Abstract
The lipophilic and hydrophilic antioxidants were evaluated in eight plants: safflower (Carthamus tinctorius), viper’s bugloss (Echium vulgare), quince (Cydonia vulgaris), evening primrose (Oenothera biennis), rose mosqueta (Rosa affinis rubiginosa), black seed (Nigella sativa), sea buckthorn (Hippophae rhamnoides) and borage (Borago officinales). The highest amounts of tocopherols were contained in seeds of borage and sea buckthorn (66.9 mg/100 g and 45.9 mg/100 g, respectively). The sea buckthorn seed lipids had the highest amount of total sterols (10.4 mg/g of lipids). The predominant form was campesterol. Sitosterol was the major sterol in the lipids of other tested seeds. The content of phenolic compounds ranged from 736.5 mg/100 g dry matter (d.m.) (evening primrose) to 74.8 mg/100 g d.m. (safflower). The highest antioxidant activity, expressed in % scavenged DPPH· free radicals, was observed for evening primrose (91.2%), while the lowest for safflower (36.2%). The correlation coefficient between the level of phenolic compounds and antioxidant activity was 0.53.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, much attention has been focused on exploring different plants as alternative sources of functional compounds [1]. Recent epidemiological studies have suggested that increased consumption of whole grains, legumes, fruits, and vegetables is inversely associated with the risk of chronic diseases [2]. This association may be attributed to natural antioxidants such as tocopherols, polyphenols and phytosterols, which prevent free radical damage [3, 4]. Increasing interest has been observed in safflower, viper’s bugloss, quince, evening primrose, rosa mosqueta, black seeds, sea buckthorn and borage seeds as objects of research because of their antioxidant and anticarcinogenic potential and ability to avert or ameliorate degenerative ailments [1, 5–7].
Safflower (Carthamus tinctorius L.) is an important type of oil crop because of the high fat content (27–32%) in the seeds and seed oil being rich in linoleic acid (70–87%). Moreover, safflower seed oil is also a rich source of vitamin E. Recently, safflower seed oil has gained interest as an excellent health care product, because it is effective in the treatment of hyperlipemia, coronary heart disease and it can enhance microcirculation [8]. Past and modern day herbalists recommend viper’s bugloss (Echium vulgare L.) for a wide range of medical applications, including treatment of colds, coughs, fevers, headache, water retention, kidney stones, inflammation, pain, as well as promotion of wound healing, treatment of reddened skin and boils, and melancholia [50]. Nectar produced by flowers of this plant has been reported as a good source of wild honey [9]. Several studies have indicated that quince (Cydonia vulgaris) is an excellent natural source of phenolic acids and flavonoids, which are considered potent antioxidants. Quince fruit is recognized as an important dietary source of health promoting compounds, due to its antioxidant, antimicrobial and antiulcerative properties [10, 11]. Sea buckthorn (Hippophae rhamnoides L.) is a temperate, hardy bush that grows wild in Central Asia and Europe and produces nutritious and delicious berries. The soft tissue of the berries contains 3–5% fat, whereas fat content of the seeds is 12–13%. Sea buckthorn berries are an excellent source of phytochemicals such as ascorbic acid, tocopherols, unsaturated fatty acid (PUFA), phenols, and carotenoids [12–15]. Berries have been used for the treatment of radiation damage, burns, oral inflammation, and gastric ulcers [16]. Other claimed positive health effects include reduction of plasma cholesterol level, inhibition of platelet aggregation, and regulation of immune function [17].
The seeds of borage (Borago officinalis L.) and evening primrose (Oenothera biennis L.) plants are good sources of the essential fatty acid (EFA) γ-linolenic acid (18:3, n-6). Evening primrose seeds contain from 10 to 17% fat, and γ-linolenic acid may constitute up to 10% of the fatty acids (FA) [18]. The content of fat in borage seeds, containing up to 25% γ-linolenic acid, ranges from 17 to 25% [19]. Although this FA is quite prone to oxidation, the oil in these seeds resists oxidation. This suggests that these oleaginous seeds must contain some potent antioxidants [20]. Lu and Foo [21] and Shahidi et al. [22] demonstrated that the antioxidant activity in evening primrose may be due to the presence of phenolic compounds. Blasinska and Troszynska [23] reported that phenolics extracted from evening primrose seeds may protect lipids from oxidation caused by free radicals. Therefore, meals after extraction of oils may be potential sources of natural food antioxidants.
Black seed or black cumin (Nigella sativa) is an important medicinal herb. In many Arabian, Asian and African countries, black seed oil is used as a natural remedy for a wide range of diseases, including various allergies. Moreover, black seed oil proved to be an effective adjuvant for the treatment of allergic diseases [24]. The plant’s mechanism of action is still largely unknown. Seeds of black cumin are used as a spice in cooking and in a wide range of traditional medicinal uses. The seed volatile oil and its main active constituent, thymoquinone, are extensively reported to exhibit a protective effect against many diseases due to its high antioxidant activity [25, 26]. The antioxidant effects of tocols and rosa mosqueta (Rosa affinis rubiginosa, a rose hip growing in the Andes) shell extract added to antioxidant-stripped canola oil (TCO) have been evaluated. Polar compound formation, degradation of tocols and carotenoid pigments have been studied. The addition of Rosa mosqueta shell extract (rose hip seed oil) provided great stability to TCO, with a low polar compound formation and a high retention of α-tocopherol compared with other TCO samples, which suggested the protective action of minor components present in the extract [27].
Although most of the above mentioned fruits have been investigated for their nutritional and medicinal properties, no research has been conducted on the phytochemical profiles of their seeds.
Therefore, the objective of the present research was to evaluate the composition of tocochromanol, sterol and phenolic compounds in seed oils from eight plants. The information obtained from this study can be used to evaluate the potential use of these berry seed oils in food products and to confirm product authenticity.
Materials and Methods
Material
Seeds of eight plants were selected for analyses: safflower (Carthamus tinctorius), viper’s bugloss (Echium vulgare), quince (Cydonia vulgaris), evening primrose (Oenothera biennis), rose mosqueta (Rosa affinis rubiginosa), black seed (Nigella sativa), sea buckthorn (Hippophae rhamnoides) and borage (Borago officinales). The seeds were supplied by the Department of Plant Raw Materials Processing and Chemistry, the Warmia–Mazurian University of Olsztyn, Poland.
Determination of Water Content
Water (moisture) in a sample was measured gravimetrically as recommended in the Current Protocols in Food Analytical Chemistry [28].
Extraction and Measurement of Total Lipids
The measurement of total lipids content in seeds is typically made by Soxhlet extraction. Gravimetric determination of total lipids content was determined by multiple continuous sample extraction with n-hexane (for 2 h). Extraction was performed using an automatic Soxhlet Büchi Extraction System B-811 (Büchi Labortechnik AG, Flawil, Switzerland).
Tocochromanol Contents
In order to determine tocopherol content, samples of seeds (2 g) were saponified using 60% KOH (2 ml), ethanol (20 ml) and pyrogallol (0.5 g). The saponification was carried out at the ethanol boiling point temperature (78 °C) for 30 min. After saponifications, unsaponifiable substances were extracted using 50 ml n-hexane/ethyl acetate (90:10 v/v). Tocopherols and tocotrienols were qualitatively and quantitatively identified using liquid chromatography HPLC (Waters 600 Asc. Milford, MA, USA) [29, 30]. A LiChrosorb Si60 column (250 × 4.6 mm; 5 μm) and an LiChrospher Si60 precolumn was used. The mobile phase consisted of n-hexane and 1,4-dioxane (97:3 v/v) at a flow rate of 1.5 ml/min. The fluorometric detector (Waters 474 Asc. Milford, MA, USA) worked at excitation (λ = 290 nm) and emission (λ = 330 nm) for tocochromanols and PC-8 [31, 32]. Standards of α-, β-, γ- and δ-tocopherol and tocotrienol (99%) were purchased from Merck (Darmstadt, Germany).
Sterol Contents
The GC method described by Rudzińska et al. [33] was applied for sterol determinations. Following this method, lipids were extracted from 1 g of ground seeds using Folch solution (solution containing chloroform and methanol 2:1 v/v). Next, 500 μg of 5-α-cholestane were added to the extracted fat as an internal standard. The sample was then saponified using methanolic 1 M KOH at room temperature for 18 h. The unsaponified fraction was extracted using diethyl ether. Plant sterols were analyzed as TMS derivatives using a Hewlett-Packard 6890 gas chromatograph and a DB5 column (30 m × 0.32 mm × 0.25 μm, J, W Scientific). Injector and detector temperature was 310 ± 1 °C, and samples were injected in split mode (1: 25). The analyses were performed at a constant temperature of 290 ± 1 °C at a constant helium flow of 1.6 ml/min. Phytosterols were identified based on their retention times according to the standards.
Methanol Extracts of Phenolic Compounds
All samples were defatted using an automatic Soxhlet Büchi Extraction System B-811 (Büchi Labortechnik AG, Flawil, Switzerland).
The extraction with n-hexane was carried out for 2 h. To obtain rapeseed phenols, each sample was extracted three times with 80% methanol. The samples were mixed with the solvent (1:3), shaken for 30 min, filtered through anhydrous sodium sulfate, and vacuum-evaporated. The residue was dissolved in 80% methanol.
Total Phenolic Contents
The content of total phenolic compounds in methanol extracts was determined by the Folin–Ciocalteu method. An aliquot (0.025 ml) of the methanolic extract was placed in a volumetric flask (10 ml). Water (5 ml) and Folin–Ciocalteu reagent (0.5 ml) were added. After 3 min, saturated sodium carbonate (1 ml) was added. The flask was filled with water up to 10 ml. After 1 h, the solution absorbance was measured at λmax 725 nm against a reagent blank using a UV–Vis spectrophotometer SP 8001 (Metertech Inc. Taipei, Taiwan). Total phenolic content was determined after preparation of a standard curve and on that basis total phenolic compounds were measured as caffeic acid equivalents (CAE).
Antioxidant Activity Determination
The method consisted of spectrophotometric measurement of the intensity of the color change in solution depending on the amount of DPPH·. The reaction was initiated by mixing 1 ml of the methanolic extract with 3 ml methanol and then adding 1 ml of DPPH·(0.012 g/100 ml). Absorbance at λmax of 517 nm (UV–Vis spectrophotometer SP 8001, Metertech Inc.) was checked at 0, 0.5, and at every 0.5 min until the reaction reached a stable state. This plateau was reached within 15 min. The activity of the extract in scavenging DPPH· was calculated as follows:
Statistical Analysis
Results are presented as the mean ± standard deviation from three replicates of each experiment. A P-value <0.05 was used to denote significant differences between mean values determined by the analysis of variance (ANOVA) with the assistance of Statistica 7.0 (StatSoft, Inc., Tulsa, OK) software.
Results and Discussion
Tested seeds may constitute raw materials for the production of oils due to their high fat contents (over 20%), with the exception of sea buckthorn (12.1% fat) (Table 1). The highest amount of the lipid was found for Rosa mosqueta (52.0%), followed by borage (35.6%) and evening primrose (30.0%). The water content in quince seeds was 15.6%, while in the other seeds it did not exceed 9.0% (Table 1).
Fat-soluble substances, such as tocochromanols, sterols and phenolic compounds, exhibit antioxidant action and influence oil stability. In the tested plant material the presence of four tocopherol homologs (α-, β-, γ-, δ-T) was recorded, while tocotrienols were not detected (Table 2). The highest amounts of total tocopherols were contained in seeds of borage (66.9 mg/100 g), sea buckthorn (45.9 mg/100 g) and viper’s bugloss (20.2 mg/100 g). In the other seeds their level did not exceed 16.0 mg/100 g.
Individual tocopherol homologs differ in their antioxidant potential and biological activity of vitamin E. The best antioxidant properties were found for δ-T, followed by γ-, β- and α-T, whereas the highest activity of vitamin E was exhibited by α-tocopherol [34]. Among tested plants in this work the highest amount of δ-T was recorded in seeds of borage (61.8 mg/100 g). The presence of this homolog was not detected in seeds of black seed, quince or safflower. Seeds of sea buckthorn and quince were rich sources of α-T (27.5 mg/100 g and 16.0 mg/100 g, respectively). β-T was found in slight amounts (up to 2.5 mg/100 g), while the highest content of γ-T was found for seeds of viper’s bugloss (19.10 mg/100 g). Literature data confirm that oil from seeds and fruits of sea buckthorn is a rich source of lipophilic vitamins, particularly vitamin E [35], while oil from borage is rich in δ-T [36]. Content of α-tocopherol in oil from sea buckthorn seeds ranges from 121 to 223 mg/100 g [37], while that of δ-T in borage oil ranges from 115 to 140 mg/100 g, respectively [36].
Phytosterols are of a great interest due to their antioxidant activity and impact on health. They are key components of the unsaponifiable matter of plant oils and fats. The sterol profiles of lipids in analyzed seeds are shown in Table 3. The sea buckthorn seed lipids had the highest amount of total sterols (10.4 mg/g of lipids) followed by viper’s bugloss at 7.4 mg/g, evening primrose (7.1 mg/g) and borage (5.1 mg/g). Safflower and quince seed lipids contained 3.5 and 3.6 mg of phytosterols in 1 g of oils, respectively. The smallest amounts of phytosterols were detected in seed lipids of rose mosqueta (2.4 mg/g) and black seed (1.0 mg/g). The contribution of the most prevalent sterol, β-sitosterol, to the total sterol content in each seed was the highest in seed lipids of rose mosqueta (82%), evening primrose (79%), quince (73%) and sea buckthorn (56%). Compared to the other seeds, contribution of β-sitosterol, to the total sterol content in borage and viper’s bugloss seed lipids were lower at 31 and 19%, respectively. Sterol fractions of the tested seeds consisted also of campesterol (3–41%), avenasterol (9–30%) and stigmasterol (1–6%). Small amounts of Δ7-stigmasterol, campestanol, cycloartenol, 24-methylenecycloartanol and citrostadienol were also detected (Table 3). Phytosterol plant extracts, and specifically those rich in β-sitosterol, might significantly reduce LDL cholesterol levels. Studies have shown that people with a diet high in β-sitosterol, at the levels of 60–130 mg/day, have lower incidence of prostate cancer. Phytosterols also appear to play a role in modulating immune function and inflammation as a result of its effects on the production of inflammatory cytokines [38]. Moreover, Yoshida and Niki [39] reported antioxidant effects of the phytosterols β-sitosterol, stigmasterol, and campesterol against lipid peroxidation.
A wide spectrum of physiological effects of berries and berry products of sea buckthorn has been shown [40]. The sterol content and composition showed little variation between subspecies and collection sites [14]. In this research, sea buckthorn had the highest levels of total phytosterols, which is important for its utilization in functional food and natural medicines.
Viper’s bugloss is reported to contain toxic secondary metabolites [41], therefore an excessive or prolonged use of this plant should be avoided, even if its content of the toxic compounds is low. However, in this work, seed lipids of viper’s bugloss had the highest levels of avenasterol. White and Armstrong [42] demonstrated that avenasterol, which is found in high levels in oats, may have valuable antioxidant activity. Additional studies have confirmed that avenasterol and other phytosterols that contain an ethylidene group possess antioxidant and antipolymerization properties, especially valuable during frying [43]. Whether these antioxidant properties have any significance to human health remains to be seen.
The sterol fraction of evening primrose is comprised of 90% β-sitosterol, with the remainder being 4-methyl sterols (citrostadienol, 5%; obtusifoliol, 1%; gramisterol, 1.5%) [18]. The lowest level of phytosterols was detected in lipids from black seed (Table 3). The content of total phytosterols in Tunisian and Iranian Nigella seed ranged from 2.58 mg/g to 2.81 mg/g [44], but in German seeds it was 3.66.mg/g [45]. Such variation in sterol content among species and varieties may be related to the variations of cultivated regions, storage conditions, maturity stage and also geographical and climatic differences.
Antioxidant activity of phenolic acids and their derivatives depend on the number of hydroxy groups in the molecule [46]. Phenolic compounds as antioxidants may exhibit multifaceted functions, e.g., by a direct reaction with free radicals, scavenging of free radicals or chelation of pro-oxidant metals. Table 4 presents total contents of phenolic compounds and antioxidant activity of methanol extracts obtained from tested seeds. Total contents of phenolic compounds were given as a caffeic acid equivalent, while antioxidant activity was determined in relation to scavenging of DPPH radicals. The highest total phenolic content was recorded for evening primrose (736.5 mg/100 g dry matter (d.m.)). In the other seeds, the content of phenolic compounds ranged from 140.5 (rosa mosqueta) to 74.8 mg/100 g d.m. (safflower). According to investigations conducted by Kraczek et al. [47], the main phenolic acids contained in evening primrose include gallic, caffeic, p-hydroxybenzoic, vanillic, ferulic and salicylic acids. Protocatechinic, p-coumaric, pyrocatechinic, o-coumaric, syringic and 2-hydroxy-4-methoxybenzoic acids were found in smaller amounts.
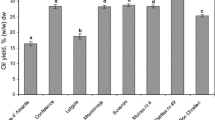
Since phenolic compounds are natural antioxidants, their content in analyzed seeds affected their antioxidant activity. The highest antioxidant activity, expressed in % scavenged DPPH free radicals, was observed for evening primrose (91.2%), while the lowest for safflower (36.2%). The correlation coefficient between the level of phenolic compounds and antioxidant activity was 0.53. Conforti et al. [48] reported a low correlation between antioxidant activity and contents of phenolic compounds (R 2 = 0.24). The lack of high correlation between total phenolic content and antioxidant activity may be caused by the nonspecific assay such as Folin–Ciocalteu reagent used in our study. The total phenolic contents assay has several disadvantages including low specificity for phenols, lack of relevance to biological oxidative processes, and interferences. It measures reducing capacity of phenols or other reducing agents present in samples (e.g., alanine, cysteine, fructose, glycine, sucrose, tryptophan, xanthine) that can react with the FC reagent [49]. Moreover, Schmidt et al. [50] did not show this relationship for extract of evening primrose flour. They also found that antioxidant properties of plant extracts are first of all affected by the composition of phenolic compounds, but not their amounts.
Conclusions
The role of natural antioxidants in disease prevention and treatment has gained interest by the health community. Among the plants analyzed in this study, rich sources of biologically active compounds was borage, sea buckthorn, viper’s bugloss and evening primrose. Borage seeds were exceptional in terms of high contents of tocopherols. Sea buckthorn was exceptional for the high levels of tocopherols and phytosterols, while evening primrose and viper’s bugloss were high in phenolic compounds and phytosterols, respectively.
References
Anwar F, Przybylski R, Rudzińska M, Gruczyńska E, Bain J (2008) Fatty acids, tocopherols and sterols profiles of seed lipids of selected Canadian prairie fruits. J Am Oil Chem Soc 85:953–959
Hu FB (2002) Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 13:3–9
Diplock AT, Charleux JL, Crozier-Willi G, Kok FJ, Rice-Evan C, Roberfroid M, Stahl W, Viña-Ribes J (1998) Functional food science and defense against reactive oxidative species. Br J Nutr 80S:77–112
Shahidi F (2004) Functional foods: their role in health promotion and disease prevention. J Food Sci 69:146–149
Eskin NAM (2008) Borage and evening primrose oil. Eur J Lipid Sci Technol 110:651–654
Larmo PS, Yang B, Hurme SAM, Alin JA, Kallio HP, Salminen EK, Tahvonen RL (2009) Effect of a low dose of sea buckthorn berries on circulating concentrations of cholesterol, triacylglycerols, and flavonols in healthy adults. Eur J Nutr 48:277–282
Sabzalian MR, Saeidi G, Mirlohi A (2008) Oil content and fatty acid composition in seeds of three safflower species. J Am Oil Chem Soc 85:717–721
Lee YC, Oh SW, Chang J, Kim JH (2004) Chemical composition ans oxidative stability of safflower oil prepared from safflower seed roasted with different temperatures. Food Chem 84:1–6
Klemow KM, Clements DR, Threadgill PF, Cavers PB (2002) The biology of Canadian weeds. 116. Echium vulgare L. Can J Plant Sci 82:235–248
Silva BM, Andrade PB, Valentăo P, Ferreres F, Seabra RM, Ferreira MA (2004) Quince (Cydonia oblonga Miller) fruit (pulp, peel, and seed) and jam: antioxidant activity. J Agric Food Chem 52:4405–4712
Silva BM, Valentăo P, Seabra RM, Andrade PB (2008) Quince (Cydonia oblonga Miller): an interesting dietary source of bioactive compounds. In: Papadopoulos KN (ed) Food chemistry research developments. Nova Science Publishers, New York, pp 243–266
Beveridge T, Li TSC, Oomah BD, Smith A (1999) Sea buckthorn products: manufacture and composition. J Agric Food Chem 47:3480–3488
Gao X, Ohlander M, Jeppsson N, Bjork L, Trajkovski V (2000) Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J Agric Food Chem 48:1485–1490
Yang B, Kallio HP (2001) Fatty acids composition of lipids in sea buckthorn (Hippophae rhamnoides L.) berries of different origins. J Agric Food Chem 49:1939–1947
Zadernowski R, Nowak-Polakowska H, Lossow B, Nesterowicz J (1997) Sea buckthorn lipids. J Food Lipids 4:167–172
Li TSG, Wang LCH (1998) Physiological components and health effects of ginseng, Echinacea, and sea buckthorn. In: Mazza G (ed) Functional foods, biochemical and processing aspects. Technomic, Lancaster, pp 329–356
Yang B, Karlsson RM, Oksman PH, Kallio HP (2001) Phytosterols in sea buckthorn (Hippophae rhamnoides L.) berries: identification and effects of different origins and harvesting time. J Agric Food Chem 49:5620–5629
Hudson BJF (1984) Evening primrose (Oenothera spp.) oil and seed. J Am Oil Chem Soc 61:540–543
Whipkey A, Simon JE, Janick J (1988) In vivo and in vitro lipid accumulation in Borago officinalis L. J Am Oil Chem Soc 65:979–984
Amarowicz R, Raab B, Karamac M (1999) Antioxidative activity of an ethanolic extract of evening primrose. Nahrung 43:216–217
Lu F, Foo LY (1995) Phenolic antioxidant components of evening primrose. In: Niki E, Packer L (eds) Nutrition, lipids, health, and disease. AOCS Press, Champaign, pp 86–95
Shahidi F, Wettasinghe M, Amarowicz R, Khan MA (2000) Antioxidants of evening primrose. In: Shahidi F, Ho CT (eds) Phytochemicals and phytopharmaceuticals. AOCS Press, Champaign, pp 278–295
Blasinska B, Troszynska A (1998) Total antioxidative activity of evening primrose (Oenothera paradoxa) cake extract measured in vitro by liposome model and murine L1210 cells. J Agric Food Chem 46:3558–3563
Kalus U, Pruss A, Bystron J, Jurecka M, Smekalova A, Lichius JJ, Kiesewetter H (2003) Effect of Nigella sativa (Black Seed) on subjective feeling in patients with allergic diseases. Phytotherapy Res 17:1209–1214
El-Dakhakhny M, Barakat M, El-Halim M, Aly SM (2000) Effects of nigella sativa oil gastric secretion and ethanol-induced ulcer in rats. J Ethnopharm 72:299–304
Nagi MN, Mansour MA (2000) Protective effect of thymoquinone against doxorubicin induced cardiotoxicity in rats: a possible mechanism of protection. Pharmacol Res 41:283–289
Romero N, Robert P, Masson L, Ortiz J, González K, Tapia K, Dobaganes C (2007) Effect of α-tocopherol, α-tocotrienol and Rosa mosqueta shell extract on the performance of antioxidant-stripped canola oil (Brassica sp.) at high temperature. Food Chem 104:383–389
Current Protocols in Food Analytical Chemistry (2001) Gravimetric determination of water by drying and weighing. Wiley, New York, pp A1.1.1–A1.1.6
Gogolewski M, Nogala-Kałucka M, Szeliga M (2000) Changes of the tocopherol and fatty acid contents in rapeseed oil during refining. Eur J Lipid Sci Technol 102:618–623
Nogala-Kałucka M, Gogolewski M, Jaworek M, Siger A, Szulczewska A (2002) Determination of some components as indicators of the quality of rapeseed produced in different regions in Poland. Oilseed Crops 23:447–459
Eitenmiller RR, Landen WO (1999) Vitamin analysis for the health and food sciences. CRC Press, Boca Raton
Ryynänen M, Lampi AM, Salo-Väänänen P, Ollilainen V, Piironen V (2004) A small-scale sample preparation method with HPLC analysis for determination of tocopherols and tocotrienols in cereals. J Food Comp Anal 17:749–765
Rudzińska M, Kazuś T, Wąsowicz E (2001) Sterols and their oxidized derivatives in refined and cold pressed seed oils. Oilseed Crops 22:477–494
Eitenmiller R, Lee J (2004) Nutritional and health implications of vitamin E. In: Eitenmiller R, Lee J (eds) Vitamin E—food chemistry, composition and analysis. Marcel Dekker, New York, pp 39–89
Kallio H, Yang B, Peippo P, Tahvonen R, Pan R (2002) Triacylglycerols, glycerophospholipids, tocopherols, tocotrienols in berries and seeds of two species (ssp. sinensis and mongolica) of sea buckthorn (Hippophae rhamnoides). J Agric Food Chem 50:3004–3009
Zadernowski R, Nowak-Polakowska H, Pieńkowska H, Czaplicki S (2002) Effect of the method of fat extraction from seeds of evening primrose and borage on selected physicochemical properties and stability of oils. Oilseed Crops 23:471–480
Cenkowski S, Yakimishen R, Przybylski R, Muir WE (2006) Quality of extracted sea buckthorn seed and pulp oil. Can Biosys Eng 48:3.9–3.16
Awad AB, Fink CS, Williams H, Kim U (2001) In vitro and in vivo (SCID mice) effects of phytosterols on the growth and dissemination of human prostate cancer PC-3 cells. Eur J Cancer Prev 10:507–513
Yoshida Y, Niki E (2003) Antioxidant effects of phytosterol and its components. J Nutr Sci Vitaminol 49:277–280
Ge XY, Shi GF, Zhang YM, Wang TB (1985) Medical application of sea buckthorn. Shanxi Med Res 2:9–14
Bruneton J (1999) Toxic plants dangerous to humans and animals. Lavoisier Publishing, Paris, p 206
White PJ, Armstrong LS (1986) Effect of selected oat sterols on the deterioration of heated soybean oil. J Am Oil Chem Soc 63:525–529
Moreau RA, Whitaker BD, Hicks KB (2002) Phytosterols, phytostanols, and their conjugates if foods: structural diversity, quantitative analysis, and health-promoting uses. Prog Lipid Res 41:457–500
Cheikh-Rouhou S, Besbes S, Lognay G, Blecker C, Deroanne C, Attia H (2008) Sterol composition of black cumin (Nigella sativa L.) and Aleppo pine (Pinus halepensis Mill.) seed oils. J Food Comp Anal 21:162–168
Ramadan MF, Mörsel JT (2004) Oxidative stability of black cumin (Nigella sativa L.), coriander (Coriandrum sativum L.) and Niger (Guizotia abyssinica Cass.) crude seed oils upon stripping. Eur J Lipid Sci Technol 106:35–43
Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T (2005) Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mut Res 579:200–213
Kraczek T, Bogucka-Kocka A, Śnieżko R (1995) The phenolic acids of some species of the Oenothera L. genus. Acta Soc Bot Poloniae 64:41–44
Conforti F, Sosa S, Marrelli M, Menichini F, Statti GA, Uzunov D, Tubaro A, Menichini F (2009) The protective ability of Mediterranean dietary plants against the oxidative damage: the role of radical oxygen species in inflammation and the polyphenol, flavonoid and sterol contents. Food Chem 112:587–594
Moore J, Yu L (2008) Methods for antioxidant capacity estimation of wheat and wheat-based food products. In: Yu L (ed) Wheat antioxidant. Wiley, New Jersey, pp 118–173
Schmidt S, Niklová I, Pokorný J, Farkaš P, Sekretár S (2003) Antioxidant activity of evening primrose phenolics in sunflower and rapeseed oils. Eur J Lipid Sci Technol 105:427–435
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nogala-Kalucka, M., Rudzinska, M., Zadernowski, R. et al. Phytochemical Content and Antioxidant Properties of Seeds of Unconventional Oil Plants. J Am Oil Chem Soc 87, 1481–1487 (2010). https://doi.org/10.1007/s11746-010-1640-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-010-1640-8