Abstract
Since its introduction in the early 1990s, the on-line coupling of field-flow fractionation to inductively coupled plasma-mass spectrometry (FFF/ICP-MS) has evolved from a “niche” method into an established technique, especially in the field of natural-colloid analysis. Around the turn of the millennium engineered nanomaterials became prominent in research as a result of new properties, and in recent years FFF/ICP-MS has been revealed to be a promising tool for their analysis. Given the beneficial properties of this technique (e.g., no stationary phase, high separation power, multi-elemental capabilities, and high sensitivity) further applications, especially in the field of biomolecule analysis, will be discovered in the near future, and FFF will evolve further as a complementary tool to well-established chromatographic techniques (e.g. high-performance liquid chromatography, size-exclusion chromatography). The focus of this article is on recent application trends of FFF/ICP-MS, revealing the applicability of this technique within several fields of research, especially natural colloids and engineered nanoparticles. Possible future application trends, based on the author’s opinion, are outlined in the “Concluding remarks and outlook” section.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
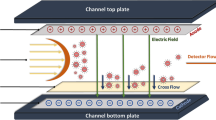
Field-flow fractionation, commonly designated as FFF, is a flow-based fractionation method, which was invented and theoretically described by John Calvin Giddings in 1966 [1]. Fractionation takes place within a trapezoidal channel without a stationary phase; hence, FFF does not belong to the class of chromatographic separation techniques. The separation channel is continuously filled with a carrier, developing a parabolic flow profile. The velocity of the carrier-flow stream varies as a function of distance from the channel walls (with the lowest velocity close to the channel walls). After the injection of a sample it is focused to produce a small sample band, and in consequence the sample accumulates at the bottom of the channel. Depending on the sample’s diffusion coefficient, sample fractions expand into different channel heights. A perpendicular field of adjustable strength is applied contrary to the sample diffusion. Upon the establishment of equilibrium, the different sample fractions move in different channel heights and interact with different flow-profile velocities; thus separation and/or fractionation is achieved (Fig. 1). Fractions containing constituents with a small diameter and/or molar weight elute before larger ones (normal mode); although if the diameter and/or molar weight exceeds a given value the elution order is reversed (steric or hyperlayer mode). A detailed theoretical consideration is outside the scope of this article, and the reader is referred to the relevant literature, e.g. Refs. [2, 3].
Depending mainly on the type of perpendicular field applied, several FFF sub-categories are defined, e.g. sedimentation (sd-FFF; centrifugal force), flow (Fl-FFF; flow force), electrical (El-FFF; electrical force), thermal (Th-FFF; temperature gradient), and magnetic-FFF (Mg-FFF; magnetic force) [2].
At first, FFF (mainly sd-FFF and Fl-FFF, mostly with UV–visible detection) was primarily used in biomolecule research (e.g. DNA [4], human and animal cells [5], and albumin [6]) because of its non-destructive and relatively impact-free separation properties. After the introduction of multi-angle light-scattering detectors in the 1990s, several applications in polymer research (mainly coupled to Th-FFF) were developed, enabling fractionation and molar-mass determination [7]. At the same time, scientists working in the field of water chemistry and aquatic colloids discovered the potential of FFF as a powerful analytical technique helping to fill knowledge gaps regarding composition, behavior, and interaction of natural solid matter with numerous compounds (e.g. metals, nutrients, and pollutants) in the natural-colloidal size range of 1 nm–1 μm [8]. However, neither UV–visible nor light-scattering detection was selective and sensitive enough to answer questions regarding, e.g., interaction of natural aquatic colloids and trace metals. In the 1990s, inductively coupled plasma-mass spectrometry (ICP–MS) emerged as a popular field because of its high sensitivity, multi-element capability, and its ability to act as an on-line detector for suitable separation methods [9]. In 1991, Ronald Beckett et al. proposed the on-line coupling of FFF with ICP–MS [10]. In 1993, Murphy et al. established the on-line coupling of sd-FFF and ICP–MS [11], and the on-line coupling of Fl-FFF and ICP–MS was described for the first time by Hassellöv et al. in 1999 [12]. The coupling of FFF and ICP–MS combines benefits from both techniques. FFF offers:
-
1.
A wide size-application range, from 1 nm to 50 μm, with high separation resolution (depending on fractionation mode);
-
2.
Continuous size information regarding fractions (in contrast with standard filtration) obtained via channel calibration or FFF theory;
-
3.
Availability of a wide range of carriers, enabling carrier-matching with respect to the sample’s stability (“soft” separation);
-
4.
Reduced interaction of sample with the separation system because of the absence of a stationary phase;
-
5.
The possibility of “on-channel” concentration and adjacent fractionation, avoiding perturbation of the sample;
-
6.
The possibility of on-line coupling to a wide range of detectors and, if needed, off-line fraction collection [13].
An ICP–MS detector provides:
-
1.
High sensitivity;
-
2.
Elemental selectivity;
-
3.
A species and/or fraction-unspecific response, enabling quantification (valid for small particles < ~2 μm [14]);
-
4.
Isotope information enabling the use of isotopically enriched tracers and isotope dilution.
Thus, the coupling of FFF and ICP–MS is a powerful tool for applications in several fields. The number of publications on FFF/ICP-MS has been steadily rising since its introduction in the 1990s, as shown in Fig. 2, which is based on the results of a Web of Science search.
Number of publications dealing with FFF coupled to ICP–MS by year and application, from Web of Science search (search finalized: 14th July 2014). Search terms (excerpt): “(flow/sedimentation) field flow fractionation”, “ICP–MS”, “inductively coupled plasma(-)mass spectrometry”, “single particle ICP–MS”, “colloids”, “nanoparticles”. Upper pie chart on right-hand side: FFF-techniques applied; Lower pie chart: Engineered nanoparticles (ENPs) investigated, according to material
The objective of this article is to reveal trends and developments in FFF/ICP-MS, with special focus on applications. To identify application trends, Fig. 2 compiles all fields of application, referring to the year of publication: natural-colloid analysis has been an ongoing topic since the mid-1990s; engineered nanoparticle (ENP) analysis first emerged just a few years ago (around 2010; mainly silver, titanium, and gold ENPs; cf. lower pie chart in Fig. 2). An emerging trend is the use of stable isotopes acting as tracers in, e.g., ENP studies. A prediction regarding future application trends, based on the author’s opinion, is given in the “Concluding remarks and outlook” section of this article.
To date only sd and Fl-FFF have been coupled on-line to ICP–MS (cf. upper pie chart in Fig. 2) [15], and these techniques are currently the most common. In sd-FFF the separation channel is spanned on a centrifugal apparatus and the field correlates to the centrifugal force, whereas in Fl-FFF an independent cross-flow stream that either permeates from the top of the channel through the bottom (symmetrical) or only through the bottom (asymmetrical) is applied. An ultrafiltration membrane with a specific cut-off is placed on the bottom of the channel. Fractions smaller than the cut-off pass through the membrane and are no longer accessible to the fractionation process. Fl-FFF is a beneficial separation tool, especially for small particles. In contrast, sd-FFF is suitable for high-density particles of larger size, whereas small, low-density particles are not sufficiently fractionated [16]. However, the devices complement each other, together covering a broad range of particle sizes and densities.
The pre-selection was based upon a thorough literature search; the author finds some applications in the field of FFF/ICP-MS particularly interesting, and regrets any inadvertent omission of reference to relevant papers. A comprehensive compilation of literature related to FFF/ICP-MS is outside the scope of this Trend article. Hence, the reader is also referred to review articles, e.g. Ref. [17].
Application trends
Natural (aquatic) colloids; trace-metal interaction
Natural colloids are defined as organic or inorganic entities, naturally occurring in the (aquatic) environment with at least one dimension in the size range 1 nm–1 μm [18]. In water analysis, filtration of the sample through 0.45 μm filters is a common approach. Aquatic colloids smaller than 0.45 μm easily pass through a filter of this pore size and hence are treated as the “dissolved” fraction (by definition). One of the most important functions of natural colloids (in the nanorange; i.e. up to 100 nm in diameter) is their capacity to bind and transport large amounts of, e.g., (anthropogenic) organic compounds, trace and toxic metal(loid)s, radionuclides, and nutrients [19], functioning as carriers for these substances and hence affecting their bioavailability. Furthermore, speciation [20] of trace elements is affected by natural colloids, also determining their toxicity and bioavailability. Natural colloids in the nanorange are of particular interest, because of their high binding capacity resulting from their high surface area. Typically 40–60 % of organic carbon, 50–100 % of Fe, and 30–70 % of Al in natural freshwater has been found to be associated with colloids, with a major fraction within the size range below 100 nm [13]. It is also known that up to 60 % of several elements is associated with this size fraction [21]. However, the fraction domain <0.45 μm has remained largely uninvestigated because of challenges in size fractionation and determination.
Since its introduction in the 1990s, FFF/ICP-MS has evolved as a powerful and indispensable tool in the field of natural colloids, helping to discover the distribution of elements among different colloidal size fractions (in the range 1 nm to 1 μm) in a qualitative and quantitative manner without sample filtration through a 0.45 μm membrane. The high separation power of FFF and the absence of a stationary phase, in combination with the multi-element capabilities and high sensitivity of ICP–MS detection, have helped to achieve a deeper insight into interactions of (trace) metal(loid)s and natural colloids and their distribution among different size fractions. It has also enabled investigation and understanding of the underlying principles of, e.g., (trace) metal(loid) and pollutant transport and/or biodistribution and correlation with relevant variables, e.g. salinity, pH, and temperature.
Hassellöv et al. [12] were the first to use Fl-FFF/ICP-MS for the simultaneous determination of colloidal size distributions and metal content for 28 elements in natural waters from a small creek in Sweden, revealing the outstanding multi-element capabilities of this method. The approach includes an on-line pre-concentration step enabling detection even at ultra-trace elemental levels (within the ng L−1 range). Figure 3 displays Fl-FFF/ICP-MS fractograms which include five metals (Pb, La, Mo, Ni, Fe) and their distribution among two main different size fractions: a small UV-absorbing organic-rich colloid fraction (2–5 nm) and a larger iron-rich colloid fraction (5–25 nm). It was revealed that most of the elements investigated were associated with the organic-rich fraction and only a few elements (e.g. Pb) were associated with the iron-rich fraction. The distribution of (trace) metals among different size fractions strongly affects their mobility and biodistribution.
Size-fraction distributions (hydrodynamic diameter; molecular weight) for six elements in a natural-water sample upon Fl-FFF/ICP-MS analysis. (Reprinted with permission from Ref. [12]; Copyright 1999 American Chemical Society)
Differences in associations of metal(loid)s, whether to organic or iron-rich colloids, can often be explained on the basis of their chemical properties. The sizes and concentrations of the organic-rich and iron-rich colloids are not fixed but vary strongly depending on, e.g., seasons, the matrix of the water (lake, river), and variations in flow.
However, the dominance of two colloidal fractions (organic-rich and iron-rich), as identified by Hassellöv et al. upon Fl-FFF/ICP-MS (and simultaneous UV–visible detection) analysis, has been revealed for several rivers and streams and seems to be a typical characteristic of most fresh watercourses.
Further advanced studies conducted by Lyvén et al. helped to categorize elements regarding their distribution between carbon and iron-rich fractions [22]. By means of Fl-FFF/ICP-MS they revealed that the smaller alkaline-earth and lanthanide elements have a clear tendency towards higher proportions in the organic-carbon colloids (humic substances (HS)) than larger ones, as a result of electrostatic binding. The cations of the first transition series have complex formation with HS (high for Co, Ni, and Cu, least for Mn and Zn). Pb was found to be mainly adsorbed to the iron-rich fraction. These findings obtained by means of Fl-FFF/ICP-MS analysis will help to fill knowledge gaps regarding transport and biodistribution of (trace) metal(loid)s in aquatic systems. Figure 4 outlines the distribution of several elements among the two colloidal fractions.
Percentage of elements found in the iron-rich fraction as a function of atomic number (Reprinted with permission from Ref. [22]; Copyright 2003 Elsevier Ltd.)
Additionally, Fl-FFF/ICP-MS studies conducted by Stolpe et al. have revealed that the ratio among the fractions is not static and is strongly affected by several variables. Fe-rich colloids occur rarely and are larger in size in a moderate-dissolved-organic-carbon (DOC), high-alkalinity river than in a high-DOC, low-alkalinity river, probably because colloid stability depends on DOC concentration, pH, and Ca2+ concentration [23].
A further factor affecting the stability of colloids is the salt concentration of the surrounding medium. Increased salt concentration is especially observed in estuaries; it leads to transformation of non-settling colloids into settling particles through aggregation, and affects the solubility of (trace) metal(loid)s because of a shift in complexation with colloidal ligands [21]. Hence, it is most likely that transportation of (trace) metal(loid)s is affected. Stolpe and Hassellöv studied the behavior of colloidal matter and associated elements in seawater matrix by means of Fl-FFF/ICP-MS [21]. Upon a salinity increase of 2.5 %, a 50 % decrease of the Fe-rich fraction was observed. By means of Fl-FFF/ICP-MS, a contrasting behavior was revealed: Fe-rich colloids tend to aggregate to larger fractions, whereas the small carbon-rich fraction is resistant towards increased salinity. Thus, Fl-FFF/ICP-MS made a valuable contribution to a deeper understanding of the metal-transportation behavior of colloids, and thus of the bioavailability of metal(loid)s in the interfaces between fresh and seawater.
An effect of climate change (global warming) on the mobilization of (trace) metal(loid)s in aquatic and soil systems is currently very probable. Fl-FFF/ICP-MS can make a valuable contribution to the elucidation of this correlation, as recently revealed by Stolpe et al. [24]. They investigated the occurrence of and changes to organic and iron-rich colloids in Alaskan rivers in the watersheds affected by permafrost. Under permafrost conditions the rivers investigated contain highly aromatic dissolved organic matter (DOM) and most of the total dissolved trace elements associate with the small DOM fractions. During summer, DOM with lower aromatic content is present and trace elements associate with the larger organic fractions and with iron-rich colloids. This temperature-induced fraction-size shift will most probably affect the mobility of trace elements.
As well as transporting essential (trace) metal(loid)s, (aquatic) natural colloids also transport (anthropogenic) contaminants. A notable class of contaminant is radionuclides released into the aquatic environment, e.g. via contamination by nuclear weapon tests and/or use, the nuclear fuel cycle, and nuclear accidents. An environmental contaminant of much concern is depleted uranium (DU) from civilian (e.g. colored glassware, gamma-radiation shielding) and military (e.g. DU-bearing ammunition) uses. DU consists mainly of 238U, which is weakly radioactive. Standard radio-analytical techniques are used to detect radionuclides; however, during the last two decades ICP–MS has become an important tool in radionuclide research, because of its lower detection limits, straightforward, and universal applicability [25]. A promising technique to elucidate DU transport in aquatic systems is Fl-FFF/ICP-MS. Interaction of radionuclides with aquatic colloids greatly alters their mobility; elucidation of the long-term mobility of actinides in groundwater is important for, e.g., siting nuclear-waste facilities and managing waste-rock piles at uranium mines. Geckeis et al. used FFF/ICP-MS to study the distribution of DU in aquatic environmental samples via natural organic and inorganic colloids. They investigated groundwater from a salt stock used as a disposal zone for nuclear waste (Gorleben aquifer, Lower Saxony, Germany) and alkaline sediment extracts. It was revealed that U in groundwater is partly bound to large inorganic colloids stabilized by humic and/or fulvic coatings and, to a lesser extent, attached to humic and/or fulvic acids. The results indicate that inorganic colloids are released from the sediment into the groundwater, and hence mobilization of, e.g., U is enabled [26].
These examples clearly reveal that FFF/ICP-MS has become a powerful tool for investigating the contribution of natural aquatic colloids (1 nm–1 μm) to (trace) metal(loid) and metal-pollutant transport in freshwater systems. Because of its high separation power, excellent limits of detection, and elemental selectivity, FFF/ICP-MS has become an inherent part of colloid analysis.
Over the last few years there has been increased public awareness of engineered nanomaterials as a result of the constantly increasing amount of new products containing nanomaterials. Engineered nanomaterials cover the same size range as natural colloids, and hence the application of FFF/ICP-MS to the field of engineered nanomaterials was identified as a further trend, which will be discussed in the next section.
Engineered nanoparticles (ENPs)
Engineered nanoparticles (ENPs) have gained much interest in the scientific community over the last decade, resulting in a “nano-hype”. The cause of interest in ENPs is an expanding nanomaterial industry, inventing and designing new materials and coatings in the nanometer range, e.g. daily-life products (functionalized clothing, cosmetics), food and food-contact materials, electronic devices, and medicine. However, detailed knowledge explaining the properties of these new materials is incomplete. Furthermore, the possible effect on humans and the environment, and the fate and quantity of ENPs being emitted to the environment, is not yet fully understood. Hence, recent legislation has identified a deficit in regulation of ENPs and evaluation of potential risks. Therefore, in 2011 the European Commission (EC) launched a nanomaterial-definition recommendation which is already partially implemented in some regulations, e.g. for cosmetic products and for biocides (Regulation (EC) no. 1223/2009 and no. 528/2012, respectively):
“[…] ‘Nanomaterial’ means a natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and where, for 50 % or more of the particles in the number size distribution, one or more external dimensions is in the size range 1 nm - 100 nm. In specific cases and where warranted by concerns for the environment, health, safety or competitiveness the number size distribution threshold of 50 % may be replaced by a threshold between 1 % and 50 %.” [27].
However, analytical techniques helping to decide whether a material can be described as “nano” by this definition, especially in complex (real) matrices, are still not fully developed.
Because of its valuable contribution to the study of natural colloids and a comparable size range to ENPs, FFF/ICP-MS was supposed to be a powerful tool for metal-based ENPs analysis. With regard to the EC definition, FFF/ICP-MS enables size determination and mass and number-based quantification.
In 2002, Siripinyanond and Barnes [28] published one of the first papers using Fl-FFF/ICP-MS for ENP analysis. They used the technique to characterize commercial, mechanical polishing slurries (used in the semiconductor industry) to reveal the suitability of Fl-FFF/ICP-MS for ENP analysis. Sizing of the fractions was enabled and, because of the multi-elemental capabilities of ICP–MS detection, the observation of co-elution of different elements became possible and an unwanted contamination of the slurries with Fe, Tl, and Zr was revealed. Mean particle sizes determined for alumina slurries were in the range 150–350 nm, and those for silica slurries were in the range 110–220 nm. The work clearly revealed the applicability of Fl-FFF/ICP-MS in engineered metallic-(nano) particle characterization.
ENP materials that are currently the focus of investigation are summarized in Table 1. Publications dealing with ENP analysis by means of FFF/ICP-MS are mainly related to Au < TiO2 < Ag ENPs (Fig. 2, lower pie chart).
Au ENPs
The history of Au ENPs can be dated back to the fifth century. The synthesis of Au ENPs is well established and they can be successfully stabilized in suspension. Au ENPs have reactive surfaces and unique optical properties, and their applicability in biology and medicine as carriers of bioactive molecules is of great interest. Surface modification of Au ENPs is possible and they can be conjugated with functional groups, e.g., citrate, transferrin, amino acids, peptides, antibodies, and lipids. The conjugates have mainly been used as labels for imaging techniques, and recently Au-ENP bioconjugates have received interest as “drug carrier vehicles” for therapeutic purposes. However, safe use can only be guaranteed if appropriate analytical techniques are available to investigate the behavior of the ENPs in biological matrices. Schmidt et al. developed an Fl-FFF/ICP-MS-based method for Au-ENP separation and size and quantity determination in rat-liver digests. Au ENPs were intravenously administered to rats and extracted from liver, and afterwards digested by means of tetramethylammonium hydroxide (TMAH). Fl-FFF/ICP-MS analysis of rat-liver digests enabled the identification of a possible Au ENP–tissue interaction [30]. From this work and on the basis of further studies, a mode of action of Au ENPs in organisms can be revealed.
The first results of ENP analysis by means of FFF/ICP-MS are promising, but such investigations are still in the early stages. Hence, Gray et al. conducted more general investigations of the applicability of Fl-FFF/ICP-MS to Au ENPs, and revealed some aspects that need to be taken into account. A size-dependent detection limit and recovery was observed and, when dealing with Au ENPs in real matrices, it is necessary to bear in mind surface properties of Au ENPs which most probably affect the elution behavior and size determination [31]. Also, Hagendorfer et al. conducted basic investigations regarding Fl-FFF/ICP-MS method development for Au-ENP analysis using NIST-certified Au ENPs (RM-8011, 8012, 8013). They scrutinized the effect of membrane–particle interactions on peak shape and recovery, the effect of charge repulsion between ENPs and of membrane-enhanced recovery, and the need for careful method optimization (e.g., cross flow, solvent composition) to maximize separation power [32].
TiO2 ENPs
TiO2 ENPs are used in large scale in a variety of consumer products, in particular in sunscreens. There is an increasing need for analytical methods enabling reliable characterization of quantitative particle-size distribution in these goods; especially in terms of quality control, because sun protection factors (SPF) are related to particle-size distribution. Samontha et al. used sd-FFF/ICP-MS to investigate the TiO2 size and mass distribution in several sunscreens of different SPF from different manufacturers [33]. After removal of organic and/or fatty constituents via extraction, in most cases a correlation between SPF and particle size was identified: higher SPF was associated with larger TiO2 particle size. Furthermore, Ti quantification was achieved. For ENP analysis, sample preparation is a crucial step and appropriate procedures are missing. Hence, a more systematic study regarding sample preparation was performed recently by Nischwitz and Goenaga-Infante [34]. An extraction procedure using hexane for defatting, followed by re-extraction and suspension in water assisted by bath sonication, was developed and characterized. Commercial sunscreens (also with different SPF) were spiked with TiO2 reference materials to optimize the extraction procedures. Analysis of sunscreen extracts was performed by Fl-FFF/ICP-MS. An increasing amount of Ti was observed to correlate with increasing SPF. Size determination of the TiO2 fractions was in the range 16–39 nm.
Ag ENPs
One of the fastest growing products in the nanotechnology industry is Ag ENPs. They have antimicrobial properties and low manufacturing costs, and thus are frequently used for many applications, e.g. cosmetics, wall paints, biocide sprays, textiles, laundry detergents, food-contact materials, and dietary supplements [35]. However, although they have many beneficial uses, concerns related to release, fate, and toxicity to the environment have been raised and analytical techniques are needed for their investigation.
The high separation power and applicability of Fl-FFF for Ag ENPs was revealed by Geiss et al. [36], who developed a size and mass-determination method. Upon optimization of the separation conditions (solvent composition, pH, and cross-flow gradient) separation of several Ag-ENP mixtures was achieved (Fig. 5, upper part: Fl-FFF/UV-visible; lower part: Fl-FFF/ICP-MS):
Overlaid fractograms from Ag-ENP mixtures analyzed by Fl-FFF with UV-visible or ICP–MS detection. Numbers above the peaks represent the hydrodynamic diameter of each fraction (nm). (Reprinted with permission from Ref. [36]; Copyright 2013 Elsevier Ltd.)
On the basis of Fig. 5 a particle-size calibration curve was obtained, enabling size determination of Ag ENPs (being a basic requirement of the nano-definition; see above). To enable quantification the authors proposed a pre-channel approach using Ag particles for quantification. However, because of the lack of (metal) ENP reference materials certified in size and fraction-related concentration [37], quantification of separated fractions is crucial and only a few other quantification approaches have been described in the literature. One of the first quantification approaches, conducted by Bolea et al., relies on a post-channel (flow) injection approach with ionic standards and either integration of the obtained peak, or comparing the counts from a flat-topped peak of a known concentration with the signal height within the fractogram [38]. Another quantification approach described recently by Meermann et al. [39] is based on species-unspecific post-channel on-line isotope dilution for fraction-related quantification. The approach was applied to a mixture of two Ag-ENP fractions (30 nm and 75 nm) and enabled an on-line fraction-related quantification.
However, most publications on FFF/ICP-MS and ENPs are related to “artificial” matrices, and mandatory methods applied to real matrices are scarce. Recently two papers were published revealing the applicability of Fl-FFF/ICP-MS for the analysis of Ag ENPs in real matrices. Loeschner et al. described the first method for the analysis of Ag ENPs in a chicken-meat food matrix [40]. Upon Ag-ENP spiking to simulate the migration of ENPs from a food container into the meat, Proteinase K digestion was conducted. Recovery values of approximately 80 % were obtained, making enzymatic digestion a promising sample-preparation method for inorganic ENPs in protein-based sample matrices. Fl-FFF/ICP-MS revealed a slight elution-time shift compared with pristine Ag ENPs. Further investigations upon fraction collection revealed an effect of the meat matrix.
To study the toxicity of ENPs, well designed and adapted toxicity tests are necessary. However, standard toxicity tests have mostly been used and discovering whether ENPs or dissolved species are responsible for any toxic effects is hardly possible. Therefore, Bolea et al. investigated the applicability of Fl-FFF/ICP-MS to Ag ENPs in toxicity-test culture medium and associated cells [41]. They revealed that Ag ENPs became oxidized within the culture medium, and “free” Ag+ ions were formed in a time-dependent manner and underwent interactions with proteins which were accelerated by the presence of cells. Association of Ag ENPs with cells was also confirmed. The ability to detect and distinguish Ag+ protein species from “free” Ag ENPs in culture media and from cell–Ag ENPs associates clearly reveals the benefits of FFF/ICP-MS analysis. Hence, FFF/ICP-MS analysis can make a valuable contribution to understanding and interpreting the toxicity of ENPs within toxicity tests.
Stable-isotope tracer
The more nanotechnology develops, the more likely the release of ENPs into the (aquatic) environment becomes, and it has become evident that natural and engineered nanoparticles have intrinsic similarities. To enable further investigation of the fate of ENPs in real matrices, analytical strategies have to be developed enabling a distinction between ENPs and, e.g., natural colloids. A promising strategy suggested by Gulson and Wong is based on the application of stable (metal) isotope tracing of ENPs [42]. Stable (metal) isotope tracers, which have been used widely in earth sciences and in metabolic and other health-related studies for several decades, are also promising in the field of ENPs, because many of the metals used in nanotechnology have more than one stable isotope and therefore fulfill the prerequisite of a minimum of two stable isotopes (Table 2).
Two main approaches to the use of isotope tracing exist, either: (i) measuring naturally occurring differences between stable isotopes (resulting from isotope fractionation), or (ii) incorporation of a stable, non-radioactive isotope or tracer whose abundance is different from that occurring naturally in the respective product. The FFF/ICP-MS coupling is ideally suited to measure differences in the isotope ratio. Approach (ii) in particular has great potential for use in nanotechnology; “labeling” of ENPs with stable isotopes enables, e.g., exposure monitoring during production, fate, transport, and toxicity studies of ENPs within lab-scale studies using real matrices. It also enables quantification of metal ENPs at realistic low-exposure concentrations, despite high background levels of the respective metals. However, as far as the author is aware, only one paper exists using isotopically modified ENPs (Ag ENPs) as tracers in combination with Fl-FFF/ICP-MS to elucidate their fate under environmental conditions, published recently by Gigault and Hackley [43]. After synthesis and characterization the 109Ag-enriched ENPs were spiked into an estuarine sediment and natural organic matter (NOM) in an aqueous suspension. On the basis of the shifted isotope ratio of the 109Ag-enriched ENPs (107Ag < 1 %, 109Ag > 99 %, Fig. 6), the Ag-ENP fraction is clearly allocable within the spiked real matrix. The authors also observed a ratio shift towards the natural Ag ratio (107Ag = 51.8 %, 109Ag = 48.2 %), indicating a possible interaction of the 109Ag ENPs with the solid sediment phase and Ag exchange with natural Ag. ICP–MS detection is ideally suited to precise measurement of isotope ratios, and the high potential of stable-isotope tracers was revealed, helping to elucidate the environmental and/or biological fate of ENPs.
Isotope-ratio (109Ag/107Ag) fractograms of Ag-enriched ENPs (red fractogram) and unspiked Ag ENPs (blue fractogram) analyzed by Fl-FFF–ICP–MS. Ag ENPs are clearly detectable as a result of isotope enrichment and significant change of the isotope ratio. (Reprinted, with permission, from Ref. [43]; Copyright, 2013, Elsevier)
From published studies, it is evident that FFF/ICP-MS is a powerful, applicable tool for ENP analysis. However, some challenges have been identified, especially regarding sample preparation and studies conducted in real matrices. Here, further basic investigations concerning, e.g., ENP stability and interaction of ENPs with the fractionation system [44] are necessary. Additional “basic” studies on, e.g., membrane-ENPs interactions within Fl-FFF, as addressed by Hagendorfer et al. [32], are also highly necessary. One crucial point not yet fully addressed is the need for stable, certified “nano” reference materials, certified in size and fraction-related quantity for method calibration and validation. However, institutions (NIST, IRRM, and BAM) are working on this requirement and will most probably overcome existing challenges in the near future.
Concluding remarks and outlook
Application trends in FFF/ICP-MS were discussed in this article. Since its introduction in the early 1990s, FFF/ICP-MS has evolved as an indispensable method in aquatic chemistry which has helped in obtaining deeper insights into the function of natural colloids as (trace) metal(loid) transporters. Around the turn of the millennium, nanotechnology became an important component of daily-life products; however, several questions, e.g. the fate and toxicity of the particles, are not yet fully answered and FFF/ICP-MS has been revealed as a promising tool in engineered-nanomaterial (ENM) analysis helping to address these problems. The high separation power of FFF and the high sensitivity and multi-elemental capabilities of ICP–MS detection are particularly crucial to the suitability of FFF/ICP-MS for ENM analysis. The suitability of ICP–MS for the determination of isotope ratios is particularly beneficial in terms of tracing and further elucidation of the fate of ENMs in complex matrices; applications in this area will most probably increase. However, some critical challenges are not yet fully solved, especially with regard to:
-
1.
Missing sample-preparation and method-development procedures for ENMs in complex matrices [44];
-
2.
Interaction of ENMs with the FFF channel; and
-
3.
The lack of CRMs certified in size and mass and/or number-based distribution for method validation, requiring further fundamental research.
As aforementioned, natural colloids have a crucial function in (trace) metal(loid) and pollutant transport, biodistribution, and speciation. It is well known that elemental species determine the properties of the elements [20]. However, applications combining standard speciation methods based on, e.g., high-performance liquid chromatography (HPLC), gas chromatography (GC), or capillary electrophoresis (CE) and FFF to investigate an interrelation between speciation and fraction-related distribution are scarce in literature. Investigations performed by Dubascoux et al. [45] clearly revealed coherences, focusing on the example of several tin species to reveal that a fraction-related species distribution exists which strongly affects the mobility and bioavailability of species. Because of limits to technical feasibility, only off-line coupling upon fraction collection is possible. To avoid potential species transformation and species degradation, an on-line coupling of FFF and standard speciation methods and further research in this area are needed.
A further trend that has increased in importance during recent years and will become more important in the near future is the complementary use of FFF/ICP-MS and single-particle ICP–MS (sp-ICP–MS), especially in ENM analysis. Sp-ICP–MS, introduced in 2003 by Degueldre and Favarger, is based on the analysis of ion clouds generated by the introduction of single particles into the ICP–MS. On the basis of the signals obtained and appropriate data assessment, a direct determination of size and number-size distribution of ENMs is possible [46]. Sp-ICP–MS and FFF/ICP-MS complement each other: FFF/ICP-MS can fractionate and detect smaller particles, providing greater size resolution, whereas sp-ICP–MS offers the unique ability to differentiate dissolved and nanoparticulate fractions, which is beneficial in terms of closing mass-balances. The high capability of the combined approach has been revealed recently [40, 47] (cf. Fig. 2) and will most probably gain in importance in the near future, especially given the impetus provided by recent ICP–MS instrumental developments enabling multi-element detection within single particles [48].
FFF is not only applicable to natural colloids and ENMs, but also to larger biomolecules, e.g. proteins. Because of its “soft” and very adaptable separation conditions (adaptable carrier; absence of stationary phase), FFF is an ideal tool for proteomics [49]. Instrumental developments and/or improvements to high-resolution organic mass spectrometry as a powerful detection system have promoted applications of FFF in the field of functionalized proteomics. The large molar-size coverage of FFF (compared with chromatographic separation methods) and on-line coupling with high-resolution organic mass spectrometry enables protein identification even in highly complex mixtures [50]. Many proteins contain metal ions and sulfur atoms as an essential component which, e.g., determines their structures or establishes active binding sites. ICP–MS is well known and is often used for protein quantification, mostly because of its species-independent response (enabling quantification without authentic standards), elemental selectivity, and high sensitivity [51]. Surprisingly, (complementary) applications of FFF–ICP–MS for proteomics are scarce in literature, but the author believes a promising complementary tool to have been revealed by Siripinyanond and Barnes [52]. They examined several metalloproteins, mostly containing Cu, Zn, and Cd, by means of Fl-FFF/ICP-MS. High element sensitivity and selectivity and species-independent response, combined with high separation power under “soft” conditions, emphasized the applicability of the complementary FFF/ICP-MS method for protein analysis, which will certainly evolve in the near future to become another application trend.
Since its introduction more than two decades ago FFF/ICP-MS has evolved from a “niche” method into an established technique, especially in the field of natural colloids. The “nano-hype” occurring a few years ago strongly promoted FFF/ICP-MS. The beneficial properties of this technique (e.g. no stationary phase, high separation power, multi-elemental capabilities, and high sensitivity) justified its existence as a complementary tool to chromatographic techniques including HPLC and size-exclusion chromatography (SEC).
In the author’s opinion FFF/ICP-MS will not become as important as chromatographic techniques, but will become an indispensable complementary tool, especially in (metallo)proteomics, nanosciences, and environmental studies.
References
Giddings JC (1966) Sep Sci 1:123–125
Schimpf M, Caldwell K, Giddings JC (2000) Field-flow fractionation handbook. Wiley-Interscience, New York
Wahlund K-G (2013) J Chromatogr A 1287:97–112
Schallinger LE, Yau WW, Kirkland JJ (1984) Science 4660:434–437
Caldwell KD, Cheng ZQ, Hradecky P, Giddings JC (1984) Cell Biophysics 6:233–251
Caldwell KD, Karaiskakis G, Myers MN, Giddings JC (1981) J Pharm Sci 70:1350–1352
Thielking H, Roessner D, Kulicke WM (1995) Anal Chem 67:3229–3233
Ledin A, Karlsson S, Duker A, Allard B (1995) Analyst 120:603–608
Engelhard C (2011) Anal Bioanal Chem 399:213–219
Beckett R (1991) At Spectrosc 12:228–232
Murphy DM, Garbarino JR, Taylor HE, Hart BT, Beckett R (1993) J Chromatogr 642:459–467
Hassellov M, Lyven B, Haraldsson C, Sirinawin W (1999) Anal Chem 71:3497–3502
Baalousha M, Stolpe B, Lead JR (2011) J Chromatogr A 1218:4078–4103
Krystek P, Ulrich A, Garcia CC, Manohar S, Ritsema R (2011) J Anal At Spectrom 26:1701–1721
Dubascoux S, Le Hecho I, Hassellov M, Von der Kammer F, Gautier MP, Lespes G (2010) J Anal At Spectrom 25:613–623
Kato H, Nakamura A (2014) Anal Methods 6:3215–3218
Pornwilard M-M, Siripinyanond A (2014) J Anal At Spectrom 29:1739–1752
Slomkowski S, Aleman JV, Gilbert RG, Hess M, Horie K, Jones RG, Kubisa P, Meisel I, Mormann W, Penczek S, Stepto RFT (2011) Pure Appl Chem 83:2229–2259
Ranville JF, Chittleborough DJ, Shanks F, Morrison RJS, Harris T, Doss F, Beckett R (1999) Anal Chim Acta 381:315–329
Templeton DM, Ariese F, Cornelis R, Danielsson LG, Muntau H, Van Leeuwen HP, Lobinski R (2000) Pure Appl Chem 72:1453–1470
Stolpe B, Hassellov M (2007) Geochim Cosmochim Acta 71:3292–3301
Lyven B, Hassellov M, Turner DR, Haraldsson C, Andersson K (2003) Geochim Cosmochim Acta 67:3791–3802
Stolpe B, Guo LD, Shiller AM, Hassellov M (2010) Mar Chem 118:119–128
Stolpe B, Guo LD, Shiller AM, Aiken GR (2013) Geochim Cosmochim Acta 105:221–239
Boulyga SF (2011) Int J Mass Spectrom 307:200–210
Geckeis H, Manh TN, Bouby M, Kim JI (2003) Colloids and Surfaces A - Physicochem Eng Aspects 217:101–108
European Commission (2011) Commission recommendation of 18 October 2011 on the definition of nanomaterial (2011/696/EU). Off J
Siripinyanond A, Barnes RM (2002) Spectrochim Acta Part B 57:1885–1896
da Silva BF, Perez S, Gardinalli P, Singhal RK, Mozeto AA, Barcelo D (2011) Trends Anal Chem 30:528–540
Schmidt B, Loeschner K, Hadrup N, Mortensen A, Sloth JJ, Koch CB, Larsen EH (2011) Anal Chem 83:2461–2468
Gray EP, Bruton TA, Higgins CP, Halden RU, Westerhoff P, Ranville JF (2012) J Anal At Spectrom 27:1532–1539
Hagendorfer H, Kaegi R, Traber J, Mertens SFL, Scherrers R, Ludwig C, Ulrich A (2011) Anal Chim Acta 706:367–378
Samontha A, Shiowatana J, Siripinyanond A (2011) Anal Bioanal Chem 399:973–978
Nischwitz V, Goenaga-Infante H (2012) J Anal At Spectrom 27:1084–1092
Hagendorfer H, Kaegi R, Parlinska M, Sinnet B, Ludwig C, Ulrich A (2012) Anal Chem 84:2678–2685
Geiss O, Cascio C, Gilliland D, Franchini F, Barrero-Moreno J (2013) J Chromatogr A 1321:100–108
Linsinger TPJ, Roebben G, Solans C, Ramsch R (2011) Trends Anal Chem 30:18–27
Bolea E, Jimenez-Lamana J, Laborda F, Castillo JR (2011) Anal Bioanal Chem 401:2723–2732
Meermann B, Fabricius AL, Duester L, Vanhaecke F, Ternes TA (2014) J Anal At Spectrom 29:287-296
Loeschner K, Navratilova J, Kobler C, Molhave K, Wagner S, Von der Kammer F, Larsen EH (2013) Anal Bioanal Chem 405:8185–8195
Bolea E, Jimenez-Lamana J, Laborda F, Abad-Alvaro I, Blade C, Arola L, Castillo JR (2014) Analyst 139:914–922
Gulson B, Wong H (2006) Environ Health Perspect 114:1486–1488
Gigault J, Hackley VA (2013) Anal Chim Acta 763:57–66
Ulrich A, Losert S, Bendixen N, Al-Kattan A, Hagendorfer H, Nowack B, Adlhart C, Ebert J, Lattuada M, Hungerbuhler K (2012) J Anal At Spectrom 27:1120–1130
Dubascoux S, Heroult J, Le Hecho I, Potin-Gautier M, Lespes G (2008) Anal Bioanal Chem 390:1805–1813
Degueldre C, Favarger PY (2003) Colloids and Surfaces A - Physicochem Eng Aspects 217:137–142
Kim ST, Kim HK, Han SH, Jung EC, Lee S (2013) Microchem J 110:636–642
Borovinskaya O, Hattendorf B, Tanner M, Gschwind S, Gunther D (2013) J Anal At Spectrom 28:226–233
Roda B, Zattoni A, Reschiglian P, Moon MH, Mirasoli M, Michelini E, Roda A (2009) Anal Chim Acta 635:132–143
Reschiglian P, Moon MH (2008) J Proteomics 71:265–276
Sanz-Medel A, Montes-Bayon M, Bettmer J, Fernandez-Sanchez ML, Encinar JR (2012) Trends Anal Chem 40:52–63
Siripinyanond A, Barnes RM (1999) J Anal At Spectrom 14:1527–1531
Acknowledgment
The German Federal Ministry of Transport and Digital Infrastructure (BMVI) is gratefully acknowledged for funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meermann, B. Field-flow fractionation coupled to ICP–MS: separation at the nanoscale, previous and recent application trends. Anal Bioanal Chem 407, 2665–2674 (2015). https://doi.org/10.1007/s00216-014-8416-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8416-1