Abstract
Phenolic profiles of nine red fruit commercial juice concentrates were characterised by means of high -performance liquid chromatography–diode array detection–mass spectrometry–mass spectrometry. Flavonoids such as anthocyanins, flavonols, hydroxycinnamic acid derivatives, stilbenoids, flavan 3-ols, ellagic acid derivatives, and other phenolic acids, were both identified and quantified in chokeberry, elderberry, blackcurrant and redcurrant, strawberry, red grape, cherry, plum, and raspberry commercial juice concentrates. Once the characterisation was carried out, the antioxidant capacity of each concentrate was assessed in vitro by means of two different methods: the 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid) (Trolox equivalent antioxidant capacity) method and the 2,2-diphenyl-1-picrylhydrazyl radical method. Elderberry, chokeberry and blackcurrant concentrates were the richest in total phenolics and they had the strongest antioxidant capacity; therefore, these three juices may have huge interest as ingredients in the design of functional juices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The consumption of fruits and vegetables has shown protection against cancer [1], cardiovascular disease [2], and cerebrovascular disease [3]. Their antioxidant constituents seem to be responsible for these health effects. The antioxidant properties have been attributed to vitamins, such as ascorbic acid, tocopherol, and β-carotene. During the last few years, other kinds of compounds have been suggested to posses even more biological effects than vitamins in terms of health-promoting effects as antioxidants [4] and anticarcinogens [1]. Several analyses have shown that this capacity is due primarily to non-vitamin C phytochemicals, particularly flavonoids, belonging to the polyphenols group.
Flavonoids are a broad class of low molecular weight, secondary plant phenolics characterised by the flavan nucleus. These compounds are present in plant tissues, where they are synthesised and perform several important functions: protection against UV radiation, pathogens, and herbivores; providing flavour and plant colour in flowers, fruits, and leaves, and contributing to many aspects of plant physiology. The flavonoid group comprises anthocyanins, flavonols, flavones, catechins, flavanones, and chalcones [5].
Epidemiological studies have reported a correlation between high consumption of flavonoids and reduced risk of cardiovascular diseases and reduction in some types of cancer [6]. Moreover, as shown by Ishige et al. [7], flavonoids and flavonoid-containing foods may have multiple beneficial effects in the treatment of neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases, owing to their capacity to protect neuronal cells from oxidative stress.
Berries and red fruits are two of the most important dietary sources of polyphenols such as anthocyanins, flavonols, flavan 3-ols and benzoic and cinnamic acid derivatives [5]. Numerous in vitro studies have now reported various health effects that these fruits have when they are part of the human diet, among those the high antiradical activity of berries [8] and the capacity to inhibit the human low-density lipoprotein and liposome oxidation [9]. It has been reported that addition to the diet of large amounts of catechin caused a reduction of total cholesterol and low-density lipoprotein fraction in serum [10]. Other authors have reported some positive therapeutic effects of anthocyanins, such as the maintenance of normal vascular permeability [11], vasoprotective and antiinflammatory properties [12], anticancer activity [13], and prevention of age-related decline of neurological function [3]. Biochemical and pharmacological activities have been attributed to quercetin and its glycosides, such as free-radical scavenging [14], effects on immune and inflammatory cell functions [15], and even anticarcinogenic and antitumour properties [16].
However, little is known about the antioxidant properties and bioactivity of these compounds in vivo. All the flavonoid properties in vivo will be dependent on the metabolism, absorption, and excretion within the body after ingestion and the reducing properties of the resulting metabolites.
In foods, most flavonoids are present as glycosides, bound to a sugar moiety. Initially it was thought that only flavonoid aglycones (without bound sugars) were able to pass through the gut wall, and that enzymes capable of cleaving the β-glycosidic bonds were not secreted into the gut or the intestinal wall. However, various reports have demonstrated that flavonoid glycosides can enter into the circulation without modification [17].
In the case of the bioavailability of anthocyanins, it has been found that they are incorporated from the digestive tract into the blood circulation system in mammals, keeping structurally intact glycoside forms. This conclusion was reached by means of both human and rat [18, 19] in vivo assays. It was also possible to quantify the presence of anthocyanins excreted unchanged in human urinary samples [19]. No remainder of the aglycone in plasma after oral administration of the anthocyanin glycosides has been found [18].
Fruit juice concentrate is the result of an industrial process in which a fruit juice is submitted to a heat process of evaporation in order to remove the majority of the water, and a product with much better conditions for storage, transport, and preservation is obtained. The original juice undergoes a heating process in which some of its properties and components change. Some authors have affirmed that processing of food products can increase flavonol levels in foods [20]. The accumulation of quercetin in processed foods can occur as a consequence of enzymatic hydrolysis of quercetin conjugates during pasteurisation or other processing procedures [20, 21]. Fruit juice processing can increase the flavonoid content because extraction processes can release flavonoids from the rind [22], and these flavonoids might be better absorbed than those in fresh fruits. Therefore, red fruit juice concentrates could be very suitable ingredients for the production of functional foods.
Many reports have been written about the phenolic profile in different fruit and berry samples, from fresh, freeze-dried, and frozen fruits [23, 24, 25], as well as from juices and fruit extracts as raw materials [26, 27]. However, only a few reports have been based on commercial fruit juice concentrates [28].
The main objective of this work was the evaluation of selected commercial red fruit juice concentrates as ingredients for “functional” fruit juice mixtures, regarding their antioxidant capacity, polyphenol content and their potential bioavailability.
Materials and methods
Reagents
Chlorogenic acid, gallic acid, ellagic acid, (+)-catechin, and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were purchased from Sigma (St. Louis, MO, USA). Quercetin 3-rutinoside, and cyanidin 3-rutinoside were supplied by Merck (Darmstadt, Germany) and by Polyphenol (Sandnes, Norway), respectively. Folin–Ciocalteu reagent was obtained from Sigma (St. Louis, MO, USA). For the antiradical activity assays 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•), and activated manganese dioxide (MnO2), were purchased from Sigma (St. Louis, MO, USA). All other reagents were of analytical grade and supplied by Merck (Darmstadt, Germany). Milli-Q (Millipore, Bedford, MA) ultrapure water was used throughout this research.
Juice concentrates
Chokeberry (Aronia melanocarpa, Michx., Spain, Juver), blackcurrant (Ribes nigrum, L., Bayernwald, Germany), redcurrant (Ribes rubrum, L., Bayernwald, Germany), elderberry (Sambucus nigra, L., Böcker Iberian, Germany), strawberry (Fragaria ananassa, Duch., Iprona, Italy), red grape (Vitis spp., Iprona, Italy), cherry (Prunus avium, L., Jahnke, Germany), plum (Prunus domestica, L., Iprona, Italy), and raspberry (Rubus idaeus, L., Bayernwald, Germany) frozen commercial juice concentrates were kindly provided by Juver (Murcia, Spain). They were thawed and stored at refrigeration temperature for the analyses.
High-performance liquid chromatography analysis
The majority of these concentrated juices are very rich in phenolic compounds. For this reason it was necessary to dilute in purified water some of them before the high-performance liquid chromatography (HPLC) analysis. Thus, chokeberry, and elderberry were diluted (sample-to-water ratio) 1:10, and blackcurrant, and raspberry concentrated juices were diluted (sample-to-water ratio) 1:2. No dilutions were needed for the rest of the concentrates. All the samples were filtered through a Millex-HV13 0.45-μm membrane filter (Millipore) before injection into the high-performance liquid chromatograph. Twenty-microlitre samples of each concentrated juice were analysed using an HPLC system equipped with a model L-7100 (Merck Hitachi) pump and a model L-7455 (Merck Hitachi) photodiode array UV--vis detector. The samples were injected by means of a model L-7200 (Merck Hitachi) autosampler. Separations were achieved on a LiChroCART column (Merck) (RP-18, 25×0.4 cm; 5-μm particle size). The mobile phase was water with 5% formic acid (v/v) (solvent A) and HPLC grade methanol (solvent B) at a flow rate of 1 mL min−1. The linear gradient started with 3% solvent B in solvent A reaching 5% solvent B in solvent A at 5 min, 8% solvent B at 10 min, 13% solvent B at 15 min, 15% solvent B at 19 min, 40% solvent B at 47 min, 65% solvent B at 64 min, 80% solvent B at 65 min, 98% solvent B at 66 min at isocratic elution until 69 min. At 70 min the gradient reached the initial conditions again.
Identification and quantification of phenolic compounds
Chromatograms were recorded at 520, 360, 320, 280, and 255 nm. Phenolic compounds were identified by their UV spectra recorded with a diode array detector, by HPLC–mass spectrometry (MS)–(MS) analysis and wherever possible by chromatographic comparisons with authentic samples. Anthocyanins were quantified as cyanidin 3-rutinoside in the HPLC chromatograms recorded at 520 nm, using an external standard. The same procedure was followed for flavonols, hydroxycinnamic acid derivatives, flavan 3-ols, and ellagic acid derivatives, which were quantified in the chromatograms recorded at 360, 320, 280, and 255 nm, respectively. They were quantified using the following external standards: rutin (quercetin 3-rutinoside) for flavonols, chlorogenic acid for hydroxycinnamic acid derivatives, (+)-catechin for flavan 3-ols, gallic acid for other phenolic compounds, and ellagic acid for its derivatives. The repeatability of the quantitative analysis was ±4%. Analyses were replicated (n=3), and the contents are given as mean values plus or minus the standard deviation. The results were expressed in milligrams of each standard per litre of juice concentrate.
HPLC–diode array detection–MS–MS
The identification of some of the flavonoids present in the juice concentrates was carried out by means of their molecular weight and their fragments. For this task a HPLC–diode array detection (DAD)–MS–MS was required. The analytical conditions used were those used in the HPLC analysis described previously, but solvent A was water with 1% formic acid (v/v) in this case. The HPLC system equipped with a DAD detector and a mass detector in series consisted of a binary pump (G1312A), an autosampler (G1313A), a degasser (G1322A), and a photodiode array detector (G1315B) controlled by software (v. A08.03) from Agilent Technologies (Waldbronn, Germany) equipped with an electrospray ionisation (ESI) system and controlled by software (v. 4.0.25). The heated capillary and voltage were maintained at 350 °C and 4 kV, respectively. Mass scan (MS) and daughter (MS–MS) spectra were measured from m/z 100 up to m/z 1,500. Collision-induced fragmentation experiments were performed in the ion trap using helium as the collision gas, and the collision energy was set at 50%. Mass spectrometry data were acquired in both negative and positive ionisation modes for all the phenolic compounds.
Determination of total phenolics
The amount of total phenolics in the concentrated juices was determined by two different methods. First, the total phenolics were calculated as the addition of all the different phenolic compounds quantified by means of the HPLC analysis. The other way was according to the Folin–Ciocalteu method [29] and the amount was expressed as gallic acid equivalents, milligrams per litre of concentrated juice. The Analyses were also replicated (n=3), and the contents are given as mean values plus or minus the standard deviation. The coefficient of variation was always less than 5%.
Antioxidant activity
DPPH• assay
Free-radical scavenging activity using the free radical DPPH• [30] was evaluated by measuring the variation in absorbance at 515 nm after 1 h of reaction in Parafilm-sealed glass cuvettes (to avoid methanol evaporation) at 25 °C [31]. The concentrated juices were diluted in purified water previously to the assay in order to get a good result, owing to the high antioxidant capacity of each one. So, chokeberry, elderberry, and blackcurrant concentrated juices were diluted (sample-to-water ratio) 1:100, strawberry and redcurrant were diluted (sample-to-water ratio) 1:40, and the rest of the concentrated juices were diluted (sample-to-water ratio) 1:20. The reaction started by adding 10 μL of the corresponding sample to the cuvette containing 80 μM (990 μL) of the free radical (DPPH•). The final volume of the assay was 1 mL. The reaction was followed with an Shimadzu UV-1603 spectrophotometer (Tokyo, Japan). The DPPH• assay was repeated three times. The coefficient of variation was always less than 5%.
ABTS•+ assay
The ABTS•+ radical cation was chemically generated with MnO2 as described by Espín and Wichers [32]. The concentrated juices were also diluted with Milli-Q water at different dilutions depending on the antioxidant capacity of each one. Thus, the concentrated juices with highest antioxidant capacity, chokeberry, elderberry, and blackcurrant, were diluted (sample-to-water ratio) 1:500. The rest of the juices were diluted (sample-to-water ratio) 1:100. The reaction was started by adding 10 μL of the corresponding sample to the cuvette containing 32 μM (990 μL) of the radical (ABTS•+). The final volume of the assay was 1 mL. The disappearance of ABTS•+ was determined by measuring the decrease of absorbance at 414 nm (in the previously-described spectrophotometer) for 60 min at 25 °C [32].
Antiradical activity was expressed as milligrams of Trolox equivalent antioxidant capacity (TEAC) following the nomenclature of Rice-Evans and Miller [33]. The coefficient of variation was always less than 5%.
Graphs and data analysis
Plots and fittings were carried out by using the Sigma Plot 6.0 program (SPSS Science, Chicago, IL).
Results and discussion
Phenolic composition of juice concentrates
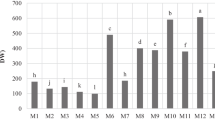
The phenolic content of the selected juice concentrates was evaluated by HPLC and by the Folin–Ciocalteu method (Table 1). As a general rule, the content determined by the Folin–Ciocalteu method was considerably higher than that determined by HPLC. This is in agreement with previous reports [34] and indicated that the Folin–Ciocalteu method overestimates the real phenolic content, as it also quantifies polymeric phenolics and other nonphenolic metabolites. In addition, the HPLC analyses allowed the quantification of different phenolic groups as anthocyanins, flavonols, hydroxycinnamic acid derivatives (caffeic, p-coumaric and ferulic derivatives), flavan-3-ols, and ellagic acid derivatives. All these phenolics had characteristic UV spectra that allowed their quantification as different phenolic groups. Other unidentified compounds were quantified as gallic acid and are included in the table as other phenolics.
Chokeberry juice concentrate was the richest in phenolic compounds, both evaluated by HLPC and by the Folin–Ciocalteu method, with 27.3 and 46.8 g L−1, respectively. This extract was very rich in anthocyanins, hydroxycinnamic acid derivatives, and flavan-3-ols, and also contained significant amounts of flavonols. No ellagic acid derivatives were detected in this juice concentrate.
Elderberry concentrate was the richest in anthocyanin pigments (11.5 g L−1) and it was also quite rich in flavonols. Flavan-3-ols and hydroxycinnamates were also present in smaller amounts. No ellagic acid derivatives were detected in elderberry juice concentrate.
Blackcurrant juice concentrate was also very rich in phenolic compounds (15.4 g L−1), mainly anthocyanins, but also flavan-3-ols, and flavonols were relevant constituents (Table 1).
Ellagic acid derivatives (free ellagic acid and O-glycosides) were only present in strawberry and raspberry juice concentrates. These compounds were present in both concentrates in similar amounts (about 600 mg L−1).
The amount of anthocyanins was also very small in the case of plum juice concentrate, although in this case other phenolics like hydroxycinnamate derivatives and flavan-3-ols were detected in the HPLC chromatograms in significant amounts, and the difference in total phenolics between the HPLC and the Folin–Ciocalteu quantification was very small (Table 1).
The rest of the red fruit juice concentrates were much poorer sources of phenolic compounds. Red grape juice concentrate was the richest in anthocyanins. Stilbenoids were only detected in the commercial red grape concentrate analysed.
In vitro antioxidant capacity
In order to assess the potential antioxidant activity of the red fruit juice concentrates studied, two in vitro antioxidant assays were assayed. Once these values were obtained, the antioxidant activity of each concentrate was correlated with their total phenolic content as well as with both their anthocyanin and flavonol contents.
All the juice concentrates showed strong free-radical scavenging activity against both DPPH• and ABTS•+ radicals (Table 2), although this activity was different depending on the method used and the radical model. Against ABTS•+, the activity was in general higher than that obtained against the DPPH• radical by factors between 1.5 (redcurrant) and 2.3 (elderberry). These assays demonstrated that the three richest concentrates in phenolic content (chokeberry, blackcurrant, and elderberry) were those showing the highest values in free-radical scavenging activity against both radicals (Table 2).
The linear correlation between antiradical activity (TEAC) against the ABTS•+ radical and total phenolic concentration of each juice concentrate was calculated, as well as the correlation with the anthocyanin and flavonol content. The graphics, together with their corresponding statistical parameters R and P, are shown in Fig. 1. These results indicate that the antiradical activity was linearly correlated with total phenolic, anthocyanin, and flavonol contents in each one of the juice concentrates assayed. The best correlation was obtained between the TEAC values and the anthocyanin content, as could be expected as anthocyanins are the main antioxidant polyphenols in the juice concentrates assayed, with a regression factor of 0.95 (Fig. 1b). In this figure it is clear that two different groups of juice concentrates are present in this sample. The richest group includes chokeberry, elderberry, and blackcurrant, and the second group, with much lower phenolic concentration, includes the rest of the juice concentrates assayed. When considering only these two groups independently, the linear correlation is lost in almost all instances. Only in the case of anthocyanins in the group of concentrates with lower phenolic content, some correlation is still observed (r 2=0.87) (Fig. 1c). The TEAC when the total phenolic content was zero was 8.2 mg and could be interpreted as the theoretical scavenging activity against ABTS•+ present in the red fruit juice concentrate, which is due to other nonphenolic compounds (e.g., vitamin C).
These results indicate that the most interesting juice concentrates were those showing the highest antioxidant capacity and the highest phenolic content. Therefore, chokeberry, elderberry, and blackcurrant juice concentrates were selected for a more detailed study using HPLC–MS to assess the content in phenolic compounds that were more bioavailable according to previous studies.
HPLC–MS analysis of the three juice concentrates selected
The most important phenolic compounds in the three richest concentrates (chokeberry, elderberry, and blackcurrant) were identified by their UV spectra and their molecular weights and their fragments, by using HPLC–DAD–MS–MS.
The main phenolics of chokeberry concentrate (Fig. 2) are described in Table 3. Four cyanidin glycosides were present in the anthocyanin fraction (A1–A4), the main one being cyanidin 3-galactoside, in a concentration of 4.3 g L−1. These results are in agreement with those reported by other authors [35]. The concentration of flavonols in this juice concentrate was lower and the main one was quercetin 3-rutinoside, accounting for almost 50% of the total flavonols in chokeberry concentrate. A little amount of quercetin aglycone appeared at the end of the chromatogram (F5 in Fig. 2). The results for flavonols F3 and F4 are in agreement with the results reported by Häkkinen and Auriola [25], although the flavonols appeared in the opposite elution order, owing to the different mobile phase used for the HPLC analysis. Concerning the composition of the hydroxycinnamic derivative, the total amount of these compounds was similar to that of anthocyanins. Two caffeic acid derivatives were detected by their characteristic UV spectrum (Fig. 2), and were identified by mass spectrometry as neochlorogenic acid (HC1, 3-O-caffeoyl-quinic acid) and chlorogenic acid (HC2, 5-O-caffeoylquinic acid) [36].
Three different anthocyanins were detected in the anthocyanin fraction of elderberry concentrate in the 520-nm chromatogram (Fig. 3, Table 4). However, after the MS–MS analysis, it could be demonstrated that five anthocyanins were present in this fraction. Cyanidin sambubioside was the most abundant anthocyanin (A2), eluting together with cyanidin 3-glucoside and preceded by A1, which consisted on cyanidin 3-glucoside-5-glucoside and cyanidin 3-sambubioside-5-glucoside, in agreement with the results reported by Hong and Wrolstad [28]. Another anthocyanin derivative appeared in the last position of the 520-nm chromatogram (A3 in Fig. 3). This last anthocyanin occurred in trace amounts and, according to the molecular ion (MS) and fragments (MS–MS) found in our analysis (A3 in Table 4), it seems to be cyanidin 3-rutinoside. Quercetin and kaempferol glycosides were also found (Table 4). The HPLC–MS–MS analysis of the main flavonol peak (F2, 2.45 g L−1) revealed the coelution of two different glycosides, where quercetin 3-rutinoside was the major product (above 99%), and quercetin 3-glucoside was detected in trace amounts. Kaempferol rutinoside was also detected (F3) in trace amounts, showing a clear MS spectrum. Compound F4 was identified as quercetin aglycone. Two glycosides of quercetin and kaempferol, not yet identified, form the first peak (F1). According to their mass spectra we can conclude that they are hexosides of their respective flavonols. Further analysis will be carried out for the total identification of these flavonols. The UV spectra of HC1 and HC2 were typical of caffeic acid derivatives. Their identification was carried out by means of HPLC–DAD–ESI–MS–MS analysis, resulting in HC1 as neochlorogenic acid (3-O-caffeoyl-quinic acid) and HC2 as chlorogenic acid (5-O-caffeoylquinic acid) [36].
The HPLC chromatograms of blackcurrant concentrate are shown in Fig. 4. Among the anthocyanin compounds, only five of them could be identified by means of their molecular weights and fragments (Table 5). The MS–MS fragment at m/z + 303 indicated that the aglycone of the three first anthocyanins isolated, corresponding to peaks A1 and A2, was delphinidin. In agreement with Hong and Wrolstad [28], delphinidin 3-rutinoside was identified as the main anthocyanin (A2), and it appeared after the glucoside of the same aglycone (A1). The following anthocyanins (A3 and A4) were clearly identified according their mass spectra as cyanidin 3-glucoside and cyanidin 3-rutinoside, respectively, also in agreement with earlier studies [28]. The total flavonol amount was not as high as the anthocyanin one; however, the wide variety of the flavonol composition was remarkable. Myricetin, quercetin, and kaempferol were identified by means of their UV spectra and their MS–MS analyses: fragments at m/z - 317 and 301, respectively. Myricetin glycosides were the most abundant, followed by quercetin glycosides, in agreement with other authors who previously studied blackcurrant flavonol composition [31, 32]. The quantification of glycosides of myricetin was achieved by means of the mass spectrometry analysis, and gave the following relative amounts: 98% of rutinoside and 2% of glucoside. HC1 and HC2 showed characteristic UV spectra of caffeic acid derivatives, but their identification was not possible through HPLC–DAD–MS–MS, because of their poor ionisation under the analytical conditions used.
Conclusions
The red-fruit juice concentrates showing a higher in vitro free-radical scavenging activity were those showing the highest polyphenol content, namely chokeberry, elderberry, and blackcurrant.
When looking at the structure of the individual phenolic compounds, and their reported ability to be absorbed in vivo, it seems clear that those extracts containing more glucosides, galactosides, and aglycones and less rutinosides and other glycosidic combinations linked to the flavonol nucleus will provide a larger amount of flavonols than could be absorbed in the small intestine [37]. This does not mean that the flavonoid compounds that are not absorbed in the small intestine are not biologically relevant, as they can exert a function locally in the large intestine, can be metabolised by the colon microflora, and their metabolites can absorbed in the distal part of the intestine [38].
On the other hand, several authors have demonstrated the bioavailability of the anthocyanins of elderberry, which appear in both plasma and urine samples, keeping their unchanged glycosylated forms, although in very small proportion from the amount ingested [18, 19].
Thus, if all the anthocyanins are able to be absorbed in the small intestine but only the flavonol aglycones and the corresponding glucosides and galactosides have this ability, the chokeberry and elderberry concentrates provide more flavonoids to be potentially absorbed in the small intestine than blackcurrant concentrate (Tables 3, 4, 5). Chokeberry contained about 8 g L−1anthocyanins and 0.88 g L−1 quercetin glucoside, galactoside, and quercetin aglycone (also readily absorbable). Elderberry concentrate contained 11.3 g L−1 anthocyanins and 0.53 g L−1 flavonols potentially absorbable in the small intestine, such as quercetin and kaempferol glucosides and other not identified hexosides, as well as quercetin aglycone.
Since anthocyanins are scarcely absorbed through the stomach and small intestine walls [39], we can suggest that chokeberry juice concentrate would be the most appropriate ingredient for the preparation of functional juices if flavonoid absorption in the small intestine is required. This should be proved with clinical bioavailability studies.
Other concentrates, which are not as rich in antioxidant phenolics, contain specific compounds that can provide suitable biological properties as is the case of the ellagic acid derivatives of strawberry and raspberry concentrates or the stilbenoids present in red grape concentrate.
These results show that red fruit concentrates are a very good source of antioxidant phenolic metabolites, which in the case of chokeberry and elderberry, are also potentially absorbed in the stomach and small intestine. Moreover, the results also confirm that the other juice concentrates are complementary sources of specific phenolics with marked biological activity.
References
Dragsted LO, Strube M, Larsen JC (1993) Pharmacol Toxicol 72:116–135
Ness AR, Powels JW (1997) Int J Epidemiol 26:1–13
Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC (1999) J Neurosci 19:8114–8121
Rice-Evans CA, Miller NJ (1998) In: Rice-Evans CA, Packer L (eds) Flavonoids in health and disease. Dekker New York, pp 199–219
Macheix JJ, Fleuriet A, Billot J (1990) Fruit phenolics. CRC, Boca Raton, FL
Hertog MGL, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A, Nedeljkovich S, Pekkarinen M, Simic BS, Toshima H, Feskens EJ, Hollman PCH, Katan MB (1995) Arch Intern Med 155:381–386
Ishige K, Schubert D, Sagrara Y (2001) Free Rad Bio Med 30:433–446
Wang SY, Jiao H (2000) J Agric Food Chem 48:5672–5676
Heinonen IM, Meyer AS, Frankel EN (1998) J Agric Food Chem 46:4107–4112
Zdunczyk Z, Frejnagel S, Wroblewska M, Juskiewicz J, Oszmianski J, Estrella I (2002) Food Res Int 35:183–186
Detre Z, Jellinek H, Miskulin M, Robert AM (1986) Clin Physiol Biochem 4:143–149
Lietti A, Cristoni A, Picci M (1976) Arzneim-Forsch 26:829–832
Gasiorowski K, Szyba K, Brokos B, Kolaczynska B, Jankowiak-Wlodarczyk M, Oszmianski J (1997) Cancer Lett 119:37–46
Duthie SJ, Dobson VL (1999) Eur J Nutr 38:28–34
Middleton E, Kandaswami C (1992) Biochem Pharmacol 43:1167–1179
Ellatar TMA, Virji AS (2000) Anticancer Res 20:1733–1738
Shimoi K, Okada H, Furugori M, Goda T, Takase S, Susuki M, Hara Y, Yamamoto H, Kinac N (1998) FEBS Lett 438:220–224
Miyazawa T, Nakagawa K, Kudo M, Muraishi K, Someya K (1999) J Agric Food Chem 47:1083–1091
Milbury PE, Cao G, Prior RL, Blumberg J (2002) Mech Ageing Dev 123:997–1006
Stewart A, Bozonnet S, Mullen W J (2000) Agric Food Chem 48:2663–2669
Price KR, Rhodes MJC (1997) J Sci Food Agric 74:331–339
van der Sluis A, Dekker M, Jongen WMF (1997) Cancer Lett 114:107–108
Häkkinen SH, Heinonen IM, Kärenlampi S, Mykkänen H, Ruuskanen J, Törrönen AR (1999) Food Res Int 32:345–353
Häkkinen SH, Kärenlampi SO, Heinonen IM, Mykkänen HM, Törrönen AR (1999) J Agric Food Chem 47:2274–2279
Häkkinen SH, Auriola S (1998) J Chromatogr A 829:91–100
Netzel M, Strass G, Kaul C, Bitsch I, Dietrich H, Bitsch R (2002) Food Res Int 35:213–216
Kähkönen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, Heinonen M (1999) J Agric Food Chem 47:3954–3962
Hong V, Wrolstad RE (1990) J Agric Food Chem 38:698–708
Singleton VL, Rossi JA (1965) Am J Enol Vitic 16:144–158
Brand-Williams W, Cuvelier ME, Berset C (1995) Lebensm Wiss Technol 28:25–30
Espín JC, Soler-Rivas C, Wichers HJ (2000) J Agric Food Chem 48:648–656
Espín JC, Wichers HJ (2000) J Food Biochem 24:225–250
Rice-Evans CA, Miller NJ (1994) Methods Enzymol 234:279–293
Chapuis-Lardy L, Contour-Ansel D, Bernhard-Reversat F (2002) Plant Sci 163:217–222
Chandra A, Rana J, Li Y (2001) J Agric Food Chem 49:3515–3521
Clifford MN, Johnston KL, Knight S, Kuhnert N (2003) J Agric Food Chem 51:2900–2911
Aherne SA, O’Brien NM (2002) Nutrition 18:75–81
Bokkenheuser VD, Shackleton CHL, Winter J (1987) Biochem J 248:953–956
Passamonti S, Vrhovsek U, Vanzo A, Mattivi F (2003) FEBS Lett 544:210–213
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
J. Bermúdez-Soto, M., A. Tomás-Barberán, F. Evaluation of commercial red fruit juice concentrates as ingredients for antioxidant functional juices. Eur Food Res Technol 219, 133–141 (2004). https://doi.org/10.1007/s00217-004-0940-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-004-0940-3