Abstract
Lipophilic bioactive compounds in oils recovered from the kernels of seven sweet cherry (Prunus avium L.) cultivars, harvested at single location in 2013, were studied. Oil yield in sweet cherry ranged between 30.3–40.3 % (w/w) dw. The main fatty acids were oleic acid (39.62–49.92 %), linoleic acid (31.13–38.81 %), α-eleostearic acid (7.23–10.73 %) and palmitic acid (5.59–7.10 %), all four represented approximately 95 % of the total detected fatty acids. The ranges of total tocochromanols and sterols were between 83.1–111.1 and 233.6–419.4 mg/100 g of oil, respectively. Regardless of the cultivar, the γ-tocopherol and β-sitosterol were the main lipophilic minor bioactive compounds. The content of the carotenoids and squalene were between 0.38–0.62 and 60.9–127.7 mg/100 g of oil, respectively. Three significant correlations were found between oil yield and total contents of sterols (r = −0.852), tocochromanols (r = −0.880) and carotenoids (r = −0.698) in sweet cherry kernel oils. The oil yield, as well as the content of lipophilic bioactive compounds in oil was significantly affected by the cultivar.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The global production of sweet cherries (Prunus avium L.) continues to increase with demands from the fruit and food industry, and in 2012 amounted to 2.3 million tons [1]. Sweet and sour cherries are not only consumed in fresh form, but also in large scales to be processed into juice, jams and candied fruits, consequently generating vast amounts of by-products (e.g., fruit pits) with limited utilization. Fruit kernels are valuable sources of oils that are rich in functional bioactive compounds such as essential fatty acids, tocochromanols, carotenoids, phytosterols and squalene [2–8].
Fatty acids are concentrated sources of energy, however some fatty acids, for instance α-eleostearic acid have been reported to possess antitumor activity in cancer cells [9].
Tocopherols and tocotrienols (vitamin E) are synthesized only by photosynthetic organisms and their content in the plant tissue ranges from below 1 μg/g to above 1 mg/g dry weight basis in different vegetative parts [10]. Tocochromanols have reported unique physicochemical activity in model and biological [11–15] systems.
Carotenoids are natural isoprenoid pigments synthesized mainly in photosynthetic, but also in some non-photosynthetic organisms such as fungi and bacteria, with over 700 different known compounds, some of which have pro-vitamin A activity [16]. Certain plasma carotenoids and tocopherols were inversely related to prostate-specific antigen levels at various points of time, suggesting that greater intake of foods containing these micronutrients could benefit men with prostate-specific antigen-defined recurrence of prostate cancer [17].
Phytosterols belong to the group of cholesterol analogues which occur in plants and vary with the carbon side chains. β-Sitosterol along with stigmasterol and campesterol are the most common forms of phytosterols in the plant world with the ability to reduce levels of cholesterol in blood serum, which contributed to the rapid application in numerous food stuffs as a functional ingredient [18].
Squalene is a 30 carbon isoprenoid, precursor of steroid hormones, vitamin D and cholesterol. Squalene has reported antioxidant activity against oxidative destruction of DNA and consequently damage of mammary epithelial cells in humans [19].
Since the cultivar is often associated with the significant different concentrations of bioactive compounds [20, 21], the present study has focused on the oil yield and its composition of lipophilic compounds (fatty acids, tocochromanols, carotenoids, sterols, squalene) recovered from selected cultivars of sweet cherry kernels.
Materials and Methods
Reagents
Methanol, 2-propanol, tert-butyl methyl ether, n-hexane (HPLC grade) and 5α-cholestane (≥97 %, GC) were purchased from Sigma-Aldrich (Steinheim, Germany). Tocopherol and tocotrienol homologues (α, β, γ and δ) (> 95 %, HPLC) were obtained from Merck (Darmstadt, Germany) and LGC Standards (Teddington, Middlesex, UK), respectively. The Sylon BTZ and fatty acid methyl ester mix were received from Supelco (Bellefonte, PA, USA) and (Steinheim, Germany), respectively.
Plant Material
The kernels were recovered from fruit pits (by removing the outer shells) of seven cultivars of mature sweet cherries (Prunus avium L.) harvested in July 2013 in Dobele, at the Latvia State Institute of Fruit-Growing. Undamaged kernels were frozen and subsequently freeze dried using a FreeZone freeze-dry system (Labconco, Kansas City, MO, USA) at a temperature of −51 ± 1 °C under vacuum of 0.055–0.065 mBar for 48 h. Kernels were selected (~20 g) and milled with a Knifetec™ 1095 universal laboratory mill (Foss, Höganäs, Sweden) to pass through a sieve of 0.75 mm mesh size to finally obtain a powder. Dry weight basis (dw) in the studied samples was measured gravimetrically.
Oil Extraction
Oil was extracted according to an earlier introduced method [3].
Fatty Acid, Sterols and Squalene Composition
The fatty acids, sterols and squalene were determined according to a method previously described [20].
Tocopherol and Tocotrienol Homologues Determination
The oils samples were diluted in 2-propanol as was described by Górnaś [22] and determined by RP-HPLC/FLD according to the method previously developed and validated [23].
Total Carotenoids Determination
Total carotenoids were estimated according to Górnaś et al. [20].
Statistical Analysis
The results were presented as means ± standard deviation (n = 3) from three batches of ground kernels. The p-value ≤0.05 was used to denote significant differences between mean values determined by one-way analysis of variance (ANOVA). The Bonferroni post-hoc test was used to denote statistically significant values at p ≤ 0.05. The association between analysed variables was assessed by Pearson’s correlation coefficient. Its significance was evaluated by Student’s t-test. Linear regression model (y = ax + b) was calculated additionally for the analysis of significant relationships between parameters. All analyses were performed with the assistance of Statistica 10.0 (StatSoft, Tulsa, OK, USA) software.
Results and Discussion
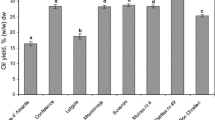
The cultivar selection was found to significantly impact the recovered oil yield for crab, cider and dessert apple seeds [3, 4]. The investigated kernels of different sweet cherry cultivars resulted in the oil yield ranging from 30.3 to 40.3 % (w/w) dw in cvs. ‘Krupnoplodnaya’ and ‘Tyutchevka’, respectively (Table 1). With the exception of the lowest oil yield cv. ‘Krupnoplodnaya’, the oil yield was comparable for the majority of studied samples (37.5–40.3 % (w/w) dw and in most cases were statistically insignificant (p ≤ 0.05). The average oil yield in seven tested samples amounted to 37.7 % (w/w) dw. A previous study included the impact of the cultivar on the oil yield for four Iranian cultivars extracted with petroleum ether in Soxhlet apparatus, the reported oil yields were between 22.2–37.9 % [24], comparable to the results obtained in the present study, however with a wider range. Another study by Bernardo-Gil et al. [25] quantified a low 9 % oil yield in sweet cherry seeds (fruit shell and kernel) extracted with supercritical carbon dioxide. Non-varietal factors such as growing conditions, horticultural practice and climate may also account for the differences among the same cultivars [4]. Nonetheless, previous studies have clearly indicated that genetic factors have a greater impact on oil yield and quality than environmental ones, for instance in corn (Zea mays L.). It has been highlighted that the oil yield and its composition can vary considerably from location to location as well as year to year, however the relative rank of genotype will be similar under various environmental conditions [26]. Additionally, the influence of external factors may have different impacts for each crop [27].
The composition of fatty acids in kernel oils recovered from seven sweet cherry cultivars was significantly different (p ≤ 0.05) in most cases (Table 1). From nine identified fatty acids, the oleic (C18:1) (39.62–49.92 %), linoleic (C18:2) (31.13–38.81 %), α-eleostearic (α-ESA C18:3) (7.23–10.73 %) and palmitic (C16:0) (5.59–7.10 %) acid together represented approximately 95 % of the total detected fatty acids. Levels over 1 % were detected for stearic (C18:0) (2.43–3.41 %) and arachidic (C20:0) (0.88–1.09 %) acid. Values below 1 % were recorded for palmitoleic (C16:1) (0.33–0.53 %), α-linolenic (α-C18:3) (0.07–0.14 %) and gondoic (C20:1) (0.27–0.58 %) acid (Table 1). Similar identification and concentration of fatty acids in sweet cherry kernel oils in three cultivars grown in Slovenia and Norway, where the cultivar factor had higher statistical significance on the variability of fatty acids profile than location, was reported previously [28]. The range and average (%) of the different types of fatty acids in kernel oils recovered from various sweet cherry cultivars were as follows: saturated (9.6–11.0, 10.2 %), monounsaturated (40.6–50.7, 46.6 %) and polyunsaturated (39.7–49.4, 43.2 %). The sum of unsaturated/saturated fatty acids (ΣUFA/ΣSFA) ratios in sweet cherry kernel oils were in the range of 8.1–9.4. The fatty acids ΣPUFA/(ΣSFA + ΣMUFA) ratios in sweet cherry kernel oils were in the range of 0.7–1.0. The ΣPUFA/(ΣSFA + ΣMUFA) ratios shows a majority of the ΣSFA + ΣMUFA were found in all the studied samples, with the exception of the oil sample ‘Krupnoplodnaya’, in which the amount of ΣPUFA and ΣSFA + ΣMUFA was equal (Table 2).
Four homologues of tocopherol (α, β, γ and δ) and two of tocotrienol (α and γ) were detected in all studied samples (Fig. 1, Table 3). γ-T was a predominant tocochromanol with the concentration between 73.7–97.4 mg/100 g oil in cvs. ‘Bryanskaya Rozovaya’ and ‘Krupnoplodnaya’, respectively, and consisted of 86–89 % of all determined tocochromanols in the analysed samples of oil. γ-T was reported to be a predominant homologue of tocopherols in other fruit seeds and kernels of plants belonging to the Rosaceae family, where the impact of the cultivar on the tocochromanols concentration was taken into consideration: plums (Prunus domestica L. and Prunus cerasifera Ehrh.) 78–89 % [29], apricots (Prunus armeniaca L.) 91–95 % [30] and pears (Pyrus communis L.) 84–88 % [31]. However, some exceptions were reported, for instance in the apple seeds (Malus domestica Borkh.) [22] and in Japanese quince seed oil (Chaenomeles japonica (Thunb.) Lindl. ex Spach) [32] where the γ-T consisted of a low percentage of the total detected tocochromanols (1–24 % and 2 %, respectively). Significantly lower concentrations in sweet cherry kernel oils were noted for α-T and δ-T (5.2–8.1 and 3.1–5.3 mg/100 g oil, respectively). Very low levels were noted for α-T3 (0.4–1.2 mg/100 g oil) and only minor amounts for β-T and γ-T3 (0.1–0.2 and 0.1–0.3 mg/100 g oil, respectively) (Table 3). The amount of total tocochromanols in sweet cherry kernel oils was in the range of 83.1–111.1 mg/100 g oil, comparable to levels reported for grape (Vitis vinifera L.) and watermelon (Citrullus lanatus (Thunb.) Matsum. & Nakai) seed oils (97.49 and 117.87 mg/100 g oil, respectively) [7]. To the best of our knowledge this is the first report of the tocopherols and tocotrienols composition in kernel oils of different sweet cherry cultivars. In the previous studies, tocochromanols were determined in sweet cherry kernels, but not in their extracted oils [33].
The content of carotenoids in sweet cherry kernel oils ranged from 0.36 to 0.62 mg/100 g oil (in cvs. ‘Iedzenu Dzeltenais’ and ‘Krupnoplodnaya’, respectively). A comparable range of carotenoids concentrations (0.10–1.58 mg/100 g oil) was reported previously in seed oils recovered from six apples cultivars [8].
Nine sterols (campesterol, β-sitosterol, Δ5-avenasterol, 24-methylene-cycloartanol, cholesterol, gramisterol, Δ7-stigmasterol, Δ7-avenasterol and citrostadienol) were identified in sweet cherry kernel oils (Table 4). The β-sitosterol was the predominant phytosterol in each studied cultivar (201.5–333.1 mg/100 g oil) and consisted of 75–86 % of the total detected sterols. A similar composition of sterols for sweet cherry kernel oil was reported previously, however the impact of the cultivar was not taken into consideration [25]. The percentage of β-sitosterol in oils recovered from different plant cultivars varied considerably, for instance in apple seed oils the levels ranged from 51 to 94 % [3]. For the other detected sterols, the concentrations were in the range of 0.0–32.5 mg/100 g oil (Table 4). The total amount of sterols ranged from 233.6–419.4 mg/100 g oil in cvs. ‘Tyutchevka’ and ‘Krupnoplodnaya’, respectively. A comparable range of total sterols was noted in seed oils recovered from six crab and five dessert apple cultivars (113–314 and 182–780 mg/100 g oil, respectively) [3].
The content of squalene in sweet cherry kernel oils ranged from 60.9 to 127.7 mg/100 g oil (in cvs. ‘Gårdebo’ and ‘Iedzenu Dzeltenais’, respectively). Squalene concentrations in sweet cherry kernel oils are significantly higher to those reported in dessert apple seed oils (9.0–34.0 mg/100 g oil) [3] and comparable to reported levels in pumpkin seeds (89.0 mg/100 g) [34] and pomegranate seed oil (82.8–144.9 mg/100 g) [35]. To the best of our knowledge this is the first report about the carotenoids and squalene concentration in sweet cherry kernel oils and therefore, direct comparison was not possible.
Three significant correlations between oil yield in seeds of different sweet cherry cultivars and the total content of sterols (r = −0.852, p ≤ 0.05), tocochromanols (r = −0.880, p ≤ 0.01) and carotenoids (r = −0.698, p ≤ 0.1) were found (Electronic supplementary material). Demonstrating that a higher oil yield in sweet cherry kernels results in a lower concentration of minor lipophilic bioactive compounds. These phenomena can provide valuable information as preliminary estimates of the total concentration of sterols, tocochromanols and carotenoids, based on known oil yields in sweet cherry kernels. A similar observation was reported previously in cases that focused on seed oils recovered from different cultivars of crab and dessert apples, recording two negative correlations between the oil yield and the content of β-sitosterol (r = −0.931) and total sterols (r = −0.901) [3]. Nevertheless, as was stated previously, a larger amount of different cultivars is required to confirm this phenomenon.
Conclusion
The oils recovered from sweet cherry cultivars are rich sources of lipophilic compounds, namely, tocopherols, carotenoids, phytosterols, and essential fatty acids. Our findings have demonstrated that the content of bioactive molecules in sweet cherry kernel oils is significantly affected by the cultivar, since the abiotic factors were the same for all studied varieties. Correlations between oil yield in sweet cherry kernels and the amount of minor lipophilic compounds were observed, cv. ‘Krupnoplodnaya’ had the highest total content of tocochromanols, carotenoids and sterols, and the lowest oil yield. Hence, in recovered oils from different sweet cherry cultivars, the oil content may be considered as a preliminary indicator of the concentration of lipophilic bioactive molecules. The impact of multiple non-varietal factors, which were not the subject of the present study, should be also taken into consideration, since the oil yield and its composition for the same cultivar grown even in the same location, year to year, can have a tendency to variability.
Abbreviations
- DW:
-
Dry weight basis
- T:
-
Tocopherol
- T3:
-
Tocotrienol
- RP-HPLC/FLD:
-
Reverse-phase high-performance liquid chromatograph/fluorescence detector
References
FAOSTAT (2014) FAO statistical database http://www.fao.org. Accessed 10 Dec 2014
Nyam KL, Tan CP, Lai OM, Long K, Che Man Y (2009) Physicochemical properties and bioactive compounds of selected seed oils. LWT-Food Sci Technol 42:1396–1403
Górnaś P, Rudzińska M, Segliņa D (2014) Lipophilic composition of eleven apple seed oils: a promising source of unconventional oil from industry by-products. Ind Crop Prod 60:86–91
Fromm M, Bayha S, Carle R, Kammerer DR (2012) Comparison of fatty acid profiles and contents of seed oils recovered from dessert and cider apples and further Rosaceous plants. Eur Food Res Technol 234:1033–1041
Górnaś P, Pugajeva I, Segliņa D (2014) Seeds recovered from by-products of selected fruit processing as a rich source of tocochromanols: RP-HPLC/FLD and RP-UPLC-ESI/MSn study. Eur Food Res Technol 239:519–524
Górnaś P, Segliņa D, Lācis G, Pugajeva I (2014) Dessert and crab apple seeds as a promising and rich source of all four homologues of tocopherol (α, β, γ and δ). LWT-Food Sci Technol 59:211–214
Górnaś P, Soliven A, Segliņa D (2015) Seed oils recovered from industrial fruit by-products are a rich source of tocopherols and tocotrienols: rapid separation of α/β/γ/δ homologues by RP-HPLC/FLD. Eur J Lipid Sci Technol 117:773–777
Fromm M, Bayha S, Kammerer DR, Carle R (2012) Identification and quantitation of carotenoids and tocopherols in seed oils recovered from different rosaceae species. J Agric Food Chem 60:10733–10742
Zhuo R-J, Wang F, Zhang X-H, Zhang JJ, Xu J, Dong W, Zou Z-Q (2014) α-Eleostearic acid inhibits growth and induces apoptosis in breast cancer cells via HER2/HER3 signaling. Mol Med Rep 9:993–998
Munné-Bosch S, Alegre L (2002) The function of tocopherols and tocotrienols in plants. Crit Rev Plant Sci 21:31–57
Dwiecki K, Górnaś P, Jackowiak H, Nogala-Kałucka M, Polewski K (2007) The effect of D-alpha-tocopherol on the solubilization of dipalmitoylphosphatidylcholine membrane by anionic detergent sodium dodecyl sulfate. J Food Lipids 14:50–61
Dwiecki K, Górnaś P, Wilk A, Nogala-Kałucka M, Polewski K (2007) Spectroscopic studies of D-α-tocopherol concentration-induced transformation in egg phosphatidylcholne vesicles. Cell Mol Biol Lett 12:51–69
Nogala-Kałucka M, Dwiecki K, Siger A, Górnaś P, Polewski K, Ciosek S (2013) Antioxidant synergism and antagonism between tocotrienols, quercetin and rutin in model system. Acta Aliment 42:360–370
Neunert G, Górnaś P, Dwiecki K, Siger A, Polewski K (2015) Synergistic and antagonistic effects between alpha-tocopherol and phenolic acids in liposome system: spectroscopic study. Eur Food Res Technol 241:749–757
Aggarwal BB, Sundaram C, Prasad S, Kannappan R (2010) Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem Pharmacol 80:1613–1631
Aust O, Sies H, Stahl W, Polidori MC (2001) Analysis of lipophilic antioxidants in human serum and tissues: tocopherols and carotenoids. J Chromatogr A 936:83–93
Antwi SO, Steck SE, Zhang H, Stumm L, Zhang J, Hurley TG, Hebert JR (2015) Plasma carotenoids and tocopherols in relation to prostate-specific antigen (PSA) levels among men with biochemical recurrence of prostate cancer. Cancer Epidemiol 39:752–762
Chen Z-Y, Jiao R, Ma KY (2008) Cholesterol-lowering nutraceuticals and functional foods. J Agric Food Chem 56:8761–8773
Warleta F, Campos M, Allouche Y, Sánchez-Quesada C, Ruiz-Mora J, Beltrán G, Gaforio JJ (2010) Squalene protects against oxidative DNA damage in MCF10A human mammary epithelial cells but not in MCF7 and MDA-MB-231 human breast cancer cells. Food Chem Toxicol 48:1092–1100
Górnaś P, Rudzińska M, Raczyk M, Mišina I, Soliven A, Lācis G, Seglina D (2016) Impact of species and variety on concentrations of minor lipophilic bioactive compounds in oils recovered from plum kernels. J Agric Food Chem 64:898–905. doi:10.1021/acs.jafc.5b05330
Górnaś P, Mišina I, Olšteine A, Krasnova I, Pugajeva I, Lācis G, Siger A, Michalak M, Soliven A, Segliņa D (2015) Phenolic compounds in different fruit parts of crab apple: dihydrochalcones as promising quality markers of industrial apple pomace by-products. Ind Crop Prod 74:607–612
Górnaś P (2015) Unique variability of tocopherol composition in various seed oils recovered from by-products of apple industry: rapid and simple determination of all four homologues (α, β, γ and δ) by RP-HPLC/FLD. Food Chem 172:129–134
Górnaś P, Siger A, Czubinski J, Dwiecki K, Segliņa D, Nogala-Kalucka M (2014) An alternative RP-HPLC method for the separation and determination of tocopherol and tocotrienol homologues as butter authenticity markers: a comparative study between two European countries. Eur J Lipid Sci Technol 116:895–903. doi:10.1002/ejlt.201300319
Farrohi F, Mehran M (1975) Oil characteristics of sweet and sour cherry kernels. J Am Oil Chem Soc 52:520–521
Bernardo-Gil G, Oneto C, Antunes P, Rodrigues MF, Empis JM (2001) Extraction of lipids from cherry seed oil using supercritical carbon dioxide. Eur Food Res Technol 212:170–174
Jellum MD, Marion JE (1966) Factors affecting oil content and oil composition of corn (Zea mays L.) grain. Crop Sci 6:41–42
Górnaś P, Rudzińska M (2016) Seeds recovered from industry by-products of nine fruit species with a high potential utility as a source of unconventional oil for biodiesel and cosmetic and pharmaceutical sectors. Ind Crop Prod 83:329–338. doi:10.1016/j.indcrop.2016.01.021
Vidrih R, Hribar J, Sekse L (2014) Cherry seeds as a source of nutritionally important fatty acids. Acta Hortic 1020:165–172. doi:10.17660/actahortic.2014.1020.23
Górnaś P, Mišina I, Grāvīte I, Lācis G, Radenkovs V, Olšteine A, Segliņa D, Kaufmane E, Rubauskis E (2015) Composition of tocochromanols in the kernels recovered from plum pits: the impact of the varieties and species on the potential utility value for industrial application. Eur Food Res Technol 241:513–520
Górnaś P, Mišina I, Grāvīte I, Soliven A, Kaufmane E, Segliņa D (2015) Tocochromanols composition in kernels recovered from different apricot varieties: RP-HPLC/FLD and RP-UPLC-ESI/MSn study. Nat Prod Res 29:1222–1227
Górnaś P, Mišina I, Lāce B, Lācis G, Segliņa D (2015) Tocochromanols composition in seeds recovered from different pear cultivars: RP-HPLC/FLD and RP-UPLC-ESI/MSn study. LWT-Food Sci Technol 62:104–107
Górnaś P, Siger A, Juhņeviča K, Lācis G, Šnē E, Segliņa D (2014) Cold-pressed Japanese quince (Chaenomeles japonica (Thunb.) Lindl. ex Spach) seed oil as a rich source of α-tocopherol, carotenoids and phenolics: a comparison of the composition and antioxidant activity with nine other plant oils. Eur J Lipid Sci Technol 116:563–570
Górnaś P, Mišina I, Ruisa S, Rubauskis E, Lācis G, Segliņa D (2015) Composition of tocochromanols in kernels recovered from different sweet cherry (Prunus avium L.) cultivars: RP-HPLC/FLD and RP-UPLC-ESI/MSn study. Eur Food Res Technol 240:663–667
Ryan E, Galvin K, O’Connor TP, Maguire AR, O’Brien NM (2007) Phytosterol, squalene, tocopherol content and fatty acid profile of selected seeds, grains, and legumes. Plant Foods Hum Nutr 62:85–91
Caligiani A, Bonzanini F, Palla G, Cirlini M, Bruni R (2010) Characterization of a potential nutraceutical ingredient: pomegranate (Punica granatum L.) seed oil unsaponifiable fraction. Plant Foods Hum Nutr 65:277–283
Acknowledgments
I would like to kindly acknowledge Dr. Arianne Soliven for her assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Electronic supplementary material
ESM 1
(DOCX 30 kb)
Rights and permissions
About this article
Cite this article
Górnaś, P., Rudzińska, M., Raczyk, M. et al. Impact of Cultivar on Profile and Concentration of Lipophilic Bioactive Compounds in Kernel Oils Recovered from Sweet Cherry (Prunus avium L.) by-Products. Plant Foods Hum Nutr 71, 158–164 (2016). https://doi.org/10.1007/s11130-016-0538-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-016-0538-5