Abstract
Jabuticaba (Myrciaria jaboticaba) is a dark-colored fruit native to Brazil that has a desirable flavor and high anthocyanin content. In the present study, jabuticaba juice was produced by steam-extraction and the phenolic compound profile, antioxidant capacity, instrumental color, and microbiological quality were evaluated during 90 days of storage at 25 °C. Cyanidin-3-O-glucoside represented 45% of the total phenolic content of the juice, which reduced with extent of 80% during storage. Total phenolic content of the juice increased by 59% during 90 days of storage, which entailed, average 4.4-fold increase in the content of gallic and ellagic acids. FRAP assay was most sensitive for measuring gallic and ellagic acids, while the TEAC assay was the most sensitive for measuring anthocyanins. Although \( a^{*} \) and \( b^{*} \) values of jabuticaba juice decreased and \( \Delta E^{*} \) increased during storage. Jabuticaba juice remained microbiologically stable during storage, and did not support the growth of inoculated Escherichia coli and Salmonella enteritidis, suggesting antimicrobial activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Jabuticaba (Myrciaria jaboticaba) is a fruit that has a purple to black peel and whitish sweet pulp with a slightly tannic and acid taste, and is native to the Brazilian Atlantic Forest. Recent research has suggested that this fruit possesses high antioxidant activity and phenolic compound content, especially anthocyanins, which have been associated with reduced risk of chronic diseases. Jabuticaba is popularly consumed fresh, because it is highly perishable, with a shelf life of only up to 3 days after harvest, as a result of intense water loss, microbiological deterioration, and pulp fermentation. Therefore, this fruit is widely used for producing artisanal products such as juices, jellies, wines, and liquors (Wu et al. 2012; Inada et al. 2015).
The tropical juice industry is expanding because of high demand for healthy and functional food products that are convenient to consume (Vidigal et al. 2011). Juice production involves an increased used of fruits and permits fruit consumption throughout the year, even in the intercrop period. Different methods are used to produce juices, with heat treatments used to extend their shelf-life. Steam extraction has been used for small- and medium-scale red grape juice production, and adds value to the raw materials, thus increasing the potential income (Lopes et al. 2016).
As a consequence of processing conditions and storage for long periods, the multiplication of spoilage and pathogenic microorganisms should be considered, as they can result in the deterioration of fruit juices or the emergence of food-borne diseases. Additionally, losses or changes in bioactive compounds may occur because of enzymatic and chemical reactions such as oxidation, rearrangements of atoms, and conjugation between compounds (Amodio et al. 2014).
The stability of polyphenols may be influenced by several parameters such as pH, temperature, and light exposure, and the loss of these compounds, particularly anthocyanins, may cause a reduction in bioactivity and can also significantly affect sensory properties, especially the color (Jimenez-Garcia et al. 2013; Wang et al. 2015). The aim of this study was to produce an innovative jabuticaba juice using steam extraction, and evaluate the effect of storage on its microbiological quality, physicochemical parameters, antioxidant capacity, and phenolic compound content. In addition to these parameters, the ideal sweetness, sensory acceptance, and purchase intent of this juice were evaluated.
Materials and methods
Standards and chemicals
2,4,6-tris(2-pyridyl)-S-triazine (TPTZ), 2,2′-azino-bis(2-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), potassium persulfate, (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Iron (II) sulfate was purchased from Merck KGaA (Darmstadt, Germany). Anhydrous sodium acetate and iron chloride were purchased from Vetec Química Fina Ltda. (Duque de Caxias, RJ, Brazil). Anthocyanin and non-anthocyanin standards were purchased from Indofine Chemical Co. (Hillsborough, NJ, USA) and Sigma-Aldrich Chemical Co. (St. Louis, MO, USA), respectively. All solvents were HPLC grade and purchased from Tedia (Fairfield, OH, USA). HPLC grade water (Milli-Q system, Millipore, Bedford, MA, USA) was used throughout the experiments.
Jabuticaba juice processing
Jabuticaba fruits (Myrciaria jaboticaba, cv. Sabará) cultivated in Minas Gerais, Brazil, were purchased at Rio de Janeiro’s agricultural trading center. Fruits were selected, washed, and sanitized in sodium hypochlorite (100 ppm solution) for 15 min. Jabuticaba juice was produced by steam extraction using a stainless steel steam juicer (Cook N Home, City of Industry, CA, USA), pre-sanitized with sodium hypochlorite (200 ppm), and rinsed with boiling water. Steam extraction was conducted in a single batch for 30 min using 4 kg of thawed and macerated fruit and 2 L of water. The juice was bottled while hot (extraction temperature 75–85 °C) in 50 mL sterile glass flasks, which were filled completely and stored in the dark at 25 °C. Samples were analyzed at 0, 30, 60 and 90 days.
Phenolic compound content determination by HPLC–DAD
Steam-extracted jabuticaba juice (2.0 mL) was centrifuged (11,300×g, 10 min) and filtered through a 0.45 µm cellulose ester membrane (Millipore®, Barueri, Brazil) prior to analysis by High-Performance Liquid Chromatography (HPLC). For anthocyanin analysis, the samples were diluted with 1% aqueous formic acid. Each sample was analyzed in triplicate.
The liquid chromatography system (Shimadzu®, Kyoto, Japan) included a quaternary pump LC-20AT, automatic injector SIL-20AHT, diode array detector (DAD) SPD-M20A, system controller CBM-20A, and degasser DGU-20A5. A reverse phase column was used for chromatographic separation of anthocyanins (C18, 5 µm, 250 mm × 4.6 mm, Kromasil®) and non-anthocyanin phenolic compounds (C18, 5 µm, 250 mm × 4.6 mm, Phenomenex®), according to Inada et al. (2015). Anthocyanins were monitored at 530 nm, and non-anthocyanin phenolic compounds were monitored from 190 to 370 nm. Identification of all phenolic compounds was performed by comparison with retention time and absorption spectra of the respective representative standard. Quantification was performed by external calibration. Data were acquired by LC solution software (Shimadzu Corporation®, version 1.25, 2009). Results were expressed as mg of compound per liter.
Antioxidant capacity
The antioxidant capacity (AC) of steam-extracted jabuticaba juice was determined in centrifuged juice (11,300×g, 10 min) using FRAP (Ferric Reducing Antioxidant Power) and TEAC (Trolox Equivalent Antioxidant Capacity) assays.
The FRAP assay was performed according to Benzie and Strain (1996) with slight modifications. FRAP reagent was prepared by mixing 2 mL of 10 mM TPTZ solution in 6 M HCl with 2 mL of 20 mM FeCl3 solution and 20 mL of 300 mM acetate buffer (pH 3.6), and warmed to 37 °C prior to analysis. Aliquots of juice (20 µL) were pipetted into a 96-well microplate, which was placed in a Victor3 1420 multilabel counter (PerkinElmer, Turku, Finland) with an automatic injector. FRAP reagent (180 µL) was automatically dispensed into each well, and the reagents were mixed by shaking and then allowed to stand at 37 °C for 6 min. The absorbance was then read at 593 nm. Quantification was performed using a calibration curve prepared with FeSO4. Results were expressed as µM of Fe2+ equivalent per liter. Each sample was analyzed in triplicate.
The TEAC assay was performed according to Re et al. (1999) with slight modifications. The ABTS radical cation stock solution was generated by reacting K2S2O8 and ABTS for 12–16 h prior to use. The ABTS radical cation stock solution was diluted in water (1:50) to an absorbance of 0.70 ± 0.02 at 720 nm. Aliquots of juice (10 µL) were pipetted into a 96-well microplate, which was placed in a Victor3 1420 multilabel counter with an automatic injector. Aliquots of the ABTS radical cation solution (190 µL) were automatically dispensed into each well, and the reagents were mixed by shaking and then allowed to stand at 37 °C for 6 min. Sample absorbance was read at 720 nm and subtracted from solvent blank absorbance. Quantification was performed using a calibration curve prepared with Trolox. Results were expressed as mmol of Trolox equivalent per liter. Each sample was analyzed in triplicate.
Physicochemical analyses
The pH of steam-extracted jabuticaba juice was determined in triplicate, according to official methods (Association of Official Analytical Chemists 2000). The total soluble solid contents (TSS) were measured using a portable refractometer (Model PAL-1; Atago, Tokyo, Japan). One drop of juice was placed on the refractometer glass prism, and the TSS was obtained as °Brix.
Instrumental color measurement
Instrumental color parameters of steam-extracted jabuticaba juice were measured in triplicate using a Konica Minolta CR-400 colorimeter (Konica Minolta, Tokyo, Japan). The equipment was set to illuminant D65 (2° observer angle) and calibrated using a standard white reflector plate. The CIELab color space was used to determine the color components: \( L^{*} \) (brightness or lightness; 0 = black, 100 = white), \( a^{*} \) (−\( a^{*} \), greenness; +\( a^{*} \), redness) and \( b^{*} \) (−\( b^{*} \), blueness; +\( b^{*} \), yellowness). The total color difference (\( \Delta E^{*} \)) during storage was calculated as follows:
where \( L^{*} \), \( a^{*} \), and \( b^{*} \) were the color coordinates at the initial time (t 0 ) and at the nth day of storage (t i ).
Microbiological analysis
Twenty-five grams of fresh fruit or 25 mL of steam-extracted jabuticaba juice were mechanically homogenized in 225 mL of sterile 0.1% peptone water (w/v) and inoculated into culture media (Table 1) as described by Downes and Ito (2001). All experiments were performed in triplicate.
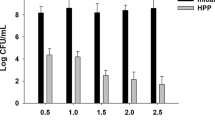
Survival of Salmonella enteritidis and Escherichia coli inoculated into steam-extracted jabuticaba juice was also evaluated. Aliquots of 20 mL of steam-extracted jabuticaba juice were inoculated to obtain approximately 1 × 106 colony forming units per milliliter (CFU/mL) of S. enteritidis (American Type Culture Collection—ATCC, 13076) or E. coli (ATCC, 25922), which were pre-cultivated for 18 h at 37 °C in brain heart infusion agar (BHI agar, Himedia Laboratories, Mumbai, India) and then suspended in saline solution. At different intervals, 1 mL aliquots of the suspension were removed, plated onto BHI agar, and incubated at 37 °C for 24 h for enumeration of viable surviving bacterial cells. The identity of isolated cultures was confirmed by Gram staining and biochemical tests.
Sensory analysis
Ideal sweetness determination
The present study received authorization to proceed from the Universidade Federal do Rio de Janeiro ethics committee (approval number: 191.694), and all participants provided written informed consent.
Eighty untrained consumers (45 females and 35 males) between 17 and 60 years of age were enrolled, and participated in sensory analysis of the ideal sweetness determination of steam-extracted jabuticaba juice. All consumers had reported that they consume fruit juice at least once a week. The ideal sweetness was determined using an unstructured (9.5 cm) just-about-right-scale, with intensity ranging from 0 (“extremely less sweet than ideal”) to 9.5 (“extremely sweeter than ideal”), with the “ideal” set at 4.75 cm. Steam-extracted jabuticaba juice was sweetened with sucrose at five concentrations: 1.0, 3.5, 6.0, 8.5, and 11%. Samples (25 mL) of each juice were presented at 10 °C in 50 mL plastic cups coded with three-digit random numbers. The samples were presented in a monadic sequential balanced order. The ideal sweetness was determined using a linear regression equation and the ideal sucrose concentration founded was used to sweeten steam-extracted jabuticaba juice for sensory acceptance and purchase intent tests.
Sensory acceptance and purchase intent tests
Sensory acceptance and purchase intent of sweetened steam-extracted jabuticaba juice were carried out with a different group of 80 untrained consumers (49 females and 31 males) aged between 17 and 79 years, with habitual consumption of fruit juice at least once a week. The following acceptance attributes were evaluated: overall impression, aroma, flavor, appearance, and texture, using an unstructured (9 cm) line scale, with intensity ranging from 0 (“extreme dislike”) to 9 (“extreme like”) and the “indifferent” response set at 4.5 cm. Samples (25 mL) of each juice were presented at 10 °C in 50 mL plastic cups coded with three-digit numbers, according to a sequential monadic presentation. The purchase intent was evaluated using a five-point structured scale, anchored at opposite ends with the expressions “I would definitely not buy” and “I would definitely buy” (Meilgaard et al. 2006).
Statistical analysis
Data were expressed as mean ± standard deviation. Linear regression of the ideal sweetness determination was performed using Excel software (Microsoft Office 2007, Redmond, Washington). Analysis of variance (one-way ANOVA) followed by Tukey’s multiple comparison post hoc test was performed for comparing steam-extracted jabuticaba juice stored at different days, using GraphPad Prism software for Windows, version 5.04 (GraphPad Software, San Diego, CA). The data was considered to be statistically significantly different where p < 0.05.
Results and discussion
Phenolic compounds and antioxidant capacity
Nine phenolic compounds were identified in steam-extracted jabuticaba juice: two hydroxybenzoic acid derivatives (gallic and ellagic acids), one hydroxycinnamic acid derivatives (m-coumaric acid), four flavonols (myricetin, myricetin-3-O-rhamnoside, quercetin and quercetin-3-O-rutinoside), and two anthocyanins (cyanidin-3-O-glucoside and delphinidin-3-O-glucoside) (Table 2). This was in accordance with previously published data for jabuticaba fruit (Wu et al. 2012; Inada et al. 2015), indicating that the phenolic compounds found in fresh fruit were also present in the steam-extracted jabuticaba juice.
The total phenolic contents of jabuticaba juice at day 0 (150.4 mg/L) was similar to that of orange juice (142.3–167.0 mg/L) (Klimczak et al. 2007) and grape juice produced by maceration (86.4–179.6 mg/L) (Lima et al. 2015). Cyanidin-3-O-glucoside, ellagic acid, quercetin-3-O-rutinoside, delphinidin-3-O-glucoside, and gallic acid were the predominant phenolics identified in jabuticaba juice, representing 45, 21, 14, 8.7, and 8.6% of total phenolic content, respectively (Table 2). m-Coumaric acid, myricetin, myricetin-3-O-rhamnoside, and quercetin were the minor phenolic compounds and together accounted for only 3.4% of the total phenolic compounds in jabuticaba juice.
A increase of 58.7% in the total phenolic compound content after 90 days of storage was observed. This may be due to 4.4-fold increase, on average, in the content of gallic and ellagic acids, as well as of 67% decrease, on average, in the content of cyanidin-3-O-glucoside and delphinidin-3-O-glucoside (Table 2; Fig. 1). The increasing contents of ellagic and gallic acids may have caused by the depolymerization of ellagitannins and gallotannins, which have previously been found in jabuticaba fruit (Wu et al. 2012; Plaza et al. 2016), but were not identified in the present study. The depolymerization of ellagitannins to ellagic acid during storage at 25 °C in processed berry products has previously been reported (Häkkinen et al. 2000; Zafrilla et al. 2001; Hager et al. 2010). Considering ellagitannins and gallotannins are not absorbed in their intact forms, the depolymerization of ellagitannins and gallotannins to ellagic and gallic acids may be favorable for consumer health. The increase in the gallic acid content may also be associated with the reduction of the content of delphinidin-3-O-glucoside, since gallic acid is a degradation product formed by the cleavage of the delphinidin B-ring (Sinela et al. 2017).
A significant decrease (70–90%) in the anthocyanin content of berry juices stored at room temperature has previously been reported (Hellström et al. 2013; Lopes et al. 2016; Mäkilä et al. 2016). A reduction in the anthocyanin content was expected, since these compounds were known to be highly unstable in aqueous solutions (Castañeda-Ovando et al. 2009), especially when stored at room temperature (Hellström et al. 2013; Mäkilä et al. 2016). Anthocyanins can be oxidized by copper and iron, and in a model medium containing a mixture of iron, copper and manganese, Sinela et al. (2017) observed a significant increase in the degradation rate of anthocyanins. The presence of these metals in jabuticaba fruit has previously been reported (Inada et al. 2015), and could explain the anthocyanin degradation observed during storage.
The antioxidant capacity (AC) of steam-extracted jabuticaba juice was 25.4 mmol Fe+2/L and 67.6 mmol Trolox/L as evaluated by FRAP and TEAC assays, respectively. While the AC, as evaluated by FRAP assay, increased by 39% after 90 days of storage, when evaluated by TEAC assay, an apparent decrease of 49% was observed (Table 2). Reque et al. (2014) also observed a 40% reduction in AC evaluated by TEAC assay in blueberry juice during storage. The disparity between AC evaluated by FRAP and TEAC assays may be a result of different assay mechanisms. The AC compounds can deactivate free radicals by two main mechanisms: HAT (hydrogen atom transfer) and SET (single electron transfer). While the FRAP method measures antioxidant activity via the HAT mechanism, the TEAC method measures both HAT and SET (Prior et al. 2005).
These results can be further explained by changes in the phenolic profile observed during storage, since each compound has its own specific AC, which varies according to its chemical structure (Rice-Evans et al. 1996). Recently, Plaza et al. (2016) observed that, despite cyanidin-3-O-glucoside being the major phenolic compound in jabuticaba peel (63%), only 25% of its AC was contributed to the total AC, whereas hydrolysable tannins (including ellagic acid and its derivatives), which represented only 25% of the total analyzed compounds, contributed to 49% of the total AC. Therefore, the increase in AC, as determined by the FRAP assay, may be associated with increasing gallic and ellagic acid content, and decrease in AC, as determined by TEAC assay, may be associated with the decreasing anthocyanin content during storage (Table 2). In fact, when the sum of the gallic and ellagic acids content vs. AC was plotted, the slope of the linear regression for the FRAP assay (0.0359 mmol Fe2+/L) was higher than that of TEAC assay (−0.1026 mmol Trolox/L), suggesting that the FRAP assay was more sensitive to the presence of these compounds. On the other hand, for the sum of the anthocyanin content, the TEAC assay was more sensitive with higher slope (0.5092 mmol Trolox/L) in comparison to that of the FRAP assay (−0.0868 mmol Fe2+/L).
Physicochemical analysis
The pH of steam-extracted jabuticaba juice (2.92–3.09) (Table 3) was similar to those described in the literature for different berry juices (Rein and Heinonen 2004; Verberic et al. 2014). After 30 days of storage, a slight increase in pH was observed (5.8%), with no further changes observed after 90 days (Table 3). Similar results were observed for juices produced from frozen berries stored for 7 months (Verberic et al. 2014). The pH of jabuticaba juice may contribute to undesirable conditions for the growth of microorganisms.
Total soluble solids (TSS) in steam-extracted jabuticaba juice (6.8% on average) (Table 3) were lower than those reported for raspberry (11%) and strawberry (10%) juices, and higher than those of lingonberry (5%) and cranberry (3%) juices (Rein and Heinonen 2004). The TSS of jabuticaba juice was not affected by storage for 90 days.
Instrumental color
The luminosity (\( L^{*} \)) of steam-extracted jabuticaba juice decreased (5.5–11%) after 60 days of storage, followed by a slight increase (2%) during storage up to 90 days (Table 3), indicating that the juice became lighter during storage. Similarly, Rein and Heinonen (2004) reported an increase in \( L^{*} \) of strawberry, raspberry, lingonberry, and cranberry juices after 103 days of storage. These authors also observed that the addition of anthocyanin-rich extracts to these juices decreased luminosity, making the juice darker due to the incremental increase of pigment molecules. Therefore, the increase of \( L^{*} \) in jabuticaba juice could be related to the degradation of anthocyanins observed during storage, as mentioned above.
During storage, a progressive decrease in \( a^{*} \) (6.9–35.5%) and \( b^{*} \) (11.2–36.7%) values (Table 3) was observed, indicating that the juice became greener and bluer, which could be due to the degradation of anthocyanins. These results were in accordance with those reported by Rein and Heinonen (2004), who observed a decrease in \( a^{*} \) and \( b^{*} \) values in different berry juices after 103 days of storage.
Based on the \( \Delta E^{*} \) values, which corresponds to the total color difference between the initial and final time points, steam-extracted jabuticaba juice had slight color differences during storage (Table 3). After 30 days of storage, the \( \Delta E^{*} \) of jabuticaba juice indicates a change in color that was not distinguishable by the human eye, and after 60 and 90 days of storage, the \( \Delta E^{*} \) of jabuticaba juice (3.6 on average) indicated a barely perceptible change in color (Obón et al. 2009).
Microbiological analysis
The microbiological quality of fresh jabuticaba fruit was evaluated, since heat-stable compounds, such as enzymes and toxins, produced by microorganisms can cause undesirable changes in the final product and/or contribute to the risk of illness for consumers. The presence of Salmonella was measured as an indicator for the presence of other pathogenic and coliform bacteria, which indicate poor sanitary conditions. Salmonella was not detected in samples of jabuticaba fruit, or in steam-extracted jabuticaba juice.
In the present study, the heat applied during steam-extraction ensured that the juice was microbiologically stable when stored at 25 °C for 90 days (Table 4). Although low numbers of microbes were detected, especially in the first days of storage, the steam-extracted jabuticaba juice was microbiologically stable up to 90 days. This suggested that the jabuticaba juice may contain constituents that contributed to unsatisfactory conditions for the multiplication of microorganisms, which include low pH (as mentioned above) and/or antimicrobial factors.
After inoculation with indicators and pathogenic microorganisms, the steam-extracted jabuticaba juice did not promote growth of E. coli or S. enteritidis, and after 2 and 6 h, respectively, the inoculated bacteria were not detectable (data not shown), suggesting potential antimicrobial activity. Although the inhibitory effect of Myrciaria jaboticaba against Salmonella growth has not been previously observed (Mohanty and Cock 2008; Silva et al. 2014), in the present study, the growth of S. enteritidis was inhibited after it was inoculated at a concentration simulating massive contamination. The antimicrobial activity of M. jaboticaba has been investigated against other microorganisms to explore the potential biotechnological applications of this fruit. The in vitro bactericidal, antifungal, and antimicrobial activity of M. jaboticaba against Streptocooccus mitis, S. oralis, S. salivarius, Lactobacillus casei, Candida albicans, C. krusei, Staphylococcus aureus, Listeria monocytogenes, and E. coli associated with the presence of phenolic compounds has been reported (Macedo-Costa et al. 2009; Diniz et al. 2010; Silva et al. 2014).
Sensory analysis
Ideal sweetness determination
The astringency provided by condensed tannic compounds extracted from the jabuticaba peel during steam-extraction of juice contrasts the sweet and slightly acid taste of the jabuticaba pulp (Wu et al. 2012). It is known that the addition of sucrose in tannic acid solutions causes an increase in sweetness and viscosity and thus can attenuate the perception of astringency (Lyman and Green 1990). Therefore, we performed an analysis to determine the ideal sweetness of steam-extracted jabuticaba juice to identify the best concentration of added sucrose.
The linear regression equation obtained for ideal sweetness (y = 0.3185x + 2.8271; R2 = 0.9654) allowed the determination of the optimum sucrose concentration of 6.05%. Despite the astringency of steam-extracted jabuticaba juice, this result was lower than that of other fruit juices, including mango (7.5%) and peach (10%) (Cardoso and Bolini 2007; Cadena and Bolini 2012). This may be due to the high concentration of simple sugars present in jabuticaba pulp (11.6 mg/100 mL of fresh pulp) (Inada et al. 2015).
Sensory acceptance and purchase intent
The 9-point hedonic scale is the most used scale to evaluate consumer acceptability and preference. However, there are some disadvantages to this scale, such as unequal interval spacing, lack of a zero point, and central tendency, because consumers avoid scoring extreme categories and there is little freedom for subjects to express their sensory opinion due to defined responses categories (Lim 2011). Therefore, in the present study, we used an unstructured line scale, which, while being more difficult to understand and use, offers greater freedom for consumers to express their sensory perceptions (da Silva et al. 2013).
The 9-point hedonic scale varies from 1 to 9 with a range of 8 possible scores, while the unstructured 9 cm scale ranges from 0 to 9 and therefore presents a 9-point interval. Thus, to evaluate the results obtained by unstructured linear scale evaluation using the terms of the 9-point hedonic scale, the values obtained in the sensory analysis were converted using the equation described by da Silva et al. (2013) [i.e. adjusted unstructured value = 1 + unstructured × (8/9)].
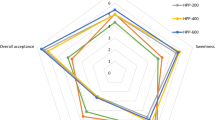
The highest scoring attributes of steam-extracted jabuticaba juice were appearance and texture (7.6 ± 1.2 and 7.4 ± 1.3, respectively), and correspond to the hedonic parameters “moderately like” and “like very much”. The high acceptance of appearance may be associated with the high anthocyanin content in jabuticaba juice (as described above), which contributes to its purplish color. The aroma of the juice scored reasonably high (6.5 ± 1.3), corresponding to the parameters “slightly like” to “moderately like”.
Taste scored the lowest (6.1 ± 1.9), corresponding to the hedonic parameter “slightly like”. The amount of sugar added to the juice may not have been sufficient to completely eliminate the astringency imparted by the jabuticaba peel tannins. Laaksonen et al. (2013) performed sensory and chemical analysis of blackcurrant juice and observed that all juice samples were perceived as remarkably sour and astringent. These authors observed a positive correlation by partial least squares regression between low pH and the presence of phenolic compounds with astringency.
In general, steam-extracted jabuticaba juice had a satisfactory overall impression, scoring 6.6 ± 1.7, which corresponds to the hedonic parameters “slightly like” to “moderately like”, as well as reasonable purchase intent average (3.9 ± 0.9), which corresponds to the expressions “I might buy” and “I would probably buy”. In addition, 60% of the consumers stated “I would probably buy” and “I would definitely buy” the steam-extracted jabuticaba juice if it was commercially available.
Conclusion
The anthocyanins cyanidin-3-O-glucoside and delphinidin-3-O-glucoside were the major phenolic compounds detected in steam-extracted jabuticaba juice, and during storage, the content of both anthocyanins progressively decreased. Despite this, the total phenolic compound content increased, which was caused by an increase in the contents of gallic and ellagic acids. The instrumental color changed during storage, which can be attributed to the loss of anthocyanins. Nevertheless, after 90 days of storage the \( \Delta E^{*} \) value of jabuticaba juice indicated that the color change would be barely distinguishable. Jabuticaba juice had high scores for sensory attributes and purchase intent, and remained microbiologically stable for 90 days. Moreover, the physicochemical characteristics of the juice resulted in a reduction in the number of inoculated E. coli and S. enteritidis. Our results indicate that the steam-extraction method may have potential application in the food industry. However, further studies are necessary in order to optimize the storage conditions for steam-extracted jabuticaba juice to best preserve its functional properties, and to identify the mechanisms involved in phenolic compound modifications during storage.
References
Amodio ML, Derossi A, Colelli G (2014) Modeling phenolic content during storage of cut fruit and vegetables: a consecutive reaction mechanism. J Food Eng 140:1–8. doi:10.1016/j.jfoodeng.2014.04.006
Association of Official Analytical Chemists (2000) Official methods of analysis, 17th edn. AOAC International, Gaitherburg
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76. doi:10.1006/abio.1996.0292
Cadena RS, Bolini HMA (2012) Ideal and relative sweetness of high intensity sweeteners in mango nectar. Int J Food Sci Technol 47:991–996. doi:10.1111/j.1365-2621.2011.02932.x
Cardoso JMP, Bolini HMA (2007) Different sweeteners in peach nectar: ideal and equivalent sweetness. Food Res Int 40:1249–1253. doi:10.1016/j.foodres.2007.08.004
Castañeda-Ovando A, Pacheco-Hernández ML, Páez-Hernández ME, Rodríguez JA, Galán-Vidal CA (2009) Chemical studies of anthocyanins: a review. Food Chem 113:859–871. doi:10.1016/j.foodchem.2008.09.001
da Silva AN, da Silva RCSN, Ferreira MAM, Minim VPR, da Costa TMT, Perez R (2013) Performance of hedonic scales in sensory acceptability of strawberry yogurt. Food Qual Prefer 30:9–21. doi:10.1016/j.foodqual.2013.04.001
Diniz DN, Macêdo-Costa MR, Pereira MSV, Pereira JV, Higino JS (2010) In vitro antifungal effect of leaves and bark of Myrciaria cauliflora berg. Extracts upon oral microorganisms. Rev Odontol UNESP 39:151–156
Downes FP, Ito K (2001) Compendium of methods for the microbiological examination of foods, 4th edn. American Public Health Association, Washington, D.C
Hager TJ, Howard LR, Prior RL (2010) Processing and storage effects on the ellagitannin composition of processed blackberry products. J Agric Food Chem 58:11749–11754. doi:10.1021/jf102964b
Häkkinen SH, Kärenlampi SO, Mykkänen HM, Heinonen IM, Törrönen AR (2000) Ellagic acid content in berries: influence of domestic processing and storage. Eur Food Res Technol 212:75–80. doi:10.1007/s002170000184
Hellström J, Mattila P, Karjalainen R (2013) Stability of anthocyanins in berry juices stores at different temperatures. J Food Comp Anal 31:12–19. doi:10.1016/j.jfca.2013.02.010
Inada KOP, Oliveira AA, Revorêdo TB, Martins ABN, Lacerda ECQ, Freire AS, Braz BF, Santelli RE, Torres AG, Perrone D, Monteiro MC (2015) Screening of the chemical composition and ocurring antioxidants in jabuticaba (Myrciaria jaboticaba) and jussara (Euterpe edulis) fruits and their fractions. J Funct Foods 17:422–433. doi:10.1016/j.jff.2015.06.002
Jimenez-Garcia SN, Guevara-Gonzalez RG, Miranda-Lopez R, Feregrino-Perez AA, Torres-Pacheco I, Vazquez-Cruz MA (2013) Functional properties and quality characteristics of bioactive compounds in berries: biochemistry, biotechnology, and genomics. Food Res Int 54:1195–1207. doi:10.1016/j.foodres.2012.11.004
Klimczak I, Małecka M, Szlachta M, Gliszczysńka-Swigło A (2007) Effect of storage on the content of polyphenols, vitamin C and the antioxidant activity of orange juices. J Food Comp Anal 20:313–322. doi:10.1016/j.jfca.2006.02.012
Laaksonen O, Mäkilä L, Tahvonen R, Kallio H, Yang B (2013) Sensory quality and compositional characteristics of blackcurrant juices produced by different processes. Food Chem 138:2421–2429. doi:10.1016/j.foodchem.2012.12.035
Lim J (2011) Hedonic scaling: a review of methods and theory. Food Qual Prefer 22:733–747. doi:10.1016/j.foodqual.2011.05.008
Lima MS, Dutra MCP, Toaldo IM, Corrêa LC, Pereira GE, de Oliveira D, Bordignon-Luiz MT, Ninow JL (2015) Phenolic compounds, organic acids and antioxidant activity of grape juices produced in industrial scale by different processes of maceration. Food Chem 188:384–392. doi:10.1016/j.foodchem.2015.04.014
Lopes MLM, Miguel MAL, Fialho E, Valente-Mesquita VL (2016) Grape juice obtained using steam extraction and other small-scale extraction methods: phenolic content, antioxidant capacity and stability during storage. Int J Food Sci Tech 51:1696–1702. doi:10.1111/ijfs.13144
Lyman BJ, Green BG (1990) Oral astringency: effects of repeated exposure and interactions with sweeteners. Chem Senses 15:151–164. doi:10.1093/chemse/15.2.151
Macedo-Costa MR, Diniz DN, Carvalho CM, Pereira MSV, Pereira JV, Higino JS (2009) Eficácia do extrato de Myrciaria cauliflora (Mart.) O. Berg. (jabuticabeira) sobre bactérias orais. Rev Bras Farmacogn 19:564–571. doi:10.1590/S0102-695X2009000400010
Mäkilä L, Laaksonen O, Alanne A-L, Kortesniemi M, Kallio H, Yang B (2016) Stability of hydroxycinnamic acid derivatives, flavonol glycosides, and anthocyanins in black currant juice. J Agric Food Chem 64:4584–4598. doi:10.1021/acs.jafc.6b01005
Meilgaard MC, Carr BT, Civille GV (2006) Sensory evaluation techniques, 4th edn. CRC Press, Boca Raton
Mohanty S, Cock I (2008) Evaluation of the antibacterial activity and toxicity of Myrciaria caulifloria methanolic leaf and fruit extracts. Internet J Microbiol 7:1–21
Obón JM, Castellar MR, Alacid M, Fernández-López JA (2009) Production of red-purple food colorant from Opuntia stricta fruits by spray drying and its application in food model systems. J Food Eng 90:471–479. doi:10.1016/j.jfoodeng.2008.07.013
Plaza M, Batista ÂG, Cazarin CB, Sandahl M, Turner C, Östman E, Maróstica Júnior MR (2016) Chacterization of antioxidant polyphenols from Myrciaria jaboticaba peel and their effects on glucose metabolism and antioxidant status: a pilot clinical study. Food Chem 211:185–197. doi:10.1016/j.foodchem.2016.04.142
Prior RL, Wu X, Schaich K (2005) Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 53:4290–4302. doi:10.1021/jf0502698
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237. doi:10.1016/S0891-5849(98)00315-3
Rein MJ, Heinonen M (2004) Stability and enhancement of berry juice color. J Agric Food Chem 52:3106–3114. doi:10.1021/jf035507i
Reque PM, Steffens RS, Jablonski A, Flôres SH, Rios AO, Jong EV (2014) Cold storage of blueberry (Vaccinium spp.) fruits and juice: anthocyanins stability and antioxidant activity. J Food Comp Anal 33:111–116. doi:10.1016/j.jfca.2013.11.007
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956. doi:10.1016/0891-5849(95)02227-9
Silva MC, Souza VB, Thomazini M, Silva ER, Smaniotto T, Carvalho RA, Genovese MI, Favaro-Trindade CS (2014) Use of the jabuticaba (Myrciaria cauliflora) depulping residue to produce a natural pigment powder with functional properties. LWT Food Sci Technol 55:203–209. doi:10.1016/j.lwt.2013.08.026
Sinela A, Rawat N, Mertz C, Achir N, Fulcrand H, Dornier M (2017) Anthocyanins degradation during storage of Hibiscus sabdariffa extract and evolution of its degradation products. Food Chem 214:234–241
Verberic R, Stampar F, Schmitzer V, Cunja V, Zupan A, Koron D, Mikulic-Petkovsek M (2014) Changes in the contents of anthocyanins and other compounds in blackberry fruits due to freezing and long-term frozen storage. J Agric Food Chem 62:6926–6935. doi:10.1021/jf405143w
Vidigal MCTR, Minim VPR, Carvalho NB, Milagres MP, Gonçalves ACA (2011) Effect of a health claim on consumer acceptance of exotic Brazilian fruit juices: Açaí (Euterpe oleracea Mart.), Camu-camu (Myrciaria dubia), Cajá (Spondias lutea L.) and Umbu (Spondias tuberose Arruda). Food Res Int 44:1988–1996. doi:10.1016/j.foodres.2010.11.028
Wang Z, Zhang M, Wu Q (2015) Effects of temperature, pH, and sunlight exposure on the color stability of strawberry juice during processing and storage. LWT Food Sci Technol 60:1174–1178. doi:10.1016/j.lwt.2014.09.015
Wu S-B, Dastmalchi K, Long C, Kennelly EJ (2012) Metabolite profiling of jaboticaba (Myrciaria cauliflora) and other dark-colored fruit juices. J Agric Food Chem 60:7513–7525. doi:10.1021/jf301888y
Zafrilla P, Ferreres F, Tomás-Barberán FA (2001) Effect of processing and storage on the antioxidant ellagic acid derivatives and flavonoids of red raspberry (Rubus idaeus) jams. J Agric Food Chem 49:3651–3655. doi:10.1021/jf010192x
Funding
The authors gratefully thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Universidade Federal do Rio de Janeiro (UFRJ), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (Brazil) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inada, K.O.P., Duarte, P.A., Lapa, J. et al. Jabuticaba (Myrciaria jaboticaba) juice obtained by steam-extraction: phenolic compound profile, antioxidant capacity, microbiological stability, and sensory acceptability. J Food Sci Technol 55, 52–61 (2018). https://doi.org/10.1007/s13197-017-2769-3
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-017-2769-3