Abstract
Results relating to the first original application of an analytical approach combining asymmetric flow field-flow fractionation (As-Fl-FFF) with multi-detection and chemical speciation for determination of organotins in a landfill leachate sample are presented. The speciation analysis involved off-line head-space solid-phase microextraction (HS-SPME)–gas chromatography with pulsed-flame photometric detection (GC–PFPD) performed after three consecutive collections of five different fractions of interest from the As-Fl-FFF system and cross-flow part (assumed to be representative of the <10 kDa phase). After 0.45 μm filtration and without preconcentration before fractionation and speciation analysis, limits of detection (LOD) were 4–45 ng (Sn) L−1 in the sample, with relative standard deviations (RSD) of 3–23%. The As-Fl-FFF fractionation of this sample enables characterization of two distinct populations—organic-rich and inorganic colloids with gyration radius up to 120 nm. Total Sn and mono and dibutyltins (MBT and DBT) appear to be distributed over the whole colloidal phase. Tributyl, monomethyl, monooctyl, and diphenyltins (TBT, MMT, MOcT, and DPhT) were also detected. Quantitative speciation analysis performed on the two colloidal populations and in the <10 kDa phase revealed concentrations from 130 ± 10 (MMT) to 560 ± 50 ng (Sn) L−1 (DPhT).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The term “colloidal” usually covers the 1 nm–1 μm size range [1, 2]. Macromolecules, molecule aggregates, particles, or microorganisms are included among colloidal materials. The role of colloids is especially crucial since they are ubiquitous in the environment. The variety of colloids of various chemical compositions and their size distribution involves many different physicochemical processes such as aggregation or adsorption. Colloidal fractionation is considered to be one of the key parameters controlling transport and bioavailability of chemical pollutants in the environment [3–5].
Although colloids have been known for a long time, they often remain neglected in environmental studies due to the lack of convenient analytical tools. In recent decades significant evolution of instrumentation has taken place, offering real possibilities to perform physicochemical work at the nano-scale. Filtration-based methods have been widely used for determining the size distribution and physicochemical properties of associated size fractions [6, 7]. Among these methods, field-flow fractionation (FFF) is promising, because of its numerous advantages compared with chromatographic techniques, especially its high resolution in size distribution. Today, most of this work is based on asymmetric flow field-flow fractionation (As-Fl-FFF) as an efficient and versatile sub-technique of FFF. Its potential for on-line fractionation and hyphenation to various detectors such as UV spectrometry or multi-angle laser light scattering (MALLS) is of the highest interest [5, 8]. Coupling of As-Fl-FFF with inductively coupled plasma–mass spectrometry (ICP–MS) is especially useful for determining metal distribution according to the size distribution of the colloidal phase. Some authors have demonstrated the potential of multi-detector approaches for metal monitoring, mainly in the aquatic environment [2, 4, 9]. However, in other environmental compartments severely affected by human activity, studies remain much less numerous. Waste management is today a crucial societal challenge. Especially since domestic solid waste production is increasing every day, it is necessary to understand its environmental impact. Among the different scientific problems generated, the role of landfill leachate remains an open question. The answer is mainly based on more complete physicochemical characterisation, including the colloidal phase. At the same time, waste regulation requires regular monitoring. Until now, the major part of the studies deals with colloidal fractionation using filtration and ultrafiltration of landfill leachates [10–14]. In these work, each size fraction was analysed separately. Very few papers refer to colloid fractionation using As-Fl-FFF coupled with different detectors [15, 16]. Klein et al. used As-Fl-FFF coupled on-line with UV and off-line with an enzyme-linked immunosorbent assay (ELISA) and atomic absorption spectroscopy (AAS) in order to characterize colloids and monitor heavy metals linked to these colloids in seepage water samples [15]. In this study, Klein et al. only applied As-Fl-FFF to low nanometre range colloids. Prestel et al. used As-Fl-FFF coupled on-line with UV, fluorescence, and ICP–MS to study sewage plant hydrocolloids [16]. All these works deal with total metal concentrations on different colloidal fractions without paying special any attention on chemical speciation of these metals. However, knowledge of total metal concentration is well known to be insufficient for comprehending the fate of metals. Because chemical speciation is undeniably considered as a key parameter controlling trace metal behaviour, chemical speciation analysis is required as a crucial tool to provide information about accumulation and toxicity. No study has ever reported combination of on-line colloidal fractionation with determination of chemical speciation as a new analytical approach to evaluate metal distribution in a given medium. This lack is probably partly due to the difficult analytical challenges involved in such an approach.
Previous works on leachates from municipal landfill sites highlighted the presence of organotins at concentrations from about 0.1 to 4 μg (Sn) L−1 [17, 18]. These compounds are recognized as ubiquitous in the environment due to their extensive use in many human activities [19]. They are also considered as priority pollutants, endocrine disrupters, and highly toxic to aquatic and terrestrial organisms [20]. As organotin compounds (OTC) are widely used in many domestic products, among the numerous diffuse sources of OTC contamination, leaching from rubbish dumps contributes to water and soil pollution by organotins [19]. However, analysis is generally performed on the whole material with no special attention given to chemical speciation on the colloidal phase.
In this work the potential of on-line As-Fl-FFF–UV–MALLS-ICP–MS combined with off-line speciation analysis was investigated. For the first time, by characterisation of sample size, the colloidal distribution of various elements was determined according to the type of the fractions in order to complete the information. The challenge was to evaluate the combined fractionation and chemical speciation approach on an environmental sample. Global speciation (i.e. chemical speciation of the different physical fractions) was thus determined. Thus important information concerning the pollutant nanovector roles of colloids in environmental processes could be obtained. Landfill leachate was chosen for this study as domestic waste is of major concern [21]. Chemical speciation involved collection of fractions from As-Fl-FFF and individual analysis of these fractions by using gas-chromatographic (GC) separation with specific pulsed-flame photometric detection (PFPD).
Experimental
Apparatus
The asymmetric flow field-flow fractionation instrument used was an Eclipse 2 (Wyatt Technology, Dernbach, Germany). The channel dimensions were 26.5 cm length and from 2.1 to 0.6 cm width. Its thickness was defined by a spacer of 350 μm height. The As-Fl-FFF principle is now well described [7]. Briefly, colloids are carried through the channel by use of a mobile phase. They are separated inside by a crossflow field induced by the different pressures on both sides of the membrane, as in on-line ultrafiltration. In this study, separation as a function of colloidal size was achieved in normal mode, i.e. “smaller” colloids being eluted first because of their higher diffusion coefficient in the mobile phase and the channel flow profile.
Ultrafiltration membranes used were 10 kDa (cut off) regenerated cellulose (Wyatt Technology, Dernbach, Germany). Flows were controlled with an Agilent 1100 series isocratic pump equipped with an Agilent series 1100 micro vacuum degasser (Agilent Technologies, Tokyo, Japan).
The UV detector was an adjustable wavelength 1100 series (Agilent Technologies). The wavelength usually selected for environmental samples (254 nm) was used in this study. The multi-angle laser light scattering detector was a DAWN DSP-F (Wyatt Technology, Santa Barbara, USA) with 18 angular detectors ranging from about 10 to 160°. The MALLS signals presented later correspond to the 90° angle detector. As MALLS signal varies as a function of size and concentration of colloids, this detector allows the direct measurement of gyration radius, r g (from about 20 nm to 500 nm) with a precision of 1–3%. Gyration radius is not a commonly used concept with regard to geometric radius (r). It allows evaluation of the colloid size as a function of the mass distribution within the colloid. Despite the fundamental differences between the r g and r concepts, the direct measurement of r g remains very useful as a direct approach to colloid size. For particles with a constant shape, evaluation of the r g during As-Fl-FFF fractionation enables control of the quality of the fractionation during elution, i.e. a normal mode with a linear relationship between retention time and r g.
The differential refractometer (DR) (Wyatt Technology, Santa Barbara, USA) was an Optilab DSP. Data from the UV, MALLS, and DR detectors were collected and treated with Astra 5.3.1.5 software (Wyatt Technology, Santa Barbara, USA). The ICP–MS was a 7500ce model (Agilent Technologies). The hyphenation between As-Fl-FFF and ICP–MS was similar to conventional HPLC–ICP–MS coupling. It consisted in using a simple PEEK tube linking the last detector of the As-Fl-FFF–UV–MALLS (i.e. MALLS) to the Micromist nebulizer of the ICP.
Gas-chromatography (GC) was performed with a 3800 gas chromatograph (Varian, Walnut Creek, CA, USA) equipped with a pulsed flame-photometric detector (PFPD) and a 1079 split/splitless injector (cf. Table 1).
Reagents
Tripropyltin (TPrT, 98%), mono, di, and trimethyltin (MMT, 97%, DMT, 97%, TMT, 100%), mono, di, and tributyltin (MBT, 95%, DBT, 97%, TBT, 96%), mono, di, and triphenyltin (MPhT, 98%, DPhT, 96%, TPhT, 95%), and trioctyltin (TOcT, 95%), as the chlorides, were purchased from Aldrich (Saint-Quentin Fallavier, France). Mono (MOcT, 97%) and dioctyltin (DOcT, 97%) were obtained from Lancaster (Strasbourg, France). Methanol and sodium ethanoate were obtained from Prolabo (Paris, France). Hydrochloric, nitric, and ethanoic acids were from Merck (Nogent-sur-Marne, France) and isooctane from Fluka (Saint-Quentin Fallavier, France). Sodium tetraethylborate (NaBEt4) was purchased from Strem Chemical (Bischeim, France). Deionised water used was 18 MΩ Milli-Q water.
Stock solutions (1000 mg (Sn) L−1) of each organotin species were prepared by dissolving the different powders in methanol. Stock solutions are stable for at least 1 year and were stored in darkness at +4 °C. Diluted solutions (10 mg (Sn) L−1 and 100 μg (Sn) L−1) were prepared weekly in methanol and water, respectively, and stored in the dark at +4 °C. A 2% (w/v) NaBEt4 solution was prepared daily in deionised water and stored under the same conditions.
Glassware and extraction materials were rinsed with deionised water, decontaminated for 2 days in 10% (v/v) nitric acid solution, and rinsed again before use.
Samples
For evaluating the coupling between As-Fl-FFF and the different detectors (i.e. UV, MALLS and ICP–MS), a test sample was used. This test sample was a soil extract and its preparation has already been described elsewhere [5, 22]. It contains 120 mg L−1 mineral colloids, mainly consisting of iron–manganese-coated clay-like platelets in a colloidal size range mainly <500 nm. The soil extract was kindly given by Frank von der Kammer. The landfill leachate was obtained from a French landfill receiving municipal wastes only. Sample collection, storage, and pretreatment before analysis are precisely described elsewhere [18, 21]. Briefly, the sample was collected, via a pipe, in polyethylene container and stored at +4 °C in the dark until analysis. The pH of the leachate was measured at 8.5 and the total organic carbon (TOC) was 812 mg (C) L−1. For speciation and total determination, the leachate was frozen at −20 °C in the dark until analysis. Its metal and metalloid content had previously been found to be: Fe, Sr, Al, (concentrations over 1000 μg L−1), Cr, Mn, Sn, Zn (100–1000 μg L−1), Pb, Cu, Sb, As (10–100 μg L−1), Se, and Cd (0.5–10 μg L−1) [18, 21]. In this study this sample was simply filtered at 0.45 μm before As-Fl-FFF-based analysis.
Operating procedure
All the operating conditions are summarized in Table 1.
The As-Fl-FFF operating conditions were optimised to fractionate large colloids (typical radius >20 nm) according to the nature of the sample. The operating conditions were:
-
1.
mobile phase: 30 mmol L−1 CH3COONH4 in Milli-Q water (pH 9),
-
2.
injected volume: 100 or 400 μL at 0.20 mL min−1 during the focus step,
-
3.
elution rate: 1 mL min−1 ; cross-flow-rate : 0.5 mL min−1 during the fractionation.
The crossflow rate was kept constant during the analysis to maintain a simple linear relationship between the hydrodynamic radius and the retention time of the colloids during the fractionation [7]. The samples were filtered at 0.45 μm before analysis.
Sample was introduced into the ICP–MS by means of a Micromist nebulizer. The ICP–MS was used in collision-cell mode, with a H2 flow rate of 4 mL min−1. These conditions were found to remove isobaric interferences and allowed the monitoring in time-resolved mode of twenty different elements.
Speciation analysis was carried out by using headspace (HS) solid-phase microextraction (fibre coated with a 100-μm apolar polydimethylsiloxane (PDMS) film; Supelco, Saint-Quentin Fallavier, France) (SPME)–GC–PFPD. This coupling was previously developed and validated in our laboratory [23, 24]. Briefly, before analysis, organotins were derivatised in order to give volatile and thermostable species for GC. The derivatisation step was performed at pH 4.5 by using an aqueous solution of 2% sodium tetraethylborate (NaBEt4). Simultaneously, SPME extraction of apolar ethylated OTC was carried out in the headspace volume of the hermetically closed derivatisation reactor. After ethylation and extraction, the SPME device was directly introduced into the injection port of the GC where the OTC were thermally desorbed and then separated and quantified. For the GC separation, nitrogen was used as carrier gas.
Following fractionation of the colloids by As-Fl-FFF, the different fractions of interest were collected (volume = 2 to 5 mL) and then analysed off line by HS-SPME–GC–PFPD. The standard addition method was used for quantification [23–25]. All analyses were replicated.
Results and discussion
Analytical evaluation
The multi-detection approach based on As-Fl-FFF–UV–MALLS–ICP–MS coupling was evaluated in order to estimate its suitability for the determination of metal distribution on the colloidal phase. Owing to the multi-detection and the different complementary signals, characterisation of the different types of colloid and the associated metal distribution was expected. The test sample was analysed and the results are presented in Figs. 1 and 2. In Fig. 1, the UV and MALLS signals of the test sample are presented. A shift of the peak maximum can be noticed. In fact, for the inorganic particles from the test sample the weak UV response is not due to absorbance but to turbidity only. Thus, the UV detector could be used as a concentration detector whereas the MALLS detector responds to both concentration and the size of the particles. With regard to the size characteristics of these particles, their r g were found to range from 30 to 300 nm (right scale of Fig. 1, where radius information is given directly by MALLS). The linearity of r g variation as a function of elution time shows the efficiency of the fractionation. In Fig. 2 the ICP–MS signals corresponding to the major elements (i.e. aluminium, iron, and manganese) and some trace elements (i.e. arsenic, lead, and tin) present in this mixture are reported. The aluminium signal is slightly shifted to the left compared with the MALLS signal and could be considered as an estimate of clay particle mass distribution. The iron distribution is shifted to the left compared with the aluminium signal and could indicate iron coating on the clays. The manganese signal follows the aluminium one, which indicates that manganese could be an intrinsic component of the particles. Arsenic and tin are distributed similarly to iron whereas lead distribution corresponds more closely to both aluminium and iron. These different fractograms obtained from the complementary detectors confirm the convenience of coupling As-Fl-FFF, UV, MALLS, and ICP–MS for environmental study.
In order to evaluate the suitability of the operating conditions of HS-SPME–GC–PFPD for organotin determination in colloidal materials, the analytical performance of the speciation method was determined in the mobile phase (volume = 5 mL) during calibration. Limits of detection (LOD) and quantification (LOQ) were evaluated by using the calibration curve method. The accuracy (trueness and precision) was tested by use of reference solutions prepared in the mobile phase, at concentrations equal to 1 to 2 times the calculated LOQ [26]. The results are presented in Table 2. Trueness was evaluated by the mean of recovery calculation. Precision was estimated by means of a repeatability test. The absolute LOD are in accordance with those previously obtained by using HS-SPME–PFPD for water analysis (range over 6–800 pg (Sn), depending on the compound) [25]. They are in agreement with environmental concentrations previously found in the same type of sample (100–4000 ng (Sn) L−1) [17]. DMT and TMT have higher LOD and LOQ because of poor analytical repeatability (around 20%). Their quantification curve is also associated with lower determination coefficients (R 2). These phenomena are because of their high volatility—their boiling points are substantially lower than those of the other organotins, leading to losses during HS-SPME [25]. The higher LOD and LOQ of DPhT can be related to its low extraction yield due to its poor volatility [24, 25]. Despite these values, the accuracy tests provided satisfactory results: the determined concentrations were found to be statistically equal to target ones for all the compounds, with suitable recovery and precision. From these results, the analytical speciation method was validated.
As-Fl-FFF fractionation combined with organotin speciation of the collected fraction
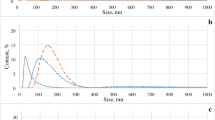
The multi-detection approach previously evaluated was then applied on the landfill leachate sample. From Fig. 3a it can be noticed that the sample clearly contains two different types of colloid, identified by two peaks called F1 and F2. F1 corresponds to the strongest UV absorbance of the fractogram. Generally, such an intense signal is attributed to organic matter (OM). This fraction probably contains OM-rich colloids, which is in accordance with previous studies demonstrating that the TOC is mainly contained in the lowest nanometre size range (sometimes regarded as humic colloids) [14, 15, 27]. F2 appears to be mainly inorganic (according to the UV signal), with gyration radius ranging from 50–110 nm. According to the DR signal, F2 has a maximum concentration corresponding to a population of colloids with r g around 60 nm. F1 and F2 DR peaks are not completely separated and the F1 UV peak is tailing, which indicates that:
-
organic matter could be coated on inorganic particles with sufficient size to be detected by MALLS, or
-
organic and/or inorganic colloids of similar range of size (r g over 30–50 nm from MALLS information) are present in the sample, or
-
the UV tailing signal is due to the turbidity of the particles.
This kind of result with two different populations, i.e. small OM-rich colloids and larger particles, has already been observed in a previous study reported by Stolpe et al. on natural water samples [28]. However, the colloidal size reached in their work did not exceed 20 nm diameter whereas the colloidal size range observed in the current landfill leachate study is larger with gyration radius up to 100 nm.
According to Fig. 3a, fractionation of the landfill leachate shows an r g variation with a particular shape. Satisfactory fractionation generally gives a regular and sufficiently slow r g variation as a function of the elution time, as shown in Fig. 1. Similar non-regular shape has already been observed for river colloids and was attributed to possible colloid–membrane interactions [29]. In the current case, these interactions occurred between 400 and 600 s, corresponding to a gyration radius around 60 nm. This phenomenon could also be the result of the high heterogeneity and complexity of the leachate.
Qualitative organotin speciation
Tin appears to have a particular colloidal distribution (Fig. 3b). Thus, by comparison with the other elements, which are mainly bound to the OM-rich F1 fraction, Sn is on both the F1 and F2 fractions. Moreover, according to the relative intensity of the ICP–MS signal, tin seems in significant concentrations in the colloidal phase. The presence of tin can be related to its inorganic (Sn+IV mainly) and organic forms (organotin compounds, OTC), these last species being highly toxic.
In order to complete the information and assess the potential environmental impact of organotins from landfill leachate, speciation analysis was performed considering the profile of the fractionation (Fig. 3b). First, mobile phase eluting from the As-Fl-FFF channel was regularly collected (five sub-fractions, Fig. 4a) and each sub-fraction was analysed (Fig. 4b). Monomethyl, monooctyl, diphenyl, and the three butyltins were detected in these different sub-fractions, as shown by the chromatograms in Fig. 4b. MBT and DBT appear distributed over the whole colloidal phases, i.e. organic and inorganic sub-fractions. MMT is only present in sub-fraction 1, attributed to “small” OM-rich colloids. TBT is mainly present in sub-fraction 2. MOcT and DPhT are specifically linked to this same sub-fraction, which was previously attributed to OM-containing colloids with r g < 50 nm. The presence of all the detected organotins in these two first sub-fractions confirms the strong affinity of OM-rich materials for these species [30].
The different OTC distribution may be partly due to their different origins (TBT from biocides, MMT, MBT, DBT, and DOcT originally present mainly in plastics, DPhT a degradation product of TPhT, this species also used as biocide). Thus, the similar colloidal distribution of MBT and DBT could be simply explained by their common origins. Moreover MBT and DBT could also be the result of TBT degradation [19]. On the other hand, the different physicochemical properties of the organotin compounds can also explain the distribution differences shown in Fig. 4. It is especially well known that the environmental behaviour of the organotins depends on the degree of substitution and on the nature of their organic groups [19]. Other works highlighted methylation, which occurs as a result of microbial activity [18]. These phenomena and plastic leaching lead to the presence of methyltins. Under these conditions, simple attribution of the organotin origins to a particular type of waste component can be critical. Nevertheless, the OTC colloidal distribution can give some interesting information about the potential mobility of organotins, some colloids being known to be more mobile than others, depending on their physicochemical nature [2].
Quantitative organotin speciation
Because of the low concentrations and the small volumes collected, collection was then performed in order to obtain fractions for quantitative speciation analysis. Thus F1, F2 and also the crossflow (CF, i.e. the part crossing the membrane) were collected and analysed. CF was considered representative of the sample part with a molar mass lower than the membrane cut off (i.e. 10 kDa). In order to ensure that the crossflow is representative of the whole sample, collection was realized from the beginning of the introduction/focusing step to the end of the elution. The results of speciation analysis performed on these three fractions are presented in Fig. 5. Organotins appear as mainly associated with the colloidal phase, only butyltins (and the most toxic TBT) being in significant concentrations in the <10 kDa part. These results highlight that speciation analysis on colloidal distribution is really important in order to evaluate the fate of contaminants.
In a previous speciation study the 3 methyl and 3 butyltins were found, whereas the other OTC (octyl and phenyltins) were not studied [18]. Considering the uncertainties, the total concentrations of MMT, MBT, DBT, and TBT measured in the current work are generally in agreement with values found by Pinel-Raffaitin et al. (respectively, 150 ± 20, 310 ± 30, 110 ± 20, and 300 ± 40 ng (Sn) L−1) [18]. DMT and TMT were not detected in any fraction, probably because of excessive dilution of the sample after collection of the different As-Fl-FFF fractions, LOD and LOQ of these compounds being not sufficiently low. MOcT and DPhT were also found in significant concentrations. These species appear distributed on F1 fraction only. The current species found and their respective concentrations are in the same range (100–1000 ng (Sn) L−1) as values presented by Mersiowsky et al. for the same type of samples [17]. In this previous study, MBT and DBT were also detected in all the landfill leachate samples analysed. TBT and DOcT were detected in 25 to 30% of the cases, whereas no phenyltin seemed to be present.
According to these results, the approach proposed to perform quantitative speciation analysis appears to be suitable for determination of organotins in the different fractions—<10 kDa and colloidal—of complex environmental samples. Despite the high dilution effect involved in collection of different As-Fl-FFF fractions, the analysis was precise and repeatable.
Conclusions
This application illustrates the potential of on-line fractionation combined with speciation analysis for determination of organotin compounds in a complex sample of environmental interest. As-Fl-FFF–UV–MALLS–ICP–MS on-line coupling has been shown to be a powerful technique giving relevant information about the dependence of metal distribution on the nature and size of colloids. In this work it enabled elucidation of the dependence of the distribution of the tin on the nature of the different colloidal fractions. From the fractograms obtained, appropriate collection of different fractions and their SPME-GC-PFPD speciation analysis completed the information by giving the organotin distribution over the colloidal phases and their concentration on the two main fractions previously identified. These first results highlight the different behaviour of the organotins with regard to their nature and their origin. By showing that organotins are mainly associated with the colloidal phase, the major importance of speciation analysis on the colloid phase is confirmed. For this purpose, on-line fractionation appears especially useful.
References
IUPAC (1999) Compendium of chemical terminology, 2nd edn. Blackwell Scientific, Oxford
Lyven B, Hassellöv M, Turner DR, Haraldson C, Andersson K (2003) Geochim Cosmochim Acta 67:3791–3802
Citeau L, Lamy I, Van Oort F, Elsass F (2003) Colloids Surf A 217:11–19
Geckeis H, Ngo Manh T, Bouby M, Kim JI (2003) Colloids Surf A 217:101–108
Kammer FVD, Baborowski M, Friese K (2005) Anal Chim Acta 552:166–174
Buffle J, Perret D, Newman M (1993) The use of filtration and ultrafiltration for size fractionation of aquatic particles, colloids and macromolecules. In: Buffle J, Leeuwen HP (eds) Environmental particles, vol 1. Lewis, Boca Raton
Beckett R, Hart BT (1993) Use of field-flow fractionation techniques to characterize aquatic particles, colloids and macromolecules. In: Buffle J, Leeuwen HP (eds) Environmental particles, vol 2. Lewis, Boca Raton
Gimbert LJ, Andrew KN, Haygarth PM, Worsfold PJ (2003) Trends Anal Chem 22:615–633
Hassellöv M, Lyven B, Haraldson C, Sirinawin W (1999) Anal Chem 71:3497–3502
Baumann T, Fruhstorfer P, Klein T, Niessner R (2006) Water Res 40:2776–2786
Baun DL, Christensen TH (2004) Waste Manag Res 22:3–23
Gustafsson Ö, Gschwend PM (1997) Limn Ocean 42:519–528
Jensen DL, Christensen TH (1999) Water Res 33:2139–2147
Jensen DL, Ledin A, Christensen TH (1999) Water Res 33:2642–2650
Klein T, Niessner R (1998) Mikrochim Acta 129:47–55
Prestel H, Schott L, Niessner R, Panne U (2005) Water Res 39:3541–3552
Mersiowsky I, Brandsch R, Ejlertsson J (2001) J Environ Qual 30:1604–1611
Pinel-Raffaitin P, Ponthieu M, Le Hecho I, Amouroux D, Mazeas L, Donard OFX, Potin-Gautier M (2007) J Anal Atom Spectrom 22:258–266
Hoch M (2001) Appl Geochem 16:719–743
Commission des Communautés Européennes COM (2001) 262 final Bruxelles
Pinel-Raffaitin P, Ponthieu M, Le Hecho I, Amouroux D, Mazeas L, Donard OFX, Potin-Gautier M (2006) J Environ Monit 8:1069–1077
Baalousha M, Kammer FVD, Motelica-Heino M, Hilal HS, Le Coustumer P (2006) J Chromatogr A 1104:272–281
Bancon Montigny C, Lespes G, Potin-Gautier M (2000) J Chromatogr A 896:149–158
Bravo M, Lespes G, De Gregori I, Pinochet H, Potin-Gautier M (2005) Anal Bioanal Chem 383:1082–1089
Le Gac M, Lespes G, Potin-Gautier M (2003) J Chromatogr A 999:123–134
ISO Accuracy (trueness and precision) of measurements methods and results Part 1. General principles and definitions (ISO 5725-1) (1994) AFNOR Paris
Gounaris V, Anderson PR, Holsen TM (1993) Environ Sci Technol 27:1381–1387
Stolpe B, Hassellov M, Anderson K, Turner DR (2005) Anal Chim Acta 535:109–121
Baalousha M, Kammer FVD, Motelica-Heino M, Le Coustumer P (2005) J Chromatogr A 1093:156–166
Hoch M, Alonso-Azcarte J, Lischick M (2003) Environ Pollut 123:217–227
Acknowledgements
The authors gratefully thank Dr F. v. d. Kammer for providing the reference sample and Dr P. Pinel-Raffaitin for the landfill leachate sample. They also thank Wyatt Technology (MALLS) and Varian (GC–PFPD) for their technical support and instrument loan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dubascoux, S., Heroult, J., Le Hécho, I. et al. Evaluation of a combined fractionation and speciation approach for study of size-based distribution of organotin species on environmental colloids. Anal Bioanal Chem 390, 1805–1813 (2008). https://doi.org/10.1007/s00216-008-1913-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-008-1913-3