Abstract
Recently, legume protein isolates are increasingly of interest as ingredients for the food industry; however, in spite of their health benefits, there is a limited information about the presence of bioactive compounds in the protein isolates. The objective of this study was to establish the phytochemical composition and selected techno-functional properties of pea and bean flours and their protein isolates obtained applying different drying methods. Regarding proximate composition, bean flour contained higher amounts of total protein (23%) and fat (44%) than pea flour; bean protein isolate (BPI) contained higher total and soluble protein, fat and starch than the pea protein isolate (PPI). Both protein isolates showed a similar emulsifying capacity (around 27%). Emulsion stability and foaming capacity were higher in the PPI (around 36%). Bean flour contained lower amounts of α-galactosides (31.64 mg/g) but a higher trypsin inhibitors content (21.95 TIU/mg) than pea flour. The preparation procedure of the protein isolates affected the bioactive compound content. The PPI showed a reduction of inositol phosphates (13%), galactosides (76%), trypsin inhibitors (90%) and total phenolic compounds (35%) compared to its whole flour. The BPI contained higher amounts of inositol phosphates (137%) and total phenolic compounds (135%) than its flour, while it showed a lower content of galactosides (54%) and a similar amount of trypsin inhibitors. Thus, the bioactive compound content and the functional properties studied indicate that protein isolates can be used as ingredients with added-value in the development of new formulated food products, allowing their increasing use in the food industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, consumers’ requirements for convenient, highly nutritional, and healthy foodstuffs of vegetable origin has been growing rapidly worldwide. It is widely known that the consumption of a plant-based diet has numerous health benefits. From an economic, environmental, nutritional and health point of view, legumes have been identified as important food ingredients of plant origin [1]. Legumes have been recognized as a source of proteins, complex carbohydrates, and soluble and insoluble fibers, as well as vitamins and minerals. Legumes also contain some phytochemicals and antioxidants considered to be bioactive substances, which include phytoestrogens, isoflavones, alkaloids, phytates, saponins, protease inhibitors, and α-galactosides [1, 2]. These molecules play an important role in the prevention of cancer, cardiovascular disease, chronic degenerative diseases and osteoporosis. In spite of these health benefits, however, there has been a significant decrease in legume consumption over recent years [3]. A possible remedy to reverse this trend could be the development of new products aimed to satisfy consumer preferences [1, 4, 5]. The development of legume-based food products can help to rise pulse consumption, allowing consumers to profit from the bioactive constituents of these food products [4]. In this way, legumes can provide high protein meat-substitutes for celiac and vegetarians/vegans, as well as low fat substitutes for health-conscious individuals; they can also be the basis for the development of many functional foods [1]. In this sense, pea and bean flours have been employed to generate either gluten-free snack or breakfast cereal-like products [4], as well as heat-induced gels with acceptable attributes [6, 7] that can offer both health benefits and convenience to consumers. The utilization of legume flours and their protein isolates are increasingly of interest to the food industry as ingredients due to their low cost, availability, and nutritional value [7]. Traditionally, soybean protein has been used in the food industry for developing food products aimed at both vegan and non-vegan people. Of legumes, peas and beans appear to have the greatest potential as a substitute for soybean protein not only because of their availability, but also because they are neither genetically modified nor classified as allergens, as soybean is [8]. Moreover, peas and beans are traditionally consumed all over the world, making their adoption by consumers as easy process. However, to determine the usefulness of pulse flours or pulse protein as an ingredient within a food matrix, it is necessary to know their techno-functional properties. According to different authors [9,10,11,12,13], pulse functionality depends on the protein, lipid or carbohydrate content as well as their interaction with other food components (e.g., pectins, phenols, etc.), and the processing conditions. Moreover, it is important to note that several pulse varieties may differ in their techno-functional properties, which could affect their applications in the food industry [11]. Karaca et al. [9] reported that the legume source, the method of obtaining the isolate, and the conditions used to study the techno-functional properties, affected the performance of the protein isolate. Therefore, detailed studies of different pulses and their varieties are needed in order to identify their possible applications in the food industry. Although there is abundant information on the effect of food processing methods on the physiochemical properties of peas and their isolates [6, 8, 9, 14,15,16,17], it is more limited for bean protein isolates [7, 11, 12]. Regarding legume protein isolates, most of the scientific studies are focused on their protein and carbohydrate content, their functional properties, such as gelation, and their water and oil absorption capacity or emulsifying properties. However, there is little information about the phytochemicals present in protein isolates capable of bringing health benefits. Moreover, the different processing methods like thermal treatment, fermentation, and enzyme hydrolysis can reduce the levels of bioactive compounds of various legumes [18]. Likewise, the process of legume protein isolates can greatly affect the content of some phytochemicals [16,17,18]. It is important to note that Phaseolus vulgaris lectin (PHA) is considered a toxic compound to humans [19], an aspect that limits its use as an ingredient in some food products (e.g., when processed at low temperature). For this reason, in this study, the Almonga variety has been used since it has been previously characterized as a very low lectin variety that can be used safely as an ingredient in the development of new food products; moreover, its consumption has been shown to improve the triglyceridemic response in humans [19]. Therefore, the target of this study was to determine the bioactive compounds content (inositol phosphates, α-galactosides, trypsin inhibitors and phenolic compounds) and the techno-functional properties of the whole flour and protein isolate from pea (Pisum sativum L.) and bean (Phaseolus vulgaris L. var. Almonga), that could form the basis for generating new food products.
Materials and Methods
Plant Material and Preparation of Protein Isolates
A commercial pea protein isolate (PPI) was used in this study, and it was kindly supplied by the company Pevesa Biotech (Sevilla, Spain) and was obtained from pea (Pisum sativum L.) flour by alkaline extraction followed by isoelectric precipitation and drum drying. White bean seeds (Phaseolus vulgaris var. Almonga) were obtained from a local farmer (Benjamín Rodríguez Álvarez SA, León, Spain). The bean protein isolate (BPI) was prepared in our laboratory by alkaline extraction followed by isoelectric precipitation according to the method of Kaur and Singh [10] with slight modifications. Briefly, whole bean seeds were ground into flour and passed through a 1 mm sieve. The flour was dispersed in distilled water (5% w/v), and the pH was adjusted to 9 (with NaOH solution) to facilitate protein solubilization. The resulting slurry was stirred for 1 h at room temperature, and then was centrifuged (9,000 g for 20 min at 5 °C). The supernatant was collected and the process was repeated one more time with the pellet. The combined supernatants were adjusted to pH 4.5 (with HCl solution) to precipitate proteins (overnight at 4 °C). The precipitate was recovered by centrifugation (9,000 g for 10 min at 5 °C), washed with distilled water and then freeze dried. Protein isolates were stored at 4 °C in sealed plastic bags until further analysis.
Proximate Composition and SDS-Protein Electrophoretic Profile
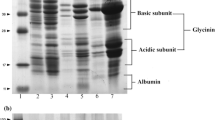
The proximate composition of flours and protein isolates was determined following the corresponding AOAC method [20]. The protein content was calculated by using a nitrogen/protein conversion factor of 5.45. Total carbohydrate content was calculated by difference. Total starch was measured using a Megazyme kit (Wicklow, Ireland) based on AOAC method 996.11 and 76.1. Legumin and vicilin fractionation was carried out by isoelectric precipitation and fractionation based on differential protein solubility according to Moreno et al. [7] and reference number 1 in the online material (ESM-1)] The SDS-PAGE electrophoresis profiles of pea and bean flours and isolates were analyzed using SDS-PAGE analysis, 4–15% acrylamide (Biorad, California, USA). The samples were dissolved in Laemmli buffer 1x. The mixture was agitated at room temperature and heated at 95 °C during 10 min. The final protein concentration was 10 μg per well in the case of isolates and 30 μg per well for flours. The ChemiDoc XRS+ system controlled by Image Lab™ 5.1 (Bio Rad, California, USA) was used for processing of the SDS-page images.
Techno-Functional Properties
The techno-functional properties were analyzed in both whole flours and protein isolates at the native pH (roughly 5.33–6.40). Bulk density (BD) and foaming capacity (FC) were analyzed according to Bencini [21]; for the foaming capacity 1 g of flour or protein isolate was dispersed in 50 mL of distilled water and whipped using a homogenizer (T-25, ultraturrax) at 5,000 g for 5 min. The BD results were expressed as g/mL and the foam capacity was expressed as the percentage increase in volume. Swelling capacity (SC) was analyzed by the method described by Aguilera et al. [5]: 100 mg of flour or protein isolate was hydrated in a known volume of distilled water (10 mL) in a calibrated cylinder at room temperature. After equilibration (18 h), the result was expressed as the volume occupied by the sample per gram of the initial sample dry weight. Emulsifying capacity (EC) and emulsion stability (ES) were determined using 1 g of flour or protein isolate and 25 mL of sunflower oil according to Bencini et al. [21]. EC was expressed as follows: \( \frac{Emulsion\ layer\ volume}{whole\ layer\ in\ the\ centrifuge\ tube}\times 100 \).
The ES of the above described emulsion was determined after 30 min and was expressed as the percentage ratio of initial to 30 min volume layer.
Determination of Bioactive Compounds
Individual inositol phosphates (IP3-IP6) were analyzed in flours and protein isolates following the method described by Pedrosa et al. [22], using an HPLC (Beckman System Gold Instrument, Los Angeles, CA, USA) equipped with a macroporous polymer PRP-1 column (150 × 4.1 mm i.d., 5 μm, Hamilton, Reno, Nevada, USA), and a refractive index detector. The different inositol phosphates were quantified by comparison with an external standard of IP6 (sodium phytate, Sigma-Aldrich, St. Louis, MO, USA). The concentration of soluble sugars and α-galactosides in the flours and protein isolates was determined as described Pedrosa et al. [22], using an HPLC (Beckman System Gold instrument, Los Angeles, CA, USA), equipped with a Spherisorb-5-NH2 column (250 × 4.6 mm i.d., Waters, Milford, MA, USA), and with a refractive index detector. The different sugars were quantified by comparison with their external standards (Sigma-Aldrich, St. Louis, MO, USA). Ciceritol was purified and kindly supplied by Dr. A. I. Piotrowicz-Cieslak (Olsztyn-Kortowo, Poland). Pea and bean flours and their protein isolates were assessed for the content of trypsin inhibitors as described Pedrosa et al. [19]. For this purpose, 25 mg of the samples were extracted using 1 mL of 50 mM HCl by stirring for 1 h at 4 °C, and centrifuged at 14,500 g for 10 min. The supernatants were stored at −20 °C until analysis. To determine the TI content α-N-benzoyl-DL-arginine-p-nitroanilide hydrochloride (Sigma-Aldrich, St. Louis, MO, USA) was used as the trypsin substrate. One trypsin unit (TIU) was defined as that which gave a reduction in A410 nm of 0.01 relative to trypsin control reactions, using a 10 mL assay volume. Phenolic compounds were extracted from whole flours and protein isolates and the quantification of different groups of phenolic compounds present in the extract was carried out according to Oomah et al. [23] . Anthocyanins, flavonols, tartaric esters and total phenolic compounds were monitored at 520, 360, 320 and 280 nm, respectively, on a Beckman spectrophotometer (Beckman DU-640, Los Angeles, CA, USA). Standards of cyanidin-3-glucoside (C3Glc) from Extrasynthese (Genay, France), and quercetin (Q), caffeic acid (CA) and catechin (CE) form Aldrich (Munich, Germany) were used to quantify anthocyanins, flavonols, tartaric esters, and total phenolic compounds, respectively.
Statistical Analysis
Results are expressed in a dry weight basis (mean ± standard deviation (SD)) and they were obtained in quadruplicate (n = 4). The statistically significant differences (p < 0.05) were established by a one-way ANOVA, and a Duncan’s multiple range test was applied. Correlations were analyzed by Pearson’s test. The Statgraphics Centurion XVI computer package (Graphics Software System, Rockville, MD, USA) was used.
Results and Discussion
Proximate Composition and Electrophoretic Profile
Table 1 shows the proximate composition of pea and bean flours and their corresponding protein isolates. It is important to note that the protein and carbohydrate content, as well as other compounds (such as fat) present in the flours and the protein isolates, affect their techno-functional properties. The proximate composition of pea and bean flour is within the ranges found in the literature [5, 12, 19]. As shown in Table 1, bean flour is characterized by a higher amount of total protein and fat, but a lower content of soluble protein (Table 2), carbohydrate and starch than the pea flour. Regarding the protein isolates, the higher amount of total and soluble protein, fat and starch was found in the bean protein isolate (BPI). The differences found in the protein isolates could be due to the differences in the extraction methods and the differences in seed composition. Moreover, the higher fat content found in BPI in comparison to bean flour and PPI could be associated to lipid-protein binding during protein extraction, producing lipid concentration in this bean isolate (reference number 2 in online resource ESM-1); while the higher soluble protein determined in BPI could be related to the different drying method used [6]. The total carbohydrates are reduced between 65 and 76% in the PPI and BPI, respectively, compared to the carbohydrates determined in their flours due to their solubilization in the extraction water. The total protein, fat and total carbohydrate content of PPI and BPI are similar or slightly higher than that reported by different authors for pea and bean isolates [8, 11]. The major pulse storage proteins are legumin (11S) and vicilin (7S), and they can show different techno-functional behavior; for example, it has been reported that vicilin shows better heat-induced gelation behavior; while, as legumin has more protein surface hydrophobicity may lead to more absorbed oil/protein [15, 24]. Therefore, it is important to know not only the protein content but also their ratio due to the natural variation in globulin composition of different pulses and their varieties. Bean and pea flour showed a similar legumin/vicilin ratio; however, the BPI showed a higher proportion of legumin than PPI, probably due to differences in the method used to obtain the isolates. It has been reported that the lower legumin/vicilin ratio improved the emulsifying capacity and stability of the legume isolates. However, there are some controversial results since some authors reported that the lower ratio vicilin/legumin result in a higher stability of emulsions [12, 15]. The SDS-PAGE protein profiles in the flours and protein isolates of pea and bean is shown in Fig. 1 (online resource ESM-2). The profile of the flours and isolates showed large similarities indicating that the isolation procedure did not reduce the presence of the main proteins in the isolates. In the bean samples, it can be observed the higher intensity of legumin bands (around 40 kDa) compared to the pea samples. Under reducing conditions, the predominant fractions corresponded to vicilins (around 25–28, 37–40 and 50–70 kDa), α- and β-subunits of the legumins (around 41 and 22 kDa) were clearly observable. Similar result were reported in the literature [11, 25, 26].
Techno-Functional Properties of the Pea and Bean Flours and their Protein Isolates
Techno-functional properties of flours and protein isolates are those that affect their utilization as ingredients in the development of a new food product [25]. The techno-functional properties of the pea and bean flours and their protein isolates are shown in Table 2. Both legume flours showed similar (p > 0.05) bulk density (BD) values (0.63 g/mL); they were lower than that reported for lentil (0.91 g/mL) and chickpea flours (0.71 g/mL), but similar to other bean flours (0.62 g/mL) and higher than soybean flour (0.47 g/mL) [5]. BD of the flours was higher than that obtained for their corresponding protein isolates. This property is important in relation to its packaging; thus, pulse flours will provide less total volume than the protein isolates for the same amount of weight. Adenekan et al. [27] reported low bulk densities (0.3–0.64 g/mL) for pigeon pea protein isolates and considered that these BD values would be important in relation to the formulation of weaning foods. The BPI showed higher bulk density (0.57 g/mL) than the PPI (0.48 g/mL) and these values were close to that reported for other legume protein isolates [25]. The differences found could be related to the differences in the starch composition as well as to the extraction processes, highlighting the drying stage [21, 28]. The swelling capacity (SC) is a hydration property related to the protein and polysaccharide (starch and dietary fiber) content in the samples as well as the size of particles and the processing methods. There were no significant differences in the swelling capacity (SC) of both flours and were higher than those found in the literature for raw flours such as lentil (2.30 g/mL), chickpea (1.70 g/mL) and soybean (5.78 g/mL) [5]. The protein isolates showed higher swelling capacity than their counterpart flours, probably due to the increase in the protein content (on average 3.2-fold) and the reduction of total carbohydrates (around 70%), together with the structure modification of the protein during the isolate elaboration [12, 28]. The higher SC value being attributed to PPI (8.49 mL/g). This could be due to a higher degree of protein unfolding produced during the drum-drying of the PPI [6, 28]. So, greater protein denaturation is associated with higher number of exposed side chains with hydrophilic groups able to retain a greater amount of water [6]. The high SC obtained for both isolates would indicate that they could be used as an ingredient in the formulation of sausages, pasta or custards [28].
The emulsifying capacity (EC) value is a measure of the surface activity of proteins and carbohydrates to lower the interfacial tension between two immiscible phases. The emulsion stability (ES) indicates the quality of the emulsion or its ability to resist long-term interfacial changes [29]. EC was higher (1.4-fold) in bean flour than in pea flour. This fact indicates that proteins in bean flour show more hydrophobic amino acids on their surface, so higher amount of hydrophobic areas, i.e., more flexible structures to enhance the short-term stabilization by interfacial action [29]. The EC of bean flour was lower than that reported for flours of white and black bean varieties (32.5 and 30.0%, respectively) but similar to Cajanus cajan flours (27.5%), while pea flour showed similar or slightly higher values to those reported for other peas (9.27–21%), but lower to those reported for soybean (28.0–35.60%) [9, 30]. Both protein isolates showed similar (p > 0.05) EC (around 27%). Although EC is mainly related to the protein content, the differences in the soluble protein content, the legumin/vicilin ratio, and the presence of carbohydrates, can play an important role in the EC of the protein isolates [14] . In this study, the EC of the flours is positively correlated with the trypsin inhibitors (R2 = 0.99, p = 0.001), and with the total inositol phosphates (IP) (R2 = 0.99, p = 0.009). However, no correlation was found between the bioactive compounds studied and the EC of the isolates. PPI has a lower total and soluble protein content than BPI (68.9 vs 75.4% and 25.4 vs 44.1%, respectively, Table 1), but showed a higher proportion of vicilin (lower legumin/vicilin ratio) and a higher amount of carbohydrates (26.6% vs 16.0%, Table 1), which may improve their emulsifying capacity [14]. Unlike pea samples, the EC of bean flour and BPI did not show significant differences (p > 0.05), probably due to the fact that BPI was obtained in our laboratory using freeze-drying that induces fewer changes in the protein structure [14, 28]. The EC of the PPI and BPI was similar or lower than that found in the literature [8, 14] for other pea (25.8–46%), bean (27.91–48.92%) and soybean (20–45%) protein isolates. Regarding emulsion stability, both pea samples showed better stability than the bean samples. The lowest ES value corresponded to bean flour, which retained 22% of the emulsion after 30 min, whereas PPI retained 35% of the emulsion over the same period. This fact could be related to the higher content of carbohydrates in the PPI that can increase the viscosity of the disperse phase, thus improving emulsion stabilization [29]. Shevkani et al. [7] reported that unfolded proteins have a higher surface area available that might enhance the emulsifying properties. Moreno et al. [6] reported a high degree of protein denaturation in the same commercial pea protein isolate, which could explain the better ES observed in the PPI. Moreover, different authors have reported that the legumin/vicilin ratio influences the emulsifying stability of legume flours and their protein isolates, since vicilin is less compact and less rigid and it could be related to a better emulsifying stability than that of legumin [8, 14, 24]. The legumin/vicilin ratio of the PPI is 8.30 while BPI showed a ratio of 15.24 (Table 1), which would be in accordance with the better ES observed for the PPI. In relation to the bioactive compounds studied, it was observed that the ES of the flours was negatively correlated with total inositol phosphates content (IP) and trypsin inhibitors (TI) (R2 = −0.95, p = 0.040 and − 0.96, p = 0.035, respectively), whereas is positively correlated with the total galactosides and phenols content (R2 = 0.95, p = 0.018 and 0.95, p = 0.042, respectively). No correlations were found between ES and the bioactive compounds in the protein isolates. The ES values obtained are lower than those reported in the literature for pea (43.00–100%), bean (42.23%) and soybean (72.33–100%) protein isolates, probably due to the differences in the method used to obtain the emulsion [8, 14], but they could still be used in the food industry as emulsifiers in sausages, salad dressings, frozen desserts or soups [15]. The foaming capacity (FC) was high (47.0–74.0%) for all samples, although both pea samples showed higher FC than the bean samples. The FC is related not only to the amount of protein but also to the processing method that can modify its conformation since protein unfolding enhances foam formation [26]. The FC of pea and bean flours were higher than that found in the literature for other pea (16.40–18.20%), bean (31.70–56%), and soybean (28.40%) samples [30]. The FC of PPI (74.0%) was higher than that of the counterpart pea flour (64.3%), but, in contrast, BPI showed lower FC (47.0%) than the corresponding bean flour (53.7%). PPI showed better FC than the BPI, and this could be due to its higher amount (about 38%) of carbohydrates. So, carbohydrates change the air-water interface, reducing its surface tension and consequently the resistance to spread so, the exposed area to retain air becomes increased, thereby increasing the FC also increased [29]. The higher amount of lipids present in the BPI could reduce its foam ability because lipids capture part of protein hydrophobic-groups available for interacting at the oil-water interface altering the equilibrium between both oil-water and air-water interfaces decreasing consequently the FC data [29]. In the flours the correlations found between FC and the studied bioactive compounds were negative for total IP and trypsin inhibitors (R2 = −0.95, p = 0.040 and − 0.98, p = 0.012) and positive for total galactosides and phenols (R2 = 0.98, p = 0.012 and 0.98, p = 0.017). In the protein isolates the correlation between FC and IP, trypsin inhibitors and total phenols was negative (R2 = −0.99, p = 0.005; −0.99, p = 0.005 and − 0.99, p = 0.004, respectively), while no correlation was found with the total galactosides content. The FC values obtained for PPI and BPI were higher than those found in the literature for some pea (57.10%), bean (18.81%), pigeon pea (33.30%), soybean (46.39%) and chickpea (19.61%) isolates [12, 14, 26, 27]. In contrast, the FC values were lower than those reported by Shevkani et al. [7] for protein isolates obtained from different lines of kidney bean (83–121%) and field pea (87–12%). These variations between different studies could be due to differences in protein composition and to the conditions of the foaming test used. The FC values obtained for PPI and BPI indicate that they could be used as foaming agents in the elaboration of baby foods, ice cream, meringues, angel cakes and desserts [15, 26, 27]. The FC was better than the emulsifying activity of both protein isolates. Although no significant correlation was observed between FC and EC in the bean samples, a strong positive correlation (R2 = 0.96, p = 0.039) was observed between both parameters in the pea samples. This correlation indicates that there are similar factors affecting foam and emulsifying capacities in both types of pea samples [15].
Bioactive Compounds
It is known that pulse flours and their protein isolates contain different amounts of bioactive compounds capable of bringing health benefits [2, 6]. Some of these bioactive compounds are affected both by the food matrix and the manufacturing process, increasing or decreasing their content in the protein isolate. Regarding the bioactive compounds content of the whole flours (Table 3), bean flour showed the highest amount of inositol phosphates (IP4-IP6), and protease inhibitors (21.9 TIU/mg), whereas pea flour showed the highest amount of α-galactosides (6.43 mg/100 mg), with verbascose being the main sugar detected (4.00 mg/100 mg). Regarding the different groups of phenols studied, pea flour showed the highest amount of anthocyanins, flavonols and total phenolic compounds. This may be related to the fact that pea is a yellow variety while the Almonga bean variety is a white seed. The content found in the flours was similar to that found in the literature for other pulses [2, 4, 17, 19]. The content of most of the bioactive compounds in the PPI was lower than that in its flour, since most of the compounds (highlighting α-galactosides and phenols) are water soluble and during the extraction procedure they are solubilized into the extracting water that is discarded. A similar trend is observed in soaked or canned legumes where the soaking water or the canning liquid is discarded [19]. Moreover, inositol phosphates are thermolabile compounds (>35 °C) and PPI is a commercial isolate, which has been drum-dried (>80 °C). In contrast, BPI contained, on average, 2.4-fold higher total inositol phosphates and IP6 than its corresponding flour. This could be due to the complex protein-phytic acid formation during the extraction procedure in the laboratory and to the freeze-drying used. The amount of inositol phosphates found in the PPI was similar or lower to that reported for other commercial and experimental pea (9.64–20.19 mg/g) and soybean protein isolates (8.4 mg/g) [31, 32], while the amount found in the BPI was higher than that reported for pea and soybean protein isolates but lower than faba bean isolates (57.10 mg/g) [16]. Traditionally, phytate (IP6) has been considered as an anti-nutritive compound due to its ability to bind minerals impairing their bioavailability; however, the less phosphorylated forms (IP3-IP5) have important roles in human health such as preventing kidney stone formation and colon cancer, promoting absorption of minerals, and improving type II diabetes, hypercholesterolemia and irritable bowel syndrome [2]. Thus, the IP content found in the PPI and BPI suggests that they could keep the health benefits related to the less phosphorylated isoforms. In addition, iron absorption can be improved when IP6 is below 10 mg/g protein in one meal serving [31]. Thus, assuming the consumption of 25 g of protein isolate (a similar amount of protein necessary for the cardiovascular health claim approved for soy protein), the PPI and BPI would supply 0.38 and 1.30 IP6 mg/g protein, respectively, improving iron absorption. Moreover, a portion of 60 g (similar weight of a cup of pulses) would supply less than 10 mg of IP/g protein. Regarding the content of α-galactosides, in comparison to the flour samples, the protein isolates showed a significant (p < 0.05) reduction of total α-galactosides of about 76 and 55% for the PPI and BPI, respectively; ciceritol was not detected in the PPI, probably because the small amount found in the pea flour lixiviate completely to the extracting water, however, for the galactosides content there were no significant differences (p > 0.05) between both protein isolates. This may be due to the high reduction (88%) found for verbascose and stachyose (81%) in the PPI. In the BPI, the main sugar detected was stachyose, although it was reduced by about 63% compared to its corresponding flour. These values were higher than those reported for commercial pea protein isolates (0.04 mg/100 mg) and those of pea, faba bean and lentil protein isolates (0.12, 0.39 and 0.60 mg/100 mg, respectively) obtained in the laboratory [33]. The flatulence, abdominal pain and/or diarrhea traditionally associated with pulse consumption are related to the presence of α-galactosides. Notably, at present, these sugars are considered as bioactive compounds with prebiotic effects promoting the growth of beneficial intestinal microflora, thereby enhancing the immune system. There is not a recommended dietary intake of α-galactosides, although Martinez-Villaluenga et al. [34] described that a dose of 3 g/day produced an increase in the intestinal bifidobacterias, bacteroides and eubacteria. Thus, the consumption of 60 g of pea or bean protein isolate would contribute to supply the half of the daily dose recommended. Therefore, both flours and protein isolates contained enough galactosides to be used as an added-value ingredient in the elaboration of new food products with some health functions. The trypsin inhibitors content in the PPI was 10-fold lower than that found in its flour. In contrast, BPI showed a slightly significant higher (p < 0.05) content of trypsin inhibitors than its corresponding flour. These differences are due to the different drying stage used to produce the protein isolates and the thermolabile-nature of this protease inhibitor. The commercial PPI was drum-dried (>80 °C), which causes the denaturation of the trypsin inhibitors, while the BPI was produced in the laboratory by freeze-drying that allows the preservation of its bioactivity. The trypsin inhibitors content determined in the bean protein isolates was higher than that reported in the literature for pea, faba bean and soybean protein isolates (5.91, 6.48 and 8.80 TIU/mg, respectively) [7, 17]. Even though protease inhibitors reduce protein digestion, in a Western’ funded diet, there have not been reported toxic problems related to the intake of these compounds. In addition, over the past two decades, protease inhibitors have been found to be effective in preventing or reducing cancer progression [1, 2]. Although there is no a recommended amount of TI consumption, noteworthy, that the traditional Japanese diet contains about 420 protease inhibitor unit/day; moreover, the consumption of purified protease inhibitor at 25–800 TIU per day during 12 weeks exerted a protective effect against cancer development and doses up to 2,000 protease inhibitor units/day do not cause health problem in the humans [3, cites herein and references number 3 and 4 in online resource ESM-1] Then, the consumption of a 60 g portion of bean isolate would supply less than the maximum recommended dose. Moreover, it has been reported that the autolysis of some protein matrices such as surimi gels can be reduced by trypsin inhibitors (>2 TIU/mg of isolate) from legumes [6]. Thus, the trypsin inhibitors present in the BPI would be enough to have this protective effect. Phenolic compounds are recognized as natural antioxidants able to reduce lipid oxidation, extending the shelf life of food products. The industrial process to obtain the PPI reduced (p < 0.05) the total phenolic compound (35%) and flavonols (19%) content, but increased the amount of anthocyanins (16%) and tartaric esters (42%); although the BPI showed a higher (p < 0.05) content of total phenolic compounds (135%) and tartaric esters (21%) than its corresponding whole flour (Table 3). These differences could be due to the different composition of the food matrix that allows phenols to bind to proteins, or to the rest of the cell walls that prevent the lixiviation of phenols to the extracting water; in addition, the conditions used to produce the BPI were not severe enough to break these bonds [35]. From a health point of view, different studies found in the literature reported phenolic compounds as bioactive molecules with antimicrobial, antioxidant and anti-inflammatory properties, lowering the risk of colon cancer and antihypertensive activities [19, 23]. Nowadays, there is no a recommended daily intake of phenolic compounds, mainly due to the differences in their total intake for the overall population; although some reports have recommended a minimum daily dose of 300 mg of total phenolic compounds to benefit from their health properties [13].
Conclusions
The pea and bean flours and their protein isolates presented good techno-functional properties and showed a great health potential due to their bioactive compounds content. The swelling, emulsifying and foaming capacity were improved in the protein isolates. The procedure used to obtain the protein isolates affected to varying degrees the content of bioactive compounds. Thus, in comparison to the pea protein isolate, the bean protein isolate contained a higher amount of inositol phosphates, trypsin inhibitors and total phenolic compounds. In the flours, there were found some correlations, either positive or negative, between the bioactive compounds and the emulsifying and the foaming capacity; in the isolates the content of inositol phosphates, protease inhibitors and phenols correlated negatively with the foaming capacity. Compared to the flours, the improvement of the functional properties observed in the protein isolates, their nutritive and bioactive compounds contents make them a suitable added-value ingredient to develop new food products such as fish or meat analogues suitable for vegans, or to be incorporated into sausages, seasonings, bakery and pasta products, etc. Even though there is not a recommended intake of these bioactive compounds, according to the literature, the content of the studied bioactive constituents in the protein isolates would be adequate to deliver their health giving (anticarcinogenic, anti-inflammatory, prebiotic) properties. In addition, the consumption of a 60 g portion (similar weight of a cup of pulses) would supply less than 10 mg/g of inositol phosphates improving the iron absorption, less than 3 g/day of galactosides, avoiding flatulence discomfort and less than the safe amount of trypsin inhibitors (<2,000 units).
References
Maphosa Y, Jideani VA (2017) The role of legumes in human nutrition. In: InTechOpen, Maria Chavarri Hueda (ed). Functional food - improve health through adequate food. https://doi.org/10.5772/intechopen.69127
Muzquiz M, Varela A, Burbano C, Cuadrado C, Guillamón E, Pedrosa MM (2012) Bioactive compounds in legumes: pronutritive and antinutritive actions. Implications for nutrition and health. Phytochem Rev 11:227–244. https://doi.org/10.1007/s11101-012-9233-9
FAO (2016) Resolution 68/231. International year of pulses, 2016. http://www.fao.org/. Accessed 20th May, 2020
Arribas C, Cabellos B, Cuadrado C, Guillamón E, Pedrosa MM (2019) The effect of extrusion on the bioactive compounds and antioxidant capacity of novel gluten-free expanded products based on carob fruit, pea and rice blends. Innov Food Sci Emerg Technol 52:100–107. https://doi.org/10.1016/j.ifset.2018.12.003
Aguilera Y, Esteban RM, Benitez V, Molla E, Martín-Cabrejas MA (2009) Starch, functional properties, and microstructural characteristics in chickpea and lentil as affected by thermal processing. J Agric Food Chem 57:10682–10688. https://doi.org/10.1021/jf902042r
Moreno HM, Domínguez-Timón F, Díaz MT, Pedrosa MM, Borderias AJ, Tovas CA (2020) Evaluation of gels made with different commercial pea protein isolate: rheological, structural and functional properties. Food Hydrocoll 99:105375. https://doi.org/10.1016/j.foodhyd.2019.105375
Shevkani K, Singh N, Kaur A, Rana JC (2015) Structural and functional characterization of kidney bean and field pea protein isolates: a comparative study. Food Hydrocoll 43:679–689. https://doi.org/10.1016/j.foodhyd.2014.07.024
Lu ZX, He JF, Zhang YC, Bing DJ (2019) Composition, physicochemical properties of pea protein and its application in functional foods. Crit Rev Food Sci Nutr 60:1–13. https://doi.org/10.1080/10408398.2019.1651248
Karaca AC, Low N, Nickerson M (2011) Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res Int 44:2742–2750. https://doi.org/10.1016/j.foodres.2011.06.012
Kaur M, Singh N (2007) Characterization of protein isolates from different Indian chickpea (Cicer arietinum L.) cultivars. Food Chem 102:366–374. https://doi.org/10.1016/j.foodchem.2006.05.029
Rui X, Boye JI, Ribereau S, Simposn BK, Prasher SO (2011) Comparative study of the composition and thermal properties of protein isolates prepared from nine Phaseolus vulgaris legume varieties. Food Res Int 44:2497–2504. https://doi.org/10.1016/j.foodres.2011.01.008
Sathe SK (2002) Dry bean protein functionality. Crit Rev Biotechnol 22:175–223. https://doi.org/10.1080/07388550290789487
Del Bo’ C, Bernardi S, Marino M et al (2019) Systematic review on polyphenol intake and health outcomes: is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern?. Nutrients 11(6):1355. https://doi.org/10.3390/nu11061355
Aluko RE, Mofolasayo OA, Watts BM (2009) Emulsifying and foaming properties of commercial yellow pea (Pisum sativum L.) seed flours. J Agric Food Chem 57:9793–9800. https://doi.org/10.1021/jf902199x
Barac M, Pešić M, Stanojevic S, Kostic A, Cabrilo S (2015) Techno-functional properties of pea (Pisum sativum) protein isolates: a review. Acta Period Technol 46:1–18. https://doi.org/10.2298/APT1546001B
Fernández-Quintela A, Macarulla MT, del Barrio AS, Martínez JA (1997) Composition and functional properties of protein isolates obtained from commercial legumes grown in northern Spain. Plant Foods Hum Nutr 51:331–341. https://doi.org/10.1023/A:1007936930354
Roy F, Boye JI, Simpson BK (2010) Bioactive proteins and peptides in pulse crops: pea, chickpea and lentil. Food Res Int 43:432–442. https://doi.org/10.1016/j.foodres.2009.09.002
Boye J, Zare F, Pletch A (2010) Pulse proteins: processing, characterization, functional properties and applications in food and feed. Food Res Int 43:414–431. https://doi.org/10.1016/j.foodres.2009.09.003
Pedrosa MM, Cuadrado C, Burbano C, Muzquiz M, Cabellos B, Olmedilla-Alonso B, Asensio-Vegas C (2015) Effects of industrial canning on the proximate composition, bioactive compounds contents and nutritional profile of two Spanish common dry beans (Phaseolus vulgaris L.). Food Chem 166:68–75. https://doi.org/10.1016/j.foodchem.2014.05.158
AOAC (1995) Official methods of analysis
Bencini MC (1986) Functional properties of drum-dried chickpea (Cicer arietinum L.) flours. J Food Sci 51:1518–1521. https://doi.org/10.1111/j.1365-2621.1986.tb13849.x
Pedrosa MM, Cuadrado C, Burbano C, Allaf K, Haddad J, Gelenser E, Takács K, Gullamón E, Muzquiz M (2012) Effect of instant controlled pressure drop on the oligosaccharides, inositol phosphates, trypsin inhibitors and lectins content of different legumes. Food Chem 131:862–868. https://doi.org/10.1013/j.foodchem.2011.09.061
Oomah BD, Cardador-Martínez A, Loarca-Piña G (2005) Phenolics and antioxidative activities in common beans (Phaseolus vulgaris L). J Sci Food Agric 85:935–942. https://doi.org/10.1002/jsfa.2019
Singhal A, Karaca AC, Tyler R, Nickerson M (2016) Pulse proteins: from processing to structure-function relationships. In: Goyal AK (ed) Grain legumes IntechOpen.https://doi.org/10.5772/64020
Zhao H, Shen C, Wu Z, Wu Z, Zhang Z, Xu C (2020) Comparison of wheat, soybean, rice, and pea protein properties for effective applications in food products. J Food Biochem 44:e13157. https://doi.org/10.1111/jfbc.13157
Adebiyi AP, Aluko RE (2011) Functional properties of protein fractions obtained from commercial yellow field pea (Pisum sativum L.) seed protein isolate. Food Chem 128:902–908. https://doi.org/10.1016/j.foodchem.2011.03.116
Adenekan MK, Fadimu GJ, Odunmbaku LA, Oke EK (2018) Effect of isolation techniques on the characteristics of pigeon pea (Cajanus cajan) protein isolates. Food Sci Nutr 6:146–152. https://doi.org/10.1002/fsn3.539
Joshi M, Adhikari B, Aldred P, Panozzo JF, Kasapis S (2011) Physicochemical and functional properties of lentil protein isolates prepared by different drying methods. Food Chem 129:1513–1522. https://doi.org/10.1016/j.foodchem.2011.05.131
Sahin S, Sumnu G (2006) Surface properties of foods. In: Sahin S, Sumnu SG (eds) Physical properties of foods. Springer. Food Science Text Series, New York, pp. 229–248
Sangronis E, Rodríguez M, Cava R, Torres A (2006) Protein quality of germinated Phaseolus vulgaris. Eur Food Res Technol 222:144–148. https://doi.org/10.1007/s00217-005-0137-4
Fredrikson M, Alminger ML, Carlsson N-G, Sandberg AS (2001) Phytate content and phytate degradation by endogenous phytase in pea (Pisum sativum). J Sci Food Agric 81:1139–1144. https://doi.org/10.1002/jsfa.918
Hurrell RF, Juillerat MA, Reddy MB, Lynch SR, Dassenko SA, Cook JD (1992) Soy protein, phytate, and iron absorption in humans. Am J Clin Nutr 56:573–578
Bhatty RS, Christison GI (1984) Composition and nutritional quality of pea (Pisum sativum L.), faba bean (Vicia faba L. spp. minor) and lentil (Lens culinaris Medik.) meals, protein concentrates and isolates. Plant Foods Hum Nutr 34:41–51. https://doi.org/10.1007/BF01095071
Martínez-Villaluenga C, Frias J, Vidal-Valverde C (2008) Alpha-galactosides: antinutritional factors or functional ingredients? Crit Rev Food Sci Nutr 48:301–316. https://doi.org/10.1080/10408390701326243
Arntfield SD, Ismond MAH, Murray ED (1985) The fate of antinutritional factors during the preparation of a Fababean protein isolate using a micellization technique. Can Inst Food Sci Technol J 18:137–143. https://doi.org/10.1016/S0315-5463(85)71771-3
Acknowledgments
The Spanish Ministry of Economy and Competitiveness funded this work (RTA2015-00003-C02).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pedrosa, M.M., Varela, A., Domínguez-Timón, F. et al. Comparison of Bioactive Compounds Content and Techno-Functional Properties of Pea and Bean Flours and their Protein Isolates. Plant Foods Hum Nutr 75, 642–650 (2020). https://doi.org/10.1007/s11130-020-00866-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-020-00866-4