Abstract
In this study, effects on the yield and the quality of product properties of carrot juice produced with lactofermentation and added citric acid, and with and without total enzymatic liquefaction (Pectinex Ultra SP-L) were investigated. Samples were stored at room temperature for 6 months and analytical changes which might occur were determined at day 0 and after 2, 4 and 6 months by quality control tests. In enzyme-treated samples, the yield and the quality of mineral incrementation were determined. Accordingly, ash and water-soluble dry matter contents were increased in the samples. Galacturonic acid formed after enzymatic breakdown of pectin in the raw material increased the total acidity of the samples. Sensorial tests of the product were also carried out. According to the test results, the products produced with enzyme treatment at day 0, after 2 and 4 months storages were found to be the ones mostly preferred. However the samples without enzyme treatment were preferred by the panelists after long-term (6 months) storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carrot juice has a high nutritional value as it is an important source of carotene. Conventional carrot juice production with mechanical pressing of the mash results in a slightly cloudy juice and a carotene-rich pomace, as the carotene insoluble in water remains in the carrot cells. Mash treatments with pectolytic enzymes should not only increase the yield, but also the carotene content of carrot juice [1]. With this process protopectin and pectin are degraded into galacturonic acid, thereby increasing uronic acid, methanol content and the total acidity of product [2]. During the mash enzyming, high- and low-esterified pectin are depolymerized and cellulose, hemicellulose, starch and protein are also partially hydrolysed, thus decreasing the viscosity to a greater extent [3].
It is difficult to preserve vegetable juices because of low acidity and high concentration of spoilage and spore-forming bacteria. Therefore vegetable juice could be produced either fermented or acidified with citric acid. With this process, the products have a desirable taste and low pH. At the same time, the thermal preservation of the product would be easy and the shelf life increased. However the analytical characteristics and the nutritional value of the product can be different from each other [4, 5]. The desirable properties of fermented vegetable juices can be achieved by choosing Lactobacillus strains suitable for the lactic acid fermentation of individual raw materials. The criteria used to gauge a strain’s suitability are as follows: rate and total production of acids, change of pH, and loss of nutritional substances etc. [6]. With the lactofermentation process, a controlled fermentation of the vegetable mash is obtained by using a starter culture such as Lactobacillus plantarum, Lactobacillus casei, Lactobacillus brevis, Lactobacillus xylosus and Lactobacillus delbrueckii [4, 7].
In this paper, results are presented from a study on the determination of storage effects on the chemical and sensorial properties of the products after 2, 4 and 6 months storage at room temperature.
Material and methods
Starter culture
RSKK 1062 L. plantarum was supplied from Refik Saydam Institute in Ankara-Turkey. L. plantarum RSKK 1062 was used to obtain homofermentative fermentation. For that purpose, L. plantarum culture with an initial concentration of 3×105 CFU/g was inoculated onto carrot mash and incubated for the lactofermentation at 35 °C for 16 h.
Enzyme
Pectinex Ultra SP-L from Aspergillus aculeatus was obtained from Novo Nordisk (Switzerland) and stored at 4 °C. Pectinex Ultra SP-L pectolytic enzyme was used for the enzymatic maceration of carrot mashes. Enzyme dosage and incubation time were within the recommended range at 0.1% Pectinex Ultra SP-L in the mash and 60 min at 35 °C.
Carrot juices
Vegetable juices were produced using carrots (Daucus carota L.) from markets in Ankara, Turkey. Batches of 15 kg of carrots were used. After the removal of the undesirable parts of the carrot, the material was ground using a food processor and carrot mash was obtained. Heat treatment of the mash was carried out for 5 min at 85–90 °C and the mash was cooled to 32 °C. Juices were processed on a laboratory scale as shown in the flow diagram (Fig. 1).
To assess the effect of acidification, citric acid (50% w/v) was added into the mash to a final juice pH of 4.5. To obtain the lactofermented carrot juice, the mash was inoculated with L. plantarum RSKK 1062 (3×105 CFU/g) and incubated at 35 °C for 16 h. Carrot juices were filled into the bottles to 180 mL and the lids were closed. These bottles were then pasteurized for 30 min in boiling water. Bottles were stored for 6 months at room temperature.
Reagents
Trans-β-carotene was purchased from Sigma (St. Louis, USA) and other chemicals from Merck (Germany).
Characterization of juices
HPLC analysis of β-carotene was performed according to Bushway [8]. A Varian model 9010 liquid chromatograph was used. It was equipped with a Rheodyne model 7125 injector, a 10 μL sample loop and a Hewlett-Packard Series 1100 diode array detector set at 458 nm and 487 nm. A stainless, 250×4.6 mm (i.d.) C18–4115 (5 μm) column (Hicrom NC 120) operating at ambient temperature was used. Acetonitrile-methanol-stabilized tetrahydrofuran (20:65:15) was used as the mobile phase with a flow rate of 1.0 mL/min.
The lactic acid [D(-), L(+) and DL lactic acid] contents of the samples were determined enzymatically following the manufacturer’s recommended procedure [9]. The samples were measured by means of absorbance readings taken at 340 nm (Shimadzu UV-2101 PC).
The mineral contents (K+, Na+, Ca2+, Mg2+ and Fe2+) of samples were measured using atomic absorbtion spectrophotometry (Shimadzu Model AA-660) according to Ross and Price [10].
Total acidity [11], pH value [12], reducing sugar, total sugar and sucrose contents [12], soluble solids [12], color intensity [13] and ash [14] were determined. Sensory evaluation was performed by describing the odor, appearance and flavor of various samples direct after the filling and after 2, 4 and 6 months of storage periods. The panel consisted of ten judges.
Results and discussion
Table 1 shows the analytical properties of carrot juice samples. Generally, enzymatic liquefaction of mash increased the juice yield. In the mash liquefaction, the breakdown of pectin and cellulose together are improved by polygalacturonase, pectinlyase, pectinesterase and cellulase activity [7, 15]. By the effects of enzymes on carrot cell wall, neutral sugars such as arabinose, galactose, rhamnose and xylose, which are attached to pectic substances, are released with the pectic substances of the cell wall and become soluble [16]. However, undissolved polysaccharide fragments are also found in the mash [15]. Özdemir-Alper and Acar [1] found that the yield of carrot juice produced with total liquefaction was increased about 8–14% and the mineral contents (Ca2+, K+, Na+ and Mg2+) of the product were also increased. Magnesium contents of lactofermented juices were found to be lower than other samples because lactic acid bacteria use magnesium for their growth. When the samples were examined individually with respect to their components, the ash contents of carrot juices produced by pectolytic enzyme treatment were found to be higher than the samples produced without enzyme treatment (Table 1). In enzyme treatments, it was determined that minerals in raw materials pass to the vegetable juices in higher amounts due to the breakdown of vegetable tissue after liquefaction of the mash, leading to this increase in the ash content of in enzyme-treated samples. The effect of enzyme treatment on ash and mineral contents was found to be significant.
The enzyme treatment, either with fermented or acid-added samples had a negative effect on β-carotene contents of samples. However, it is thought that this is a side-effect as a result of the increase in juice yield and soluble solids of the enzyme-treated samples.
Total acidity has been found to be higher in enzyme-treated samples (Table 1). The increase in total acidity, represented the main variation in the juice contents; this was due to the increase in the galacturonic acid content. This increase corresponded to the extraction of galacturonic acid present in the form of pectin chains in the cell walls [17].
Because of some components in carrot juice that show buffer properties no apparent changes were observed in pH values of the samples (Table 1).
Some of the sucrose in carrot is inverted because of the enzymatic liquefaction. Therefore, the sucrose amount in enzyme-treated samples was found to be lower (Table 1).
β-carotene concentrations were found to be higher in fermented carrot juices, thus lactic acid fermentation was found to have a positive effect on β-carotene extraction from the mash (Table 1).
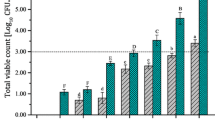
According to the sensory evaluation of samples, in 0, 2 and 4 months, the samples produced with enzyme treatment were preferred by the panelists (Fig. 2). However after 6 months, the enzyme-treated samples were not preferred to the non-enzyme-treated samples.
The project was completed with the determination of the storage effect on the chemical and sensorial properties of the products after 2, 4 and 6 months of storage at room temperature. While the production methods did not affect the β-carotene degradation, the storage period for 2, 4 and 6 months were found todo so (Fig. 3, Fig. 4, Fig. 5).
In conclusion, the use of total liquefying enzyme such as Pectinex Ultra SP-L in the production of lactofermented and citric-acid-added carrot juices increases the yields. By the effects of enzymes on carrot cell wall, neutral sugars such as arabinose, galactose, rhamnose and xylose, which are attached to pectic substances, are released with the pectic substances of the cell wall and become soluble. In fact, the yield in carrot juice produced with enzymatic treatment was increased from about 5.65% to 8.52% and the mineral substances (Ca2+, Mg2+, Fe2+, K+, Na+) of carrot juices were also increased.
It is difficult to preserve vegetable juices such as carrot juice because of their low acidity. The acidity of carrot juice (pH~6.2) can be adjusted by the addition of citric acid or the lactofermentation method to pH 4.5. With these processes carrot juice can be preserved by pasteurization as occurs for low pH fruit juices, increasing shelf life.
Enzymatic treatment did not show a positive effect on β-carotene content. However, increasing juice yield as a result of degradation of some components in carrot cell wall such as pectin and cellulose with enzymatic treatment caused a relative decrease in β-carotene. It is thought that, carrot tissue is softened with the growth of lactic acid bacteria, thus making the β-carotene extraction from tissue easier.
It was determined that storage caused a decrease in β-carotene. In our experiments, since carrot juices were produced under laboratory conditions, the air in the head space could not be removed. It is thought that oxygen in head space caused the degradation of β-carotene and that for this reason, color intensity decreased during storage.
While there were no significant changes in other analytical properties during storage, the panelists’ preferences decreased for carrot juices produced with enzymatic treatment and stored for 6 months.
References
Özdemir-Alper N, Acar J (1996) Flüssiges Obst 63:521–523
Fauquembergue P, Grassin C (1996) Fruit Process 12:490–494
Schmitt R (1988) Flüssiges Obst 55:309–310
Liepe HU, Junker M (1984) Flüssiges Obst 50:304–307
Acar J (1998) Flüssiges Obst 4:196–198
Karovičová J, Drdák M, Greif G, Hybenova E (1999) Eur Food Res Technol 210:53–56
Schobinger U (1987) Frucht Gemuesesaefte, 2. Auflage, Verlag Eugen, Ulmer
Bushway RJ (1986) J Agric Food Chem 34:409–412
Boehringer-Mannheim(1997) Boehringer-Mannheim enzymatic bioanalysis. Determination of D- and L-lactic acid in foodstuffs
Ross JHT, Price WJ (1970) J Sci Food Agric 21:506–507
Anonymous (1968) Bestimmung der Titrierbaren Sauren. IFU Analysen, No.3
Cemeroğlu B (1992) Meyve ve Sebze İşleme Endüstrisinde Temel Analiz Metodları. Biltav Yayınları, Ankara
Tanner H, Brunner HR (1979) Getränke-Analytik. Verlag Heller, Schwabisch Hall, Germany
Anonymous (1988) TS 1880, Sirke Standardı. TSE, Ankara
Ekşi A (1988) Meyve suyu durultma tekniği. Gıda Teknolojisi Derneği, Yayın No:9, Ankara
Grassin C, Fauquembergue P (1993) Fruit Process 7:242–245
Schobinger U, Dürr P, Akersson A (1981) Alimenta 20:37–42
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Demir, N., Acar, J. & Bahçeci, K.S. Effects of storage on quality of carrot juices produced with lactofermentation and acidification. Eur Food Res Technol 218, 465–468 (2004). https://doi.org/10.1007/s00217-004-0883-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-004-0883-8