Abstract
The composition of lipophilic and hydrophilic components in cultivated (C. tinctorius) and wild (C. oxyacantha) safflower seed oils was studied. By LC–HRMS/MS2, a total of seven highly abundant bioactive compounds with hydrophilic nature, a lignan glycoside (tracheloside), two flavonoids (acacetin–glucuronide pentoside and acacetin-7-O-D-glucuronide), and four alkaloids (N-coumaroylserotonin glucoside, N-feruloylserotonin glucoside, N-coumaroylserotonin, and N-feruloylserotonin), in seeds of both species, were identified. Only a minor part of the hydrophilic compounds (≤ 0.05%) present in the seeds was transferred into the seed oil during the extraction. The linoleic (~ 78%), oleic (~ 15%), palmitic (~ 5%), and stearic (~ 2%) acids—constituted 99% of all detected fatty acids in both species. α-Tocopherol was a main form of tocochromanols (over 94%) in both safflower seed oils. β-Sitosterol was the predominate form (over 36%) of phytosterols, while high levels were also recorded for gramisterol (17.1%) and avenasterol (19.6%) in C. oxyacantha and C. tinctorius seed oils, respectively. Zeaxanthin was a predominated form of carotenoids (over 37%), while high levels were recorded for lutein and β-carotene 15 and 25%, mainly in C. oxyacantha. The total amount of minor lipophilic compounds such as tocochromanols, carotenoids and sterols in C. oxyacantha vs. C. tinctorius seed oil was 57.9 vs. 58.2, 0.76 vs. 0.5, and 185.5 vs. 274 mg/100 g oil, respectively. The presence of squalene was detected only in C. oxyacantha (10.4 mg/100 g oil). Despite the similar composition and levels of fatty acids and tocochromanols, species differed by the phytosterols, carotenoids, and bioactive compounds with hydrophilic nature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The genus Carthamus (Asteraceae) includes thistle-like plants cultivated and wild grown in the Mediterranean region, and central as well as western Asia. Carthamus tinctorius L. (cultivated safflower) is grown mainly due to its oil-rich seeds and is the best known representative of the genus and the only cultivated species. While Carthamus oxyacantha M. Bieb. (wild safflower) is the most known wild species of the genus Carthamus. C. oxyacantha, it is assumed to be the ancient male parent of C. tinctorius [1]. In addition, both species have the same chromosome number and have been successful in reciprocal crosses [2]. The wild safflower is wide spread in northwest India, Turkey, Uzbekistan, Turkmenistan, Kazakhstan, Iran, and subtropical regions of western Iraq [1], while most of world’s production of cultivated safflower is located in the United States, México, Kazakhstan, and India. Safflower is an important seed oil crop with a wide range of applications in such industrial branches as pharmaceutics, cosmetics, food, and feed products [3]. It is worthy to mention that the C. oxyacantha and C. tinctorius seed oils have high similarity in fatty acid composition which indicates that hybridization between these species for genetic improvement of agronomic traits in cultivated safflower may have no adverse effects on its oil quality [1]. However, the existence of two lines of cultivated safflower, one with high linoleic acid content (most common and well-studied) and second with a high content of oleic acid, should be taken into account [3]. The world production of safflower nearly doubled from 540 thousands of tons in 2006–941 thousands of tons in 2016 [4]. An increasing trend of safflower world production observed in the last decade confirms that the safflower is a valuable and important oilseed crop. While C. oxyacantha and C. tinctorius seed oils are well-studied in terms of fatty acid composition, there is a limited amount of information about the presence of minor lipophilic compounds such as tocochromanols, phytosterols, carotenoids, and squalene, as well as bioactive compounds with hydrophilic nature in the seeds, especially in C. oxyacantha species. Therefore, the aim of this study was to determine the lipophilic and hydrophilic compounds in the seeds and seed oils of both species, more specifically the fatty acid, phytosterol, tocopherol, tocotrienol, carotenoid, phenolic, and alkaloid profiles to evaluate the difference in a composition of bioactive compounds between these two species.
Materials and methods
Chemical material
Methanol, n-hexane, 2-propanol, tert-butyl methyl ether (HPLC grade), 5α-cholestane (≥ 97%, GC), all-trans-β-carotene (≥ 97%, UV) were obtained from Sigma-Aldrich (Taufkirchen, Germany). The α-, β-, γ- and δ-homologues of tocopherol and tocotrienol (> 95%, HPLC) were purchased from Merck (Darmstadt, Germany) and LGC Standards (Teddington, Middlesex, UK), respectively. The Sylon BTZ and fatty acid methyl ester mix were received from Supelco (Bellefonte, PA, USA) and Supelco (Taufkirchen, Germany), respectively. All other reagents were of analytical grade acquired by Sigma-Aldrich (Taufkirchen, Germany).
Plant material
Wild (C. oxyacantha M. Bieb.) and cultivated (C. tinctorius L.) safflower seeds, originated from India and Ukraine, respectively. The received seeds were randomly separated into three batches (30 ± 5 g of each batch), and residual moisture was removed by freezing and sequential freeze-drying using a freeze-dry system (FreeZone, Labconco, Kansas City, MO, USA) for 24 h to reached moisture content 5.5 ± 0.5%. Then, the seeds were milled with a MM 400 mixer mill (Retsch, Haan, Germany). Dry weight basis (dw) for the obtained powder was measured gravimetrically.
Oil extraction
Oil was extracted according to the method described previously [5]. Briefly, a sample of ground seeds (5 g) was weighted into the centrifuge tube (50 mL) and mixed for 1 min with n-hexane (25 mL) via a vortex mixer REAX top (Heidolph, Schwabach, Germany). Then, the homogenized material was subjected to an ultrasonic bath Sonorex RK 510 H (Bandelin electronic, Berlin, Germany) for 5 min at 35 °C and again mixed for 1 min. The supernatant was collected, while the remaining solid residue was re-extracted twice as described above. The combined supernatants were vacuum evaporated at 40 °C until constant weight was achieved. The oil yield was expressed as %, w/w of recovered oil to seeds dry weight.
Fatty acid determination
The fatty acid composition of obtained oil samples was determined via gas chromatography (GC) using a flame ionization detector (FID) in line with official method of the American Oil Chemists’ Society AOCS [6]. Briefly, a Hewlett-Packard 5890 II GC system (Hewlett-Packard, Palo Alto, CA, USA) equipped with a Supelcowax 10 capillary column (30 m × 0.20 mm × 0.20 µm) (Supelco, Bellefont, PA, USA) and an FID detector under programmed temperature conditions: from 60 to 200 °C increased at a rate of 12 °C/min, then, held at 200 °C for an additional 25 min, using hydrogen as a carrier gas (1.0 mL/min), were applied. The results were expressed as a percentage of the total peak area of all the fatty acids in the oil sample. The detailed method description was reported in our previous study [7].
Tocochromanols determination
The oil samples (0.1 g) were diluted in a 10 mL volumetric flask with 2-propanol, mixed, filtrated through a nylon syringe filter (0.22 μm), and directly injected under reverse phase (RP) conditions on an HPLC system (Shimadzu, Kyoto, Japan) [8]. Tocochromanols (tocopherol and tocotrienol homologues) were identified according to a previously validated RP–HPLC method [9] using a Luna pentafluorophenyl (PFP) column (150 × 4.6 mm, 3 µm) (Phenomenex, Torrance, CA, USA) protected by a guard column (4 × 3 mm) and fluorescence detector (RF-10AXL) at an excitation wavelength of 295 nm and an emission wavelength of 330 nm. Tocochromanols were separated in isocratic conditions using a mobile phase contained methanol:water (93:7; v/v) at a flow rate of 1.0 mL/min and the column oven and room temperature set at 40 °C and 22 ± 1 °C, respectively.
Carotenoids’ determination
Prior to the carotenoid determination, the oil sample (0.5 g) was saponified according to Rodriguez-Amaya and Kimura [10]. The carotenoids’ separation was performed using the Agilent Technologies 1200 Rapid Resolution system (Waldbronn, Germany) equipped with Agilent 1260 Infinity DAD VL + and a Poroshell 120 SB-C18 (150 × 4.6 mm, 5 µm) column held at 25 °C (Agilent Technology, Palo Alto, USA). The mobile phase, with the constant flow rate of 0.5 mL/min, was composed of 0.5 g/L of triethylamine in acetonitrile (A), methanol (B), and ethyl acetate (C). Initial mobile phase composition was 95(A):5(B):0(C), which was gradually changed to 60(A):20(B):20(C) in 40 min and held for 10 min. The identification of the carotenoids was based on the comparison of UV/VIS data [maximum absorption wavelengths (λmax), spectroscopic fine structures (% III/II)] and molecular weight according to a guide to carotenoid analysis in foods [11] and our previous report [12]. The mass spectrometric characteristics performed on Waters Micromass ZQ mass spectrometer (Waters Corporation, Milford, MA, USA) in positive electrospray mode under following conditions: capillary voltage, 3000 V; cone voltage, 30 V; ion source temperature, 120 °C; desolvation temperature, 300 °C; desolvation gas flow 300 L/h; cone gas flow 20L/h. Scanning diapason was from m/z 100 to 1000. The quantification of individual carotenoids was done using a calibration curve for all-trans-β-carotene at 454 nm.
Phytosterols’ determination
Plant sterols were determined according to the procedure recommended by AOCS [13] via GC. Briefly, a Hewlett-Packard 6890 GC system (Hewlett-Packard, Palo Alto, CA, USA) equipped with a DB-35MS capillary column (25 m × 0.20 mm × 0.33 μm) (J&W Scientific, Folsom, CA, USA) and an FID detector under programmed temperature conditions: at 100 °C was held for 5 min, then, programmed to 250 °C at 25 °C/min, held for 1 min, then, further programmed to 290 °C at 3 °C/min, and held for 20 min, using hydrogen as a carrier gas (1.5 mL/min), were applied. An internal standard, 5α-cholestane, was used for sterols and squalene quantifications. The detailed method description was reported in our previous study [14].
Extraction, separation, and isolation of bioactive compounds with hydrophilic nature
The bioactive compounds from safflower seeds and seed oil were extracted according to the previously described protocols, with minor modifications [15, 16]. Briefly, 0.1 g of the defatted seeds was extracted with 10 mL of 80% (v/v) aqueous methanol solution containing 0.1% formic acid using a vortex mixer REAX top and an ultrasonic bath, with the identical parameters as reported previously [15]. The supernatant was centrifuged, filtered through a nylon syringe filter (0.45 μm), and directly analyzed by HPLC, as described below. In the case of seed oil, the bioactive compounds were extracted according to Górnaś et al. [16]. Briefly, 2.5 g of oil was diluted in 60 mL of n-hexane and extracted three times with a fresh portion of 35 mL of 60% (v/v) aqueous methanol solution by shaking in a funnel separator for 5 min, each time. The combined extracts of aqueous methanol solution were evaporated to dryness in a vacuum rotary evaporator at 40 °C, then dissolved in 1 mL 90% (v/v) aqueous methanol solution; after that, it was filtered through 0.45 μm syringe filters, and directly analyzed by HPLC, as described below. The bioactive compounds with hydrophilic nature were separated and quantitated using Shimadzu HPLC system equipped with a Kinetex XB-C18 (250 × 4.6 mm, 5 μm) column held at 25 °C and protected by a guard column Synergi Fusion-RP phase (4 × 3 mm) (Phenomenex, Torrance, CA, USA). The chromatographic separation was performed using 3 mobile phases [water with 1% formic acid (A), methanol (B) and acetonitrile (C)], and the elution gradient was programmed as follows: 0 min 4% (B) and 7.5% (C); 5 min 20% (B) and 13.5% (C); 17 min 30% (B) and 11% (C); 19 min 80% (B); 21 min 90% (B); 22 min 90% (B); 25 min 4% (B); and 7.5% (C) (total analysis time 30 min). The bioactive compounds were monitored at wavelength 200–600 nm by the DAD detector (SPD-M20A). To identify the detected unknown bioactive compounds, seven peaks with the highest peak area were collected at the exit of the capillary and identified using high-resolution mass spectrometry (HRMS). Due to the absence of the standards, provided values were calculated based on the areas of the individual compound, at the wavelength of 280 nm, in relation to their sum obtained for each species. The distribution of isolated bioactive compounds with hydrophilic nature in seeds and seed oil (%), and percentage of their transfer into the seed oil during the ultrasound-assisted extraction (%) was estimated.
Identification of bioactive compounds with hydrophilic nature by LC–HRMS/MS2
The chromatographic separation of isolated earlier bioactive compounds was carried out using a Dionex UltiMate 3000 UHPLC system (Thermo Fisher Scientific, San Jose, CA, USA) on a Phenomenex Luna C18(2) 100A (100 × 2 mm, 3 μm) analytical column. Binary mobile-phase gradient was used, consisting of 0.1% formic acid in water (A) and acetonitrile (B). The flow rate was 0.4 mL/min, while injection volume was set to 5 μL. The gradient program was as follows: 10% (B) from 0 to 2 min, 5–99% (B) from 2 to 9 min, 99% (B) from 9 to 13 min, and 10% (B) from 13 to 17 min. Column compartment and autosampler temperatures were maintained at 30 °C and 14 °C, respectively. High-resolution mass spectrometry (HRMS) acquisition was performed on a 7T Bruker SolariX ICR-FT-HRMS system (Bruker Daltonics, Germany) equipped with an electrospray ionization source (ESI). Both ionization polarities were used during measurement series. Main ESI source parameters were set as follows: nebulizer pressure: 0.9 bar (N2), dry gas flow: 8 L/min (N2), and dry temperature: 220 °C. MS and MS2 scan range was set from 100 to 1500 m/z. Spectra were acquired using a transient size of 512 k (transient length: 244.7 ms), which corresponds to a resolving power (FWHM) of 30,000 and 60,000 at m/z 500 and m/z 250, respectively. Automated acquisition of MS2 spectra was conducted with an auto-MS/MS mode. Three MS2 spectra were acquired per precursor using an isolation window of 3 m/z. Intensity threshold for precursor selection was set to 50,000 cps. Collision energies for each m/z value were calculated with a built-in linear function: CE (V) = 0.025 × (precursor m/z value) + 10. “Smart exclusion” mode was enabled during the auto-MS/MS measurement mode, which allowed to exclude selected precursor m/z values (± 10 ppm) from acquisition for 1 min after the initial trigger. Accurate mass calibration (< 1 ppm) was performed with 1 mM sodium formate solution in water. It was directly infused into the ESI source through a syringe pump with a flow rate of 150 µL/h.
Statistical analysis
The results are presented as means ± standard deviation (n = 3) from three independent batches. The p value ≤ 0.05 was used to denote significant differences between mean values determined by one-way analysis of variance (ANOVA). The Bonferroni post hoc test was used to denote statistically significant values at p ≤ 0.05. All analyses were performed with the assistance of Statistica 12.7 (Dell Software, TX, USA) software.
Results and discussion
Oil yield and fatty acids
The oil yield in the seeds of studied species C. oxyacantha and C. tinctorius was 31.5 and 29.2% dw, respectively (Table 1). Statistically significant differences (p ≤ 0.05) in terms of oil yield were not observed. In the present study, the acquired oil yield is in accordance to the values reported by other authors which ranged to 20.0–30.8% and 22.0–36.7% for C. oxyacantha and C. tinctorius, respectively [1, 17,18,19,20,21,22,23,24,25,26,27]. The oil yield in the seeds of different safflower species is largely affected by their genotype and abiotic factors [1, 17,18,19,20,21,22,23,24,25,26]. Nevertheless, sowing and harvest time is strongly associated with abiotic factors and can affect the overall yield [17,18,19]. A significantly higher amount of oil (42.3–46.4%) has been reported in the high oleic safflower (C. tinctorius) line [3]. In addition, it should be highlighted that the determined oil yield strongly depends on the applied method of extraction [28].
The presence of 8 and 11 fatty acids was detected in the C. oxyacantha and C. tinctorius seed oils, respectively. Four of them–linoleic acid (C18:2), oleic acid (C18:1), palmitic acid (C16:0), and stearic acid (C18:0)—were identified as major components and constituted almost 99% of the total fatty acids (Table 1). In both species, over three quarters of fatty acid levels was consisted by C18:2 (77.9% and 76.8%), while remaining quarter consisted of C18:1 (14.9% and 13.5%), C16:0 (5.4% and 6.4%), and C18:0 (1.3% and 2.5%). The levels of other identified fatty acids did not exceed 0.3% (individually) and 1.1% (all) in the studied samples. Similar fatty acids’ composition in both species, as found in the present study, was reported previously [1, 17,18,19,20,21,22,23,24,25,26,27]. The profile of fatty acids in seed oils of different safflower species, similar as for oil yield, is affected by the genotype and abiotic factors [1, 17,18,19,20,21,22,23,24,25,26], as well as the sown and harvest time which is strongly associated with abiotic factors [17,18,19]. In general, a similarity in the fatty acid composition of the seed oil of wild and cultivated accessions of safflower indicates that hybridization between C. oxyacantha and C. tinctorius for genetic improvement of agronomic traits in cultivated safflower may have no adverse effects on its oil quality [1]. Both species C. oxyacantha and C. tinctorius have the same chromosome number, while their hybridization has been successful in reciprocal crosses [2]. The safflower species are generally predominated with the C18:2. However, there are also genotypes of cultivated safflower with a high content of C18:1 in which the ratio between the fatty acids C18:1 and C18:2 is reversed in comparison to genotypes with high levels of C18:2 [3]. The samples, C. oxyacantha and C. tinctorius in terms of fatty acid composition, differed between each other significantly (p ≤ 0.05) only in levels of C16:0 and C18:0 (Table 1). However, taking into account the impact of genotype and abiotic factors on fatty acids composition, the recorded difference in levels of C16:0 and C18:0 between those two species in the present study cannot be considered as a future marker to distinguish an origin of oil (C. oxyacantha vs. C. tinctorius).
The total amount of saturated (ΣSFA, 7.0% and 9.4%), monounsaturated (ΣMUFA, 14.9% and 13.5%), and polyunsaturated fatty acids (ΣPUFA, 78.0% and 77.0%) in C. oxyacantha and C. tinctorius, respectively, was nearly identical to the level of four main fatty acids (C16:0, C18:0, C18:1, and C18:2), since with the exception of C16:0 and C18:0 (SFA), each of them represents another group of fatty acids and their level was 99% of the total detected. The ratio of ΣUFA/ΣSFA was 13.2 and 9.6%, while the ratio ΣPUFA/(ΣSFA + ΣMUFA) was 3.5 and 3.6% for C. oxyacantha and C. tinctorius, respectively (Table 1). Both fatty acids ratios were similar to previously reported in red currant (Ribes rubrum L.) and gooseberry (Ribes uva-crispa L.) melon seed oils [29]. The ΣPUFA/(ΣSFA + ΣMUFA) ratio may be used as an indicator of the oxidative stability of oils, since it is negatively correlated with the oxidative stability of sample (induction period). However, the presence of natural antioxidants is not taken into account for in the reported model [29].
Tocochromanols
In both safflower seed oils, the presence of four homologues (α, β, γ, and δ) of tocopherol (T) and two homologues (α and γ) of tocotrienol (T3) were found. The α-T was the predominant form reaching over 94% of total tocochromanols determined in the C. oxyacantha and C. tinctorius (Table 1). The content of β-T was at a low level of 3%, while, for the remaining tocochromanols, the content was at low level (around 1% and below). The predominance of α-T in C. tinctorius seeds and seed oil in all previous studies was reported [26, 27, 30, 31], and in general, the results of tocochromanols composition are consistent beyond the two reports [3, 32]. Vosoughkia et al. [32] did not found the presence of β-T in C. tinctorius seed oil, but δ-T in similar level as recorded for γ-T was detected. However, the tocopherols separation was done by RP-HPLC system using the C18 column, while isomers β and γ, generally, cannot be separated by the C18-bonded silica stationary phase column employed for RP–LC. Nevertheless, all tocopherols, including isomers β and γ, can be separated by employing the same C18 column via supercritical fluid chromatography (SFC) system [33]. In addition, tocochromanols were identified by the UV–Vis detection at wavelength 295 nm, which increase the possibility of misidentification and/or peaks overlapping, since a large number of compounds are detected at similar wavelength (± 25 nm). For instance, a small fraction of phenolic compounds (< 0.03% from the total amount present in seeds) can be transferred into the seed oil during the extraction by hexane and contribute to misidentification [34]. Interestingly, Salaberría et al. [3], in addition to tocopherols, detected a low level of two tocotrienols (β and γ) in two genotypes of safflower (C. tinctorius) line with a high content of oleic acid. While in the current study, the presence of δ-T, α-T3, and γ-T3 in C. tinctorius seed oil in very low level (0.1–0.2 mg/100 g oil) was detected (Table 1). To the best of our knowledge, the composition of tocochromanols in C. olitorius seed oil was not reported previously. The seed oils, C. oxyacantha and C. tinctorius regarding tocochromanols concentration, differed between each other significantly (p ≤ 0.05) only in levels of tocotrienols. The total amount of tocochromanols in the species C. oxyacantha and C. tinctorius was nearly identical and amounted to 57.9 and 58.2 mg/100 g oil, respectively (Table 1). The recorded total amount of tocochromanols in safflower oil is within the range of the levels reported in different cultivars of C. tinctorius (19.2–73.1 mg/100 g oil) [3, 26, 30, 32].
Carotenoids
From six carotenoids (neoxanthin, violaxanthin, lutein, zeaxanthin, β-cryptoxanthin, and β-carotene) identified in both safflower species, zeaxanthin was a predominated form consisting of 37 and 58% of total carotenoids in C. oxyacantha and C. tinctorius seed oils, respectively. In C. oxyacantha, seed oil high content was noted also for lutein and β-carotene 15 and 25%, respectively, while in C. tinctorius seed oil, lutein consisted of 18% of total carotenoids. Other carotenoids, in both species, accounted for 5 ± 2%. Both species differed statistically significantly (p ≤ 0.05) in the content of most carotenoids, with the exception of the main carotenoids lutein and zeaxanthin. Based on individual carotenoids concentration, β-carotene seems to be a good candidate for distinguishment between the safflower species. The total amount of carotenoids in C. oxyacantha and C. tinctorius seed oils was 0.76 and 0.5 mg/100 g oil, respectively (Table 1). To the best of our knowledge, this is the first report of carotenoid composition in cultivated and wild safflower seed oils.
Sterols and squalene
The presence of ten and six sterols was detected in C. oxyacantha and C. tinctorius seed oils, respectively. The presence of campestanol, 24-methylene cholesterol, gramisterol, 24-ethylcholest-7,24(28)dien-3beta-ol, cycloartenol, and 24-methylenecycloartanol in the C. oxyacantha and their absence in the C. tinctorius, and vice versa for avenasterol and Δ7-stigmasterol, was recorded. β-Sitosterol was a main form of phytosterols in both species and constituted 36.4 and 46.0% of the total amount of sterols in the C. oxyacantha and C. tinctorius seed oils, respectively. Over twofold lower levels, compared to β-sitosterol, for gramisterol (17.1%) and avenasterol (19.6%) in the C. oxyacantha and C. tinctorius, respectively, were obtained (Table 1). In both species (C. oxyacantha vs. C. tinctorius), similar levels of campesterol (10.7 vs. 12.0%), campestanol (10.0 vs. 7.6%) and sitostanol (6.8 vs. 6.1%) were noted. A comparable level to campesterol and campestanol was acquired for Δ7-stigmasterol (8.6%) in the C. tinctorius, while the level of remaining sterols in the C. oxyacantha (cycloartenol, 24-methylene cholesterol, 24-ethylcholest-7,24(28)dien-3beta-ol, 24-methylenecycloartanol, and campestanol) was at 5.1, 4.2, 2.9, 0.6, and 0.5%, respectively, of total sterol amount. A similar composition of sterols, with the predominance of β-sitosterol in the level comparable as found in the present study, was reported in C. tinctorius seed oil [3, 27, 30,31,32]. It was clearly shown that the percentage of a predominance of β-sitosterol is affected by the genotype and can amount between 41.7 and 53.5% [3, 30,31,32]. To the best of our knowledge, the present study for the first time reports the composition of sterols in C. oxyacantha seed oil. The total concentration of sterols in the C. oxyacantha was nearly half-fold lower in comparison with C. tinctorius (185.5 and 274.3 mg/100 g oil, respectively). The measured total amount of sterols in safflower oil is in accordance with reported levels in different cultivars of C. tinctorius (124.8–418.0 mg/100 g oil) [3, 30,31,32]. The reported difference in the sterols concentration in different cultivars of C. tinctorius seed oil shows that the observed difference in the present study between the two safflower species does not necessarily have to be the result of the species impact but of the cultivar. A similar conclusion was reported in the study, where the minor lipophilic bioactive compounds, including sterols, in the kernel oils of 28 plum genotypes of hexaploid species Prunus domestica L. and diploid plums Prunus cerasifera Ehrh. and their crossbreeds were studied [14].
The presence of squalene was detected only in the species C. oxyacantha with the amount of 10.4 mg/100 g oil (Table 1). Comparison within the same genus Carthamus cannot be provided, since the presence of squalene has not been reported previously. A similar amount of squalene as noted in C. oxyacantha seed oil can be found in sunflower [35].
Characterization of bioactive compounds with hydrophilic nature by LC–HRMS/MS2
A total of seven highly abundant bioactive compounds isolated by the HPLC system from C. oxyacantha and C. tinctorius extracts were identified (Table 2). Molecular formula assignment was performed using accurate mass information (precursor mass error < 2 ppm) and isotopic pattern fit (mSigma value < 20) from the acquired MS1 spectra on DataAnalysis 5.0 software (Bruker Daltonics, Germany). Compound tentative identification was based on MS2 spectra surveys using in silico fragmentation tools: SIRIUS 4 [36] and MetFrag [37]. All acquired MS2 spectra are presented in supplementary material (Fig. S2–S8).
In negative ionization mode (ESI−), compound 1 produced a deprotonated ion at m/z 483.1774, which suggested a molecular formula C25H28N2O8. A very likely candidate under such formula is coumaroylserotonin–glucoside, a frequently reported constituent of C. tinctorius [38]. This assignment was further supported by MS2 spectra. In particular, two abundant fragment ions were observed at m/z 321.125 (− 162 Da, [M–C6H10O5–H]−) and m/z 363.120 (− 120 Da, [M–C8H8O–H]−) which can be attributed to a loss of hexose moiety and p-vinyl phenol (from coumaric acid unit), respectively. A similar fragmentation pathway was displayed by compound 2, which produced an [M–H]− parent ion at m/z 513.1879 in ESI−. Again, an intense fragment was detected due to loss of hexose at m/z 351.135 (− 162 Da, [M–C6H10O5–H]−) and another one at m/z 363.120 (− 150 Da, [M–C9H10O2–H]−). The latter originated from loss of methoxy-vinylphenol unit, which is a likely scenario if ferrulic acid moiety is present in the parent compound. The fragment itself was also seen in MS2 spectra at m/z 149.061. Furthermore, a characteristic fragment which corresponds to deaminated serotonin unit was observed for compounds 1 and 2 at m/z 158.061; hence, compound 2 was identified as feruloylserotonin glucoside. Similar fragmentation patterns were observed for compounds 4 and 5, suggesting that they may be metabolic precursors of the detected serotonin derivatives mentioned above. In positive ionization mode (ESI +) both compounds produced the deaminated serotonin ion at m/z 160.076. Most descriptive MS2 peak for compound 4 was observed at m/z 177.102 (− 146 Da, [M–C9H6O2 + H]+) which indicated a loss of coumaric acid unit. This particular fragment was also present in MS2 spectra at m/z 147.044. Interestingly, compound 5 did not produce the ion at m/z 177.102. Instead of losing whole ferulic acid moiety, fragmentation tree indicated a cleavage of methoxy-vinylphenol at m/z 203.081 (− 150 Da, [M–C9H10O2 + H]+). This MS2 pathway was also observed for its glycosidic derivative (compound 2). Taking into account fragmentation pattern, compounds 4 and 5 were assigned as N–coumaroylserotonin and N-feruloylserotonin (moschamine), respectively. All four described above alkaloids have been found previously in C. tinctorius seeds [38].
In ESI + compound 3 predominantly yielded an [M + NH4]+ ion at m/z 568.2387. An initial loss of hexose unit was observed in MS2 spectra at m/z 389.160 (− 179 Da, [M–NH3–C6H10O5]+), while less pronounced daughter ions were formed at m/z 371.149 (− 197 Da, [M–NH3–C6H10O5–H2O]+) and m/z 343.154 (− 225 Da, [M–NH3–C6H10O5–H2O–CO]+). According to these results, compound 3 was identified as tracheloside. Tracheloside is a lignan glycoside, with the structurally similar to the lignan phytoestrogen, arctiin, previously isolated from C. tinctorius [39].
Compound 7 showed an [M + H]+ ion at m/z 461.1074, which corresponded to the molecular formula C22H20O11. A major fragment was detected at m/z 285.076 (− 176 Da, [M–C6H8O6 + H]+). Such cleavage indicates a presence of glucuronide residue. Furthermore, the fragment itself hints that aglycone of parent compound is a methoxy-dihydroxyflavone (C16H12O5). Taking into account the observed fragmentation pattern and occurrence data from other reports, compound 7 was, therefore, identified as acacetin-7-O-D-glucuronide. Acacetin-7-O-D-glucuronide is a flavonoid which presence was previously reported in C. tinctorius [38]. Similar MS2 spectra were noted for compound 6, which had an [M + H]+ precursor at m/z 593.1495 (C27H28O15). Fragment spectra revealed two major MS2 ions at m/z 461.107 (− 130 Da, [M–C5H8O4 + H]+) and at m/z 285.076 (− 308 Da, [M–C5H8O4–C6H8O6 + H]+). The first fragment corresponds to a loss of pentose, while the latter is identical as the observed precursor for compound 7. Hence, compound 6 was tentatively identified as acacetin–glucuronide–pentoside. The presence of acacetin–glucuronide pentoside flavonoid in any safflower species has not been reported previously.
Bioactive compounds with hydrophilic nature in seeds and seed oils of two safflower species
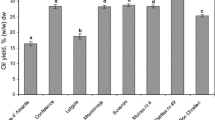
In the seeds and seed oils of both safflower species, a lignan glycoside (tracheloside), two flavonoids (acacetin–glucuronide pentoside and acacetin-7-O-D-glucuronide), and four alkaloids (N-coumaroylserotonin glucoside, N-feruloylserotonin glucoside, N-coumaroylserotonin, and N-feruloylserotonin) were detected. Based on the peak areas, it can be seen that C. oxyacantha seeds are richer in flavonoids in comparison to C. tinctorius, while an opposite relation was observed in the case of two saponins, N-coumaroylserotonin, and N-feruloylserotonin (Fig. 1). The presence of all bioactive compounds with hydrophilic nature detected in seeds was also confirmed in seed oils of both species; however, only a minor part (≤ 0.05%) of all compounds present in the seeds was transferred into the seed oil during the extraction (Table 3). The presence of hydrophilic compounds in oil can be surprising; however, frequently, it has been shown that the application of ultrasound-assisted extraction can co-extract such compounds. For instance, phenolic compounds and pyrrolizidine alkaloids with polar nature have been extracted by the non-polar hexane [34] or the highly non-polar plastochromanol-8 and β-carotene by the polar aqueous solutions of ethanol [40].
RP–HPLC–DAD chromatograms at wavelength 280 nm of bioactive compounds with hydrophilic nature in seeds of C. oxyacantha (a), and C. tinctorius (b). Both chromatograms were obtained by the injection of 5 μL of each sample extracted in the same conditions. 1–7, the assigned number of the bioactive compounds which has been identified by ICR-FT-HRMS system (Table 2, Figs. S1–S8)
Conclusion
The present study shows that both safflower seed oils—cultivated (C. tinctorius) and wild (C. oxyacantha)—have a similar profile of the lipophilic components with some exemptions. The composition and level of fatty acids and tocochromanols were nearly identical, while some differences were noted for carotenoids, sterols, and squalene, between the cultivated and wild safflower species. In case of bioactive compounds with hydrophilic nature, the C. oxyacantha seeds were richer in flavonoids, while C. tinctorius seeds in saponins. Recorded difference in the profile of phytosterols, carotenoids, and bioactive compounds with hydrophilic nature in seeds of safflower species (C. tinctorius vs. C. oxyacantha) can be a perspective tool for a distinction of these two species; however, additional studies are required taking into account different genotypes and abiotic factors.
Abbreviations
- AOCS:
-
American Oil Chemists’ Society
- DW:
-
Dry weight basis
- FID:
-
Flame ionization detector
- GC:
-
Gas chromatography
- HPLC:
-
High-performance liquid chromatography
- PFP:
-
Pentafluorophenyl
- RP:
-
Reverse phase
- SFC:
-
Supercritical fluid chromatography
- T:
-
Tocopherol
- T3:
-
Tocotrienol
References
Sabzalian MR, Saeidi G, Mirlohi A (2008) Oil content and fatty acid composition in seeds of three safflower species. J Am Oil Chem Soc 85:717–721
Ashri A, Knowles PF (1960) Cytogenetics of safflower (Carthamus L.) species and their hybrids. Agron J 52:11–17
Salaberría F, Constenla D, Carelli AA, Carrín ME (2016) Chemical composition and physical properties of high oleic safflower oils (Carthamus tinctorius, Var. CW88-OL and CW99-OL). J Am Oil Chem Soc 93:1383–1391
FAOSTAT (2019) FAO statistical database. http://www.fao.org. Accessed 05 Jun 2019
Górnaś P, Rudzińska M, Segliņa D (2014) Lipophilic composition of eleven apple seed oils: a promising source of unconventional oil from industry by-products. Ind Crops Prod 60:86–91
Ratnayake WMN, Hansen SL, Kennedy MP (2006) Evaluation of the CP-Sil 88 and SP-2560 GC columns used in the recently approved AOCS official method Ce 1h–05: Determination of cis-, trans-, saturated, monounsaturated, and polyunsaturated fatty acids in vegetable or non-ruminant animal oils and fats by capillary GLC method. J Am Oil Chem Soc 83:475–488
Górnaś P, Rudzińska M, Raczyk M, Mišina I, Soliven A, Segliņa D (2016) Composition of bioactive compounds in kernel oils recovered from sour cherry (Prunus cerasus L.) by-products: Impact of the cultivar on potential applications. Ind Crops Prod 82:44–50
Górnaś P (2015) Unique variability of tocopherol composition in various seed oils recovered from by-products of apple industry: Rapid and simple determination of all four homologues (α, β, γ and δ) by RP-HPLC/FLD. Food Chem 172:129–134
Górnaś P, Siger A, Czubinski J, Dwiecki K, Segliņa D, Nogala-Kalucka M (2014) An alternative RP-HPLC method for the separation and determination of tocopherol and tocotrienol homologues as butter authenticity markers: a comparative study between two European countries. Eur J Lipid Sci Technol 116:895–903
Rodriguez-Amaya DB, Kimura M (2004) HarvestPlus handbook for carotenoid analysis. HarvestPlus, International Food Policy Research Institute, Washington, DC
Rodriguez-Amaya DB (2001) A guide to carotenoid analysis in foods. ILSI Press, Washington
Górnaś P, Radziejewska-Kubzdela E, Mišina I, Biegańska-Marecik R, Grygier A, Rudzińska M (2017) Tocopherols, tocotrienols and carotenoids in kernel oils recovered from 15 apricot (Prunus armeniaca L.) genotypes. J Am Oil Chem Soc 94:693–699
AOCS (1997) Official Method Ch 6–91, 1997. Determination of the composition of the sterol fraction of animal and vegetable oils and fats by TLCand capillary GLC. Official methods and recommended practices of the American Oil Chemists’ Society. American Oil Chemists’ Society, Champaign, IL, USA
Górnaś P, Rudzinska M, Raczyk M, Mišina I, Soliven A, Lācis G, Seglina D (2016) Impact of species and variety on concentrations of minor lipophilic bioactive compounds in oils recovered from plum kernels. J Agric Food Chem 64:898–905
Makarova E, Górnaś P, Konrade I, Tirzite D, Cirule H, Gulbe A, Pugajeva I, Seglina D, Dambrova M (2015) Acute anti-hyperglycemic effects of an unripe apple preparation containing phlorizin in healthy volunteers: a preliminary study. J Sci Food Agric 95:560–568
Górnaś P, Siger A, Segliņa D (2013) Physicochemical characteristics of the cold-pressed Japanese quince seed oil: new promising unconventional bio-oil from by-products for the pharmaceutical and cosmetic industry. Ind Crops Prod 48:178–182
Coşge B, Gürbüz B, Kiralan M (2007) Oil content and fatty acid composition of some safflower (Carthamus tinctorius L.) varieties sown in spring and winter. Int J Nat Eng Sci 1:11–15
Senkal BC, Kiralan M, Ramadan MF (2016) Impact of harvest times on the quality characteristics of oils recovered from different safflower (Carthamus tinctorius) cultivars sown in spring and autumn. Eur Food Res Technol 242:371–381
Gecgel U, Demirci M, Esendal E, Tasan M (2007) Fatty acid composition of the oil from developing seeds of different varieties of safflower (Carthamus tinctorius L.). J Am Oil Chem Soc 84:47–54
Arslan B (2007) The determination of oil content and fatty acid compositions of domestic and exotic safflower (Carthamus tinctorius L.) genotypes and their interactions. J Agron 6:415–420
Nazari M, Mirlohi A, Majidi MM (2017) Effects of drought stress on oil characteristics of Carthamus species. J Am Oil Chem Soc 94:247–256
Ahmadzadeh S, Kadivar M, Saeidi G (2014) Investigation of oil properties and seed composition in some safflower lines and cultivars. J Food Biochem 38:527–532
Ashrafi E, Razmjoo K (2010) Effect of irrigation regimes on oil content and composition of safflower (Carthamus tinctorius L.) cultivars. J Am Oil Chem Soc 87:499–506
Yeilaghi H, Arzani A, Ghaderian M, Fotovat R, Feizi M, Pourdad SS (2012) Effect of salinity on seed oil content and fatty acid composition of safflower (Carthamus tinctorius L.) genotypes. Food Chem 130:618–625
Vosoughkia M, Hossainchi Ghareaghag L, Ghavami M, Gharachorloo M, Delkhosh B (2012) Evaluation of oil content and fatty acid composition in seeds of different genotypes of safflower. Int J Agric Sci Res 2:59–66
Matthaus B, Özcan MM, Al Juhaimi FY (2015) Fatty acid composition and tocopherol profiles of safflower (Carthamus tinctorius L.) seed oils. Nat Prod Res 29:193–196
Zlatanov M, Antova G, Angelova-Romova M, Momchilova S, Dimitrova R, Marcheva M (2015) Detailed characterization of lipids in safflower varieties grown in Bulgaria. Riv Ital Sostanze Gr 92:279–288
Górnaś P, Siger A, Rudzińska M, Grygier A, Marszałkiewicz S, Ying Q, Sobieszczańska N, Segliņa D (2019) Impact of the extraction technique and genotype on the oil yield and composition of lipophilic compounds in the oil recovered from Japanese quince (Chaenomeles japonica) seeds. Eur J Lipid Sci Technol 121:1800262
Górnaś P, Rudzińska M (2016) Seeds recovered from industry by-products of nine fruit species with a high potential utility as a source of unconventional oil for biodiesel and cosmetic and pharmaceutical sectors. Ind Crops Prod 83:329–338
Ben Moumen A, Mansouri F, Richard G, Fauconnier ML, Sindic M, Nabloussi A, Elamrani A, Serghini Caid H (2015) Variations in the phytosterol and tocopherol compositions and the oxidative stability in seed oils from four safflower (Carthamus tinctorius L.) varieties grown in north-eastern Morocco. Int J Food Sci Technol 50:2264–2270
Nogala-Kalucka M, Rudzinska M, Zadernowski R, Siger A, Krzyzostaniak I (2010) Phytochemical content and antioxidant properties of seeds of unconventional oil plants. J Am Oil Chem Soc 87:1481–1487
Vosoughkia M, Ghavamib M, Gharachorloo M, Sharrifmoghaddasi M, Omidi AH (2011) Lipid composition and oxidative stability of oils in safflower (Carthamus tinctorius L.) seed varieties grown in Iran. Adv Environ Biol 5:897–902
Urvaka E, Mišina I, Soliven A, Górnaś P (2019) Rapid separation of all four tocopherol homologues in selected fruit seeds via supercritical fluid chromatography using a solid-core C18 column. J Chem 2019:5307340
Górnaś P, Picron JF, Perkons I, Mišina I, Rudzińska M, Sobieszczańska N, Chakradhari S, Patel KS (2019) Profiling of the beneficial and potentially harmful components of Trichodesma indicum seed and seed oil obtained by ultrasound-assisted extraction. J Am Oil Chem Soc 96:249–259
Tuberoso CIG, Kowalczyk A, Sarritzu E, Cabras P (2007) Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chem 103:1494–1501
Dührkop K, Fleischauer M, Ludwig M, Aksenov AA, Melnik AV, Meusel M, Dorrestein PC, Rousu J, Böcker S (2019) SIRIUS 4: a rapid tool for turning tandem mass spectra into metabolite structure information. Nat Methods 16:299–302
Ruttkies C, Schymanski EL, Wolf S, Hollender J, Neumann S (2016) MetFrag relaunched: incorporating strategies beyond in silico fragmentation. J Cheminf 8:3
Zhou X, Tang L, Xu Y, Zhou G, Wang Z (2014) Towards a better understanding of medicinal uses of Carthamus tinctorius L. in traditional Chinese medicine: a phytochemical and pharmacological review. J Ethnopharmacol 151:27–43
Nishibe S (1972) Tracheloside from the seeds of Carthamus tinctorius. Phytochemistry 11:2629
Górnaś P, Šnē E, Siger A, Segliņa D (2014) Sea buckthorn (Hippophae rhamnoides L.) leaves as valuable source of lipophilic antioxidants: the effect of harvest time, sex, drying and extraction methods. Ind Crops Prod 60:1–7
Acknowledgements
This research was supported by the UGG, New Delhi grant Nr. F.18-1/2011(BSR) 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest regarding the publication of this article.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chakradhari, S., Perkons, I., Mišina, I. et al. Profiling of the bioactive components of safflower seeds and seed oil: cultivated (Carthamus tinctorius L.) vs. wild (Carthamus oxyacantha M. Bieb.). Eur Food Res Technol 246, 449–459 (2020). https://doi.org/10.1007/s00217-019-03414-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-019-03414-w