Abstract
Although the positive role of non-Saccharomyces yeasts on the overall quality of wine is encouraging research into their oenological potential, current knowledge on the topic is still far from satisfactory. This work analyzes the contribution of starter cultures of Torulaspora delbrueckii, inoculated sequentially with Saccharomyces cerevisiae (multi-starter fermentation), on the fermentation and aromas of two different white style wines, i.e., dry and sweet wines. Chemical analysis of Soave and Chardonnay wines (dry wines) showed that multi-starter fermentation greatly affected the content of several important volatile compounds, including 2-phenylethanol, isoamyl acetate, fatty acid esters, C4–C10 fatty acids and vinylphenols. Moreover, strain-specific contributions have been shown by testing two different T. delbrueckii strains. Evidence of the positive impact of T. delbrueckii activity on wine quality was also demonstrated in Vino Santo, a sweet wine. Due to its low production of acetic acid, this non-Saccharomyces yeast is recommended for the fermentation of high sugar grapes. T. delbrueckii also influenced the content of different variety of chemical groups, including lactones. From a sensory perspective, all wines produced by multi-starter fermentation have greater aromatic intensity and complexity than wines resulting from a monoculture fermentation. These results emphasize the potential of employing T. delbrueckii, in association with S. cerevisiae, for the production of white wines of different styles with improved and enhanced flavour.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, utilization of non-Saccharomyces yeasts has been considered for industrial wine production as several have shown high oenological potential (Ciani and Maccarelli 1998; Romano et al. 2003; Renault et al. 2009; Suarez-Lepe and Morata 2012). Several studies have reported that some species, such as Torulaspora delbrueckii, Lachancea (Kluyveromyces) thermotolerans, Metschnikowia pulcherrima, Pichia kluyveri, Hanseniaspora uvarum, Hansenula anomala, Rhodotorula mucillaginosa and Candida spp., when used as a pure culture or in association with Saccharomyces cerevisiae, were able to enhance the flavour properties in different types of wine (Anfang et al. 2009; Izquierdo Cañas et al. 2011; Calabretti et al. 2012; Sadoudi et al. 2012; Andorrà et al. 2012; Cordero-Bueso et al. 2013; Gobbi et al. 2013). In particular, the importance of the contribution of non-Saccharomyces yeasts to the aromatic complexity of the wine, when used to drive multi-starter fermentations, has been shown (Calabretti et al. 2012; Azzolini et al. 2012; Sadoudi et al. 2012).
The success of non-Saccharomyces yeasts has led to the industrial production of selected cultures for winemaking. Currently, T. delbrueckii and Lachancea thermotolerans are commercially available as active dry yeasts, P. kluyveri as frozen form. T. delbrueckii was the first non-Saccharomyces species produced for that purpose, about half a century after the introduction of the first S. cerevisiae commercial wine starter cultures, and different strains of T. delbrueckii are now available for winemaking. The impact of some of these strains on the fermentation and aroma of different wines has been documented (Izquierdo Cañas et al. 2011; Azzolini et al. 2012; Takush and Osborne 2012; van Breda et al. 2013).
Despite the increasing number of studies on non-Saccharomyces yeasts, the level of current knowledge on their impact on wine quality is still far from satisfactory. Indeed, the effects of pure and mixed cultures on wine aroma have been often evaluated in conditions (e.g., laboratory-scale trials carried out on synthetic media or sterilized grape musts) very different from those normally found in the winery (Andorrà et al. 2012). Moreover, unlike S. cerevisiae, knowledge about strain heterogeneity within a single non-Saccharomyces species, like T. delbrueckii, and about the impact of strain-specificity on wine aromas is still limited. Some studies underline the importance of intra-species heterogeneity within non-Saccharomyces yeasts for wine production (Ciani and Maccarelli 1998; Renault et al. 2009; van Breda et al. 2013). Given the interest in these yeasts from winemakers, and the possibility of widespread use encouraged by the availability of commercial active dry yeasts, further investigation is recommended to evaluate their behaviour under different winery conditions.
The objective of this study was to investigate the effects of T. delbrueckii, in association with S. cerevisiae (multi-starter fermentation) on the aroma of different types of white wine. Experiments in winery conditions were carried out to produce two dry table wines, one international (Chardonnay) and one local (Italian Soave), and one sweet white wine (Vino Santo, an Italian passito wine). Bely et al. (2008) reported that a mixed T. delbrueckii and S. cerevisiae culture was the best combination of yeasts for improving the analytical profile of sweet wine, particularly in terms of volatile acidity. Moreover, in our previous investigation (Azzolini et al. 2012) the use of mixed cultures of T. delbrueckii and S. cerevisiae had a relevant impact on the fermentation and aroma of Amarone wine, a dry red passito wine. The chemical and sensory profiles of wines obtained by multi-starter fermentation were analyzed and compared to those traditionally produced with S. cerevisiae cultures alone or by spontaneous fermentation with indigenous microflora.
Materials and methods
Starter strains
Two strains of T. delbrueckii and one of S. cerevisiae yeasts, all commercially available, were used in this study. The T. delbrueckii strains were Zymaflore® Alpha (Laffort, Bordeaux, France) and BIODIVA® (Lallemand, Montreal, Canada); the S. cerevisiae strain was Lalvin EC1118® (Lallemand). In this study, the three strains are called Td1, Td2 and Sc, respectively. These strains were used in the form of active dry yeast according to the manufacturer’s instructions.
Vinifications
Three vinifications were carried out to produce the Soave (Verona, Italy) and Chardonnay wines, as the dry white wines, and Vino Santo (Trent, Italy), as the sweet white wine.
The Soave and Chardonnay wines were obtained in the winery through traditional winemaking methods using Garganega and Chardonnay grapes, respectively. After crushing the grapes with 40 mg L−1 of SO2, the juices were separated from the pomace and carefully divided among 150 L steel tanks to obtain homogenous trials each containing 80 L of must. The Garganega juice was pH 3.35, reducing sugars 21.4° Brix, titratable acidity 5.95 g L−1 as tartaric acid. The Chardonnay was pH 3.20, reducing sugars 22.5° Brix, titratable acidity 6.72 g L−1 as tartaric acid. The musts for the multi-starter fermentations (Td1 + Sc and Td2 + Sc) were inoculated sequentially with T. delbrueckii and S. cerevisiae, added when the ethanol content was about 3–4 % v v−1. Musts inoculated with S. cerevisiae (Sc) only were used as control. T. delbrueckii and S. cerevisiae strains were inoculated at the concentration approximately of 3–4 × 106 and 2–3 × 106 cfu mL−1, respectively. Alcoholic fermentation (AF) was carried out in a cellar room without temperature controls ranging from 16 to 20 °C.
To produce the Vino Santo wine, at laboratory level, withered Nosiola grapes were crushed and sulphited (SO2 50 mg L−1), then the resulting juice (pH 3.87, reducing sugars 42.7° Brix, titratable acidity 8.38 g L−1 as tartaric acid) was divided into trial lots of 500 mL each. Multi-starter fermentation (Td2 + Sc) was induced by adding the T. delbrueckii strain Td2, then S. cerevisiae when the weight loss corresponded to theoretical ethanol content of about 3 % v v−1. The other trial batches were inoculated with S. cerevisiae only (Sc) or not inoculated at all (Spn), in which fermentation was carried out by spontaneous indigenous microflora. AF was carried out in a cellar room without temperature controls ranging from 14 to 17 °C, and was stopped in order to have wines with ethanol and residual sugars in compliance with the regulations for Vino Santo wine production.
All three trials were carried out in triplicate. After fermentation, the wines were removed from the containers and allowed to settle for 2 days through natural sedimentation.
Isolation and identification of yeasts
Grape juices and fermenting juice samples were collected to count the yeasts by plate counting on WL agar (Fluka, Seelze, Germany) and lysine agar medium (Oxoid Ltd., London, UK). Colonies were isolated (about 50 for each trial) according to their morphology (texture, surface, margin, elevation and colour) and frequencies. Analysis of cell morphology was performed using a phase-contrast microscopy at a magnification of ×1,000. All isolates were preserved on YPD agar (1 % w v−1 yeast extract, 2 % w v−1 glucose, 2 % w v−1 peptone and 1.5 % w v−1 agar), stored at 4 °C and also kept at −80 °C with 25 % w v−1 glycerol. Yeast identification was carried out by ITS-RFLP analysis. The 5.8S-ITS region was amplified using ITS1 and ITS4 primers (White et al. 1990) and the PCR conditions were identical to those reported by to Esteve-Zarzoso et al. (1999). Microsatellite multiplex PCR was carried out according to Vaudano and Garcia-Moruno (2008) to discriminate strains among S. cerevisiae isolates. PCR product (ITS and microsatellite) and ITS restriction fragments were separated by electrophoresis on 2.0 % w v−1 agarose gels with 1 × TAE buffer (40 mM Tris, 20 mM acetic acid and 1 mM EDTA, pH 8) strained with ethidium bromide.
Analysis of must and wine
Ethanol was analyzed by near-infrared (NIR) spectroscopy using an Alcolyzer Wine Analysis System (Anton Paar GmbH, Graz, Austria). Sugar content and titratable acidity were determined according to standard analysis methods. Acetic acid was quantified using an enzyme kit (La Roche, Basel, Switzerland).
Analysis of the volatile composition of wines was been performed using gas chromatography-mass spectrometry (GC–MS) Solid Phase Extraction (SPE), as described by Tosi et al. (2013).
SPE was performed to quantitatively extract the volatile compounds with an automated Aspec™ XL solid phase extraction apparatus (Gilson Inc., Middleton, WI, U.S.) using Isolute® ENV + 1 g cartridges (IST Ltd., Mid Glamorgan, U.K.). The free volatile compounds were eluted with 9 mL of dichloromethane. The solution was dried with Na2SO4 and concentrated to 0.4 mL with a gentle nitrogen flow.
The GC–MS analysis was performed with an Agilent 6890 N Network Gas Chromatography system coupled with a 5978B inert XL EI/CI MS (Agilent Technologies, Milan, Italy), equipped with a HP-WAX Bonded PEG fused silica capillary column (60 m × 320 μm i.d. × 0.25 μm film thickness; Agilent Technologies). The MS conditions included electron impact energy 70 eV, and MS source temperature 230 °C. The temperatures of the transfer line and GC injector temperature were 200 and 250 °C respectively, and helium was used as the carrier (flow: 1.5 mL min−1). The column temperature program was 50 °C for 4 min, increased to 240 °C at 4 °C min−1, then 240 °C for 16 min.
Identification of volatile compounds was achieved by means of pure reference standard (Sigma-Aldrich, St. Louis, MO, U.S.) co-injections; when the authentic standard were not available the identification was based on the comparison with the spectral data of the NIST library.
Quantitative analysis was performed by total ion chromatograph using the response factors calculated for each compound by calibration curves in a hydroalcoholic solution, containing 12 % v v−1 ethanol with 5 g L−1 tartaric acid, like a model wine. As for compounds for which commercial standards were not available, the response factors of compounds with similar chemical structures were utilized.
The odour activity values (OAV) were calculated as the ratio between the measured quantitative concentration of a substance in the wine and its odour threshold, when available.
Sensory analysis
The Soave and Chardonnay wines were tested by a panel of six judges in one session using a total of six olfactive-gustative descriptors (aroma intensity, complexity, floral, green apple, tropical fruit, and ripe fruit) and six mouthfeel attributes (acidity, bitterness, herbaceous, persistence, freshness and body). A number from 0 to 10 was assigned to each one and the average of the two repetitions was used as final score.
Statistical treatment of data
Student t test was used for the wine compounds and sensory scores to evaluate the differences in the samples. Principal component analysis (PCA) was performed on volatile compounds determined by the SPE/GC–MS of all the wines using PAST software package (version 1.89).
Results
Fermentation of dry table wines and starter implantation analysis with Torulaspora delbrueckii
The course of fermentation was similar in both vinifications depending on the type of inoculation and T. delbrueckii strain used (Supplementary Table S1). Ethanol production was significantly faster in control trials than in the musts inoculated with both yeasts; among the latter, the Td2 + Sc wines produced higher ethanol levels than did the Td1 + Sc wines (Supplementary Figure S1).
Spontaneous microflora in both grape musts was composed by different yeasts such as H. uvarum, S. cerevisiae, M. pulcherrima, and other unidentified species (Table 1). The former two species were the most frequent and the total concentration of all wild yeasts was approximately 3.0 × 104 and 5.8 × 103 cfu mL−1 in Soave and Chardonnay must, respectively.
Representative S. cerevisiae isolated from both grape juices displayed different microsatellite profiles and were also distinguishable from EC1118 strain profile (Supplementary Figure S2). Identification of isolates collected during the vinifications was carried out to ascertain the population dynamics of indigenous yeasts and the colonization of T. delbrueckii and S. cerevisiae starter strains during AF. The evolution of cell populations in both types of wine was similar and T. delbrueckii was able to dominate rapidly the fermentation. No wild yeasts were further detected after the starter inoculation. The different profile of colonies in WL plate and different cell morphology between T. delbrueckii and S. cerevisiae allowed to carry out a reliable quantitative analysis of the two starter strain populations. Usually, the former had flat colony or lightly convex, while the latter had clearly convex or umbonate colony elevation (Table 1). At the optical microscopy T. delbrueckii cells appeared rounded, small with a large vacuole, while those of S. cerevisiae were ellipsoidal and bigger with small vacuoles (data not shown). The populations of the Td1 and Td2 strains, inoculated at the concentration of 2–4 × 106 cfu mL−1, increased rapidly and after 4 days the concentration was approximately 7–8 × 107 cfu mL−1. The inoculation of S. cerevisiae was carried out at the 5th and 6th day, when ethanol was about 3 % v v−1, in Td2 + Sc and Td1 + Sc, respectively (Supplementary Figure S1). Microsatellite multiplex PCR analysis of representative yeasts isolated after the starter inoculation confirmed that S. cerevisiae population was composed mainly by EC1118 strain. Indeed, all isolates identified as S. cerevisiae (S21–29 and C17–26, Table 1) displayed a microsatellite profile similar to that of EC1118 (Supplementary Figure S2). S. cerevisiae colonized rapidly the fermenting must and its population increased, progressively overwhelming T. delbrueckii population. Nevertheless, T. delbrueckii cells remained at relatively high concentrations (about 2–3 × 106 cfu mL−1) up to the end of AF, a cell density similar to that of S. cerevisiae population. Figure 1 shows the percentage of T. delbrueckii estimated during the AF in all sequentially inoculated trials with respect to total yeast concentration, mainly composed by S. cerevisiae.
Percentage of T. delbrueckii during the alcoholic fermentation for Soave (full line) and Chardonnay (dashed line) wine production by the sequential inoculation of T. delbrueckii and S. cerevisiae (Td1 + Sc and Td2 + Sc, circle and triangle symbol respectively). Bars are standard deviation of three independent trials
Chemical analysis of dry table wines
The volatile composition of wines produced by multi-starters differed from the control wines due to variations in the content of several molecules that greatly impact to wine aroma (Table 2). Interesting differences were also observed between wines inoculated with the Td1 and Td2 strains.
The content of several alcohols changed in one or both type of wines and relevant modifications involved benzyl alcohol, furfuryl alcohol, 1-octen-3-ol, vanillic alcohol, methionol (3-methylthio-1-propanol) and 2-phenylethanol. The increase of methionol (potato note) was particularly important in Chardonnay wine as it was detected above or near the threshold (1 mg L−1; Ferreira et al. 2000). The OAV was 1.5 and 1.0 in the wine fermented with the Td1 and Td2 strains, respectively. High OAV (3–4) were also observed for 2-phenylethanol (rose note) in both wines fermented with T. delbrueckii.
Esters and fatty acids were the chemical groups that underwent very consistent modifications related to the presence of T. delbrueckii during the fermentation. Ester acetates clearly decreased in wines that were sequentially inoculated with mixed cultures, especially due to the reduction in isoamyl acetate (banana note), detected at levels much higher than its threshold (30 µg L−1; Ferreira et al. 2000). Also, esters of C4–C10 fatty acids, responsible for the fruity scent, strongly decreased. Ethyl octanoate was detected at concentrations fourfold to fivefold lower in the wines inoculated with T. delbrueckii than in the control wines, while ethyl decanoate was found below the threshold (200 µg L−1; Ferreira et al. 2000). The OAV was <1.0 in the Td1 + Sc Soave wine and in the Td1 + Sc and Td2 + Sc Chardonnay wines.
Other noteworthy variations involved esters of hydroxyacids, lactic acid, succinic acid and malic acid, compounds that have high sensory thresholds (20–200 mg L−1; Campo et al. 2006).
The decrease in fatty acids (cheese, butter and rancid notes) was particularly marked in both types of wine inoculated with T. delbrueckii, as the total amount was two to four folds lower than in the control wines. Greater differences were observed for hexanoic and octanoic acid, the most abundant fatty acids. Decanoic acid was the only one that decreased under its threshold (1 mg L−1; Ferreira et al. 2000). The OAV was <1.0 in wines inoculated with the Td1 and in Td2 + Sc Chardonnay wine.
The carbonylic compounds involved in consistent modifications were phenylacetaldehyde, benzaldehyde, furfural, homo- and nor-furaneol, most of which occurred in the Soave wines.
T. delbrueckii also affects the content of some volatile phenols, like 4-vinylguaiacol and 4-vinylphenol, which decreased in the Td1 + Sc Soave wines and in the Chardonnay wines inoculated with both non-Saccharomyces strains. In the latter wines, 4-vinylguaiacol (pharmaceutical note) was detected below its threshold (40 µg L−1; Guth 1997). The OAV was <1.0, while in the control wine the OAV was 2.1.
PCA was applied to volatile compound data (Table 2) to discriminate the wines. The wines of both vinifications were clearly distinct from each other, including those inoculated with the two different T. delbrueckii strains (Fig. 2). About 50 % of compounds used to PCA analysis had high PC1 and PC2 loading values (<−8.0 and >8.0) in both types of wines (Supplementary Table S2). In general, compounds that described the wines inoculated with T. delbrueckii were different between Soave and Chardonnay wines, while both Sc wines were well described mainly by C4–C10 fatty acids and their esters.
a Principal component analysis of aroma composition of Soave wines obtained by the sequential inoculation of T. delbrueckii and S. cerevisiae (Td1 + Sc and Td2 + Sc) and the inoculation S. cerevisiae as control (Sc). b Principal component analysis of aroma composition of Chardonnay wines obtained by the sequential inoculation of T. delbrueckii and S. cerevisiae (Td1 + Sc and Td2 + Sc) and the inoculation S. cerevisiae as control (Sc)
The sensory properties of the Soave and Chardonnay wines produced by T. delbrueckii and S. cerevisiae were distinct from the related controls (Supplementary Figure S3). In both wines those inoculated with T. delbrueckii were significantly distinct (p < 0.05) for the descriptors of freshness and acidity, which scored lower than in Sc wines, as well as for flavour intensity, complexity and persistence, which were higher than in Sc. According to the judges’ comments, floral and tropical fruits contributed most to the complexity of the wines inoculated with non-Saccharomyces yeast, despite these two descriptors did not differ significantly among the wines. In both types of wine, no significant differences resulted in the wines produced with the two different non-Saccharomyces strains.
Fermentation of dessert wine and microbiological analysis
The kinetics of AF differed among the trial wines and those inoculated with S. cerevisiae only (the control wines), with trial wines displaying the highest rate of CO2 production, while the trial wines fermented by multi-starters and spontaneous microflora were quite similar, especially in the second half of AF (Supplementary Figure S4).
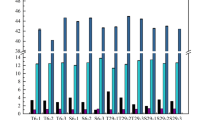
Several spontaneous yeasts were isolated on grape must and some of them were identified as H. uvarum, Zygosaccharomyces bailii, Z. rouxi, M. pulcherrima, Pichia guilliermondii, S. cerevisiae and S. pastorianus (Table 1). The total concentration of these wild yeasts was about 8.2 × 104 cfu mL−1 (Fig. 3a). Molecular analysis by microsatellite multiplex PCR of representative isolates identified as S. cerevisiae (VS9, VS26 and VS33, Table 1) displayed three profiles different from that of EC1118 starter strain (Supplementary, Figure S2).
a Total yeast cell concentration (log10 cfu mL−1) measured during the alcoholic fermentation carried out spontaneously (Spn, white circle) or induced by the sequential inoculation of T. delbrueckii and S. cerevisiae (Td2 + Sc, black square) and the inoculation of S. cerevisiae (Sc, black triangle) to produce Vino Santo wine. Bars are standard deviation of three independent trials and arrow indicates the inoculation of S. cerevisiae in Td2 + Sc wine. b Percentage of T. delbrueckii (dark grey bar) and other yeasts, mainly S. cerevisiae (light grey bar) on Td2 + Sc wine during the alcoholic fermentation. Bars are standard deviation of three independent trials. c Percentage of non-Saccharomyces (dark grey bar) and Saccharomyces (light grey bar) during spontaneous alcoholic fermentation (Spn wine). Bars are standard deviation of three independent trials
The evolution of yeast cell concentration in all trials was monitored and, at the beginning of AF, cell density on Td2 + Sc and Sc wines increased rapidly just after the inoculation of T. delbrueckii strain Td2 and S. cerevisiae strain, respectively (Fig. 3a, b). Conversely, yeast density in the non-inoculated trial (Spn wine) was more slowly than the inoculated trials. In this last vinification non-Saccharomyces species (H. uvarum, P. gulliermondii and Zygosaccharomyces spp. were the most frequent) colonized the early phase of AF but progressively they were replaced by indigenous Saccharomyces spp. (Figure 3c). In multi-starter trial (Td2 + Sc wine) the population of T. delbrueckii, inoculated in the must at the concentration of 3.7 × 106 cfu mL−1, remained high (107 cfu mL−1) throughout the fermentation and started to decline after the inoculation of S. cerevisiae strains, carried out at the 11th day (Fig. 3b). Subsequently, S. cerevisiae dominated up to the end of fermentation. S. cerevisiae strain inoculated at the concentration of 1.5 × 106 cfu mL−1 in grape must of Sc trial dominated rapidly the fermenting wine until the end of AF (data not shown). Both in Td2 + Sc and Sc wine, only S. cerevisiae yeasts (VS27–32, Table 1), that displayed the same microsatellite multiplex PCR profile of EC1118, were found after starter inoculation (Supplementary Figure S2).
Chemical analysis of dessert wines
Multi-starter fermentation produced lower amounts of acetic acid during the course of AF than fermentations carried out by S. cerevisiae only and by indigenous yeasts (Fig. 4). At devatting, the multi-starter wines contained 0.29–0.37 g L−1 of acetic acid less than the other wines.
Acetic acid (g L−1) production during alcoholic fermentation carried out spontaneously (Spn, white circle) or induced by the sequential inoculation of T. delbrueckii and S. cerevisiae (Td2 + Sc, black square) and the inoculation of S. cerevisiae (Sc, black triangle) to produce Vino Santo wine. Bars are standard deviation of three independent trials
T. delbrueckii significantly affected the volatile composition of the Vino Santo wine, and the trend to variations of several molecules agreed with the data of the Soave and Chardonnay wines (Table 3). However, the particularity of Vino Santo wine provided interesting information on the effects of this non-Saccharomyces yeast on the aroma of sweet style wines.
The major effects of T. delbrueckii on alcohols were observed on the content of benzyl alcohol, 3-methylthio-1-propanol and 2-phenylethanol. The latter was twice as high in the Td2 + Sc wine (OAV = 2.9) than in the other two wines (OAV = 1.6).
Among esters, the decrease of wine isoamyl acetate was observed in the Td2 + Sc wine, down to half compared to the other two wines. The decrease in C6–C10 fatty acid esters was similar to the dry table wines described above. Moreover, the content of ethyl 4-hydroxybutyrate, ethyl 2-hydroxy-4-methylpentanoate, diethyl 2-hydroxyglutarate and ethyl cinnamate decreased, confirming the strong impact of T. delbrueckii on volatile ester composition. It is interesting to note the high content of ethyl cinnamate that determined high OAV in all wines (OAV = 18.4, 11.5 and 12.3 in the Sc, Spn and Td2 + Sc wines, respectively) due to its low sensory threshold (1.1 µg L−1; Ferreira et al. 2000). In the Spn wine, the behaviour of these esters was similar to that observed in the Td2 + Sc wine.
A significant decrease of content of C6–C10 fatty acids was found in the Td2 + Sc wine compared to the Sc wine. This result agreed with the dry table wine data, although the decrease was not as drastic. Isovalerianic acid and hexanoic acid were detected above their thresholds, and in the wine inoculated with T. delbrueckii the isovalerianic acid was about half (OAV = 5.1) of that in the wine inoculated with S. cerevisiae (OAV = 9.2). The decrease in hexanoic acid was less marked (OAV = 1.4 vs 1.7). In contrast, in all the wines, butyric acid was above 173 1.1 µg L−1, its threshold (Ferreira et al. 2000), and consistently increased in the Td2 + Sc wine (OAV = 2.0). Similar modifications for isovalerianic acid, hexanoic acid and octanoic acid were observed in the Td2 + Sc and Spn wines.
The varietal aromas (monoterpenes and norisoprenoids) were found at particularly high concentrations compared to dry table wines, and the variations among the wines were generally slight (<30 %), with the most relevant observed for citronellol, endiol and 3-oxo-α-ionol.
More important were the changes in carbonylic compounds, in particular benzaldehyde, furfural, syringaldehyde and homo- and nor-furaneol, although their concentrations remained under their respective thresholds: 0.2, 14.1, 50.0, 0.04 and 2.0 mg L−1, respectively (Ferreira et al. 2000; Peinado et al. 2004; Campo et al. 2006; Sarrazin et al. 2007).
The effect of T. delbrueckii on vinylphenols in the Vino Santo wines supported the data of the previously produced Chardonnay wine, as both molecules decreased (especially 4-vinylphenol) in the Td2 + Sc wine. Moreover, it was observed to have consistent impacts on vanillin and homovanillic acid content in the wines.
Among the lactones, which were present in very high amounts, it is worth noting the increase of the two sherry lactones and the decrease in ethyl pyroglutamate (detected at high concentrations in all three wines), γ-butyrolactone and 4-carboetoxy-γ-butyrolactone related to the presence of T. delbrueckii during the fermentation with respect to the Sc wine. Unlike the other two wines, the wine that fermented spontaneously (Spn wine) had a γ-nonalactone (sweet, caramel note) level lower than its threshold of 30 µg L−1 (Ferreira et al. 2000). The OAV were <1.0.
Similar to the dry table wines, PCA clearly distinguished the wines according to their volatile composition (Supplementary Figure S5). More than 50 % of compounds to PCA analysis had high PC1 and PC2 loading values (<−8.0 and >8.0; Supplementary Table S2). Td2 + Sc wines were mainly described by 2-phenylethyl alcohol, 3-methylthio-1-propanol, ethyl lactate, and butyric acid, Sc wines by ethyl hexanoate, isovalerianic acid, 4-terpineol, 4-vinylphenol, while Spn wines by furfural alcohol and cis-3 hexenol.
The three different fermentation strategies used to produce the Vino Santo wines generated substantial differences regarding to the wine’s olfactive and gustative properties. In particular, the Sc wines were unanimously judged negatively, as their bouquet and mouthfeel were characterized by a pronounced acetic acid note detrimental to the wine quality. This note was also perceptible in wine obtained through spontaneous fermentation (Spn wine), but only in the mouth and to a lesser extent than the Sc wines, although that did not prejudice the overall quality like Sc wine. Due to this sensory defect of Sc and Spn wines the comparative descriptive sensory analysis of the three wines was not carried out. The Vino Santo wine produced by multi-starter fermentation (Td2 + Sc wine) was appreciated for its balance both in flavour and mouthfeel, and, at the same time, was characterized by aroma complexity, similar to the Spn wines, giving the wines a more harmonious flavour.
Discussion
The results of the fermentation kinetics agreed with the previous studies (Cabrera et al. 1988; Bely et al. 2008; Comitini et al. 2011; Azzolini et al. 2012; Cordero-Bueso et al. 2013), which reported a slower rate of ethanol production in the trials where the musts were inoculated with T. delbrueckii yeast. However, the use of T. delbrueckii cultures together with S. cerevisiae ensured outstanding fermentative performances for both styles of wines (dry and sweet). Moreover, the case of the Vino Santo wine highlighted the advantage of using T. delbrueckii, which resulted in a considerable decrease in acetic acid production, as reported by Bely et al. (2008). The consequence of such decrease can be extremely important, as the results of sensory analysis of the Vino Santo wines demonstrated. Indeed, the results of this study suggest that the use of non-Saccharomyces yeast can be recommended for the production of this type of wine, while inoculation with S. cerevisiae only could even be risky as volatile acidity can increase to unacceptable levels.
The impact of T. delbrueckii on aroma compounds affected different chemical groups, and particularly informative were the compounds that behaved similarly in all three trials.
The increase in 2-phenylethanol was a clear effect of the co-presence of T. delbrueckii and S. cerevisiae yeasts on the alcohol composition of the three wines, as previously reported (Azzolini et al. 2012; Sadoudi et al. 2012; Cordero-Bueso et al. 2013). The differences in the 2-phenylethanol content between the wines fermented by Td1 and Td2 could be strain-related, although Renault et al. (2009) did not find significant differences among several T. delbrueckii strains on the production of this alcohol. Other alcohols, like methionol, a potent volatile sulphur flavour compound, seems to be affected by T. delbrueckii activity, but its synthesis by this yeast has not yet been investigated.
The impact of T. delbrueckii on important volatile esters appears to be pronounced, as demonstrated by the strong decrease in isoamyl acetate and C6–C10 fatty acid esters in wines obtained by multi-starter fermentation. Similar behaviours were previously documented, comparing wines fermented by S. cerevisiae and co-cultures of T. delbrueckii and S. cerevisiae (Viana et al. 2008; Azzolini et al. 2012; Sadoudi et al. 2012; Cordero-Bueso et al. 2013). In this study, the contribution of T. delbrueckii on their general decrease appears to be even more evident. This is more significantly reflected at sensory level, especially in table wines, such as the Soave and Chardonnay, than in sweet wines, like the Vino Santo, due to the higher odour potency these esters generally have in young wines (Sumby et al. 2010). The difference in the scores for green apple and freshness between Td1 and Td2 + Sc and Sc wines could be related to their different fatty acid ethyl ester content. Similar observations were reported in Madeira wines submitted to oxidative aging (Câmara et al. 2006). Nevertheless, it is important to note that the perception of fruity aroma is the result of a complex interaction between various compounds of different chemical groups, including powerful mercaptans and pyrazines not detected in this study (Mateo-Vivaracho et al. 2010). Thus, further chemical analysis is necessary for a better understanding of the differences in the fruity attributes among these wines.
As fatty acid esters can also have high odour activity in aged dessert wines (Bailly et al. 2009; Bowen and Reynolds 2012), the role of T. delbrueckii on the modulation of the sensory properties of Vino Santo, due to these aromatic esters, could be relevant. Certainly, ethyl cinnamate can be considered a good candidate for be odour-potent compounds in Vino Santo wine, whose content can vary significantly depending on the yeast metabolism, as this study demonstrated.
The contribution of T. delbrueckii activity on wine aroma was also clearly evident at the level of fatty acids. Their strong decrease can be considered to be positive as they are generally responsible for negative effects on overall wine aroma, especially in young wines. Therefore, the use of co-cultures of T. delbrueckii and Saccharomyces yeasts could be strategic in order to modulate the production of ethyl esters and fatty acids, formed enzymatically during fermentation, especially in table wines such as Soave and Chardonnay.
In sweet wines, like Vino Santo, T. delbrueckii may affect the wine’s aroma profile by acting on carbonylic compounds, although most of them were detected below their threshold. Interestingly, the behaviour of these compounds was similar among the wines produced by multi-starter and spontaneous fermentations compared to those inoculated with S. cerevisiae only. The presence of various indigenous non-Saccharomyces yeasts in the natural fermentation of dessert wines provided great metabolic diversity, including those involved in the metabolism of aromatic aldehydes and ketones (Krings and Berger 1998), that contribute to the particular complexity in the aroma of these wines. Because the production of these molecules by T. delbrueckii is still unknown, this study provided some insights for its investigation.
The results for vinylphenols suggest that multi-starter fermentation may consistently decrease their content, as observed for the Chardonnay wines inoculated with T. delbrueckii where 4-vinylguiacol decreased to under its threshold. Moreover, this occurrence could reflect positively on the sensory properties considering the unpleasant flavours of these phenols, which are also precursors for ethylphenols, which have lower thresholds. Further assay of hydroxycinnamate decarboxylase activity in T. delbrueckii would be necessary to ascertain the possibility of minimizing ethylphenol as shown previous investigations (Benito et al. 2011).
The effects of T. delbrueckii on lactones were pronounced in the Vino Santo wine, and the consistent variations in sherry lactones agreed with our previous study carried out on Amarone wine, a raisin wine like Vino Santo (Azzolini et al. 2012). The same tendency for these lactones, observed in wines produced by spontaneous and multi-starter fermentation, suggests the importance non-Saccharomyces yeasts have on sherry-like notes related to these molecules, precisely these found in sherry wines (Muller et al. 1973). Also, the sensory effects of the decrease of ethyl pyroglutamate linked to T. delbrueckii do not appear to be negligible, as this lactone is responsible for burnt, caramel and honey notes that are the key odour descriptors for Vino Santo wine, as well as for sweet fortified wines (Schneider et al. 1998; Campo et al. 2006).
In conclusion, this study of the utilization of co-cultures of T. delbrueckii and S. cerevisiae yeasts, sequentially inoculated into must to produce two different styles of white wines, demonstrates significant differences distinguishing the chemical and sensory properties, compared to the same wines obtained by traditional (monoculture or spontaneous) fermentation. Furthermore, the relevance of strain-specificity within T. delbrueckii to these differences was shown, providing insights for further investigations on the effects of strain diversity on wine quality for non-Saccharomyces yeasts. Naturally, the ability of the T. delbrueckii and S. cerevisiae co-cultures to specifically impact on volatile wine compounds should be further studied to broaden the understanding of the winemaking potential of these multi-starter fermentations.
References
Andorrà I, Berradre M, Mas A, Esteve-Zarzoso B, Guillamon JM (2012) Effect of mixed culture fermentations on yeast populations and aroma profile. LWT Food Sci Technol 49:8–13
Anfang N, Brajkovich M, Goddard MR (2009) Co-fermentation with Pichia kluyveri increases varietal thiol concentrations in Sauvignon Blanc. Aust J Grape Wine Res 15:1–8
Azzolini M, Fedrizzi B, Tosi E, Finato F, Vagnoli P, Scrinzi C, Zapparoli G (2012) Effects of Torulaspora delbrueckii and Saccharomyces cerevisiae mixed cultures on fermentation and aroma of Amarone wine. Eur Food Res Technol 35:303–312
Bailly S, Jerkovic V, Meurée A, Timmermans A, Collin S (2009) Fate of key odorants in Sauternes wines through aging. J Agric Food Chem 57:8557–8563
Bely M, Stoeckle P, Masneuf-Pomarède I, Dubourdieu D (2008) Impact of mixed Torulaspora delbrueckii–Saccharomyces cerevisiae culture on high-sugar fermentation. Int J Food Microbiol 122:312–320
Benito S, Morata A, Palomero F, Gonzales MC, Suarez-Lepe JA (2011) Formation of vinylphenolic pyranoanthocyanins by Saccharomyces cerevisiae and Pichia gulliermondii in red wines produced following different fermentation strategies. Food Chem 124:15–23
Bowen AJ, Reynolds A (2012) Odor potency of aroma compounds in Riesling and Vidal blanc table wines and icewines by gas chromatography-olfactometry-mass spectrometry. J Agric Food Chem 60:2874–2883
Cabrera MJ, Moreno J, Ortega JM, Medina M (1988) Formation of ethanol, higher alcohols, esters, and terpenes by five yeast strains in must from Pedro Ximénez grapes in various degrees of ripeness. Am J Enol Vitic 4:283–287
Calabretti A, La Cara F, Sorrentino A, Di Stasio M, Santomauro F, Rastrelli L, Gabrielli L, Limone F, Volpe MG (2012) Characterization of volatile fraction of typical Irpinian wines fermented with a new starter yeast. World J Microbiol Biotechnol 28:1433–1442
Câmara JS, Alves MA, Marques JC (2006) Changes in volatile composition of Madeira wines during oxidative ageing. Anal Chim Acta 563:188–197
Campo E, Ferreira V, Escudero A, Marqués JC, Cacho J (2006) Quantitative gas chromatography–olfactometry and chemical quantitative study of the aroma of four Madeira wines. Anal Chimica Acta 583:180–187
Ciani M, Maccarelli F (1998) Oenological properties of non-Saccharomyces yeasts associated with wine-making. World J Microbiol Biotechnol 14:199–203
Comitini F, Gobbi M, Domizio P, Romani C, Lencioni L, Mannazzu I, Ciani M (2011) Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol 28:873–882
Cordero-Bueso G, Esteve-Zarzoso B, Cabellos JM, Gil-Diaz M, Arroyo T (2013) Biotechnological potential of non-Saccharomyces yeasts isolated during spontaneous fermentations of Malvar (Vitis vinifera cv. L.). Eur Food Res Technol 236:193–207
Esteve-Zarzoso B, Belloch C, Uruburu F, Querol A (1999) Identification of yeasts by RFLP analysis of the 5.8S rRNA gene and the two ribosomal internal transcribed spacers. Int J Syst Bacteriol 49:329–337
Ferreira V, Lopez R, Cacho JF (2000) Quantitative determination of the odorants of young red wines from different grape varieties. J Sci Food Agric 80:1659–1667
Gobbi M, Comitini F, Domizio P, Romani C, Lencioni L, Mannazzu I, Ciani M (2013) Lanchancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: strategy to enhance acidity and improve the overall quality of wine. Food Microbiol 33:271–281
Guth H (1997) Quantitation and sensory study of character impact odorants of different white wine varieties. J Agric Food Chem 45:3027–3032
Izquierdo Cañas PM, Palacios Garcia AT, Garcia Romero E (2011) Enhancement of flavour properties in wines using sequential inoculations of non-Saccharomyces (Hansenula and Torulaspora) and Saccharomyces yeast starter. Vitis 50:177–182
Krings U, Berger RG (1998) Biotechnological production of flavours and fragrances. Appl Microbiol Biotechnol 49:1–8
Mateo-Vivaracho L, Zapata J, Cacho J, Ferreira V (2010) Analysis, occurrence, and potential sensory significance of five polyfunctional mercaptans in white wines. J Agric Food Chem 58:10184–10194
Muller CJ, Kepner RE, Webb AD (1973) Lactones in wines. A review. Am J Enol Vitic 24:5–9
Peinado RA, Moreno J, Bueno JE, Moreno JA, Mauricio JC (2004) Comparative study of aromatic compounds in two young white wines subjected to re-fermentative cryomaceration. Food Chem 84:585–590
Renault P, Miot-Sertier C, Marullo P, Hernandez-Orte P, Lagarrigue L, Lonvaud-Funel A, Bely M (2009) Genetic characterization and phenotypic variability in Torulaspora delbrueckii species: potential applications in the wine industry. Int J Food Microbiol 134:201–210
Romano P, Fiore C, Paraggio M, Caruso M, Capece A (2003) Function of yeast species and strains in wine flavour. Int J Food Microbiol 86:169–180
Sadoudi M, Tourdot-Maréchal R, Rousseaux S, Steyer D, Gallardo-Chacon J-J, Ballester J, Vichi S, Guérin-Schneider R, Caixach J, Alexandre H (2012) Yeast-yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol 32:243–253
Sarrazin E, Dubourdieu D, Darriet P (2007) Characterization of key-aroma compounds of botrytized wines, influence of grape botrytization. Food Chem 103:536–545
Schneider R, Baumes R, Bayonove C, Razungles A (1998) Volatile compounds involved in the aroma of sweet Fortified wines (vin doux naturel) from Grenache noir. J Agric Food Chem 46:3227–3230
Suarez-Lepe JA, Morata A (2012) New trends in yeast selection for winemaking. Trend Food Sci Technol 23:39–50
Sumby KM, Grbin PR, Jiranek V (2010) Microbial modulation of aromatic esters in wine: current knowledge and future prospects. Food Chem 121:1–16
Takush DG, Osborne JP (2012) Impact of yeast on the aroma and flavour of Oregon Pinot Noir wine. Aus J Grape Wine Res 18:131–137
Tosi E, Azzolini M, Lorenzini M, Torriani S, Fedrizzi B, Finato F, Cipriani M, Zapparoli G (2013) Induction of grape botrytization during withering affects volatile composition of Recioto di Soave, a “Passito”-style wine. Eur Food Res Tech 236:853–862
van Breda V, Jolly N, van Wyk J (2013) Characterization of commercial and natural Torulaspora delbrueckii wine yeast strains. Int J Food Microbiol 163:80–88
Vaudano E, Garcia-Moruno E (2008) Discrimination of Saccharomyces cerevisiae wine strains using microsatellite multiplex PCR and band pattern analysis. Food Microbiol 25:56–64
Viana F, Gil JV, Genovés S, Vallés S, Manzanares P (2008) Rational selection of non-Saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol 25:778–785
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Acknowledgments
The authors would like to thank Paola Vagnoli and Tiziana Nardi (Lallemand Italia, Verona, Italy) and Ann Dumont (Lallemand Inc., Montreal, Canada) for supplying yeast strains, and for their help on the manuscript. Thanks to Giacomo Citron for supplying Laffort yeast strains.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Azzolini, M., Tosi, E., Lorenzini, M. et al. Contribution to the aroma of white wines by controlled Torulaspora delbrueckii cultures in association with Saccharomyces cerevisiae . World J Microbiol Biotechnol 31, 277–293 (2015). https://doi.org/10.1007/s11274-014-1774-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-014-1774-1