Abstract
With the increasing world population and vegan diet, there has been increasing consumer demand for alternative protein sources. A substitute for animal proteins is the plant protein, for instance, leaves. The Pereskia aculeata, known as ora-pro-nóbis, is undoubtedly a leafy vegetable with great potential due to its relatively high protein content (17 to 28%). This study aimed to produce ora-pro-nóbis protein concentrate (OPNPC) from ora-pro-nóbis leaves flour (OPNF) by isoelectric precipitation at three different pH’s (3.5, 4.0, and 4.5). The protein extraction by precipitation in different pHs produced OPNPC with protein content and extraction yield ranging from 52 to 55% and 1–4%, respectively. Given the highest yield, the concentrate obtained at pH 3.5 (OPNPC3.5) was selected for further investigation and comparison to OPNF. The differences in color, techno-functional properties, in vitro protein digestibility (IVPD), and structural properties were evaluated. Most techno-functional properties were statistically higher in OPNPC3.5 than in OPNF. These included its water solubility, oil holding capacity, foam capacity and stability, and emulsifying activity and stability. OPNPC3.5 had a higher IVPD (80%) than flour (77%). Scanning electron microscopy and Fourier transform infrared spectroscopy confirmed distinct compositions of materials, which can explain the difference in techno-functional properties. The findings indicate controlling protein extraction conditions as a useful technique to maximize the yield of protein concentrate obtained from ora-pro-nóbis, which was more nutritious and had better techno-functional properties than flour. This demonstrates its potential as an alternative plant-based protein to design healthy and sustainable food products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the increasing concern of consumers for healthier and sustainable products and the growth of vegetarian, flexitarian, and vegan people, it has been necessary to find alternative protein sources to substitute or decrease the consumption of animal proteins. In recent years, countless studies have investigated vegetables, agro-industrial by-products, and algae as alternative protein sources [1]. Some leaves are a nutritious and sustainable source of alternative protein. Plant leaves present high protein content (1–8% on a wet basis or 13–28% on a dry basis) and can be considered an inexpensive raw material to derive proteins for human consumption [2,3,4,5]. Particularly, leaves from cassava, sugar beet, tobacco, moringa, and ora-pro-nóbis have drawn attention for their economic feasibility and techno-functional properties [6].
The Pereskia aculeata Miller, popularly known as ora-pro-nóbis or Barbados gooseberry, is a climbing cactus with spiny stems and large leaves that can reach up to 10 m in length [7, 8]. Native to the American tropics, this non-conventional vegetable also grows naturally in South and Southeast Africa, as well as Northeast and Southeast Australia [9]. Although its fruit and flower are edible, the most commercialized and consumed part of this plant is its leaves [8, 9]. The leaves are smooth, dark green, and succulent; their shape varies between elliptical and symmetrical and reaches up to 15 cm in length and 8 cm in width [10]. Celebrated for its outstanding adaptability to diverse climatic conditions, the ora-pro-nóbis can thrive with low soil fertility and achieve satisfactory vegetative growth [11]. This characteristic emphasizes the potential for its straightforward cultivation in various parts of the world. Typical of some regions of Brazil, it is designated in low-income communities as the “meat of the poor” due to its high protein content, which varies from 17.4 to 28.4% [7, 8]. Ora-pro-nóbis leaves have higher protein content than other plant sources, such as amaranth (14.5%) [12], corn (9–12%), wheat (8–15%), and sorghum (9–17%) [13]. Furthermore, their proteins exhibit high in vitro digestibility (approximately 85%), underscoring their significant nutritional value [8, 14]. Ora-pro-nóbis leaves are also an excellent source of essential amino acids, having amounts comparable to soybeans and relatively higher than those of other commercially available sources, such as peas and wheat grains [15,16,17].
Several studies have evaluated the use of this leaf as flour in processed foods, such as hamburgers, commercial cake premixes, pasta, ice cream, dairy drinks, and sausages [18]. Besides the flour, the rich mucilage found in this vegetable has also been extensively investigated for application as a gelling agent, texture modifier, or stabilizer in processed food products [19, 20]. Despite these studies on the characterization and application of ora-pro-nóbis mucilage and flour, no specific studies on the properties of its protein isolate or concentrate have been reported in the literature. One of the main reasons is the lack of efficient techniques for extracting proteins from the leaf’s cell matrix, as they have a rigid cell wall that makes extraction a challenge [3]. The yields and protein contents obtained from the extraction based on alkaline solubilization-acid precipitation are still unattractive [21, 22]. In this way, using a precipitation pH close to the protein’s isoelectric point (pI) is a crucial step for successful protein extraction. At the pI, the negative and positive charges of the proteins are equal, resulting in low solubility of the material and greater precipitation/recovery [3].
Exploring basic ways to overcome bottlenecks for efficient ora-pro-nóbis protein extraction and understanding its techno-functional and nutritional properties are essential for using it in the food industry. Therefore, the objective of this study was to produce protein concentrate (OPNPC) from ora-pro-nóbis leaves by acid precipitation at three different pHs, i.e., 3.5, 4.0, and 4.5. The techno-functional and structural properties of the selected OPNPC sample with the highest yield were evaluated and compared to the flour from which it was derived.
Materials and methods
Material
The ora-pro-nóbis (Pereskia aculeata Miller) flour (OPNF) was purchased from Mundo Cerealista (São Paulo, Brazil). The OPNF had a proximate composition, on a dry basis, of: 58.26 g/100 g of carbohydrates, 14.37 g/100 g ash, 5.35 g/100 g lipids, and 22.02 g/100 g protein. The moisture content was 8.53 g/100 g. All the chemicals employed in this current study were reagent grade.
Ora-pro-nóbis protein extraction
Ora-pro-nóbis proteins were extracted by the conventional method of alkaline solubilization-acid precipitation [23]. Briefly, OPNF was dispersed in water at a flour-to-water ratio of 1:10 (g/mL). The mixture was adjusted to pH 10 with NaOH (1 M) and stirred vigorously for two hours. The suspension was centrifuged at 10000g for 30 min at 20 °C. Then, the pH of the supernatant was adjusted using HCl (1 M) to precipitate the proteins. To find the best pH of protein precipitation, tests were performed with pH 3.5, 4.0, and 4.5. The precipitated proteins were collected by centrifugation at 10000g for 20 min at 5 °C. The protein precipitates were resuspended in water and neutralized (pH 7) using NaOH. After freeze-drying, the ora-pro-nóbis protein concentrates (OPNPC) obtained at different pHs were stored separately in plastic bags at -18 °C before analyses.
Characterization of ora-pro-nóbis flour and protein concentrate
Protein contents and extraction yield of ora-pro-nóbis protein
The protein content of the OPNPC was determined by total nitrogen analysis by combustion (Dumas) using a protein analyzer (model NDA 701, VELP Scientific, Italy). Nitrogen content was multiplied by the conversion factor of 6.25 to determine the total crude protein content. The yield of the OPNPCs was calculated as suggested by Karaman et al. [24]:
The OPNPC sample with the highest yield was selected for further evaluation of color, structural and techno-functional properties, and in vitro digestibility.
Color properties
The color was determined by using the colorimeter UltraScan PRO- HunterLab. The CIELab color scale was used to measure the L*, a*, and b* parameters, where L* ranges from 0 (black) to 100 (white), a* shows the variation from green (-a*) to red (+ a*) and b* varies from blue (-b*) to yellow (+ b*). The total color difference (ΔE) was calculated according to Eq. 2, and OPNF was taken as standard. All measurements were performed in quadruplicate.
where L, a, and b are the standard color parameter values and L*, a*, and b* are the color parameter values of the sample.
Structural properties
Fourier transform infrared spectroscopy (FTIR)
The FTIR specters were recorded on a spectrophotometer IRPrestige-21 model (Kyoto, Japan). The analysis was carried out in the mean infrared region with a Fourier transform wavenumber range of 4000 to 500 cm− 1, using 10 scans and with a spectral resolution of 4 cm− 1.
Scanning electron microscopy (SEM)
Scanning electron microscopy (SEM) was carried out using a Hitachi TM4000Plus scanning electron microscope (HITACHI, Japan) operating at an acceleration voltage of 10 kV. The images were captured using the software Hitachi TM4000, with a magnitude of ×100.
Techno-functional properties
Protein solubility
Protein solubility was determined according to the methodology described by Calderón-Chiu et al. [23] with few modifications. 10 mg of flour or protein concentrate was dispersed in distilled water (50 mL). The dispersion was then adjusted to pH 7 using HCl or NaOH, stirred for 30 min, and centrifuged at 7500g for 15 min. The supernatant was recovered. Protein content in the supernatant was determined using the Bradford method [25] and bovine serum albumin was used as standard. The total protein content was determined by the solubilization of the sample in NaOH (0.5 N). Solubility (%) was calculated by the Eq. 3:
Water holding capacity (WHC)
WHC was determined using Rodríguez-Ambriz et al. [26] methodology with modifications of Ogunwolu et al. [27]. Briefly, 1 mL of distilled water was added to 100 mg of the sample (OPNPC3.5 or OPNF). The mixture was vortexed for 30 s and centrifuged at 1800g for 20 min at 25ºC. After centrifugation, the supernatant was drained completely at 45º for 10 min. WHC (g/g) was calculated as:
where W2 is the weight of microtube and sample after absorbing water; W1 is the weight of microtube and sample; W0 is the initial weight of sample.
Oil holding capacity (OHC)
The OHC was determined according to the methodology used by Lin and Zayas [28] with modifications of Ogunwolu et al. [27]. 100 mg of the sample was vortex-mixed with 1 mL of sunflower oil for 30 s. The emulsion was incubated at room temperature (about 25ºC) for 30 min and then centrifuged at 13,600g for 10 min at 25ºC. OHC (g/g) was calculated as:
where W2 is the weight of microtube and sample after absorbing oil; W1 is the weight of microtube and sample; W0 is the initial weight of sample.
Emulsifying activity index (EAI) and emulsifying stability index (ESI)
EAI was determined according to the spectrophotometric method of Pearce and Kinsella [29], with some modifications as described by Saricaoglu [30]. Briefly, dispersions (pH 7) were prepared by mixing 300 mg of the sample with 30 mL of distilled water. Thus, 10 mL sunflower oil was added and homogenized in an Ultra-turrax at 20,000 rpm for 1 min. The emulsion (50 µL) was transferred immediately and after 10 min to tubes containing 12.5 mL of 0.1% sodium dodecyl sulfate (SDS). The absorbance at 500 nm was measured with a spectrophotometer (Beckman Coulter DU 800, CA, USA) and the following equations were used for the calculation of EAI and ESI, respectively:
where A0 is the absorbance at 500 nm immediately after preparation, N refers to the dilution factor (250), \(\theta\) the oil phase ratio (0.25), L cuvette thickness (0.01 m), and C the concentration of the protein in the dispersion (g/mL)
where A0 and A10 are absorbance after 0 and 10 min, respectively; and t = 10 min.
Foaming properties
Foam capacity and foam stability were determined according to the methodology described by Shevkani et al. [31] with few modifications. Each sample was dispersed in distilled water (1% w/v, 20 mL, pH 7) in a graduated cylinder (50 mL) and the dispersion was then homogenized for 1 min at 20,000 rpm using an ultra-turrax. Foaming capacity (FC) was calculated as the percent increase in the volume of the suspensions upon mixing (Eq. 8), while foam stability (FS) was estimated as the percentage of foam remaining after 15 and 30 min (Eq. 9).
where \({V}_{1}\) is the initial volume of the dispersion; and \({V}_{2}\) is the volume of the dispersion after homogenization.
Where, \({V}_{t}\) is the foam volume after 15 and 30 min.
In vitro protein digestibility (IVPD)
A 62.5 mg sample was weighed and rehydrated in 10 mL of milli-Q water at 37º C for 1 h. Then, the pH of the dispersion was adjusted to 8 using NaOH or HCl. Ten milliliters (10 mL) of a multi-enzyme solution consisting of about 16 mg of trypsin, 31 mg of chymotrypsin, and 13 mg of protease was also prepared at 37ºC and pH 8.0. Then, 1 mL of multi-enzyme solution was added to the hydrated sample, and the digestion pH was recorded after 10 min [32, 33]. The change of the pH after 10 min of digestion (ΔpH10) was used to calculate the percentage of in vitro protein digestibility (Eq. 10).
Statistical analysis
Each experiment was run in triplicate and the data were expressed as means ± standard deviation. The results were statistically analyzed by analysis of variance (ANOVA) and t-test at 5% significance using SISVAR® software, version 5.6.
Results and discussion
Characterization of ora-pro-nóbis flour and protein concentrate
Protein contents and extraction yield of ora-pro-nóbis protein
The protein content of OPNF was 22.02% on a dry basis, which was similar to the value (21.81%) reported by Maciel et al. [34]. Usually, the protein content of green leaves ranges between 16 and 29% (dry basis) [35]. The OPNPC, on the other hand, had values ranging from 52.21 to 55.33% (Table 1), which were higher than that reported by Khan and Varshney [36] for the protein concentrate of Albizia lebbeck leaves (37%), but within the range described for moringa leaf protein (40.43–75.77%) [37]. However, the content was inferior to that found in the protein concentrate of jackfruit leaves (65.82%) which used high hydrostatic pressure (HHP) technology to favor the extraction of proteins [23].
Table 1 also shows the yield of protein concentrate extracted at pH 3.5, 4.0, and 4.5. The extraction yield varied from 1.17 to 4.19%. According to Balfany et al. [38], the isoelectric point of Rubisco, the main protein in leaves, is in the pH range of 3.5-5.0. At the isoelectric point, the solubility of the protein is minimal, leading to its precipitation and allowing its recovery [3]. The extraction yield of ora-pro-nóbis proteins at pH 3.5 (4.19%) and 4.0 (2.73%) was in the range of those found in moringa leaves (2 to 6.5%), but it was much lower than the extraction of proteins from jackfruit leaves (32%) assisted by high hydrostatic pressure [23]. Protein content and recovery yields of proteins from plant foliage are not as high as those of other vegetables due to the complex interaction of proteins with a plethora of other molecules in the leaf. The extraction procedures developed so far have primarily been carried out in laboratories and have not been transferred to industry for large-scale applications [3]. Several different techniques have emerged with high potential to assist the conventional protein extraction (i.e., use of enzymes, microwave, ultrasound, pulsed electric energy, and high pressure), however, they are still in the early stages of their industrial applications [39].
Given the highest yield, the OPNPC obtained by acid precipitation at pH 3.5 (OPNPC3.5) was chosen to have its techno-functional and structural properties, as well as in vitro digestibility, evaluated and compared with those of OPNF.
Color
All the color parameters were significantly (P < 0.05) different between the OPNPC3.5 and OPNF (Table 2). The concentrate was relatively darker (< L*) than the flour. The a* parameter changed from − 0.43 (OPNF) to 0.98 (OPNPC.3.5), which was consistent with the change in flour coloration from green to brown in the concentrate (Fig. 1). This darkening after the concentration of proteins was also observed in moringa flour by Bocarando-Guzmán et al. [37]. Modifications can be associated with the removal of flour constituents and the alkalinization step used to extract the proteins. High alkali concentrations may lead to the formation of dark-brown products [40, 41]. In addition, during alkalinization, protein reactions with polyphenols, carbohydrates, lignin, or pigments (such as chlorophyll and carotenoids) can trigger darkening [23]. Both OPNPC3.5 and OPNF have positive b* values, however, the lower b* in the concentrate indicates a less yellowish material (Table 2). Ora-pro-nóbis leaves have a high content of carotenoids (190–210 µg/g) [9]. Thus, protein reactions with this pigment during the alkalinization step may decrease the yellow color of the material.
The total color difference, ΔE of the OPNPC3.5 in relation to the OPNF was 19.88. Values higher than 5 indicate color changes visually perceptible to the human eye [42, 43], as shown in Fig. 1.
Structural properties
Fourier transform infrared spectroscopy (FTIR)
FTIR spectra revealed some differences between the flour and protein concentrate (Fig. 2). It is worth noting the presence of four distinct peaks (1718, 1373, 1155, and 758 cm− 1) only in the OPNF spectrum, which can be associated with a larger number of components in the constitution of the flour, such as lipids and carbohydrates. Peaks around 1745 cm− 1 indicate lipid presence and correspond to the C = O stretching vibration of lipid ester bonds [44]. As observed by Neves et al. [45], the peak at 1373 cm− 1 suggested the presence of COO- from the carboxylic acid group of the ora-pro-nóbis protein. Peaks around 1000–1200 cm− 1 wavelength correspond to C-H stretching vibration resulting from carbohydrates such as cellulose and starch [44]. In this way, the peak observed around 1155 cm− 1 can indicate the presence of cellulose in the flour. The absorption at 758 cm− 1 is mainly originated from carbohydrate vibrations [46].
Usually, in the infrared spectra, three groups of absorption bands can be observed for protein materials: amide I (1600–1700 cm− 1), amide II (1480–1585 cm− 1), and amide III (1260–1300 cm− 1) [44, 47, 48]. It can be seen that the peaks present in the OPNPC3.5 spectrum around 1650 (Amide I), 1539, and 1483 cm− 1 (Amide II) were more intense than the OPNF peaks. The peak at 1483 cm− 1 (Amide II) besides being more intense, was also narrower. Amide I and II are related to the secondary structure of proteins (β-sheet, α-helix, random coils, β-turns, etc.) [24] and their inter- or intramolecular effects [49]. It can be also observed the shift of some peaks. The peak for amide III was detected at a wavelength of around 1286 cm− 1 for OPNPC3.5 and around 1305 cm− 1 for OPNF, i.e., a shift of 19 cm− 1. Amide III indicates the presence of interactions between protein and other macromolecules such as carbohydrates with C-N stretching and N-H bending vibrations [44]. This is indirect evidence that changes in the protein’s structure can occur during the extraction [50]. A shift of the peak from 837 (OPNF) to 929 cm− 1 (OPNPC3.5) was also observed.
Scanning electron microscopy (SEM)
According to the SEM images (Fig. 3), OPNF exhibited heterogeneous shape and size particles, possibly due to the presence of more than one macromolecule in its composition (protein, carbohydrates, and fibers) and the interactions among these components. In general, the particles present in OPNF exhibited porous characteristics, indicating void spaces or gaps within the particles.
On the other hand, the structures in OPNPC3.5 did not display any porosity. OPNPC3.5 mainly exhibited a thin and smooth lamellar structure, as also observed by Chen et al. [51] for protein isolates from cumin.
Techno-functional properties
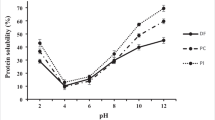
Protein solubility
Solubility relates directly to many important food properties such as emulsifying, foaming, surface-active properties, and gel-forming abilities. The solubility of OPNF and OPNPC3.5 at pH 7 was 23.37 and 87.38%, respectively (Table 3). This implies that the concentration of proteins improved the material solubility more than 3.5 times. Highly dependent on the balance of hydrophobic and hydrophilic components, the higher solubility of the concentrate can be related to the remotion of hydrophobic components, mainly fibers. Higher solubility is typically desirable as it can facilitate the incorporation of the material as an ingredient in food formulations [23] and improve other techno-functional properties of the material, such as foaming and emulsifying capacity. A study with Mahua deoiled cake flour also showed decreased solubility of proteins compared to protein isolate [52]. However, lower solubility to moringa isolate protein was reported by Bocarando-Guzmán et al. [37] compared with moringa flour (in the pH range of 4.5–7.5). According to the authors, the moringa flour was subjected to extreme conditions, which may have resulted in protein changes. Milder extraction conditions and higher protein purity in the sample improve protein solubility [3].
Water and oil holding capacities
Water holding capacity (WHC) is the ability of the protein to keep the water content remaining in its three-dimensional structure [6]. In this study, results indicated that the OPNPC3.5 has lower WHC than the OPNF (1.82 and 5.44 g/g, respectively). This can be explained by the fact that the OPNPC3.5 was almost completely dissolved in water, possibly due to the higher solubility of the concentrate (> 87%). Furthermore, another explanation for the higher WHC of OPNF could be the presence of more carbohydrates in the flour, which helps in water absorption [53]. OPNPC3.5 had WHC similar to that evaluated for some cereal proteins (1.4–1.5 g/g) [54], whereas this property was found to be higher in soy isolate protein (7.6 g/g) [55]. WHC values found for OPNF and pea protein isolate (5 g/g) were quite similar [56].
Regarding oil-holding capacity (OHC), both materials showed higher values than those reported for pea and soy isolates (0.86 and 1.43 g/g) [54]. However, the OPNPC3.5 had a greater OHC than OPNF (Table 3). According to Biswal et al. [52], the OHC3.5 of proteins increases with the presence of amino acids with nonpolar side chains, which can be the case of ora-pro-nóbis proteins that has a high amount of leucine, glycine, and phenylalanine [15]. High OHC is desirable for using in the cold meat industry since protein can bridge the fat and water in these products [57]. Besides that, a high OHC is good for improving the mouthfeel and flavor retention of specific food products [58].
Emulsifying activity index (EAI) and emulsifying stability index (ESI)
Food industries have constantly sought to develop functional ingredients with suitable emulsifying characteristics for application in different types of food. Emulsifying activity index (EAI) provides an estimation of the interfacial area stabilized per unit weight of protein based on the turbidity of a diluted emulsion [29], whereas the ESI provides a measure of the stability of the same diluted emulsion over a defined period [59]. In this study, the OPNPC3.5 showed 4.75-fold higher EAI than the flour (Table 3). EAI is directly affected by the protein content, oil absorption, and solubility of the material. The higher the protein content, OHC, and solubility, the greater the emulsifying activity [47, 54, 60]. A similar value was described for mulberry leaf protein extracted by ultrasound (22.45 m2/g) [48], while lower EAI was reported for pulse (9–11 m2/g) [58] and cereal protein (20 m2/g) [54]. The low EAI for OPNF (5.29 m2/g) was probably due to its low protein content (22%). Immediately after the homogenization step, the flour emulsion underwent an instantaneous phase separation. The low protein content and the high amount of insoluble fibers in the OPNF may be responsible for affecting the emulsion formation and decreasing the EAI. Although of significant (P < 0.05) difference between the EAI value of the concentrate and flour, their use as emulsifiers still appears to be limited at the studied pH [9].
ESI of OPNF and OPNPC3.5 were both greater than 86 min. When these results are compared to those obtained in previous studies with moringa [61] and jackfruit leaf proteins [23], 13 and 30 min, respectively, the emulsions formed by the ora-pro-nóbis samples can be considered more stable over time. One possible explanation is that ora-pro-nóbis protein has the ability to adopt a flexible structure that can interact with the oil phase through the hydrophobic amino acids exposed and with the water phase through the polar amino acids, thereby maintaining emulsion stability [37].
Foaming properties
Desirable in numerous food products, the foaming process is nothing more than the air entrapment by proteins [6]. Foaming capacity (FC) indicates an increase in the percentage of volume after whipping while foaming stability (FS) shows the ability of the protein to maintain the foam [52]. The FC of the OPNF (2.5%) was significantly (P < 0.05) lower than in concentrate (61.5%). This behavior can be related to the limited availability of proteins in flour capable of diffusing at the water-air interface and forming foam [37]. Moreover, the proteins present in flour can still be linked to non-proteinaceous material, hindering the protein migration to the interface air-water [62]. The FC of OPNPC3.5 (Table 3) was similar to that reported by Martin et al. [63] for sugar beet leaf isolate (60%). However, the FC of the pea protein isolate obtained by Pedrosa et al. [64] was slightly higher (74%). Higher FC was also observed in pea flour (64%) [64] when compared to OPNF. Regarding stability, OPNPC3.5 kept more than 88% of the initially formed foam after 30 min, showing excellent stability. OPNF, however, showed an FS value of 45% after 30 min. A low FS indicates the reduced ability of a foam to resist gravity-induced drainage and collapse [65]. The FC and FS results obtained for the flour and protein concentrate prove that the concentration of the ora-pro-nóbis proteins produces a material with improved foaming properties. Besides that, the high values of FC and FS reveal the potential of concentrate as an ingredient in the development of food products, such as ice cream, meringues, whipped cream, and leavened or fermented bread [6].
In vitro protein digestibility
Digestibility of food proteins is a measure of their susceptibility to being hydrolyzed by the enzymes present in the gastrointestinal tract, being dependent on the protein structures, the presence of other food components, and eventually processing technology [56]. IVPD was higher (P < 0.05) in the OPNPC3.5 (79.78% ± 0.72) than in OPNF (77.15% ± 0.27). Anti-nutritional components like protease inhibitors, phytic acid, cyanide, polyphenols, and tannin, may have been removed during protein concentration, increasing the digestibility of the concentrate [66]. The digestibility of OPN flour in the current study was close to that of ora-pro-nóbis leaves (75.9%) and Albizia lebbeck leaves protein concentrates (76.78–77.26%), as previously reported by Takeiti et al. [67] and Khan and Varshney [36], respectively. According to these authors, the results obtained indicate good in vitro digestibility.
Conclusions
In this study, the alkaline solubilization and isoelectric precipitation at pH 3.5 produced protein concentrate of ora-pro-nóbis (OPNPC3.5) with the highest yield compared to the other precipitation pHs. Analysis revealed distinct characteristics between OPNPC3.5 (52.43% protein) and ora-pro-nóbis flour (OPNF; 22.02% protein), including significant differences in color, structure and techno-functional properties. OPNPC3.5 exhibited enhanced characteristics compared to OPNF, such as superior water solubility, oil holding capacity (OHC), foaming properties, and in vitro digestibility. The solubility of OPNPC3.5 was 3.5 times greater than that of the flour, whereas its ability to retain oil was 3.0 times higher. Moreover, while the flour exhibited negligible foaming capacity, OPNPC3.5 reached almost 62%. The improvements in OHC, emulsifying, and foaming properties can be attributed to protein structural changes (FTIR findings) during the extraction process and increased protein solubility. These preliminary findings highlight the potential of both ora-pro-nóbis flour and protein concentrate as versatile ingredients in a variety of food products, fulfilling diverse techno-functional requirements. Further research is warranted to explore and optimize the applications of these nutritious, non-conventional sources in the food industry.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
References
J. Zhang, L. Liu, H. Liu, A. Yoon, S.S.H. Rizvi, Q. Wang, Crit. Rev. Food Sci. Nutr. 59, 3267 (2019)
A. Akyüz, S. Ersus, Food Chem. 335, 127673 (2021)
A.A. Anoop, P.K.S. Pillai, M. Nickerson, K.V. Ragavan, Compr. Rev. Food Sci. Food Saf. 473 (2022)
M. Ducrocq, M.H. Morel, M. Anton, V. Micard, S. Guyot, V. Beaumal, V. Solé-Jamault, A. Boire, Food Chem. 381, 132254 (2022)
A.G.A. Sá, Y.M.F. Moreno, B.A.M. Carciofi, Trends Food Sci. Technol. 97, 170 (2020)
M.Z. Mulla, P. Subramanian, B.N. Dar, Lwt. 158, 113106 (2022)
J.A.A. Garcia, R.C.G. Corrêa, L. Barros, C. Pereira, R.M.V. Abreu, M.J. Alves, R.C. Calhelha, A. Bracht, R.M. Peralta, and I. C. F. R. Ferreira, Food Chem. 294, 302 (2019)
V.B.V. Maciel, C.M.P. Yoshida, F.M. Goycoolea, Curr. Med. Chem. 26, 4573 (2018)
M.B. Egea, G. Pierce, Ref. Ser. Phytochem 225 (2021)
N.R. Madeira, N. Botrel, G.B. Amaro, R.A. de e Mello, C.C. Telles, A.M.R. Junqueira, and D. B. da Silva, in Espécies Nativ. Da Flora Bras. Valor Econômico Atual Ou Potencial Plantas Para o Futur. Região Nord, edited by C. ORADIN, L.; CAMILLO, J.; PAREYN, F. Germain (Ministério do Meio Ambiente, Brasília, DF, 2018), pp. 225–236
N.F.N. Silva, S.H. Silva, D. Baron, I.C. Oliveira, Neves, F. Casanova, Foods 12, 1 (2023)
D.N. López, M. Galante, M. Robson, V. Boeris, D. Spelzini, Int. J. Biol. Macromol. 109, 152 (2018)
L. Day, Trends Food Sci. Technol. 32, 25 (2013)
F.A. Lima Junior, M.C. Conceição, J. Vilela de Resende, L.A. Junqueira, C.G. Pereira, and M. E. Torres Prado, Food Hydrocoll. 33, 38 (2013)
N. Botrel, R.L. de Godoy, N.R. Madeira, G.B. Amaro, and R. A. Castro E Melo, Estudo Comparativo Da Composição Proteica E Do Perfil de Aminoácidos Em Cinco Clones de Ora-pro-Nóbis (Embrapa Hortaliças, Brasília, DF, 2019)
S.H.M. Gorissen, J.J.R. Crombag, J.M.G. Senden, W.A.H. Waterval, J. Bierau, L.B. Verdijk, L.J.C. van Loon, Amino Acids. 50, 1685 (2018)
L. Zheng, Z. Wang, Y. Kong, Z. Ma, C. Wu, J.M. Regenstein, F. Teng, Y. Li, Food Hydrocoll. 110, 106115 (2021)
C.C. Lise, C. Marques, M.A.A. da Cunha, M.L. Mitterer-Daltoé, Eur. Food Res. Technol. 247, 851 (2021)
A.M.T. Lago, I.C.O. Neves, N.L. Oliveira, D.A. Botrel, L.A. Minim, J.V. de Resende, Ultrason. Sonochem. 50, 339 (2019)
K.C.G. Silva, T.N. Amaral, L.A. Junqueira, N. de Oliveira, Leite, J.V. de Resende, South. Afr. J. Chem. Eng. 23, 42 (2017)
G. Kaur, S. Bhatia, J. Food Meas. Charact. 16, 3166 (2022)
A.T. Tenorio, J. Gieteling, G.A.H. De Jong, R.M. Boom, A.J. Van Der Goot, G.A.H. de Jong, R.M. Boom, A.J. Van Der Goot, Food Chem. 203, 402 (2016)
C. Calderón-Chiu, M. Calderón-Santoyo, E. Herman-Lara, Ragazzo-Sánchez. Food Hydrocoll. 112, 106319 (2021)
K. Karaman, H. Bekiroglu, M. Kaplan, B. Çiftci, C. Yürürdurmaz, O. Sagdic, Int. J. Biol. Macromol. 200, 458 (2022)
M.M. Bradford, Anal. Biochem. 72, 248 (1976)
S.L. Rodríguez-Ambriz, A.L. Martínez-Ayala, F. Millán, G. Dávila-Ortíz, Plants Foods Hum. Nutr. 60, 99 (2005)
S.O. Ogunwolu, F.O. Henshaw, H.-P. Mock, A. Santros, S.O. Awonorin, Food Chem. 115, 852 (2009)
C.S. Lin, J.F. Zayas, J. Food Sci. 52, 5 (1987)
K.N. Pearce, J.E. Kinsella, J. Agric. Food Chem. 26, 716 (1978)
F.T. Saricaoglu, Int. J. Biol. Macromol. 144, 760 (2020)
K. Shevkani, N. Singh, A. Kaur, J.C. Rana, Food Hydrocoll. 43, 679 (2015)
E.S. Tan, N. Ying-Yuan, C.Y. Gan, Food Chem. 152, 447 (2014)
T. Tinus, M. Damour, V. Van Riel, P.A. Sopade, J. Food Eng. 113, 254 (2012)
V.B.V. Maciel, R.Q. Bezerra, E.G.L. das Chagas, C.M.P. Yoshida, R.A. de Carvalho, Brazilian J. Food Technol. 25, 1 (2021)
A.T. Tenorio, K.E. Kyriakopoulou, E. Suarez-Garcia, C. van den Berg, A.J. van der Goot, Trends Food Sci. Technol. 71, 235 (2018)
L.H. Khan, V.K. Varshney, J. Diet. Suppl. 15, 386 (2018)
M.D. Bocarando-Guzmán, S. Luna-Suárez, A.S. Hernández-Cázares, J.A. Herrera-Corredor, J.V. Hidalgo-Contreras, M.A. Ríos-Corripio, Int. J. Food Prop. 25, 733 (2022)
C. Balfany, J. Gutierrez, M. Moncada, S. Komarnytsky, Nutrients. 15, 1327 (2023)
M. Pojić, A. Mišan, B. Tiwari, Trends Food Sci. Technol. 75, 93 (2018)
L. Amagliani, J. O’Regan, A.L. Kelly, J.A. O’Mahony, Trends Food Sci. Technol. 64, 1 (2017)
L. Shen, X. Wang, Z. Wang, Y. Wu, J. Chen, Food Chem. 107, 929 (2008)
C.L. Luchese, V.F. Abdalla, J.C. Spada, C. Tessaro, Food Hydrocoll. 82, 209 (2018)
M. Melgosa, M.M. Pérez, A. Yebra, R. Huertas, E. Hita, Opt. Pura Apl. 34, 1 (2001)
R. Gundogan, A.C. Karaca, Lwt. 130, 109609 (2020)
I.C.O. Neves, A.A. Rodrigues, T.T. Valentim, A.C.F. de Meira, S.H. Silva, L.A.A. Veríssimo, J.V. de Resende, J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1161, (2020)
F.M. Pelissari, M.M. Andrade-Mahecha, P.J.D.A. Sobral, F.C. Menegalli, Starch/Staerke. 64, 382 (2012)
S.-Y. Luo, Z. Huang, X. Chen, M.-H. Zong, W.-Y. Lou, Nat. Prod. Res. 25, 1 (2021)
L. Zhao, X. Cheng, X. Song, D. Ouyang, J. Wang, Q. Wu, J. Jia, Process. Biochem. 165187 (2023)
K. Wang, D.W. Sun, H. Pu, Q. Wei, Trends Food Sci. Technol. 67, 207 (2017)
F. Zhao, D. Zhang, X. Li, H. Dong, Molecules. 23, 1775 (2018)
J. Chen, T. Mu, M. Zhang, D. Goffin, Int. J. Food Sci. Technol. 54, 752 (2019)
A.K. Biswal, C. Lenka, P.K. Panda, J.M. Yang, P.K. Misra, Lwt. 137, 110459 (2021)
L. de Gouvêa, R. Caldeira, T. de Azevedo, M.C. Galdeano, I. Felberg, J.R. Lima, C.G. Mellinger, Food Hydrocoll. 137, 108351 (2023)
H. Zhao, C. Shen, Z. Wu, Z. Zhang, C. Xu, J. Food Biochem. 44, e13157 (2020)
K.K. Ma, M. Greis, J. Lu, A.A. Nolden, D.J. McClements, A.J. Kinchla, Foods. 11, 1 (2022)
Z. Avelar, A.A. Vicente, J.A. Saraiva, R.M. Rodrigues, Trends Food Sci. Technol. 113, 219 (2021)
C. Sun, W. Wu, Y. Ma, T. Min, F. Lai, H. Wu, Int. J. Food Prop. 20, S3311 (2018)
J. Ge, C.X. Sun, A. Mata, H. Corke, R.Y. Gan, Y. Fang, Food Hydrocoll. 112, 106288 (2021)
A.C. Karaca, N. Low, M. Nickerson, Food Res. Int. 44, 2742 (2011)
R. Mustafa, M.J.T. Reaney, in Food Wastes By-Products, edited by R. Campos-Vega, B. D. Oomah, and H. A. Vergara-Castañeda (John Wiley & Sons, 2020), pp. 93–126
Y. Cattan, D. Patil, Y. Vaknin, G. Rytwo, C. Lakemond, O. Benjamin, Innov. Food Sci. Emerg. Technol. 75, 102903 (2022)
A.M. Rayan, H.M. Swailam, Y.S. Hamed, Plant. Foods Hum. Nutr. 78, 117 (2023)
A.H. Martin, O. Castellani, G.A.H. de Jong, L. Bovetto, C. Schmitt, J. Sci. Food Agric. 99, 1568 (2019)
M.M. Pedrosa, A. Varela, F. Domínguez-Timón, C.A. Tovar, H.M. Moreno, A.J. Borderías, M.T. Díaz, Plant. Foods Hum. Nutr. 75, 642 (2020)
S.M.T. Gharibzahedi, B. Smith, Trends Food Sci. Technol. 107, 466 (2021)
T. Benhammouche, A. Melo, Z. Martins, M.A. Faria, S.C.M. Pinho, I.M.L.P.V.O. Ferreira, F. Zaidi, Food Chem. 348, 128858 (2021)
C.Y. Takeiti, G.C. Antonio, E.M.P. Motta, F.P. Collares-Queiroz, K.J. Park, Int. J. Food Sci. Nutr. 60, 148 (2009)
Funding
The authors acknowledge the financial support of Coordination for the Improvement of Higher Education Personnel (CAPES)-Finance Code 001, CAPES-PRINT (88887.694979/2022-00) and São Paulo Research Foundation (FAPESP) (2019/05578-7).
Author information
Authors and Affiliations
Contributions
Fabiana Helen Santos: Conceptualization and design of study, analysis, data acquisition and interpretation, investigation, writing-original draft. Ludmilla de Carvalho Oliveira: Conceptualization of study, supervision, data interpretation, writing review, and editing. Serafim Bakalis: Supervision, writing review, and editing. Marcelo Cristianini: Conceptualization and design, supervision, writing review, and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Compliance with ethics requirements
This study does not contain any studies with human participation or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santos, F.H., de Carvalho Oliveira, L., Bakalis, S. et al. Techno-functional properties and in vitro digestibility of ora-pro-nóbis flour and protein concentrate for assessing food application potential. Food Measure 18, 6793–6802 (2024). https://doi.org/10.1007/s11694-024-02692-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-024-02692-7