and Eckert et al. [32] where hydrolysis significantly improved the OHC of pea protein enriched flour, kidney bean protein isolate, and FBPI, respectively. Due to the unravelling of protein structures, more functional groups were allowed to bind the hydrophobic (hydrocarbon) patches of the oil via interactions such as hydrogen bonding, hydrophobic, and electrostatic interactions [32]. Protein–protein interactions may have also been promoted post hydrolysis due to the exposure of surface active groups, forming highly porous matrices and physically entrapping oil droplets within the micro-capillaries [5].
Foaming capacity and stability
Table 5 shows the FC and FS at pH 4.5 and 7.0 of the untreated, heat-treated, and hydrolyzed isolates for both pulses. FC measures the ability of the protein to incorporate and entrap air, and FS refers to the ability of the generated foam to withstand destructive factors such as gravity and coalescence. The FC and FS changed significantly as a function of DH at a given pH for both pulses, except for the FC of PPI at pH 7.0, which was unaffected. At pH 7.0, the untreated FBPI had the highest FC of 291%, while heat and enzymatic treatments lowered FC significantly to < 90%. The heat-treated FBPI had the lowest FC of 40%, whereas hydrolysis enhanced it as a function of DH until 15% (FC of 82%), and further hydrolysis to 20% DH did not improve the property. One of the major factors contributing to the lower FC of the hydrolysates was hypothesized to be the absence of the majority of the albumins (which were lost during centrifugation). Albumins exhibit superior FC compared to globulins, as the latter is associated with reduced protein solubility and interaction with water, causing lower protein unfolding at the aqueous phase for air entrapment [40]. PPI, however, showed no significant differences among the samples in FC at pH 7; all samples (untreated, heat-treated, and hydrolysed) showed FC of ~ 220–240%. There were strong positive correlations of FC with the SC at pH 7.0 for both pulses (r = 0.941, p < 0.001), indicating that very high charges (> ± 30 mV) may negatively contribute to foamability, and a reduction in the magnitude of charge (with protein becoming less negative) may enhance this ability. The SH was negatively correlated to FC at pH 7.0 (FBPI: r = − 0.395, p < 0.05; PPI: r = − 0.606, p < 0.05), suggesting that highly hydrophobic sites may have prevented the proteins to generate films and foam at the interface.
The FS at pH 7.0 for both faba bean and pea proteins followed a decreasing trend upon hydrolysis. The untreated and heat-treated samples had FS in the range of 68%-73% for FBPI, and 62−66% for PPI. The FS for faba bean decreased to 35% at 5% DH and further to 24% at 20% DH, while for pea the FS was similar for both hydrolysates (13–14% at 5–10% DH). Similar to FC, FS also had a strong positive correlation with SC at pH 7.0 (FBPI: r = 0.728, p < 0.001; PPI: r = 0.702, p < 0.05) and a negative one with SH (FBPI: r = − 0.948, p < 0.001), further confirming the importance of moderate charge and hydrophobicity of a protein in determining its foaming properties. Yin et al. [20], Vioque et al. [41], and Barać et al. [42] reported decreasing trends for FC for hemp, rapeseed, and pea proteins as hydrolysis progressed, indicating that an increase in hydrophobicity may not contribute to efficient foaming ability.
At pH 4.5, the untreated FBPI had the maximum FC of 157%, while the treated samples (heated and enzymatically modified) showed significantly lower values of < 100%. Among the treatments, the heat-treated isolate had the lowest FC (20%), while hydrolysis had a positive impact on FC (50–93%) and increased it as a function of DH up to 15%. The heat-treated PPI isolate did not foam at pH 4.5. At a pH close to pI with a lack of repulsive forces and high hydrophobicity, the heat-treated peptides (mainly globulins) could be too bulky due to protein–protein interactions that do not allow for effective migration to the interface and foam accordingly. There was an increase in FC after PPI hydrolysis with the hydrolysates having significantly higher values (174% at 5% DH and 214% at 10% DH) than the untreated isolate (153%).
The FS at pH 4.5 showed similar trends for both pulse types. The untreated samples of FBPI and PPI had minimum FS (15% and 4%, respectively), whereas after hydrolysis the FS increased above that of the respective heated controls. The FS for FBPI hydrolysates ranged between 62 and 73%, while that for PPI was 45–59%. The FS at pH 4.5 was higher than that at pH 7.0 for the hydrolysates. Samaei et al. [43] also reported that at acidic pH, FS is higher, likely because, at lower pH, there is lower electrostatic repulsion, enabling the formation of more cohesive films at the interface. Overall, opposite to pH 7.0, at pH 4.5, the more charged a protein was, the higher the FC (FBPI: r = − 0.727, p < 0.001; PPI: r = − 0.810, p < 0.05) and FS (FBPI: r = − 0.900, p < 0.001; PPI: r = − 0.872, p < 0.001).
Emulsifying activity and stability indices
The EAI and ESI at pH 4.5 and 7.0 for FBPI and PPI samples are presented in Table 5. At pH 7.0, the EAI increased in general as a function of DH for both pulses. For FBPI, although the untreated and heat-treated samples had significantly different values, the difference between the values was minimal (16.4 and 15.0 m2/g, respectively). The FBPI hydrolysates showed higher EAI, which were ~ 18–19 m2/g at 5–10% DH and ~ 24–25 m2/g at 15–20% DH. For PPI the heat-treated sample had the lowest EAI (~ 5 m2/g) compared to the untreated sample (~ 20 m2/g) and the hydrolysates (~ 21–30 m2/g). Barać et al. [42] reported an increase in EAI of PPI when treated with chymosin, which later decreased as a function of DH at pH 7.0. Eckert et al. [32] also reported a similar trend where EAI for FBPI treated by trypsin increased within 15 min of hydrolysis, while further hydrolysis lowered EAI. However, hydrolysis up to 20% DH in the present study did not show any detrimental effect on EAI. The steady increase in EAI is related to the consistently low IT of the protein samples upon processing (~ 10–13 mN/m), as IT was negatively correlated with pH 7.0 EAI for both pulses (FBPI: r = − 0.682, p < 0.016; PPI: r = − 0.703, p < 0.001). Increases to the SH profile upon hydrolysis were one of the major reasons for the enhanced EAI of FBPI (r = 0.930, p < 0.001). However, for PPI, SH was negatively correlated with PPI (r = − 0.941, p < 0.041) possibly owing to the inherent differences between the two pulses. The increase in charge density (SC) after enzyme hydrolysis for PPI contributed to the improvements in EAI (r = − 0.865, p < 0.001), which was opposite to the foaming results and attributed to the fundamental differences in the two colloidal systems (phase density difference, droplet size, dispersed phase solubility, etc.) [44].
For ESI, although there were statistical differences in the values at pH 7.0 for FBPI samples the magnitude of the difference was relatively small (~ 12–14 min). In contrast, for PPI, the heat-treated sample had a markedly higher ESI (~ 25 min) than the untreated isolate (~ 12 min) and the hydrolysates (~ 14–16 min). There was a negative correlation between ESI and SC for FBPI (r = − 0.52, p < 0.05), while for PPI, there was a negative correlation between ESI and IT (r = − 0.607, p < 0.036).
At pH 4.5, the EAI values for both pulses were lower than their corresponding values at pH 7.0, which was expected as pH 4.5 is closer to the general pI of pulse proteins where aggregation is likely and in turn prevents the efficient formation of emulsions. For FBPI, there was little difference in EAI values among samples until 15% DH was reached. The EAI for samples below 15% DH (including the controls) ranged from ~ 2 to 4 m2/g, while 15–20% DH samples had EAI of > 10 m2/g. For PPI, the untreated sample showed the highest EAI of 5.0 m2/g, which was reduced to 1.0 and ~ 1.5 m2/g upon heating and hydrolyzing, respectively. The reduced molecular size of the peptides and the enhanced rate of diffusion and interfacial flexibility of the hydrolysates as compared to the heated control may play a critical role in EAI as no correlations were found with SC at pH 4.5.
In terms of ESI, unexpectedly, the values at pH 4.5 were generally higher than those at pH 7 for both pulses. The untreated FBPI showed the maximum stability (~ 35 min) followed by the heat-treated and 5% DH samples (~ 27 min). Further hydrolysis reduced ESI with the 10–20% DH hydrolysates having similar values (~ 11–14 min). Although smaller peptides resulting from hydrolysis allow better diffusion within solution (and onto the interface), they may not be able to reduce interfacial tension as effectively as native protein particles due to the reduction in molecular size and structure rigidity. PPI behaved differently than FBPI in terms of ESI at pH 4.5. The untreated PPI control had an ESI of ~ 16 min which was significantly increased by heat and enzymatic treatments to > 40 min. Barać et al. [42] also reported the ESI of PPI at pH 5.0 to improve after hydrolysis (30 min chymosin treatment). Konieczny et al. [5] reported an increase in ESI at pH 4.0 for pea protein-enriched flour when treated with papain. Enhanced ESI of PPI post hydrolysis can be attributed to the high solubility profile of the samples (Table 4), which was greater than FBPI under each respective treatment and may have allowed for even faster migration of the particles to the interfacial area, overcoming the negative effect of reduced film thickness and elasticity.
Protein nutritional quality
To predict the protein quality, the limiting amino acid and their scores, IVPD and IVPDCAAS were determined (Table 6). Generally, the limiting amino acid profile of a pulse depends on the pulse type and variety, but it is usually the sulfur-containing amino acids, cysteine and methionine, or the hydrophobic amino acid, tryptophan [45, 46]. Most samples were first limiting in tryptophan and had scores ranging between 0.41 and 0.76 for FBPI and between 0.42 and 0.85 for PPI. The exception to this was the untreated PPI and 10% DH FBPI, which were first limiting in the sulfur-containing amino acids (cysteine and methionine, scored together) with scores of 0.85 and 0.87, respectively. There was an initial decrease in the scores of tryptophan for FBPI upon heating from 0.54 to 0.41 which was then increased by hydrolysis to > 0.63. The 10% DH FBPI had the highest amino acid score (0.87) of the hydrolysates (Table S2 and S3). The heat treatment may have resulted in the loss of tryptophan in the form of albumins. Similar to the FBPI, PPI had a decrease in amino acid scores after the heat treatment from > 0.87 to 0.42 which was then improved with hydrolysis to 0.72–0.85 but did not reach the levels of the untreated isolate. This suggests that trypsin cleaved peptides in a way that resulted in solutions containing more tryptophan which were later removed by centrifugation. The sulfur-containing amino acids were limiting in the untreated PPI (0.87) but not in the hydrolysates, opposite to the trend observed by Konieczny et al. [47], where cysteine levels decreased with hydrolysis. Conditions of enzymatic hydrolysis, including the choices of enzymes and substrate materials, temperature, mixing ratio, pH, etc., play a vital role in deciding the fate of amino acids.
The IVPD of the untreated FBPI was similar to the heat-treated isolate and the 5% DH sample (~ 84–87%), but it decreased with further hydrolysis to ~ 77% at 10% DH. As hydrolysis progressed to 15–20% DH, IVPD increased (~ 80–82%). The IVPDCAAS, which is the product of IVPD and the amino acid scores, and a measure of overall protein quality, was lowest for FBPI heat-treated sample (~ 36%) followed by the untreated sample (~ 47%). The value increased for the hydrolysates, of which the 10% DH sample had the highest IVPDCAAS of ~ 66%, followed by the 15–20% (~ 60–61%) and the 5% DH samples (~ 54%). Overall, the faba bean protein hydrolysates exhibited higher protein quality than those not modified by trypsin. The same trend was not found for PPI. The IVPD was maximum for the untreated PPI (~ 88%), while heat and 5% DH enzyme treatment reduced it to ~ 81%-83%. Further hydrolysis to 10% DH lowered the IVPD to ~ 78%. Due to the significantly lower amino acid score (0.42) for the heat-treated isolate, the IVPDCAAS for that sample was only ~ 35% while that of the untreated control was ~ 77%. Hydrolysis improved protein quality compared to the heat-treated control as a function of DH with 5% and 10% DH samples exhibiting ~ 59% and ~ 67% IVPDCAAS, respectively.
Shi et al. [48] reported the IVPD of raw FBPI and PPI to be ~ 78% and ~ 82%, respectively, which were lower than our present findings. The authors also reported the IVPDCAAS to be similar to the current values for FBPI (~ 46%) while that for PPI was much lower (~ 65%), with the limiting amino acids being methionine and cysteine for both pulses. Protein digestibility depends substantially on the species and origin of the crop. A study by Weerasooriya et al. [49] reported that protein digestibility was largely impacted by genotype for sorghums. The decrease in IVPD after heat-treatment or hydrolysis is likely due to two reasons. First, as the majority of albumins were hypothesized to be removed by centrifugation, the remaining portion in the form of globulins may be too bulky and rigid in terms of protein structure, preventing efficient attacks by the digestive enzymes. These albumin and liberated peptides are not well separated in the centrifuge conditions employed in this study, and remain in the discarded supernatant. Second, since the hydrolysates were treated with trypsin, substrate binding sites specific to this enzyme may have already been occupied and cleaved, leaving fewer pockets available for the trypsin later added during IVPD tests as part of the multi-enzyme cocktail.
Conclusions
In this study, trypsin was used to hydrolyze FBPI up to 20% DH and PPI up to 10% DH. The FTIR analysis showed changes in the secondary protein structures after hydrolysis with differences observed between pulses. The DH influenced many quality attributes, both positively (solubility, OHC, FC, FS, EAI, ESI, and IVPDCAAS) and negatively (FS, ESI, IVPD, and IVPDCAAS), depending on the pulse type and functionality pH (for foaming and emulsifying properties). Hence, no conclusion can be drawn on the level of hydrolysis to optimize protein functionality and quality based on the present findings; however, certain attributes can be tailored under controlled conditions. Improved solubility of PPI and FBPI with enzymatic hydrolysis can allow for the protein isolates to be used as liquid ingredients. The high OHC of FBPI at 5–10% DH can be exploited to make products that require improved textural properties and better mouthfeel (e.g., processed meats and meat analogs). At neutral pH, both faba bean (20% DH) and pea (10% DH) hydrolysates improved emulsifying activity by over 50% with modest improvements in emulsion stability as well. Trypsin hydrolysis can be used to improve the protein quality in terms of in vitro protein digestibility corrected amino acid score of FBPI but not PPI. As the heat-treatment without hydrolysis was detrimental to many protein properties novel methods of enzyme inactivation that are non-thermal/destructive such as ultrasonication, plasma-activated water, etc., should be researched so the full potential of enzymatic modification of plant protein ingredients can be achieved.
Data availability
Data can be made available upon request.
References
Amândio MST, Rocha JMS, Xavier AMRB (2023) Enzymatic hydrolysis strategies for cellulosic sugars production to obtain bioethanol from Eucalyptus globulus bark. Fermentation 9(3):241. https://doi.org/10.3390/fermentation9030241
Hou Y, Wu Z, Dai Z, Wang G, Wu G (2017) Protein hydrolysates in animal nutrition: industrial production, bioactive peptides, and functional significance. J Anim Sci Biotechnol 8(3):513–525. https://doi.org/10.1186/s40104-017-0153-9
Barać MB, Pešić MB, Stanojević SP, Kostić AZ, Čabrilo SB (2015) Techno-functional properties of pea (Pisum sativum) protein isolates: a review. Acta Period Technol 46:1–18. https://doi.org/10.2298/APT1546001B
Goertzen AD, Nickerson MT, Tanaka T (2021) The improvement of the functional properties of a chickpea protein isolate through proteolysis with three proteases. Cereal Chem 98(3):439–449. https://doi.org/10.1002/cche.10383
Konieczny D, Stone AK, Korber DR, Nickerson MT, Tanaka T (2020) Physicochemical properties of enzymatically modified pea protein-enriched flour treated by different enzymes to varying levels of hydrolysis. Cereal Chem 97(2):326–338. https://doi.org/10.1002/cche.10248
Martineau-Côté D, Achouri A, Karboune S, L’Hocine L (2022) Faba bean: an untapped source of quality plant proteins and bioactives. Nutrients 14(8):1–27. https://doi.org/10.3390/nu14081541
Le Gall M, Guéguen J, Séve B, Quillien L (2005) Effects of grinding and thermal treatments on hydrolysis susceptibility of pea proteins (Pisum sativum L.). J Agric Food Chem 53(8):3057–3064. https://doi.org/10.1021/jf040314w
Sim S, Akila SRV, Chiang J, Henry C (2021) Plant proteins for future foods: a roadmap. Foods 10(8):1967. https://doi.org/10.3390/foods10081967
Humiski LM, Aluko RE (2007) Physicochemical and bitterness properties of enzymatic pea protein hydrolysates. J Food Sci. https://doi.org/10.1111/j.1750-3841.2007.00475.x
Adler-Nissen J (1979) Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J Agric Food Chem 27(6):1256–1262. https://doi.org/10.1021/jf60226a042
Avramenko N, Low N, Nickerson M (2013) The effects of limited enzymatic hydrolysis on the physicochemical and emulsifying properties of a lentil protein isolate. Food Res Int 51:162–169. https://doi.org/10.1016/j.foodres.2012.11.020
AOAC (2005) Official method of analysis of AOAC international, 18th edn. Association of Official Analytical Chemists, Gaithersburg
Das PP, Xu C, Lu Y, Khorsandi A, Tanaka T, Korber DR, Nickerson MT, Rajagopalan N (2023) Snapshot of proteomic changes in Aspergillus oryzae during various stages of fermentative processing of pea protein isolate. Food Chem Mol Sci 6:100169. https://doi.org/10.1016/j.fochms.2023.100169
Kato A, Nakai S (1980) Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins. Biochim Biophys Acta BBA Protein Struct 624:13–20
Stone AK, Karalash A, Tyler RT, Warkentin TD, Nickerson MT (2015) Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res Int 76:31–38. https://doi.org/10.1016/j.foodres.2014.11.017
Can Karaca A, Low N, Nickerson M (2011) Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res Int 44:2742–2750. https://doi.org/10.1016/j.foodres.2011.06.012
Wang Y, Ghosh S, Nickerson MT (2019) Effect of pH on the formation of electrostatic complexes between lentil protein isolate and a range of anionic polysaccharides, and their resulting emulsifying properties. Food Chem. 298:125023. https://doi.org/10.1016/j.foodchem.2019.125023
Achouri A, Boye JI, Yaylayan VA, Yeboah FK (2005) Functional properties of glycated soy 11S glycinin. Food Chem and Toxicol 70:269–274. https://doi.org/10.1111/j.1365-2621.2005.tb07172.x
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Yin SW, Tang CH, Cao JS, Hu EK, Wen QB, Yang XQ (2008) Effect of limited enzymatic hydrolysis with trypsin on the functional properties of hemp (Cannabis sativa L.) protein isolate. Food Chem 106:1004–1013. https://doi.org/10.1016/j.foodchem.2007.07.030
Wilde PJ, Clark DC (1996) Methods of testing protein functionality. Blackie Academic and Professional, London
Pearce KN, Kinsella JE (1978) Emulsifying properties of proteins: evaluation of a turbidimetric technique. J Agric Food Chem 26(3):716–723. https://doi.org/10.1021/jf60217a041
FAO (Food and Agricultural Organization) (1991) The state of food and agriculture. http://www.fao.org/3/a-t0496e.pdf. Accessed 19 Jan 2023
Tinus T, Damour M, Van Riel V, Sopade PA (2012) Particle size-starch-protein digestibility relationships in cowpea (Vigna unguiculata). J Food Eng 113:254–264. https://doi.org/10.1016/j.jfoodeng.2012.05.041
García Arteaga V, Apéstegui Guardia M, Muranyi I, Eisner P, Schweiggert-Weisz U (2020) Effect of enzymatic hydrolysis on molecular weight distribution, techno-functional properties and sensory perception of pea protein isolates. Innov Food Sci Emerg Technol 65:102449
Bogdanov IV, Shenkarev ZO, Finkina EI, Melnikova DN, Rumynskiy EI, Arseniev AS, Ovchinnikova TV (2016) A novel lipid transfer protein from the pea Pisum sativum: Isolation, recombinant expression, solution structure, antifungal activity, lipid binding, and allergenic roperties. BMC Plant Biol 16(1):1–17. https://doi.org/10.1186/s12870-016-0792-6
Carbonaro M, Maselli P, Nucara A (2012) Relationship between digestibility and secondary structure of raw and thermally treated legume proteins: a Fourier transform infrared (FT-IR) spectroscopic study. Amino Acids 43(2):911–921. https://doi.org/10.1007/s00726-011-1151-4
Shevkani K, Singh N, Kaur A, Rana JC (2015) Structural and functional characterization of kidney bean and field pea protein isolates: a comparative study. Food Hydrocoll 43:679–689. https://doi.org/10.1016/j.foodhyd.2014.07.024
Bogahawaththa D, Bao Chau NH, Trivedi J, Dissanayake M, Vasiljevic T (2019) Impact of selected process parameters on solubility and heat stability of pea protein isolate. Food Sci Technol 102:246–253. https://doi.org/10.1016/j.lwt.2018.12.034
Shevkani K, Singh N, Chen Y, Kaur A, Yu L (2019) Pulse proteins: Secondary structure, functionality and applications. J Food Sci Technol 56(6):2787–2798. https://doi.org/10.1007/s13197-019-03723-8
Achouri A, Zhang W, Shiying X (1998) Enzymatic hydrolysis of soy protein isolate and effect of succinylation on the functional properties of resulting protein hydrolysates. Food Res Int 31(9):617–623. https://doi.org/10.1016/S0963-9969(98)00104-5
Eckert E, Han J, Swallow K, Tian Z, Jarpa-Parra M, Chen L (2019) Effects of enzymatic hydrolysis and ultrafiltration on physicochemical and functional properties of faba bean protein. Cereal Chem 96(4):725–741. https://doi.org/10.1002/cche.10169
Xu X, Liu W, Liu C, Luo L, Chen J, Luo S, McClements DJ, Wu L (2016) Effect of limited enzymatic hydrolysis on structure and emulsifying properties of rice glutelin. Food Hydrocoll 61:251–260. https://doi.org/10.1016/j.foodhyd.2016.05.023
Peng W, Kong X, Chen Y, Zhang C, Yang Y, Hua Y (2016) Effects of heat treatment on the emulsifying properties of pea proteins. Food Hydrocoll 52:301–310. https://doi.org/10.1016/j.foodhyd.2015.06.025
Liu C, Bhattarai M, Mikkonen KS, Heinonen M (2019) Effects of enzymatic hydrolysis of fava bean protein isolate by Alcalase on the physical and oxidative stability of oil-in-water emulsions. J Agric Food Chem 67(23):6625–6632. https://doi.org/10.1021/acs.jafc.9b00914
Yuan B, Ren J, Zhao M, Luo D, Gu L (2012) Effects of limited enzymatic hydrolysis with pepsin and high-pressure homogenization on the functional properties of soybean protein isolate. Food Sci Technol 46(2):453–459. https://doi.org/10.1016/j.lwt.2011.12.001
Wu WU, Hettiarachchy NS, Qi M (1998) Hydrophobicity, solubility, and emulsifying properties of soy protein peptides prepared by papain modification and ultrafiltration. J Am Oil Chem Soc 75(7):845–850. https://doi.org/10.1007/s11746-998-0235-0
Periago MJ, Vidal ML, Ros G, Rincón F, Martínez C, López G, Rodrigo J, Martínez I (1998) Influence of enzymatic treatment on the nutritional and functional properties of pea flour. Food Chem 63(1):71–78. https://doi.org/10.1016/S0308-8146(97)00199-4
Wani IA, Sogi DS, Shivhare US, Gill BS (2015) Physico-chemical and functional properties of native and hydrolyzed kidney bean (Phaseolus vulgaris L.) protein isolates. Food Res Int 76:11–18. https://doi.org/10.1016/j.foodres.2014.08.027
Malomo SA, Aluko RE (2015) A comparative study of the structural and functional properties of isolated hemp seed (Cannabis sativa L.) albumin and globulin fractions. Food Hydrocoll 43:743–752. https://doi.org/10.1016/j.foodhyd.2014.08.001
Vioque J, Sánchez-Vioque R, Clemente A, Pedroche J, Millán F (2000) Partially hydrolyzed rapeseed protein isolates with improved functional properties. J Am Oil Chem Soc 77(4):447–450. https://doi.org/10.1007/s11746-000-0072-y
Barać M, Čabrilo S, Pešić M, Stanojević S, Pavlićević M, Maćej O, Ristić N (2011) Functional properties of pea (Pisum sativum, L.) protein isolates modified with chymosin. Int J Mol Sci 12(12):8372–8387. https://doi.org/10.3390/ijms12128372
Samaei SP, Ghorbani M, Tagliazucchi D, Martini S, Gotti R, Themelis T, Tesini F, Gianotti A, Gallina Toschi T, Babini E (2020) Functional, nutritional, antioxidant, sensory properties and comparative peptidomic profile of faba bean (Vicia faba L.) seed protein hydrolysates and fortified apple juice. Food Chem 330:127120. https://doi.org/10.1016/j.foodchem.2020.127120
Walstra P, Vliet T (2008) Dispersed systems: Basic considerations. In: Damodaran S, Parkin KL, Fennema OR (eds) Fennema’s food chemistry. CRC Press, Boca Raton. https://doi.org/10.1201/9781420020526
Duc G (1997) Faba bean (Vicia faba L.). Field Crops Res 53(1–3):99–109. https://doi.org/10.1016/S0378-4290(97)00025-7
Nosworthy MG, Franczyk A, Zimoch-Korzycka A, Appah P, Utioh A, Neufeld J, House JD (2017) Impact of processing on the protein quality of pinto bean (Phaseolus vulgaris) and buckwheat (Fagopyrum esculentum Moench) flours and blends, as determined by in vitro and in vivo methodologies. J Agric Food Chem 65(19):3919–3925. https://doi.org/10.1021/acs.jafc.7b00697
Konieczny D, Stone AK, Nosworthy MG, House JD, Korber DR, Nickerson MT, Tanaka T (2020) Nutritional properties of pea protein-enriched flour treated with different proteases to varying degrees of hydrolysis. Cereal Chem 97(2):429–440. https://doi.org/10.1002/cche.102586
Shi D, House JD, Wanasundara JPD, Nickerson MT (2022) Comparative evaluation of the nutritional value of faba bean flours and protein isolates with major legumes in the market. Cereal Chem 99(5):1013–1029. https://doi.org/10.1002/cche.10575
Weerasooriya DK, Bean SR, Nugusu Y, Ioerger BP, Tesso TT (2018) The effect of genotype and traditional food processing methods on in-vitro protein digestibility and micronutrient profile of sorghum cooked products. PLoS ONE 13(9):1–22. https://doi.org/10.1371/journal.pone.0203005
Funding
Financial support for the present study was provided through Agriculture and Agri-Food Canada’s AgriScience Program (Canadian Pulse Science Research Cluster) under the Canadian Agricultural Partnership, the Saskatchewan Pulse Growers, the Alberta Pulse Growers, the Manitoba Pulse and Soybean Growers and the Natural Sciences and Engineering Research Council of Canada Discovery grant program. The authors gratefully acknowledge support from the Sustainable Protein Production (SPP) program of the National Research Council Canada and by the Aquatic and Crop Resource Development Research Centre as part of its contribution to the SPP program.
Author information
Authors and Affiliations
Contributions
Joy Sareen carried out the experiments, analyzed data, and wrote the first draft of the manuscript. Dai Shi and Andrea K. Stone provided critical review and editing of the draft. Caishuang Xu carried out CGE and SDS-PAGE experiments and data analysis. Brittany Polley carried out the trypsin inhibitor assay. James D. House, Pankaj Bhowmik, and Nandhakishore Rajagopalan provided technical assistance and comments on the draft. Takuji Tanaka and Michael T. Nickerson designed and supervised the research and revised the manuscript drafts. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Compliance with ethics requirements
This article does not contain any studies with human participants or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sareen, J., Shi, D., Stone, A.K. et al. Effect of enzyme hydrolysis on the physicochemical, functional, and nutritional properties of pea and faba bean protein isolates. Eur Food Res Technol 249, 3175–3190 (2023). https://doi.org/10.1007/s00217-023-04358-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-023-04358-y