Abstract
The use of non-Saccharomyces yeasts for the production of quality wine has become increasingly frequent in recent years. Several studies of the influence of Torulaspora delbrueckii and Metschnikowia pulcherrima on chemical composition have been reported, especially in aspects concerning aroma. The aim of this article was to study the influence of sequential inoculation of these non-Saccharomyces yeasts and Saccharomyces cerevisiae on the composition and quality of base wine for sparkling wine production. The results indicate that sequential inoculation with non-Saccharomyces yeasts may be an interesting tool for obtaining base wines with different characteristics. On the one hand, T. delbrueckii Biodiva™ strain increased glycerol concentration, reduced volatile acidity and exerted a positive effect on foaming properties improving foamability (Hm) and foam persistence (Hs). On the other hand, M. pulcherrima Flavia® strain also increased foam persistence (Hs) and changed the aromatic profile, increasing smoky and flowery notes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Alcoholic fermentation is the anaerobic transformation of sugars, mainly glucose and fructose, into ethanol, carbon dioxide and other secondary products. This process, which is carried out by yeast, makes it possible to turn grape juice into wine. However, grape must is a non-sterile substrate that contains several types of yeasts and bacteria which can grow and consequently affect the final wine composition and quality. The presence of different yeasts in grape juice depends on several factors, such as the grape cultivar, the grape’s maturity, pesticide treatments, the development of fungal plagues, climatic conditions and viticultural practices [1]. However, other factors are also important. All contact of grapes and must during harvest, transport and in particular, during winery operations significantly influence the final distribution of yeasts at the beginning of alcoholic fermentation [2].
Numerous studies have been performed to isolate and identify the yeasts present on the surface of grapes and winery equipment [1, 3]. Other studies have focused on the quantitative and qualitative changes in different yeast populations during alcoholic fermentation [4–6]. In general, these studies have confirmed that during spontaneous alcoholic fermentation, grape must is transformed into wine by the sequential activity of different yeast species. Under these conditions, fermentation generally begins with the growth of weakly fermentative yeast species belonging to the genera Candida, Debaryomyces, Hanseniaspora, Metschnikowia, Pichia, Torulaspora and Zygosaccharomyces [7]. These species, known collectively as non-Saccharomyces yeasts, are practically undetectable after 2 or 3 days of fermentation [2, 3]. As these yeasts disappear, highly fermentative strains of the species Saccharomyces cerevisiae begin to multiply until they become solely responsible for alcoholic fermentation [3]. Evidently, the succession of these different yeast species during alcoholic fermentation influences the final composition of the wine in some of their organoleptic key compounds in such a way that depending on which yeasts have grown, it may be positive in some cases or negative in others [8, 9]. Since some of these non-Saccharomyces yeasts can produce several negative by-products, wineries usually add sulphur dioxide to the grape juice to prevent their undesirable growth. Moreover, most wineries inoculate selected dry yeast (S. cerevisiae) in order to guarantee alcoholic fermentation without any deviation. However, other wineries, especially traditional wine cellars, still use spontaneous alcoholic fermentation, because they believe that the sequential development of non-Saccharomyces and Saccharomyces yeasts confers greater complexity on wines.
The role of non-Saccharomyces yeasts in winemaking has been reassessed in recent years [10]. Some studies have looked at the use of controlled mixed fermentations using non-Saccharomyces and Saccharomyces yeast species [11, 12]. These studies have shown that mixed fermentations using controlled inoculations of S. cerevisiae starter cultures and non-Saccharomyces yeasts are a feasible way of improving wine complexity, because this practice has been observed that increases the typicality of wines and ensures a correct alcoholic fermentation [13]. This practice has also been reported as being able to increase some desirable metabolites, such as some acetate esters [14] and glycerol [15, 16]. Moreover, some non-Saccharomyces yeasts have been reported as being able to release more polysaccharides than S. cerevisiae strains [17].
Hanseniaspora and Torulaspora genera have also been reported as improving the presence of some interesting aromas [18, 19] and reducing volatile acidity [16, 20]. A non-Saccharomyces yeast, Torulaspora delbrueckii, formerly known as Saccharomyces rosei or Saccharomyces delbrueckii, has recently received considerable attention from the wine industry. This yeast is a typical representative of the natural microbiota on the grape’s surface and just like S. cerevisiae can be found in most wine-producing regions [21].
Torulaspora delbrueckii has previously been suggested for the vinification of musts low in sugar and acidity, and it has been used for the production of red and rosé wines in Italy [22] and for Sauvignon Blanc in South Africa [12]. More recently, [19] used T. delbrueckii in pure cultures and cultures mixed with S. cerevisiae yeast to ferment botrytised musts. This study demonstrated that a mixed culture of T. delbrueckii and S. cerevisiae was the best combination for improving the analytical profile of sweet wines because it considerably reduced the production of volatile acidity (53 %) and acetaldehyde (60 %). The presence of T. delbrueckii has also been reported to increase the presence of some volatile compounds [23] because of its higher β-glucosidase activity [19]. In addition, some strains of T. delbrueckii also appeared to have a greater polysaccharide production capacity than S. cerevisiae [17, 24].
Another non-Saccharomyces yeast which is attracting attention from the wine industry is Metschnikowia pulcherrima. This yeast is generally present during the early stages of grape juice fermentation [25] and has also shown some relevant detectable effects in wine composition. In specific terms, M. pulcherrima is a high producer of β-glucosidase [26] and its presence in mixed cultures can decrease the volatile acidity and increase the production of medium-chain fatty acids, higher alcohol, esters, terpenols and glycerol [15, 27].
Some authors have also reported that M. pulcherrima can decrease the titratable acidity of the final wines [15, 28]. This effect on the total acidity can be considered positive or negative, depending on the initial acidity level of the grape juice [28]. It has also been reported that M. pulcherrima has a higher capacity to release polysaccharides during alcoholic fermentation compared to S. cerevisiae [24]. More recently, sequential fermentations using M. pulcherrima and S. cerevisiae have been reported as producing wines with a significantly lower ethanol concentration [16].
Another recently described interesting aspect is that M. pulcherrima has an antimicrobial activity. The presence of M. pulcherrima does not influence the growth of S. cerevisiae but has a broad and effective antimicrobial action on undesired wild spoilage yeasts, such as Brettanomyces/Dekkera [29].
Today, sparkling wines account for an important percentage of the high-quality wine market and they are the type of wines for which sales have increased most in recent years [30]. There are significant differences between sparkling and still wines, of which the presence of a high carbon dioxide concentration (10–12 g/L) is the most important. The persistence of the foam of sparkling wines is one of the major factors affecting their visual organoleptic characteristics [31]. The ability of sparkling wines to form a stable collar is considered a criterion of quality by consumers [32]. It has been reported that the proteins and mannoproteins composition of base wines exert a major influence on their foaming properties [31, 32]. Some authors have studied the influence of the different S. cerevisiae strains during the first and second fermentation of sparkling wines, as well as during the ageing period [33, 34]. However, to our knowledge, there are no previous specific studies of the influence of sequential inoculation of non-Saccharomyces and S. cerevisiae during the first fermentation on the chemical composition and foaming properties of base wines in sparkling wine production.
The aim of this study was to determine the effect on the organoleptic quality and analytical composition of base wines obtained by sequential inoculation of two different non-Saccharomyces (M. pulcherrima and T. delbrueckii) and S. cerevisiae during the first fermentation of base wines of the AOC Cava.
Materials and methods
Chemicals
All products were of high purity and suitable for high-performance liquid chromatography (HPLC). Absolute ethanol, hydrochloric acid, l-tartaric acid, sodium hydroxide, d-glucose and d-fructose were purchased from Panreac (Barcelona, Spain). Ammonium acetate, ammonium chloride, ammonium formate, l-glutamine, l-aspargine, l-alanine, l-isoleucine, l-methionine, l-threonine, l-cysteine, l-tyrosine, l-valine, glycine, l-phenylalanine, glutamicacid, l-serina, l-leucine, l-histidine, l-tryptophan and l-lysine were provide by Sigma−Aldrich (Steinheim, Germany). Difco yeast nitrogen base w/o amino acids and ammonium sulphate were purchased from B. D. Becton, Dickinson and Company (Franklin Lakes, USA). Yeast extract, peptone, agar and lysine agar were purchased from Oxoid (Barcelona, Spain).
A Shodex P-82 pullulan calibration kit (P-5, Mw = 5.9 kDa; P-10, Mw = 11.8 kDa; P-20, Mw = 22.8 kDa; P-50, Mw = 47.5 kDa; P-100, Mw = 112 kDa; P-200, Mw = 212 kDa; P-400, Mw = 404 kDa; P-800, Mw = 788 kDa) was obtained from Waters (Barcelona, Spain), and a pullulan 1.3 kDa and four BioChemika dextrans (12, 25, 50 and 80 kDa) were obtained from Fluka (St. Louis, MO, USA). The polysaccharides used as external standards for quantification were pectins from citrus fruit (≥90 %), and dextrans synthesised by Leuconostoc mesenteroides (≥99.9 %) were provided by Sigma-Aldrich (St. Louis, MO, USA).
The protein used as an external standard for quantification was bovine serum albumin (BSA) purchased from Sigma-Aldrich (St. Louis, MO, USA).
All solutions were filtered beforehand through 0.22 µm acetate cellulose filters (Millipore GSE).
Grape samples
The study was carried out with grapes of the Vitis vinifera cv. Macabeo. The grapes were manually picked from vineyards of Juvé & Camps SL in Espiells [AOC Cava; 41° 27′ 1.8972″ (N) and 1°49′ 6.6216″ (E)] during the 2013 vintage. The grapes were immediately transported to the experimental winery of the Oenology Faculty of the Rovira i Virgili University in Constantí (Tarragona, Spain).
Winemaking procedure
The grapes (1,200 kg) were crushed with an automatic crusher (Delta F2, Bucher Vaslin SA, Chalonnes sur Loire, France) and pressed with a pneumatic press (Marzola, Navarrete, La Rioja, Spain) to obtain a yield of 0.6 L/kg of grape juice. Since non-Saccharomyces yeasts are usually very sensitive to high concentrations of sulphur dioxide, the grape juice was immediately sulphited with a relatively low dose (30 mg/L of potassium disulphite) and filtered with a rotary vacuum filter (Della Toffola, Treviso, Italia). Seventy litres of filtered grape juice was then pumped to each of nine stainless steel tanks with a capacity of 100 L. These tanks were equipped with a jacket for temperature control. The initial density of the grape juice was 1,071. Three tanks were immediately inoculated with 250 mg/L of a commercial S. cerevisiae yeast strain considered as control (QA23®, Lallemand Inc., Montreal, Canada). Another three tanks were initially inoculated with 250 mg/L of a commercial T. delbruekii (Biodiva™, Lallemand Inc., Montreal, Canada), and 24 h later, when the density had fallen to around ten units, these tanks were reinoculated with 250 mg/L of the control S. cerevisiae yeast strain (QA23®, Lallemand Inc., Montreal, Canada). Finally, the remaining three tanks were initially inoculated with 250 mg/L of a commercial M. pulcherrima (Flavia®, Lallemand Inc., Montreal, Canada), and 36 h later, these tanks were reinoculated with 250 mg/L of the control S. cerevisiae yeast strain (QA23®, Lallemand Inc., Montreal, Canada). All the grape juices were supplemented with 400 mg/L of a yeast fermentation nutrient (Nutrient Vit Blanc, Lallemand Inc., Montreal, Canada). All vinifications were performed at 18 ± 1 °C. Once the alcoholic fermentations were finished, the wines were racked and sulphited (40 mg/L of potassium disulphite). All the wines were maintained in airtight vessels at 4 °C until the analysis, which took place three months later, when the wines were stable against potassium hydrogen tartrate precipitation [35]. No treatment with bentonite was performed because base wines were nearly stable [36], and the use of bentonite as riddling agent during second fermentation guarantees the stability of the future sparkling wines [32].
Synthetic grape juice fermentations
Similar fermentations were also performed by triplicate in a synthetic grape juice reproducing the experimental conditions developed in the natural grape juice. The aim of this experimental approach was to study the effect of sequential inoculations in a simpler matrix. A modification of the synthetic grape juice described by Riou et al. [37] was used. The only change was in the sugar concentration that was 170 g L (85 g/L glucose and 85 g/L fructose). The yeast assimilable nitrogen (YAN) content in grape must was 300 mg N/L, as well as 120 mg N/L ammoniacal nitrogen (ammonium chloride), and 180 mg N/L amino acids (4.65 mg N/L asparagine, 11.39 mg N/L glutamic acid, 10.40 mg N/L serine, 47.87 mg N/L glutamine, 3.05 mg N/L histamine, 3.40 mg N/L glycine, 8.87 mg N/L threonine, 29.60 mg N/L arginine, 22.90 mg N/L alanine, 1.51 mg N/L tyrosine, 2.41 mg N/L cysteine, 5.29 mg N/L valine, 2.93 mg N/L methionine, 11.95 mg N/L tryptophan, 3.20 mg N/L phenylalanine, 3.47 mg N/L isoleucine, 5.14 mg N/L leucine, 1.62 mg N/L lysine). This synthetic grape juice also contained 1.70 g/L of the yeast nitrogen base (YNB) w/o amino acids and ammonium and 4 g/L tartaric acid, and adjusted to pH 3.2 with sodium hydroxide.
Wine sampling and yeast isolation during fermentations
Samples were taken from each vat during the vinification process at the beginning and the end of alcoholic fermentation. The initial point was 24 h after first inoculation, and the final point was when the density was lower than 995. Fifty-microlitres sterile plastic flasks were then filled with the must/wine from the centre of the vessels, kept under refrigerated conditions and transported to the laboratory. After dilutions, the samples were spread on two culture media plates. The first was YPD (yeast extract 1 % (w/v), peptone 1 % (w/v), glucose 2 % (w/v) and agar 2 % w/v) which allows all yeasts to grow [38]. The second culture medium was lysine agar (LYS), which is unable to support the growth of S. cerevisiae [39]. The plates were incubated for 48 h at 28 °C. Yeast colonies were counted, and ten colonies were randomly selected from each medium and from each fermentation sample for identification.
DNA extraction and identification of yeast colonies
Yeast identification was carried out by means of PCR–RFLP of the 5.8S-ITS ribosomal region, as previously described [40]. Specific differentiation between C. zempellina and C. stellata was employing the restriction enzyme MboI [41]. The results are presented as the arithmetic mean of the percentage of imposition of the three replicates.
Standard wine analysis
The analytical methods recommended by the OIV (2014) [42] were used to determine the ethanol content, titratable acidity, pH, volatile acidity and glycerol.
Measurement of foaming properties
The samples were centrifuged (10 min at 12,000 g) and tempered at 18 °C for 24 h before analysis. All foam measurements were taken using the Mosalux procedure [43]. A glass cylinder placed on a glass frit was filled with 100 mL of the sample. Carbon dioxide was then injected into the glass cylinder through the glass frit, with a constant gas flow of 115 mL/min under a constant pressure of 100 kPa.
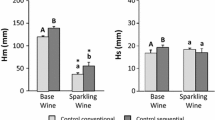
Two parameters were measured: Hm corresponding to the maximum height reached by the foam after CO2 injection through the glass frit and Hs corresponding to the stable height during CO2 injection. Hm represented the foamability (the wine’s ability to foam), and Hs represented the foam stability (persistence of the foam collar or the wine’s ability to have a stable foam). Both parameters, Hm and Hs, are expressed in mm. All measurements were determined in triplicate.
Polysaccharides extraction from samples
The samples were processed using the methodology described by [44]. Briefly, 10 mL of wine samples was centrifuged for 20 min at 10,000×g in a Biofuge Primo (Heraeus, Hanau, Germany). The supernatant was concentrated to a final volume of 2 mL using a vacuum evaporator (Univap 100ECH, Uniequip, Martinsried, Germany). The total soluble polysaccharides were precipitated by the addition of 10 mL cold acidified ethanol (HCl 0.3 M in absolute ethanol) and kept for 24 h at 4 °C. The samples were then centrifuged (10,000×g, 15 min) and the supernatants discarded. Finally, the precipitates were dissolved in 1 mL of ultra-pure water, frozen to −20 °C and freeze-dried using a lyophilizer (Christ Alpha 1–4, Martin Christ, Osterode am Harz, Germany).
Determination of polysaccharides by HRSEC-RID
The soluble fractions were analysed by high-resolution size-exclusion chromatography (HRSEC) [44] in order to determine the molecular distribution and quantify the proteins obtained from samples. The lyophilised samples were resuspended in 1 mL of 50 mM ammonium formate, filtered through 0.22 um acetate cellulose filters (Millipore GSE), and then, 100 μL was injected into the chromatographic system. The analyses were carried out in an HPLC Agilent 1200 Series system (Agilent Technologies Inc., Santa Clara, USA) with a refractive index detector. Separation was carried out at 20 °C, using two different Shodex gel permeation HPLC columns (OHpak SB-803 HQ and SB-804 HQ, 300 mm × 8 mm I.D.; Showa Denko, Japan). The mobile phase consists of an aqueous solution of 50 mM ammonium formate applied with a constant flow of 0.6 mL/min for 60 min, and the cell RID temperature was 35 °C. The molecular weight distribution of the wine fractions was followed by calibration with pullulan and dextran standards of different molecular weights (see above). The polysaccharides were quantified according to the peak area for each fraction, using the external standard method with pectin and dextran commercial standards. The calibration curve was obtained by injection of standard solutions, under the same conditions as for the samples analysed, in the range between 0 and 2 g/L.
Sample preparation for protein analysis and for enrichment of wines with colloids
Aliquots of 15 mL of white wines and synthetic wines were centrifuged (10 min at 12.000×g) in a Sorvall RC-5C (Heraeus, Hanau, Germany) and dialysed in tubes of a molecular weight cut-off of 3,500 Da (Membrane Filtration Products Inc., San Antonio, TX, USA). The dialysed samples were lyophilised (Christ Alpha 1–4, Martin Christ, Osterode am Harz, Germany) and preserved at −20 °C until the time of analysis. A similar procedure was applied to aliquots of 200 mL of synthetic wines in order to obtain a colloid extract to enrich samples of 200 mL of control white wine with the released colloids from S. cerevisiae and both sequential inoculations. These enriched wines were used to measure the foaming properties in comparison with the original control wine.
Determination of proteins by HRSEC-DAD
The soluble fractions were analysed by high-resolution size-exclusion chromatography (HRSEC) in order to determine the molecular distribution and quantify the proteins obtained from samples [45]. The lyophilised samples were resuspended in 0.6 μL of 300 mM ammonium acetate and were centrifuged at 12,000 g for 5 min. The supernatant was filtered through 0.22 µm acetate cellulose filters (Millipore GSE), and 100 μL of supernatant was then injected into the chromatographic system. The analyses were carried out in an HPLC Agilent 1200 Series system (Agilent Technologies Inc., Santa Clara, USA) with a DAD detector monitored at 230, 280 and 320 nm. Separation was carried out at 20 °C using a Shodex gel permeation HPLC columns (OHpak SB-803 HQ, 300 mm × 8 mm I.D.; Showa Denko, Japan). The mobile phase consists of an aqueous solution of 300 mM ammonium acetate applied with a constant flow of 0.6 mL/min for 70 min. The proteins were quantified according to the peak area for each fraction and using the external standard method with BSA (see above) in the range between 0 and 10 mg/mL (r 2 > 0.99).
Analysis of volatile compounds
The analysis of volatile compounds was tasked to the Laboratory for Aroma Analysis and Oenology of the University of Zaragoza (Zaragoza, Spain) according to the methods proposed and validated by Barata et al. [46].
The quantification of major compounds was carried out using the method previously described [47]. The extract was prepared in accordance with this method with adjustments [46]: in 15-mL screw-capped centrifuge tubes, containing 4.1 g of ammonium sulphate, were added 2.7 mL of wine, 6.3 mL of water, 20 µL of internal standard solution (2-butanol, 4-methyl-2-pentanol, 4-hydroxy-4-methyl-2-pentanone, heptanoic acid and 2-octanol at 200 µg/mL in ethanol), and 0.25 mL of dichloromethane. The tubes were shaken for 90 min and then centrifuged at 867×g for 10 min. Once the phases had been separated, the dichloromethane phase was recovered with a 0.5-mL syringe and transferred to a 0.3-mL vial. The extract was then analysed by GC in a Hewlett-Packard 5890 series II gas chromatograph with FID. This instrument was equipped with capillary column DB-WAX (30 m and 0.32 mm I.D. and 0.5-µl film thickness) from J&W Scientific preceded by a 2 m × 0.53 mm uncoated precolumn. Chromatographic conditions were as follows: hydrogen as the carrier gas (2.2 mL/min; split injection mode 1:10 (split relation) with 3-µL injection volume; injector temperature at 250 °C; and detector temperature at 250 °C). The initial column temperature was 40 °C for 2 min, heated to 200 at 2 °C min, and remaining at that temperature for 30 min. Quantitative data were obtained by interpolation of relative peak areas in the calibration graphs built by interpolation of relative peak areas in the calibration graphs built by the analysis of synthetic wines containing known amounts of the analytes.
This analysis was carried out using the methods proposed and validated by [48] with adjustments [46]: standard SPE cartridges (1 mL total volume) filled with 50 mg of LiChrolut EN resins were placed in the vacuum manifold extraction system, and the sorbents were conditioned by rinsing the cartridges with 6 mL of dichloromethane, 2 mL of methanol, and finally, 2 mL of a water–ethanol mixture (12 %, v/v). The cartridges were then loaded with 15 mL of wine sample and 10 µL of a surrogate standards solution containing 3-octanone, β-damascene and heptanoic acid (all at 200 µL/g of ethanol). This mixture was passed through the SPE cartridges (2 mL/min) followed by a wash step using 5 mL of 40 % water–methanol solution. The resins were then dried by letting air pass through (negative pressure of 0.6 bars, 10 min). Analytes were recovered in a 2-mL vial, by elution with 0.6 mL of dichloromethane. Twelve microlitres of an internal standard solution (300 mg/L of 4-hydroxy-4methyl-2-pentanone and 2-octanol) was added to the eluted sample. The extract was then analysed by GC with ion-trap MS detection. CP-3800 gas chromatographic analyses were performed under the conditions described in [48].
Sensory analysis
All sensory analyses were performed in the tasting room of the Faculty of Oenology of Tarragona (Rovira I Virgili University) which was designed according to UNE-EN ISO 8589:2010 [49]. Tasting was carried out with the ISO official tasting glasses (ISO 3591.1977). To evaluate the effect of sequential inoculation with T. delbrueckii or M. pulcherrima and S. cerevisiae versus the control fermented only with S. cerevisiae on wine organoleptic characteristics, all the wines were tasted by a group of nine expert tasters from the Rovira i Virgili University. Two sensory triangle tests were conducted to compare the control wine with both sequential inoculation wines according to UNE ISO 4120.1983. In all the cases, the main objective was to determine whether tasters were able to recognise the wine that was different. The second objective was to determine which wine was preferred by the panellists who correctly identified the different wines.
Statistical analysis
One-factor analysis of variance (ANOVA) was carried out using SPSS 15.0 software (SPSS Inc., Chicago, IL). The level of significance of sensory triangle tests was determined following Jackson’s method [50].
Results and discussion
Yeast population kinetic
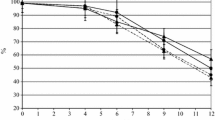
Figure 1 shows the percentage of presence of the different yeasts at the beginning and the end of alcoholic fermentation of the different experimental conditions. When the culture medium was YPD, a high presence of indigenous non-Saccharomyces yeast, Candida zemplinina (formerly C. stellata), was detected at the beginning of the fermentation in all the samples (between 50 and 57 %). The presence of C. zemplinina at the beginning of alcoholic fermentation has previously been described as very common in spontaneous and in inoculated fermentation [2, 3]. However, the presence of the initially inoculated yeast was confirmed in all cases. Specifically, S. cerevisiae was present at 50 %, whereas T. delbrueckii and M. pulcherrima were present at 50 and 43 %, respectively, in their corresponding inoculated tanks. These data confirm that in all cases, the inoculated yeasts were present at the beginning of the alcoholic fermentation despite the high presence of C. zemplinina. The initial high population of C. zemplinina as well as the presence of the corresponding inoculated yeasts was confirmed when the LYS medium was employed. In the particular case of the grape juice inoculated with S. cerevisiae, the presence of C. zemplinina was 100 % which is quite logical because only non-Saccharomyces can grow in this medium. By contrast, T. delbrueckii and M. pulcherrima were present at 43 and 40 %, respectively, in their corresponding inoculated tanks in the LYS medium at the beginning of alcoholic fermentation.
As expected, the preponderance of S. cerevisiae at the end of alcoholic fermentation was clear in all the cases in the YPD medium and was 100 % in the case of the tank inoculated only with S. cerevisiae, and also when sequential inoculation with M. pulcherrima and S. cerevisiae was carried out. This has been previously described by [51] probably due to the difficulty of surviving elevated alcoholic [52]. This preponderance was also present when the sequential inoculation with T. delbrueckii and S. cerevisiae was applied. However, in that case, a slight presence of T. delbrueckii (13 %) was detected, confirming the ability of this non-Saccharomyces yeast to survive at high ethanol concentrations [20, 48]. These data were confirmed when the LYS medium was employed. In that case, the percentage of T. delbrueckii was 100 %. By contrast, no colonies were detected in the tanks inoculated only with S. cerevisiae or with M. pulcherrima and then with S. cerevisiae. These results are quite logical, since S. cereviaise cannot grow in this medium and the other non-Saccharomyces yeast present at the initial point of the different medium, C. zemplinina and M. pulcherrima cannot survive at high ethanol concentrations [2, 4].
Taken together, these data confirm that all the inoculated yeasts were at least present in a significant level in their corresponding vats, although in all cases the presence of indigenous C. zemplinina was really high. This yeast is present in all fermentations around 50–60 %, and for this reason, it is expected that the effect of its presence on the properties of wine is comparable with control. The validity of the experimental design is consequently confirmed.
Standard parameters
Table 1 shows the standard parameters of the white wines and synthetic wines. The ethanol content, titratable acidity and pH of all the samples were very similar, indicating that no statistically significant differences (p < 0.05) were caused in these parameters by sequential inoculation of both non-Saccharomyces yeasts. M. pulcherrima has been previously described as being able to significantly decrease ethanol content [16], but this was not the case under our experimental conditions. The glycerol content and volatile acidity from sequential inoculations with M. pulcherrima were also similar to the control wines. However, the development of T. delbrueckii at the beginning of alcoholic fermentation produced some interesting differences in volatile acidity and glycerol concentration. Specifically, wines from sequential inoculation with T. delbrueckii had significantly higher glycerol content and significantly lower volatile acidity than their corresponding control wines. In the particular case of volatile acidity, this difference was drastic in the synthetic medium, in which the volatile acidity was more than three times lower. These data are consistent with previously published data, which have described T. delbrueckii as producing wines with lower volatile acidity and higher glycerol content [17, 21].
Foam parameters
Figure 2 shows the foam parameters of white wines and synthetic wines. The results for foamability (Hm) were very interesting. Sequential inoculation with the employed strain of T. delbrueckii seems to improve the foam characteristics of white wine, because both parameters, Hm and Hs, increased significantly in white wine (by 17 and 20 %, respectively). By contrast, sequential inoculation with M. pulcherrima did not improve Hm, but significantly increased Hs in white wine, and the increase was even higher than in the case of T. delbrueckii (35 %).
This tendency was also observed in the synthetic wines but to a lesser degree. Specifically, Hm was slightly but significantly higher (9 %) in sequential inoculation, whereas no differences were observed in the case of M. pulcherrima. No significant differences were found in Hs in any of the experimental conditions, possibly because Hs is not stable in that media.
Proteins and polysaccharides
Proteins and mannoproteins released by yeasts have been reported as exerting a positive effect on foam parameters [33]. Molecular exclusion HPLC of all the samples was performed for this reason (Table 2). The total protein content of white wines and synthetic wines was significantly higher in samples from sequential inoculation with T. delbrueckii than in the corresponding controls. These differences were mainly due to the lower molecular weight fraction (LMw; molecular weight < 60 kDa). Since proteins can stabilise the bubble’s film because of their surface properties [33], the increase in LMw protein fraction may be related to the improved foam parameters observed in this wine.
It has been described that it exists a relationship between foam characteristics and chemical composition of base wines and their corresponding sparkling wines [36]. Consequently, it is expected that the corresponding sparkling wines maintain these characteristics.
By contrast, the total protein content of wines from sequential inoculation with M. pulcherrima was similar to the controls. Some slight but significant differences were observed in the high molecular weight fraction (HMw; molecular weight > 80 kDa). However, no differences were found in any of the other fractions.
Table 3 shows the polysaccharide fraction of the different wines. The total polysaccharide concentration of white wine fermented by sequential inoculation with T. delbrueckii was very similar to the controls. This similar behaviour was observed in all molecular weight fractions. However, sequential inoculation with M. pulcherrima produced a white wine with a significantly higher concentration of total polysaccharides. This increase was mainly due to the high and the intermediate molecular weight fraction (HMw; 144–1,000 kDa; IMw; 40–144 kDa). M. pulcherrima and T. delbrueckii have previously been reported as releasing higher amounts of polysaccharides [17, 24]. However, this was not the case in our experimental conditions with T. delbrueckii. The oligosaccharide (Mw < 5 kDa) concentration of wines fermented with both sequential inoculations was also statistically similar to the controls. However, the oligosaccharide concentration of the wines fermented with sequential inoculation with T. delbrueckii was significantly higher than in wines fermented with sequential inoculation with M. pulcherrima.
The chromatogram profiles obtained in the synthetic wine were quite different to those obtained in the white wines, probably due to no polysaccharides from grapes being present in this medium. Only two peaks can be distinguished under these conditions—one peak for oligosaccharides, and other for polysaccharides. In this medium, the content of polysaccharides obtained by sequential inoculation with T. delbrueckii was significantly higher and the content of oligosaccharides significantly lower than in the controls. By contrast, sequential inoculation with M. pulcherrima led to similar polysaccharide concentrations and significantly lower oligosaccharide concentrations than in the control.
This dissimilar behaviour of T. delbrueckii and M. pulcherrima in synthetic wine as compared to white wine seems to indicate that the composition of the fermentation media exerts some influence on the polysaccharide and oligosaccharide release by these non-Saccharomyces yeasts. Indeed, some authors have suggested that the lower the content of colloids in the media, the higher the release of colloids by yeasts [53]. White wine comes from a grape juice containing grape polysaccharides, whereas the synthetic juice did not. Moreover, some of the original polysaccharides of the grape juice would have precipitated or been hydrolysed, making it impossible to establish a complete balance. In any case, sequential inoculation with T. delbrueckii produced larger amounts of polysaccharides than the controls in the synthetic medium. These data are consistent with those reported by other authors, who described T. delbrueckii as releasing more polysaccharides than S. cerevisiae, although this behaviour was not observed in the white wine.
The foaming properties of the different enriched control wines were measured in order to clarify whether the positive effects of sequential inoculation of T. delbreuckii and M. pulcherrima on the foam properties were due to the combination of colloids released by these yeasts during alcoholic fermentation (Fig. 3). In all cases, the enriched wines presented significantly enhanced parameters of the foam (Hm and Hs). However, the increase in Hm was significantly higher in the wines which were enriched with the colloids released by the sequential inoculation with T. delbrueckii. Consequently, these data confirm that sequential inoculation with T. delbrueckii has a clearly positive effect on this parameter. This improvement of the foam is presumably due to the released proteins included in the ensemble of colloids, which as mentioned above, exert a positive influence on bubble’s film stability [31, 32].
Volatile compounds
Table 4 shows the fermentative volatile composition of white wines. In general, the production of total acids by both sequential inoculations with non-Saccharomyces yeasts was significantly lower than in controls. However, acetic acid must be considered as accounting for more than 85 % in all cases, and the data should therefore be analysed separately.
The production of acetic acid by sequential inoculation with T. delbrueckii was significantly lower than in the controls. These data are consistent with the trends observed for the volatile acidity (Table 1) and agree with previously published data, which described acetic acid production during alcoholic fermentation as being minor when T. delbrueckii is present [19]. By contrast, sequential inoculation with M. pulcherrima originated white wines with acetic acid concentration which was similar to the controls.
Some slight but significant differences were found in some of the other acids, depending on the yeast. However, these differences were too small to provide any organoleptic difference.
The production of higher alcohols in white wines by both sequential inoculations with non-Saccharomyces yeasts was significantly higher, with 3-methyl-1-butanol being mainly responsible for this increase. In any case, these data confirm that the development of T. delbrueckii and M. pulcherrima significantly increases the production of higher alcohols, as described previously [15, 23].
The development of T. delbrueckii or M. pulcherrima during alcoholic fermentation has been reported as inducing lower production of acetaldehyde [20]. Our data confirm that this is true in sequential inoculation with M. pulcherrima, but not in the case of T. delbrueckii.
In general, the production of total major esters in wines from sequential inoculation with T. delbrueckii was similar to the controls, whereas it was lower in the wines from sequential inoculation with M. pulcherrima. However, the fact that ethyl acetate represents more than 60 % in all the samples must be considered. If ethyl acetate is not considered, no significant differences in the total major esters would be detected in any of the experimental conditions.
As was the case with higher alcohols, some slight but significant differences were found in some of the other major esters, depending on the yeast. However, these differences were too small to provide any important organoleptic difference.
With regard to the minor esters, no differences were found between the control wines and those fermented by sequential inoculation with T. delbrueckii. However, the presence of these minor esters was significantly lower in the wines from sequential inoculation with M. pulcherrima. This lower minor ester concentration was mainly due to a drastic decrease in butyl acetate concentration (of around 90 %).
Some authors have found that the development of non-Saccharomyces yeasts tends to create wines with higher ester concentrations [9, 14]. As discussed above, no major differences were found in any of the sequential inoculation with non-Saccharomyces, with the sole exception of butyl acetate, which was at much lower levels in the wines produced from sequential inoculation with M. pulcherrima.
By contrast, the production of lactones by both sequential inoculations with non-Saccharomyces yeasts was significantly higher, and g-decalactone was responsible for this increase.
In general, no great differences in the aromatic profile of fermentative volatile compounds were found in any of the experimental conditions. In the case of T. delbrueckii, these data are consistent with other studies, which found only slight differences [20].
Table 5 shows the volatile phenols of the different wines. Sequential inoculation with T. delbrueckii did not lead to major differences in volatile phenols. Only some slight but significant increases were detected in 4-ethylphenol and 4-vinylphenol. However, these compounds were in all cases far below the perception threshold, and they, therefore, have no sensory implications. By contrast, the total volatile phenols concentration of the wines from sequential inoculation with M. pulcherrima was significantly higher than in the controls. This increase was due to 4-vinylphenol, 2-methoxyphenol and especially 2,6-dimethoxyphenol. This latter compound, which has a smoky aroma [54], was present in wines from sequential inoculation with M. pulcherrima at a concentration four times higher than in the controls, and above its perception threshold (570 µg/l) [48]. Therefore, the presence of this compound should be perceived.
Sensory evaluation
Table 6 shows the sensory analysis results. Two triangular tests were performed, comparing the control with both sequential inoculation wines. Six tasters were able to distinguish the wines from sequential inoculation with T. delbrueckii from the control wines, whereas three could not. These data are statistically significant (p < 0.05) and consequently indicates the existence of sensory differences between these wines. Moreover, five of the six tasters who correctly identified the different wines preferred the wine from sequential inoculation with T. delbrueckii.
The comparison between wines from sequential inoculation with M. pulcherrima was even more significant (p < 0.01). In this case, eight tasters were able to distinguish between the wines, and five of them attributed smoky and flowery aromas to the wine fermented by sequential inoculation with M. pulcherrima. The smoky perception is probably associated with the higher production of 2, 6-dimethoxyphenol since it has been previously described that has this olfactory note [54]. However, the detected flowery notes cannot be associated with any of the measured volatile compounds. In any case, there was no clear preference because among the tasters who correctly identified the different wines four preferred the control, while the other four preferred the wine from sequential inoculation with M. pulcherrima.
Conclusion
It can, therefore, be concluded that sequential inoculation with non-Saccharomyces yeasts may be a useful tool for obtaining base wines with different characteristics. On the one hand, the T. delbrueckii Biodiva™ strain exerts a positive effect on foaming properties, improving foamability (Hm) and foam persistence (Hs). On the other hand, M. pulcherrima Flavia® strain also increases foam persistence (Hs) and changes the aromatic profile by increasing smoky and flowery notes. Further studies are needed to verify whether these different characteristics produced by sequential inoculation of non-Saccharomyces remain in the corresponding sparkling wines after the second fermentation and bottle ageing.
References
Sabaté J, Cano J, Esteve-Zarzoso B, Guillamón JM (2002) Isolation and identification of yeasts associated with vineyard and winery by RFLP analysis of ribosomal genes and mitochondrial DNA. Microbiol Res 157:267–274
Constantí M, Poblet M, Arola L, Mas A, Guillamón JM (1997) Analysis of yeast populations during alcoholic fermentation in a newly established winery. Am J Enol Vitic 48:339–344
Beltran G, Torija MJ, Novo M, Ferrer N, Poblet M, Guillamón JM, Rozès N, Mas A (2002) Analysis of yeast populations during alcoholic fermentation: a six year follow-up study. Syst Appl Microbiol 25:287–293
Schütz M, Gafner J (1993) Analysis of yeast diversity during spontaneous and induced alcoholic fermentations. J Appl Bacteriol 75:551–558
Guillamón JM, Sabaté J, Barrio E, Cano J, Querol A (1998) Rapid identification of wine yeast species based on RFLP analysis of the ribosomal internal transcribed spacer (ITS) region. Arch Microbiol 169:387–392
Esteve-Zarzoso B, Peris-Torán MJ, García-Maiquez E, Uruburu F, Querol A (2001) Yeast population dynamics during the fermentation and biological aging of sherry wines. Appl Environ Microbiol 67:2056–2061
Urso R, Rantsiou K, Dolci P, Rolle L, Comi G, Cocolin L (2008) Yeast biodiversity and dynamics during sweet wine production as determined by molecular methods. FEMS Yeast Res 8:1053–1062
Heard GM, Fleet GH (1985) Growth of natural yeast flora during the fermentation of inoculated wines. Appl Environ Microbiol 50:727–728
Plata C, Millán C, Mauricio JC, Ortega JM (2003) Formation of ethyl acetate and isoamyl acetate by various species of wine yeasts. Food Microbiol 20:217–224
Jolly NP, Varela C, Pretorius IS (2014) Not your ordinary yeast: non-Saccharomyces yeast in wine production uncovered. FEMS Yeast Res 14:215–237
Ciani M, Maccarelli F (1997) Oenological properties of non-Saccharomyces yeasts associated with wine-making. World J Microbiol Biotechnol 14:199–203
Jolly NP, Augustyn OPR, Pretorius LS (2003) The effect of non-Saccharomyces yeasts on fermentation and wine quality. S Afr J Enol Vitic 24:8–10
Amerine MA, Berg HW, Cruess WV (1972) The technology of winemaking, 3rd edn. The AVI Publishing Company Inc, Westport
Rojas V, Gil J, Piñaga F, Manzanares P (2003) Acetate ester formation in wine by mixed cultures in laboratory fermentations. Int J Food Microbiol 86:181–188
Sadineni Naresh V, Kondapalli N, Obulam V (2012) Effect of co-fermentation with Saccharomyces cerevisiae and Torulaspora delbrueckii or Metschnikowia pulcherrima on the aroma and sensory properties of mango wine. Ann Microbiol 62:1353–1360
Contreras A, Hidalgo C, Henschke PA, Chambers PJ, Curtin C, Varela C (2014) Evaluation of non-Saccharomyces yeast for the reduction of alcohol content in wine. Appl Environ Microbiol 80:1670–1678
Giovani G, Rosi I, Bertuccioli M (2012) Quantification and characterization of cell wall polysaccharides released by non-Saccharomyces yeast strains during alcoholic fermentation. Int J Food Microbiol 160:113–118
Maturano YP, Rodríguez Assaf LA, Toro ME, Nally MC, Vallejo M, Castellanos de Figueroa LI, Combina M, Vazquez F (2012) Multi-enzyme production by pure and mixed cultures of Saccharomyces and non-Saccharomyces yeasts during wine fermentation. Int J Food Microbiol 155:43–50
Bely M, Stoeckle P, Masneuf-Pomarède I, Dubourdieu D (2008) Impact of mixed Torulaspora delbrueckii–Saccharomyces cerevisiae culture on high-sugar fermentation. Int J Food Microbiol 122:312–320
Sadoudi M, Tourdot-Maréchal R, Rousseaux S, Steyerd D, Gallardo-Chacón JJ, Ballester J, Vichi S, Guérin-Schneider R, Caixach J, Alexandre H (2012) Yeast–yeast interactions revealed by aromatic profile analysis of sauvignon blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol 32:243–253
Jolly NP, Augustyn OPH, Pretorius IS (2006) The role and use of non-Saccharomyces yeasts in wine production. S Afr J Enol Vitic 27:15–38
Castelli T (1955) Yeasts of wine fermentations from various regions of Italy. Am J Enol Vitic 6:18–19
Azzolini BT, Emanuele F, Fabio V, Paola MF (2012) Effects of Torulaspora delbrueckii and Saccharomyces cerevisiae mixed cultures on fermentation and aroma of amarone wine. Eur Food Res Technol 235:303–313
Domizio P, Liu Y, Bisson LF, Barile D (2014) Use of non-saccharomyces wine yeast as novel sources of mannoproteins in wine. Food Microbiol 43:5–15
Prakitchaiwattana CJ, Fleet GH, Heard GM (2004) Application and evaluation of denaturing gradient gel electrophoresis to analyse the yeast ecology of wine grapes. FEMS Yeast Res 4:865–877
Rodríguez ME, Lopes C, Valles S, Giraudo MR, Caballero A (2007) Selection and preliminary characterization of β-glycosidases producer patagonian wild yeasts. Enzyme Microb Technol 41:812–820
Rodríguez ME, Lopes CA, Barbagelata RJ, Barda NB, Caballero AC (2010) Influence of Candida pulcherrima Patagonian strain on alcoholic fermentation behaviour and wine aroma. Int J Food Microbiol 138:19–25
Comitini F, Gobbi M, Domizio P, Romani C, Lencioni L, Mannazzu I, Ciani M (2011) Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol 28:873–882
Oro L, Ciani M, Comitini F (2014) Antimicrobial activity of Metschnikowia pulcherrima on wine yeasts. J Appl Microbiol 116:1209–1217
Ody-Brasier A, Vermeulen F (2014) The price you pay: price-setting as a response to norm violations in the market for Champagne grapes. Adm Sci Q 59:109–144
Vanrell G, Canals R, Esteruelas M, Fort F, Canals JM, Zamora F (2007) Influence of the use of bentonite as a riddling agent on foam quality and protein fraction of sparkling wines (Cava). Food Chem 104:148–155
Brissonnet F, Maujean A (1993) Characterization of foaming proteins in a Champagne base wine. Am J Enol Vitic 44:297–301
Martínez-Rodríguez A, Carrascosa AV, Barcenilla JM, Angeles Pozo-Bayón MA, Carmen Polo M (2001) Autolytic capacity and foam analysis as additional criteria for the selection of yeast strains for sparkling wine production. Food Microbiol 18:183–191
Andrés-Lacueva C, Lamuela-Raventós RM, Buxaderas S, De La Torre-Boronat MDC (1997) Influence of variety and aging on foaming properties of Cava (sparkling wine). J Agric Food Chem 45:2520–2525
Boulton RB, Singleton VL, Bisson LF, Kunkee RE (1996) Principles and practices of winemaking. Chapman & Hall, New York
Esteruelas M, Poinsaut P, Sieczkowski N, Manteau S, Fort F, Canals JM, Zamora F (2009) Comparison of methods for estimating protein stability in white wines. Am J Enol Vitic 60:302–311
Riou C, Nicaud JM, Barre P, Gaillardin C (1997) Stationary-phase gene expression in Saccharomyces cerevisiae during wine fermentation. Yeast 10:903–915
Yarrow D (1998) Methods for insolation, maintenance and identification of yeast. In: Kurtzman CP, Fell JW (eds) The yeast. A taxonomic study, 4th edn. Elsevier, Amsterdam
Angelo J, Siebert KJ (1987) A new medium for the detection of wild strains in brewing culture yeast. J Am Soc Brew Chem 45:135–140
Esteve-Zarzoso B, Belloch C, Uruburu F, Querol A (1999) Identification of yeast by RFLP analysis of the 5.8 rRNA and the two ribosomal internal transcribed spacers. Int J Syst Bacteriol 49:329–337
Spiczki M (2004) Species identification and comparative molecular and physicological molecular and physiological analysis of Candida zemplinina and Candida stellata. J Basic Microbiol 4:471–479
Organisation Internationale de la Vigne et du Vin (2014) Methods of analysis of wines and must. http://www.oiv.int/oiv/info/enmethodesinternationalesvin
Poinsaut P (1991) Le mosalux appareil de mesure du pouvoir moussant du vin. Rev Oenol 59:36–43
Ayestarán B, Guadalupe Z, León D (2004) Quantification of major grape polysaccharides (Tempranillo V.) released by maceration enzymes during the fermentation process. Anal Chim Acta 513:29–39
Canals JM, Arola L, Zamora F (1998) Protein fraction analysis of white wine by FPLC. Am J Enol Vitic 49:383–388
Barata A, Campo E, Malfeito-Ferreira M, Loureiro V, Cacho J, Ferreira V (2011) Analytical and sensorial characterization of the aroma of wines produced with sour rotten grapes using GC-O and GC-MS: identification of key aroma compounds. J Agric Food Chem 59:2543–2553
Ortega C, López R, Cacho J, Ferreira V (2001) Fast analysis of important wine volatile compounds: development and validation of a new method base on gas chromatographic-flame ionization detection analysis of dichloromethane microextracts. J Chromatogr 923:205–214
López R, Aznar M, Cacho J, Ferreira V (2002) Determination of minor and trace volatile compounds in wine by solid–phase extraction and gas chromatography with mass spectrometric detection. J Chromatogr 966:167–177
AENOR (2010) Análisis sensorial. AENOR, Madrid
Jackson RS (2002) Quantitative (technical) wine assessment. In: Taylor SL (ed) Wine tasting. A professional handbook, 1st edn. Academic Press, Hong Kong
Santos J, Sousa MJ, Cardoso H, Inácio J, Silva S, Spencer-Martins I, Leão C (2008) Ethanol tolerance of sugar transport, and the rectification of stuck wine fermentations. Microbiology 154:422–430
Parapouli M, Hatziloukas E, Drainas C, Perisynakis A (2010) The effect of Debina grapevine indigenous yeast strains of Metschnikowia and Saccharomyces on wine flavor. J Ind Mircobiol Biotechnol 37:85–93
Guilloux-Benatier M, Guerreau J, Feuillat M (1995) Influence of initial colloid content on yeast macromolecule production and on the metabolism of wine microorganisms. Am J Enol Vitic 46:486–492
Culleré L, Escudero A, Cacho J, Ferreira V (2004) Gas chromatography−olfactometry and chemical quantitative study of the aroma of six premium quality spanish aged red wines. J Agric Food Chem 52:1653–1660
Acknowledgments
The authors thank Juvé & Camps SA winery for providing the grapes and Lallemand Inc. for financial support.
Conflict of interest
None.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González-Royo, E., Pascual, O., Kontoudakis, N. et al. Oenological consequences of sequential inoculation with non-Saccharomyces yeasts (Torulaspora delbrueckii or Metschnikowia pulcherrima) and Saccharomyces cerevisiae in base wine for sparkling wine production. Eur Food Res Technol 240, 999–1012 (2015). https://doi.org/10.1007/s00217-014-2404-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-014-2404-8