Abstract
Shalgam juice is a dark red-colored and sour fermented beverage produced and consumed in Turkey. The main ingredient of shalgam juice is black carrot, which is rich in anthocyanins. In this study, commercially available shalgam juice was characterized by determining its chemical composition and antioxidant capacity and by identifying its microflora. The predominant acid in the shalgam juice was lactic acid. LC/MS/MS analysis revealed the presence of the anthocyanins cyanidin-3-galactoside, cyanidin-3-glucoside, and cyanidin-3-arabinoside. The total phenolic content (517.21 µg GAE/mL) and antioxidant capacity (in µmol Trolox equivalents/mL) determined by ABTS (3.42), DPPH (4.44) and FRAP (2.26) assays of the commercial shalgam juice were similar to other common fruit juices. A total of 21 Lactobacillus species and subspecies were identified in shalgam juice using species-specific PCR with the nucleotide sequences of some of the identified lactic acid bacteria. Shalgam juice inhibited the growth of Caco-2 cells lines in a dose-dependent manner and had significantly higher inhibition at 3200 µg/mL compared to black carrot (p < 0.05).These results suggest that in addition to the actions of it (poly)phenolic constituents, shalgam juice might have a potent as antioxidant, probiotic and antiproliferative agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
More than 3500 fermented foods and beverages are produced around the world using a variety of food ingredients, manufacturing techniques and microorganisms [1, 2]. Dairy fermented products are common and widely available in the commercial marketplace [3]. However, consumer demand for non-dairy fermented foods is increasing in the western world in parallel with the increase in health problems like lactose-intolerance or high cholesterol [1]. Recently, fermented fruit and vegetable products are preferred due to their bioactivity beyond their healthful profile of nutrients and their desirable taste and flavor [4]. Non-dairy lactic acid fermented beverages are produced mostly in a traditional manner, especially in small-scale industries or in homes and villages in African, Asian and Middle Eastern countries [2]. The production of lactic acid fermented beverages from fruits such as pomegranates, cranberries, pineapples, oranges, and tomatoes and vegetables, such as beets, cabbages, and carrots, is based on the use of bacteria from their natural microflora [5, 6].
Shalgam juice, a dark red, cloudy, and sour fermented beverage, has been a popular drink in the west and southeast regions of Turkey, where it is mostly produced and consumed [7, 8]. Shalgam juice is defined by Turkish Standards Institution (TS) in TS 11149 standard as “The product produced by lactic acid fermentation of turnip (Brassica rapa), black carrot (Daucus carota), chili powder, and extract obtained from the lactic acid fermentation of bulgur flour, sourdough, drinking water, and salt, which can be heat treated for preservation, if desired” [9]. However, black carrot, bulgur flour, dough, salt, and water are the main ingredients of shalgam juice and turnip is a minor ingredient, used only when available, though it can provide specific sensory characteristics. The dark color of the beverage is derived from the black carrot anthocyanins, and its sour taste is attributed to lactic acid produced during fermentation [10]. Black carrot anthocyanins and lactic acid bacteria in shalgam juice are well known for their presumed health benefits such as reduction in the risk of cardiovascular disease and cancer [5, 11].
There is no regulatory or other standard method for commercial or home-scale production of shalgam juice. The fermentation is spontaneous, but addition of a starter culture for fermentation is also used in controlled laboratory-scale production but not preferred in commercial production since shalgam juice fermentation cultures are not commercially available. However, the addition of 15 % (w/w) shalgam juice from a previous production is a common practice. In shalgam juice production, two main production methods are used, “traditional production” and “direct production” [7]. Traditional production consists of two stages, consisting of the initial fermentation of the dough followed by the fermentation of the carrots. Direct production consists of single stage fermentation by mixing all ingredients. Although both methods are used in commercial, small-scale, and laboratory-scale production, most commercially available products are produced with the traditional production technique [10, 12, 13].
There are many traditional beliefs about the therapeutic properties of shalgam juice. Although chemical, microbiological, sensory properties and production techniques of shalgam juice were widely studied [7, 13, 14], the potential health effects of shalgam juice as a whole product are not widely known. To understand the possible beneficial effects and bioactive properties of commercially available shalgam juice, its antioxidant capacity, the spectrum of microorganisms associated with the natural fermentation of black carrot, in relation to the chemical composition, is still needed to be studied. Furthermore, although antiproliferative activity of plants associated anthocyanin composition was studied against cancer cells, the antiproliferative activity of shalgam juice as a whole beverage against cancer cells may power to understand the health benefits. The aim of this study was to characterize the chemical and microbiological properties and antiproliferative effects of a commercially available shalgam juice on colorectal carcinoma (Caco-2) cell lines. The specific aims of this work were to investigate antioxidant and antiproliferative capacities and bacterial composition of shalgam juice in vitro as a basis for elucidating the mechanism(s) underlying its functional properties.
Materials and methods
Samples
Shalgam juice and black carrot were kindly provided from a single Turkish producer (major contributor with 99 % of the Turkish market) (Adana, Turkey). Different batches were collected at different periods over the course of three years. The juice of freshly squeezed black carrot was used as a control for the comparison of the antioxidant analysis.
Chemicals
The standards used in organic acid (l-(+)-lactic, acetic, citric, oxalic, succinic, l-ascorbic, propionic, malic and butyric acids) and sugar (d-(+)-glucose, d-(-)-fructose, d-(+)-sucrose and D(-)-arabinose) analyses were purchased from Supelco, USA. HPLC-grade methanol, acetonitrile, ethyl acetate and formic acid were from Fisher Scientific, USA. Potassium sulfate and phosphoric acid were purchased from Merck, Germany and Sigma Aldrich, Germany, respectively. LC MS/MS standards were from Extrasynthese, France. The chemicals and reagents for antioxidant and total phenolic analyses (Trolox, gallic acid, ABTS (2,2-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)), DPPH (2,2-Diphenyl-1-picrylhydrazyl), TPTZ (2,4,6-tris(2-pyridyl)-s-triazine and Folin–Ciocalteu were acquired from Sigma Aldrich Co., Germany, unless otherwise indicated. The growth media for microbial count experiments [Man Rogosa Sharp (MRS) agar, potato dextrose agar (PDA), and nutrient agar (NA)] were purchased from Conda, Spain. Gram staining kit and glycerol used for cell storage were bought from Salubris, USA and Sigma, UK, respectively. Purelink Genomic DNA Mini Kit and reagents used for PCR experiments (10× Tag reaction buffer, MgCl2, dNTPs, Taq DNA polymerase) were from Fermentas and Invitrogen, USA. Phosphate-buffered saline (PBS) and reagents for cell culture experiments (Dulbecco’s Modified Eagle Medium (DMEM); with 2 mM l-glutamine, fetal bovine serum (FBS), penicillin and streptomycin) were purchased from Gibco, USA unless otherwise indicated.
Determination of chemical composition
Proximate analysis
Chemical composition of shalgam juice was determined according to the standards of Turkish Standards Institute (TS) and compared with the TS 11149, standard for shalgam juice [9]. Carbohydrate content (g/L) was determined using phenol–sulfuric acid method of DuBois et al. [15] with glucose as the standard. Soluble solids were determined with the refractometric method using a Bellingham Stanley, sucrose indexed refractometer (Xylem, USA) according to the standard method of TS 4890 and AOAC [9, 16]. The results were expressed as % (w/v). The percent salt (w/v) and ash content (w/v) were measured with the Mohr titration method according to TS 2664 and TS EN 1135, respectively [9]. The protein content (g/L) was determined by the Kjeldahl method using an automated system (Buchi, Switzerland) according to the official AOAC method [16] for fruit and fruit juices with some modifications. During the digestion, potassium sulfate (K2SO4) was used instead of HgO or Hg. Total acidity was determined with titration according to the TS EN 12147 [9] and expressed as lactic acid [16], and the pH was measured with a pH meter (Radiometer Analytical, France) according to TS 1728 ISO 1842 [9].
Organic acids and sugars
Organic acids in the shalgam juice were quantified according to McFeeters et al. [17] with slight modifications. Briefly, shalgam juice samples were centrifuged (Hettich, Germany) for 5 min at 10,000×g, filtered through a 20 µm filter (Minisart Sartorius, Germany) and diluted with MilliQ water (1:4). Organic acids in the diluted juice were then quantified using an HPLC system (Thermo Scientific, England) equipped with a Photodiode Array (PDA) detector (Thermo Scientific, England) set at 210 nm and a Hypersil GOLD C18 column (5 µm, 250 × 4.6 mm; Thermo Scientific, England). Organic acids were eluted from the column using 0.013 mol/L phosphoric acid (pH 2.3) at 1 mL/min flow rate. Concentration of l-(+)-lactic, acetic, citric, oxalic, succinic, l-ascorbic, propionic, malic and butyric acids in the shalgam juice was calculated based on the standard curves constructed using authenticated standards.
Sugars were determined using an HPLC system (Thermo Scientific, England) equipped with a Refractive Index Detector (Thermo Scientific, England) and a HyperREZ XP Carbohydrate Ca++ Column (8 µm, 300 × 7.7 mm; Thermo Scientific, England). Sugars were eluted with the mobile phase of 0.005 mol/L H2SO4 at 0.6 mL/min flow rate [18]. Column and detector temperatures were maintained at 50 and 35 °C, respectively. Identification and quantification of sugars in the shalgam juice were accomplished based on the calibration curves of authentic standards, d-(+)-glucose, d-(−)-fructose, d-(+)-sucrose and d-(−)-arabinose.
Anthocyanins
Anthocyanins in the shalgam juice were determined both with HPLC–PDA and LC–MS/MS. The HPLC system was equipped with a PDA detector (Thermo Scientific, England) set at 520 nm and a Hypersil GOLD C18 column (5 µm, 250 × 4.6 mm; Thermo Scientific, England). Mobile Phase A was acetonitrile, and mobile phase B was 4 % phosphoric acid in MilliQ water [19]. The flow rate was set at 1.0 mL/min with the gradient condition as followed: 0 % A and %100 B at 0 min, 20 % A and 80 % B at 20 min, 40 % A and 60 % B at 25 min, and 0 % A and 100 % at 30 min. Since authentic standard for anthocyanins is not commercially available, in HPLC analysis, the peaks were identified using the information in literature [19–21].
In the LC–MS/MS analysis, anthocyanins in 250 µL shalgam juice were extracted using a 500-mg C18 Sep-Pak Vac 10-mL mini-cartridge (Waters Corporation, USA), according to Brenes et al. [22]. The juice was applied to the cartridge, which was activated by sequential elution of 10 mL each of ethyl acetate, methanol, and water. After the cartridge was washed with 4 mL of 0.01 N HCl and 20 mL ethyl acetate, anthocyanins were eluted with 4 mL methanol containing 0.1 % formic acid, dried under N2 air, and reconstituted with 300 µL of 5 % acetonitrile in 0.1 % aqueous formic acid for LC–MS/MS analysis. Six anthocyanins, including cyanidin-3-galactoside, cyanidin-3-glucoside, cyanidin-3-arabinoside, peonidin-3-galactoside, peonidin-3-glucoside, peonidin-3-arabinoside, were quantified using an Agilent 1100 Series HPLC system (USA) and an API 3000 Triple Quadrupole mass spectrometer (Applied Biosystems, USA) operating in positive electrospray ionization (ESI) and SIM mode, according to Milbury et al. [23]. The separation of anthocyanins was achieved using a linear gradient consisting of mobile phase A (water/formic acid, 99.9:0.1, v/v) and B (acetonitrile/formic acid, 99.9:0.1, v/v) at a flow rate of 300 µL/min. The gradient was set as follows: 0–20 min, 10–100 % B; 20–25 min, 100 % B; 25–26 min, 100–10 % B, and 26–30 min, 10 % B. Authentic standards that were available were administered for the quantification of anthocyanins in shalgam juice.
Determination of antioxidant activities
Sample and standard preparation
Shalgam juice was centrifuged for 5 min at 10,000×g, filtered (Minisart Sartorius, Germany), and diluted using 80 % methanol (1/10–1/200). Dilutions of Trolox standard (0.005–0.1 mg/mL) were prepared using 80 % methanol from a stock solution (1 mg/mL). Shalgam juice samples and Trolox standard were prepared daily and all analyses were run in triplicate.
ABTS radical scavenging activity
ABTS (2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)) radical scavenging assay was performed according to the method of Re et al. [24]. ABTS radical cation stock solution was prepared and kept for 12–16 h in the dark at room temperature before use. The absorbance of the working ABTS radical cation solution was adjusted to 0.70 ± 0.05 by diluting the stock solution with 80 % methanol. 50 μL of Trolox standard or diluted juice sample (1/10–1/200) were mixed with 250 μL of the working ABTS solution in a 96-well plate, and the absorbance was monitored in a microplate reader (MultiSkan Go, Thermo Scientific). Slopes of the linear portions of the absorbance versus concentration graphs of the sample and Trolox were determined. The ABTS scavenging activity was calculated by dividing the slope of the sample to that of the Trolox standard (y = 16.695 + 0.0051, R 2 = 0.995) and expressed as µmol/mL Trolox equivalents (TE).
DPPH radical scavenging activity
2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay was carried out according to Fukumoto and Mazza [25]. Diluted juice samples (1/10–1/200) or Trolox standard (22 µL) was mixed with 200 µL of 150 µmol/L DPPH solution in 80 % methanol. The absorbance at 515 nm was read in a microplate reader after 30-, 180-, and 300-min incubation at room temperature in the dark. The DPPH scavenging activity was calculated by dividing the slope of the linear portion of the absorbance versus concentration graph of the sample to that of Trolox standard (y = 5.0742–0.0006, R 2 = 0.997) and expressed as µmol/mL TE. The time interval giving the highest slope was used for the calculations (30 min for Trolox standard and 300 min for juice samples). Color interference of anthocyanin containing samples might occur during DPPH assay due to the absorbance of the colored samples at 515 nm resulting in underestimation of the antioxidant activity. In this study, color interference did not appear to be a factor in the sample dilution range used as a linear decrease in absorbance values was observed with increasing concentration upon sample addition.
Ferric reducing antioxidant power (FRAP)
FRAP was measured according to Güçlü Üstündağ and Mazza [26]. FRAP reagent was prepared daily by mixing 25 mL of 300 mmol/L acetate buffer at pH 3.6, 2.5 mL of 20 mmol/L FeCl3 and 2.5 mL of 10 mmol/L TPTZ (2,4,6-tris(2-pyridyl)-s-triazine in 40 mmol/L HCl. Trolox standard or diluted juice sample (1/10–1/200, 50 μL) were mixed with the FRAP reagent (250 μL) in a 96-well plate, and the absorbance at 593 nm was read after 4 min using a microplate reader. The FRAP value was determined by dividing the slope of the linear portion of the absorbance versus concentration graph of the sample to that of Trolox standard (y = 17.006x − 0.0049, R 2 = 0.996) and expressed as µmol/mL TE.
Total phenolic content
Total phenolic content was determined according to the method of Ainsworth and Gillespie [27]. After mixing 200 µL of 10 % Folin–Ciocalteu reagent with 100 µL diluted juice sample or gallic acid standard, 800 µL of 700 mmol/L sodium carbonate anhydrous was added. After incubation at room temperature for 2 h, the absorbance at 765 nm was read in a microplate reader. Total phenolic content was quantified using a gallic acid calibration curve (0.015–0.045 mg/mL in water, y = 3.5725x − 0.0376, R 2 = 0.995) and expressed as µg/mL gallic acid equivalents (GAE).
Determination of microbiological composition of shalgam juice
Enumeration of microorganisms in shalgam juice
The microbial load in shalgam juice was determined using classical culturing techniques. Lactobacillus species were cultivated on MRS agar and incubated at 37 °C for 48 h in a 5 % CO2 incubator (New Brunswick, UK). Yeast and molds were grown on PDA for 48 h at 25 °C. Total mesophilic aerobic bacteria (TMAB) count was determined by spreading on NA and incubated for 24 h at 37 °C. Viable counts were performed and expressed in Log10 CFU/mL.
The identity of the strains was confirmed by Gram staining and with colony morphologies. Isolated colonies were cultured and stored in appropriate growth medium containing 30 % glycerol at −80 °C.
Identification of lactic acid bacteria (LAB) in shalgam juice
Bacterial DNA isolation
DNA of the microorganisms in shalgam juice was isolated either directly from shalgam juice or from selected colonies from MRS after enumeration. For direct DNA isolation from shalgam juice, it was centrifuged for 5 min at 20,000×g (Sigma, Germany) and the pellet was suspended in PBS. For DNA isolation from colonies, the selected colonies were suspended in PBS and then centrifuged (Hettich, Germany) for 1 min at 20,000×g. The resulting cell pellets were obtained for DNA isolation using Purelink Genomic DNA Mini Kit according to manufacturer’s instructions with some modifications. Additional lysozyme enzyme (20 mg/mL) was used to disrupt the cell walls of Gram-positive bacteria. Tubes were incubated for 30 min in a 37 °C water bath before the kit protocol was applied. The isolated DNA was quantified at 260 nm using a spectrophotometer (Implen Nanophotometer, USA) and confirmed using 1 % agarose gel electrophoresis (Sigma, UK).
Species-specific PCR
Bacterial DNA samples were amplified with species-specific primers (Invitrogen, USA) based on the 16S–23S regions of lactic acid bacteria found in dough and vegetable products (Table 1). The PCR mix contained 10 mmol/L 10× Tag Reaction Buffer, 1.5 mmol/L MgCl2, 200 µmol/L of each dNTP, 1.75 U Taq DNA Polymerase, 10 pmol of each primer, and 1 µL of isolated DNA. Various PCR amplification protocols (Table 1) were used according to primers’ annealing temperatures in the PCR thermal cycler (Bio-Rad My Cycler, USA). PCR products were quantified on a 2 % agarose gel with 100–1000 bp DNA ladder (Fermentas, USA).
Sequencing analysis
Amplified fragments were diluted such that the bands can be seen clearly (30 µL) and sequenced with the primers (5 pmol) used in amplification (Refgen Company, Ankara, Turkey). Sequence results were analyzed using Sequence Scanner Software v1.0 (Applied Biosystems), and each base sequence was compared to the rDNA sequence archived in the nucleotide sequence database of National Centre for Biotechnology Information (NCBI) using the basic local alignment search tool (BLAST) search program.
Measurement of inhibition of Caco-2 cell proliferation
In vitro antiproliferative effect of shalgam juice and black carrot juice (freshly peeled and squeezed) was defined with colorectal carcinoma (Caco-2) cells using MTS assay (CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay, Promega, USA). The assay is composed of a tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS] and an electron coupling reagent (phenazine methosulfate; PMS). Caco-2 cells (Caco-2/An1) were obtained from cell culture collection of Sap Institute (HÜKÜK, Şap Enstitüsü, Turkey) and were grown in DMEM; with 2 mM l-glutamine supplemented with 10 % (v/v) FBS; 100 units/mL penicillin, 100 μg/mL streptomycin at 37 °C in 5 % CO2 incubator (New Brunswick, UK). Shalgam and black carrot juices were freeze-dried and suspended in PBS at a concentration of 6400 µg/mL. Nine dilutions were prepared from each suspension with growth media, and 50 μL of dilutions were added to the wells of a 96-well plate. The plate was equilibrated at 37 °C with 5 % CO2 humidified atmosphere while harvesting the cells for assay. Cells were suspended to a final concentration of 1 × 105 cells/mL in growth media. 50 µL of cell suspension was dispensed into all wells containing juice suspensions, and plate was incubated for 48 h at 37 °C with 5 % CO2 humidified atmosphere. After incubation, 20 μL/well MTS/PMS solution was added to wells and plate was incubated for another 3 h. The absorbance at 490 nm was measured using ELISA plate reader (Bio-Tek, USA) to determine the amount of soluble formazan produced by cellular reduction of the MTS. Inhibition of cell proliferation was calculated from the absorbance of cells treated with juice samples, control cells incubated with only growth medium without samples and blank containing only growth medium with juice suspension but not cells using the following equation:
As: sample absorbance with cells, Ab: sample absorbance without cells, Ac: control absorbance with cells, Abc: control absorbance without cells.
Statistical analysis
One-way analysis of variance (ANOVA) was performed to assess statistical significance, followed by post hoc Tukey’s HSD test. p value <0.05 was considered significant. For cell proliferation, comparison was performed by two independent test, Student’s t test for difference between shalgam and black carrot and one-way ANOVA followed by Dunnett’s test for the comparison of that of untreated cell (control) group. Minitab version 16 statistical software was used in all statistical analyses.
Results and discussion
Chemical composition of shalgam juice
The chemical composition of the commercially available shalgam juice was within the standard range [9] for total carbohydrate (0.29 g/L), soluble solid (3.4 %, w/v), salt (2.0 % w/v), ash content (1.86 %, w/v), pH 3.43, and total acidity (6.38 g/L as lactic acid) (Table 2). Soluble solids in the shalgam juice (carbohydrates, organic acids, protein, minerals and salt) were mainly derived from constituents in black carrot and bulgur dough, which were subject to the influence of quantity of black carrot (10–20 %) and the type of starter culture. Salt in shalgam juice was derived from its addition during the production to control the growth of microflora other than lactic acid bacteria (LAB), which are salt tolerant and to prevent spoilage. The ash content of shalgam juice was affected by minerals present in the water, dough and black carrots used in production [13, 28].
The proteins in shalgam juice originate from the soluble proteins of bulgur flour and black carrot. The protein content in the shalgam juice was at least 53 % larger than reported previously for commercially and laboratory-scale produced shalgam juices (0.88–1.83 g/L) [13]. This difference may be attributed to the cultivation method of the black carrots.
Lactic acid is the predominant acid in shalgam juice so that the total acidity is expressed as lactic acid. The total acidity found in this study is consistent with other reports [13, 14, 28]. Similar to shalgam juice, in lactic acid fermented vegetable juices such as from carrots and cabbage, the total acidity ranged from 4.38–7.40 g/L, which was dependent by fermentation time [29, 30].
Determination and quantification of organic acids and sugars
The pH value is associated with the production of organic acids during the fermentation of vegetables and influences organoleptic properties (flavor, aroma, color) of the final products. We found lactic acid to be the predominant acid with its content as least 5.9-fold larger than acetic, citric, and succinic acids (Table 2). The content of the 3 minor organic acids was comparable to other commercial products, while lactic acid was 23–45 % larger [7, 13, 19]. Both l(+) and d(−)-lactic acid are present in shalgam juice with the l(+) form being predominant. Malic, propionic, and butyric acids were not found in shalgam juice.
Fructose was the primary sugar in the tested shalgam juice with its concentration being at least 39 % larger than sucrose and glucose (Table 2). Arabinose was not detected. The content of fructose and sucrose in the tested shalgam juice was within the range reported in the literature (fructose 0.006–4.0 g/L, sucrose 0.01–1.14 g/L, glucose 0.09–1.902 g/L) [13]. Black carrots are rich in sucrose (4.11 g/100 g) and contain glucose (0.69 g/100 g), and fructose (0.58 g/100 g) in smaller quantities [31]. Because these sugars are utilized by LAB during fermentation, they are present in lower amounts in shalgam juice [13]. In contrast to our study, Tanguler and Erten [13] found arabinose (0.134–0.193 g/L) in shalgam juice.
Determination of anthocyanins using HPLC and LC/MS analyses
The HPLC analysis tentatively identified anthocyanins, i.e., cyanidin-3-xylosylglucosylgalactoside (cya 3-xylglcgal), cyanidin-3-xylosylgalactoside (cya 3-xylgal), and cyanidin-3-xylosylglucosylgalactoside acylated with sinapic acid, ferulic acid or coumaric acid, in shalgam juice based on their elution and 520-nm absorbance (Fig. 1a), which were consistent with those of Turker et al. [19, 20], Khandare et al. [32] and Kammerer et al. [21]. Even though the authenticated standard for these 5 anthocyanins is not commercially available, the presence in shalgam juice remains to be confirmed using LC–MS/MS in scan mode. Based on the percentage of the area under curve (AUC) of individual peak to the sum of the five AUCs, we noted that cyanidin-3-xylosylglucosylgalactoside acylated with ferulic acid was the predominate anthocyanin, followed by cyanidin-3-xylosylgalactoside. The AUC percentage of cyanidin-3-xylosylglucosylgalactoside acylated with ferulic acid was two third of all acylated anthocyanins, a value comparable to those reported in shalgam juice (73 %) [20] and in black carrot (55-99 %) [21]. Acylated anthocyanins in shalgam juice are more stable to heat, pH and other environmental factors because their ability to prevent nucleophilic attack from water [19–21] makes them more resistant to degradation compared to non-acylated ones.
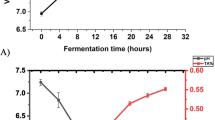
a Anthocyanins in shalgam juice were profiled using HPLC–PDA: (1) cyanidin-3-xylosyl-glucosyl-galactoside, (2) cyanidin-3-xylosyl-galactoside, (3) cyanidin-3-xylosyl-glucosyl-galactoside acylated with sinapic acid, (4) cyanidin-3-xylosyl-glucosyl-galactoside acylated with ferulic acid and (5) cyanidin-3-xylosyl-glucosyl-galactoside acylated with coumaric acid. Values are mean of three individual experiments and given as the area %. b Anthocyanin content in shalgam juice quantified using LC–MS/MS. Values are mean ± SD of three individual experiments
Six anthocyanins including cyanidin-3-galactoside, cyanidin-3-glucoside, and cyanidin-3-arabinoside, and peonidin-3-galactoside, peonidin-3-glucoside, and peonidin-3-arabinoside in the shalgam juice were quantified using LC–MS/MS. Of the 6 anthocyanins, only cyanidin-3-galactoside, cyanidin-3-glucoside, and cyanidin-3-arabinoside were detectable with their concentration at 233, 0.21, and 0.039 ng/mL, respectively (Fig. 1b). However, the 5 anthocyanins detected in the HPLC assay were not found because the LC–MS/MS was set in the multiple reaction monitoring mode for the quantification of 6 anthocyanins. These cyanidin-based anthocyanins are commonly found in blackberries, elderberries, black carrots, sweet potatoes, and red cabbages [33]. Cyanidin can be glycosylated with glucose, xylose, galactose, and sophorose or be acylated. Cyanidin-3-galactose is presented in cranberry juice with concentrations ≥10-fold larger than in the shalgam juice [23].
The literature shows that total anthocyanin content in commercial and laboratory-scale produced shalgam juice ranged from 67.5 to 168.2 mg/L as cyanidin-3-glucoside equivalents [19, 20]. This wide range might be attributed to the complex interplay between microflora and (poly)phenols during the fermentation [34]. Bacterial enzymes are capable of metabolizing (poly)phenols to phenolic acids and other smaller molecules. For example, Barthelmebs et al. [35] suggested that decarboxylase enzymes synthesized by Lb. plantarum species changed the structure of phenolic acids. With their microbial modulating properties, phenolics including anthocyanins can influence fermentation by mediating viability and growth of LAB and other microorganisms [34]. Thus, this interplay warrants future examination to help inform production of a more bioactive-rich shalgam juice.
Determination of total phenolic content and antioxidant activities
Total phenolic content of the shalgam juice was ≥71 % lower than black carrot juice (p < 0.05) (Table 3). However, our shalgam juice contained a similar TPC to apple (339 µg GAE/mL) and orange juices (755 µg GAE/mL) [36].
We employed 3 assays to assess total antioxidant capacity (TAC) of the shalgam juice. Similar to the lower TPC, the shalgam juice displayed ≥68 % lower than fresh black carrot juice (Table 3). Interestingly, the magnitude of the difference between the 2 juices in 3 assays was ~70 % even though the FRAP assay determines the reducing power of antioxidants and the other two assays measure the radical scavenging activity. The ABTS scavenging activity of the shalgam juice was similar to apple (3.6 µmol TE/mL) and orange juices (4.2 µmol TE/mL) [37]; the DPPH scavenging activity to plum juice (4.6 µmol TE/mL) [38]; and the FRAP value close to commercially available apple, apricot, orange, and peach juices ranging from 2.0 to 2.9 µmol TE/mL [39]. Black carrot juice had a comparable ABTS value to cranberry juice (10.4 µmol TE/mL) [37], DPPH to cherry (10.0 µmol TE/mL) and raspberry (13.4 µmol TE/mL) [38], and FRAP to sour cherry and strawberry juices (4.6 and 4.1 µmol TE/mL, respectively) [39].
Total antioxidant capacity of commercial shalgam juice and fresh black carrot juice has not been reported, and a few studies have reported TAC of black carrot [40–42]. Kaur and Kapoor [40] observed that assessed using a β-carotene oxidation assay, TAC of ethanol and water extracts of black carrot was 73.0 and 61.8 %, respectively. Sun et al. [42] found that water-based extract of freeze-dried black carrot exhibited the largest ABTS and DPPH scavenging activities as compared to lipophilic extract of colored varieties of the carrots. Importantly, the TAC of foods is subject to the influence of processing, e.g., enzyme treatment and drying method [32, 41]. For example, TPC and FRAP value of heated and pectinase-treated black carrot juices were almost 1- and 2-fold larger than the values found in our study. Further, such comparisons do not take into account factors such as cultivar, cultivation, and growing season and location.
Determination of microbiological composition of shalgam juice
Enumeration of microorganisms in shalgam juice
The microflora of shalgam juice is composed principally of LAB from beginning to the end of fermentation [7]. We found that the population of LAB, specifically Lactobacillus spp. was 4.09 Log CFU/mL (Table 4). Similar to previous reports where TMAB counts of shalgam juice ranged between 3.25 and 8.57 Log CFU/mL [13], in our study, total aerobic microorganisms (TMAB) count was 2.89 Log CFU/mL in shalgam juice. Yeasts and molds in shalgam juice originate from the raw materials and the environment. Total yeast and mold counts of shalgam juice found in our study were similar to the previously reported values [8, 14].
Identification of LAB in shalgam juice
Microflora profile has a great impact on flavor, aroma, and texture attributes of shalgam juice. The LAB in shalgam juice are mostly derived from bulgur dough and other ingredients as well as the tanks used in production [7]. We characterized LAB in shalgam juice using species-specific PCR and confirmed LAB species by comparing the amplified target DNAs with published sequences in the NCBI nucleotide sequence database using the BLAST search program (Table 5). We identified a total of 21 Lactobacillus species and subspecies in the shalgam juice. Of these 21, Lb. plantarum subsp. plantarum, L. casei, Lb. brevis and Lb. helveticus were detected in the isolated colonies in the shalgam juice, indicating that these species were resistant to the acidic condition created during the fermentation, and the rest of the species were identified directly from the shalgam juice. The species-specific PCR primers based on the 16S–23S rRNA intergenic spacer regions were particularly valuable in the bacteria identification in the shalgam isolates and permitted discrimination among subspecies of Lb. plantarum, Lb. casei, Lb. brevis, and Lb. delbrueckii.
Lb. plantarum group including Lb. plantarum JDM1, Lb. plantarum subsp. plantarum, Lb. plantarum subsp. argentoratensis, Lb. casei group including Lb. casei, Lb. casei subsp. casei, Lb. paracasei subsp. paracasei, Lb. paracasei subsp. tolerans and two different strains of Lb. brevis were identified both directly from shalgam juice and in the isolated colonies. Lb. plantarum isolated in this study displayed the homology of Lb. plantarum isolated in kimchi and vegetables [43], and Lb. brevis and Lb. casei had the homology of fermented plant products [44]. Lb. plantarum and Lb. brevis have been reported to be the predominant LAB at the beginning, middle, and end of shalgam juice produced at the laboratory scale [7, 14]. In contrast, Tanguler et al. [7] did not detect Lb. casei and Lb. brevis in commercially available shalgam juice. Lb. plantarum and Lb. casei species have been used as starter cultures in production of fermented vegetable juices, such as beet, cabbage, carrot and tomato, and of dairy products. They are also included in probiotic products due to their potential health benefits [5]. Lb. sharpeae, identified in the shalgam juice, is also a subgroup of Lb. casei and can be found in meat products. However, its properties remain to be characterized [45]. Lb. parabrevis, identified in the shalgam juice, is a newly proposed taxon of Lb. brevis and originated mostly from sourdough fermentation, suggesting that Lb. brevis in the shalgam juice is derived from the dough [46]. Importantly, this strain may induce spoilage in vegetable products [44]. Lb. helveticus, identified in the isolated colonies of shalgam juice, had homology of two different strains of Lb. helveticus in cheese [47]. Lb. helveticus is closely related to L. acidophilus group. This species, an obligate homofermentative LAB species, is mostly found in dairy products and dough [48] and displays immunomodulatory and antimutagenic properties due to peptides released from milk proteins during fermentation [49].
Lb. delbrueckii group including Lb. delbrueckii subsp. lactis, Lb. delbrueckii subsp. delbrueckii, Lb. delbrueckii subsp. indicus, Lb. gasseri and Lb. acidophilus were identified directly in the shalgam juice but not in the isolated colonies, indicating that they were present in the beginning of the fermentation and disappeared in later steps, probably due to the reduction in pH [50]. Similarly, Tanguler and Erten [14] found the presence of Lb. delbrueckii subsp. delbrueckii in the early fermentation of commercially available shalgam juices. Lb. acidophilus and Lb. gasseri found in the shalgam juice had been isolated from probiotic products, e.g., yogurt, milk powder, and fermented vegetables [51]. Lb. reuteri identified in the shalgam juice had homology with Lb. reuteri, which has drawn attention due to reutericyclin, an antibiotic substance produced during fermentation and proven effective against some of the Gram-negative and lactic acid bacterial species and, therefore, proposed as a preservative in bread production [52]. Lb. reuteri is mostly found in the human gastrointestinal tract and has also been identified as a predominant LAB in sourdough as well as cereal products.
Inhibition of Caco-2 cell proliferation
Phenolic contents, especially anthocyanins, are known to have an antiproliferative effect against colorectal carcinoma cells mostly due to their antioxidant capacities [11, 57, 58, 60–62]. The beneficial mechanism(s) are generally investigated by exposing cells to isolated phenolics, although the structure and properties of the latter can be affected by the complex food matrix in vivo [58]. A more reliable method to determine the potential antiproliferative activity of a product may be to investigate the effects of its phenolics as a whole rather than on an individual basis [58]. In the present study, the viability of colorectal carcinoma (Caco-2) cells was evaluated in the presence of varying concentrations of lyophilized whole shalgam or black carrot juices.
Cellular viability of shalgam-treated cells compared to untreated controls was observed to vary in a dose-dependent manner, decreasing to 97.7 % at the lowest (50 µg/mL) and to 59.3 % at the highest concentration (6400 µg/mL) of shalgam extract (Fig. 2). Viability of Caco-2 cells in the presence of black carrot extract under same conditions and respective concentrations was 96.7 and 62.1 % (Fig. 2). Shalgam juice revealed significantly higher inhibition on Caco-2 cells compared to untreated control group at the concentrations 3200 and 6400 µg/mL (p < 0.05).
Cell viability in passage 32 Caco-2 cells incubated with shalgam juice and black carrot. Data were expressed as mean values ± SD (n = 3). Single asterisk (*) indicates a significant difference in that tested concentration compared to respective untreated cell (control) group using one-way ANOVA followed by Dunnett’s test (p < 0.05). The symbol # depicts a significant difference between shalgam juice and black carrot juice at that tested concentration using Student’s t test (p < 0.05)
In a previous study, anthocyanins associated with black carrot juice (2000 µg/mL) were shown to display antiproliferative effect on HT-29 colorectal carcinoma cells [11], suggesting that the potential antiproliferative activity of shalgam juice might be associated with its black carrot-associated anthocyanins. Nevertheless, in the present study, shalgam juice exhibited statistically higher antiproliferative activities than black carrot juice at 3200 µg/mL (p = 0.029). Various mechanisms have been proposed for the antiproliferative bioactivity of anthocyanins, such as structural [59, 60] or compositional changes that may occur during fermentation [19, 20]. As opposed to black carrot juice, the organic acid concentration of shalgam juice increases during fermentation, and the resulting decrease in pH causes changes in its overall composition. Both this difference in the organic acid composition, as well as the presence of microbial species (LAB) producing the organic acids may account for the difference between the cytotoxic effect of shalgam and black carrot juices. On the one hand, cytotoxic effects of organic acids produced by anaerobic intestinal bacteria including some of the organic acids, such as lactic and acetic acids found in shalgam juice, were found to be cytotoxic and cause apoptosis in several epithelial cell lines including those lining the colon [61]. On the other hand, Lb. casei and Lb. acidophilus strains, also found in shalgam juice, were shown to have antiproliferative effect on HT-29 colorectal carcinoma cells by inhibiting cell growth 21–28 % when they were added to cell lines at the concentration of 108 CFU/mL [62]. In another study, when LAB were co-cultured with other probiotic cultures (Bifidobacterium spp.) and glucose, they reduced the proliferation of colorectal carcinoma cells more [63]. Although shalgam juice, which contains less than 106 viable bacteria, does not meet the requirements of a probiotic product, presence of LAB in shalgam juice may still confer some possible health benefits.
On the other hand, anthocyanins appear to be less potent inhibitors of cell proliferation compared to anthocyanidins. Zang et al. [64] evaluated the antiproliferative activity of five anthocyanidins (cyanidin, delphinidin, pelargonidin, petunidin, and malvidin) and four anthocyanins, (cyanidin-3-glucoside, cyanidin-3-galactoside, delphinidin-3-galactoside and pelargonidin-3-galactoside) at 12.5–200 µg/mL concentrations against human cancer cell lines [64]. While the assayed anthocyanins did not inhibit proliferation of cell lines even at the highest concentration, anthocyanidins did show strong inhibitory activity. However, currently, the anthocyanins rich extracts from blueberry and blackcurrant juice showed antiproliferative effect on three tumor cell lines at 100 µg/mL [65].
The final product of shalgam juice has high salt concentration (2 %). Although salt itself is not carcinogenic or mutagenic, it was shown to be co-carcinogenic or co-mutagenic when carcinogens and mutagens are present [66]. To observe whether salt has any effect on colorectal carcinoma cells, present salt concentration was tested and no inhibitory activity was found (data not shown). In accordance with our results, co-mutagenicity of salt has not been observed in other fermented products such as kimchi, a vegetable product [66]. Sodium benzoate, on the other hand, is a chemical preservative used in shalgam juice production to inhibit the growth of yeasts and molds, thus to prevent the spoilage [9]. However, the concentration (0.02 %) used in shalgam juice production is under the maximum allowed limit of FDA regulations (0.1 %). Previous studies showed that 0.03–0.12 % of sodium benzoate did not have any cytotoxic or carcinogenic effects in rats and on human lymphocyte cells [67, 68].
Conclusion
This study characterizes the antioxidant and microbial composition of shalgam juice. In vitro antiproliferative activity of shalgam juice associated with phenolic and microbial composition against colon cancer cells was demonstrated. Future human studies are warranted to establish bioavailability of (poly)phenolic compounds in shalgam juice and substantiate their contribution as well as that of the probiotic bacteria to the presumed health benefits of this beverage.
References
Waters DM, Mauch A, Coffey A, Arendt EK, Zannini E (2015) Lactic acid bacteria as a cell factory for the delivery of functional biomolecules and ingredients in cereal-based beverages: a review. Crit Rev Food Sci 55:503–520
Corbo MR, Bevilacqua A, Petruzzi L, Casanova FP, Sinigaglia M (2014) Functional beverages: the emerging side of functional foods commercial trends, research, and health implications. Compr Rev Food Sci F 13(6):1192–1206
Tamang JP (2012) Animal-based fermented foods of Asia. In: Hui YH, Evranuz EO (eds) Handbook of animal-based fermented food and beverage technology. CRC Press, Florida
Granato D, Branco GF, Nazzaro F, Cruz AG, Faria JAF (2010) Functional foods and nondairy probiotic food development: trends, concepts, and products. Compr Rev Food Sci F 9(3):292–302
Gobbetti M, Di Cagno R, De Angelis M (2010) Functional microorganisms for functional food quality. Crit Rev Food Sci Nutr 50:716–727
Rakin M, Sekulić MV, Mojović L (2012) Health benefits of fermented vegetable juices. In: Hui YH, Evranuz EÖ (eds) Handbook of plant-based fermented food and beverage technology. CRC Press, Florida, pp 385–406
Tanguler H, Saris PEJ, Erten H (2015) Microbial, chemical and sensory properties of shalgams made using different production methods. J Sci Food Agr 95:1008–1015
Altay F, Kanbancıoglu-Güler F, Daskaya-Dikmen C, Heperkan D (2013) A review on traditional Turkish fermented non-alcoholic beverages: microbiota, fermentation process and quality characteristics. Int J Food Microbiol 167:44–56
Anonymous (2003) Turkish Standard—Şalgam suyu/Turnip juice. Turkish Standard Institute
Tanguler H, Erten H (2013) Selection of potential autochthonous starter cultures from shalgam, a traditional Turkish lactic-acid fermented beverage. Turk J Agric For 37:212–220
Netzel M, Netzel G, Kammerer D, Schieber A, Carle R, Simons L, Bitsch I, Bitsch R, Konczak I (2007) Cancer cell antiproliferation activity and metabolism of black carrot anthocyanins. Innov Food Sci Emerg Tech 8:365–372
Erten H, Tanguler H, Canbas A (2008) A traditional Turkish lactic acid fermented beverage: shalgam (salgam). Food Rev Int 24(3):352–359
Tanguler H, Erten H (2012) Chemical and microbiological characteristics of shalgam (şalgam): a traditional Turkish lactic acid fermented beverage. J Food Qual 35:298–306
Tanguler H, Erten H (2012) Occurrence and growth of lactic acid bacteria species during the fermentation of shalgam (salgam), a traditional Turkish fermented beverage. Food Sci Technol Leb 46(1):36–41
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
AOAC (1990) Fertilizers. In: Helrich K (ed) Official methods of analysis, 15th edn, vol 1. Association of official analytical chemistry, Arlington, Virginia, p 17
McFeeters RF, Thompson RL, Fleming HP (1984) Liquid chromatographic analysis of sugars, acids and ethanol in lactic acid vegetable fermentations. J Assoc Off Anal Chem 67(4):710–714
Andersson R, Hedlund B (1983) HPLC analysis of organic acids in lactic acid fermented vegetables. Z Lebensm Unters F A 176(6):440–443
Turker N, Aksay S, Istanbullu O, Artuvan E (2007) A study on the relation between anthocyanin content and product quality: shalgam as a model beverage. J Food Qual 30:953–969
Turker N, Aksay S, Ekiz HI (2004) Effect of storage temperature on the stability of anthocyanins of a fermented black carrot (Daucus carota var. L.) beverage: shalgam. J Agric Food Chem 52(12):3807–3813
Kammerer D, Carle R, Schieber A (2004) Quantification of anthocyanins in black carrot extracts (Daucus carota ssp. sativus var. atrorubens Alef.) and evaluation of their color properties. Eur Food Res Technol 219(5):479–486
Brenes CH, Del Pozo-Insfran D, Talcott ST (2005) Stability of copigmented anthocyanins and ascorbic acid in a grape juice model system. J Agric Food Chem 53:49–56
Milbury PE, Vita JA, Blumberg JB (2010) Anthocyanins are bioavailable in humans following an acute dose of cranberry juice. J Nutr 140(6):1099–1104
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorizarion assay. Free Radic Biol Med 26(9/10):1231–1237
Fukumoto LR, Mazza G (2000) Assessing antioxidant and prooxidant activities of phenolic compounds. J Agric Food Chem 48(8):3597–3604
Güçlü-Üstündağ O, Mazza G (2009) Effects of pressurized low polarity water extraction parameters on antioxidant properties and composition of cow cockle seed extracts. Plant Food Hum Nutr 64(1):32–38
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat Protoc 2(4):875–877
Arici M (2004) Mikrobiologische und chemische Eigenschaften von Salgam. Ernährungs 51:10–11
Demir N, Acar J, Bahçeci KS (2004) Effects of storage on quality of carrot juices produced with lactofermentation and acidification. Eur Food Res Technol 218(5):465–468
Yoon KY, Woodams EE, Hang YD (2006) Production of probiotic cabbage juice by lactic acid bacteria. Bioresour Technol 97(12):1427–1430
Alasalvar C, Grigor JM, Zhang D, Quantick PC, Shahidi F (2001) Comparison of volatiles, phenolics, sugars, antioxidant vitamins, and sensory quality of different colored carrot varieties. J Agric Food Chem 49(3):1410–1416
Khandare V, Walia S, Singh M, Kaur C (2011) Black carrot (Daucus carota ssp. sativus) juice: processing effects on antioxidant composition and color. Food Bioprod Process 89(4):482–486
Stintzing FC, Stintzing AS, Carle R, Frei B, Wrolstad RE (2002) Color and antioxidant properties of cyanidin-based anthocyanin pigments. J Agr Food Chem 50(21):6172–6181
Rodríguez H, Curiel JA, Landete JM, de las Rivas B, de Felipe LF, Gómez cordovés C, Mancheño JM, Muñoz R (2009) Food phenolics and lactic acid bacteria. Int J Food Microbiol 132(2–3):79–90
Barthelmebs L, Divies C, Cavin J (2000) Knockout of the p-coumarate decarboxylase gene from Lactobacillus plantarum reveals the existence of two other inducible enzymatic activities involved in phenolic acid metabolism. Appl Environ Microb 66(8):3368–3375
Halvorsen BL, Holte K, Myhrstad MCW, Barikmo I, Hvattum E, Remberg SF, Wold A, Haffner K, Baugerød H, Andersen LF, Moskaug Ø, Jacobs DR Jr, Blomhoff R (2002) A systematic screening of total antioxidants in dietary plants. J Nutr 132(3):461–471
Seeram NP, Aviram M, Zhang Y, Henning SM, Feng L, Dreher M, Heber D (2008) Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J Agr Food Chem 56(4):1415–1422
Bermudez-Soto M, Tomas-Barberan F (2004) Evaluation of commercial red fruit juice concentrates as ingredients for antioxidant functional juices. Eur Food Res Technol 219(2):133–141
Herken EN, Guzel S (2010) Total antioxidant capacity and total phenol contents of selected commercial fruit juices in Turkey. Int J Food Prop 13(6):1373–1379
Kaur C, Kapoor HC (2002) Anti-oxidant activity and total phenolic content of some Asian vegetables. Int J Food Sci Technol 37(2):153–161
Uyan SE, Baysal T, Yurdagel U, El SN (2004) Effects of drying process on antioxidant activity of purple carrots. Die Nahrung/Food 48(1):57–60
Sun T, Simon PW, Tanumihardjo SA (2009) Antioxidant phytochemicals and antioxidant capacity of biofortified carrots (Daucus carota L.) of various colors. J Agric Food Chem 57(10):4142–4147
Wang Y, Chen C, Ai L, Zhou F, Wang L, Zhang H, Chen W, Guo B (2011) Complete genome sequence of the probiotic Lactobacillus plantarum ST-III. J Bacteriol 193(1):313–314
Axelsson L (2004) Lactic acid bacteria: Classification and physiology. In: Lahtinen S, Ouwehand AC, Salminen S, von Wright A (eds) Lactic acid bacteria microbiological and functional aspects. CRC Press, Boca Raton
Holley RA, Lamoureux M, Dussault F (2000) Identification of lactic spoilage bacteria from vacuum-packed cooked luncheon meat and induction of repairable injury by mild thermel stress. Lebensm Wiss Technol 29(1–2):114–122
Vancanneyt M, Naser SM, Engelbeen K, De Wachter M, Van der Meulen R, Cleenwerck I, Hoste B, De Vuyst L, Swings J (2006) Reclassification of Lactobacillus brevis strains LMG 11494 and LMG 11984 as Lactobacillus parabrevis sp. nov. Int J Syst Evol Microbiol 56:1553–1557
Callanan M, Kaleta P, O’Callaghan J, O’Sullivan O, Jordan K, McAuliffe O, Sangrador-Vegas A, Slattery L, Fitzgerald GF, Beresford T, Ross RP (2008) Genome sequence of Lactobacillus helveticus, an organism distinguished by selective gene loss and insertion sequence element expansion. J Bacteriol 190(2):727–735
De Vuyst L, Neysens P (2005) The sourdough microflora: biodiversity and metabolic interactions. Trends Food Sci Tech 16(1–3):43–56
LeBlanc JG, Matar C, Valdéz JC, LeBlanc J, Perdigon G (2002) Immunomodulating effects of peptidic fractions issued from milk fermented with Lactobacillus helveticus. J Dairy Sci 85(11):2733–2742
Germond JE, Lapierre L, Delley M, Mollet B, Felis GE, Dellaglio F (2003) Evolution of the bacterial species Lactobacillus delbrueckii: a partial genomic study with reflections on prokaryotic species concept. Mol Biol Evol 20(1):93–104
Azcarate-Peril MA, Altermann E, Goh YJ, Tallon R, Sanozky-Dawes RB, Pfeiler EA, O’Flaherty S, Buck BL, Dobson A, Duong T, Miller MJ, Barrangou R, Klaenhammer TR (2008) Analysis of the genome sequence of Lactobacillus gasseri ATCC 33323 reveals the molecular basis of an autochthonous intestinal organism. Appl Environ Microb 74(15):4610–4625
Gänzle MG, Höltzel A, Walter J, Hammes WP, Ho A (2000) Characterization of reutericyclin produced by Lactobacillus reuteri LTH2584. Appl Environ Microb 66(10):4325–4333
Furet J, Quénée P, Tailliez P (2004) Molecular quantification of lactic acid bacteria in fermented milk products using real-time quantitative PCR. Int J Food Microbiol 97(2):197–207
Guarneri T, Rossetti L, Giraffa G (2001) Rapid identification of Lactobacillus brevis using the polymerase chain reaction. Lett Appl Microbiol 33:377–381
Tabasco R, Paarup T, Janer C, Peláez C, Requena T (2007) Selective enumeration and identification of mixed cultures of Streptococcus thermophilus, Lactobacillus delbrueckii subsp. bulgaricus, L. acidophilus, L. paracasei subsp. paracasei and Bifidobacterium lactis in fermented milk. Int Dairy J 17(9):1107–1114
Walter J, Tannock GW, Tilsala-Timisjarvi A, Rodtong S, Loach DM, Munro K, Alatossava T (2000) Detection and identification of gastrointestinal Lactobacillus species by using denaturing gradient gel electrophoresis and species-specific PCR primers. Appl Environ Microbiol 66(1):297–303
Bermúdez-Soto MJ, Larrosa M, Garcia-Cantalejo JM, Espín JC, Tomás-Barberan FA, García-Conesa MT (2007) Up-regulation of tumor suppressor carcinoembryonic antigen-related cell adhesion molecule 1 in human colon cancer Caco-2 cells following repetitive exposure to dietary levels of a polyphenol-rich chokeberry juice. J Nutr Biochem 18(4):259–271
Cilla A, González-Sarrías A, Tomás-Barberán FA, Espín JC, Barberá R (2009) Availability of polyphenols in fruit beverages subjected to in vitro gastrointestinal digestion and their effects on proliferation, cell-cycle and apoptosis in human colon cancer Caco-2 cells. Food Chem 114(3):813–820
Suda I, Oki T, Masuda M, Kobayashi M, Nishiba Y, Furuta S (2003) Physiological functionality of purple-fleshed sweet potatoes containing anthocyanins and their utilization in foods. Jpn Agric Res Q 37:167–173
Igarashi K, Kimura Y, Takenaka A (2000) Preventive effects of dietary cabbage acylated anthocyanins on paraquat-induced oxidative stress in rats. Biosci Biotechnol Biochem 64(8):1600–1607
Sakurazawa T, Ohkusa T (2005) Cytotoxicity of organic acids produced by anaerobic intestinal bacteria on cultured epithelial cells. J Gastroenterol 40(6):600–609
Choi SS, Kim Y, Han KS, You S, Oh S, Kim SH (2006) Effects of Lactobacillus strains on cancer cell proliferation and oxidative stress in vitro. Lett Appl Microbiol 42(5):452–458
Grimoud J, Durand H, De Souza S, Monsan P, Ouarné F, Theodorou V, Roques C (2010) In vitro screening of probiotics and synbiotics according to anti-inflammatory and antiproliferative effects. Int J Food Microbiol 144(1):42–50
Zhang Y, Vareed SK, Nair MG (2005) Human tumor cell growth inhibition by nontoxic anthocyanidins, the pigments in fruits and vegetables. Life Sci 76:1465–1472
Diaconeasa Z, Leopold L, Rugină D, Ayvaz H, Socaciu C (2015) Antiproliferative and antioxidant properties of anthocyanin rich extracts from blueberry and blackcurrant juice. Int J Mol Sci 16(2):2352–2365
Park K, Kim B (2011) Lactic acid bacteria in vegetable fermentations. In: von Salminen S, von Wright A, Ouwehand A (eds) Lactic acid bacteria microbiological and functional aspects. CRC Press, Boca Raton
Nair B (2001) Final report on the safety assessment of benzyl alcohol, benzoic acid, and sodium benzoate. Int J Toxicol 20(3):23–50
Mpountoukas P, Vantarakis A, Sivridis E, Lialiaris T (2008) Cytogenetic study in cultured human lymphocytes treated with three commonly used preservatives. Food Chem Toxicol 46(7):2390–2393
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Rights and permissions
About this article
Cite this article
Ekinci, F.Y., Baser, G.M., Özcan, E. et al. Characterization of chemical, biological, and antiproliferative properties of fermented black carrot juice, shalgam. Eur Food Res Technol 242, 1355–1368 (2016). https://doi.org/10.1007/s00217-016-2639-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-016-2639-7