Abstract
Background
Nicotine addiction continues to be a health challenge across the world. Despite several approved medications, smokers continue to relapse. Several human and animal studies have evaluated the role of the endogenous opioid system as a potential target for smoking cessation medications.
Methods
In this review, studies that have elucidated the role of the mu (MORs), delta (DORs), and kappa (KORs) opioid receptors in nicotine reward, nicotine withdrawal, and reinstatement of nicotine seeking will be discussed. Additionally, the review will discuss discrepancies in the literature and therapeutic potential of the endogenous opioid system, and suggest studies to address gaps in knowledge with respect to the role of the opioid receptors in nicotine dependence.
Results
Data available till date suggest that blockade of the MORs and DORs decreased the rewarding effects of nicotine, while activation of the MORs and DORs decreased nicotine withdrawal-induced aversive effects. In contrast, activation of the KORs decreased the rewarding effects of nicotine, while blockade of the KORs decreased nicotine withdrawal-induced aversive effects. Interestingly, blockade of the MORs and KORs attenuated reinstatement of nicotine seeking. In humans, MOR antagonists have shown benefits in select subpopulations of smokers and further investigation is required to realize their full therapeutic potential.
Conclusion
Future work must assess the influence of polymorphisms in opioid receptor-linked genes in nicotine dependence, which will help in both identifying individuals vulnerable to nicotine addiction and the development of opioid-based smoking cessation medications. Overall, the endogenous opioid system continues to be a promising target for future smoking cessation medications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tobacco smoking continues to attract healthcare resources due to the considerable morbidity and mortality associated with it (USDHHS 2014). Aggressive efforts on the part of healthcare professionals and organizations have certainly helped in slowing down the tobacco epidemic. Despite these efforts, several millions of smokers continue to smoke and suffer the consequences of smoking. Nicotine is a major component of tobacco smoke that is primarily responsible for continued tobacco smoking (Stolerman and Jarvis 1995). The availability of nicotine to teenagers in the form of E-cigarettes has compounded the problem of tobacco smoking, and it appears that the battle against nicotine addiction will continue for some time in the future (Callahan-Lyon 2014). Even though the Food and Drug Administration (FDA) has approved several smoking cessation medications, long-term abstinence is still a challenge for many smokers (D’Souza and Markou 2011). Hence, there is a continuous need to identify novel targets for smoking cessation medications.
High rates of smoking are observed in disease states involving the endogenous opioid system. For example, high rates of smoking have been reported in individuals suffering from chronic pain compared to the general population (Andersson et al. 1998; Hutchinson et al. 2001; Jamison et al. 1991; Zvolensky et al. 2010). The reasons for the high rates of smoking in patients suffering from chronic pain are not fully understood. One hypothesis put forth to explain this phenomenon is that people suffering from chronic pain compared to the general population may have higher vulnerability to get addicted to drugs of abuse including nicotine (Ditre et al. 2011; Hooten 2016). The endogenous opioid system plays an important role in modulating pain (Kirkpatrick et al. 2015). So does the endogenous opioid system have a role in this increased vulnerability to nicotine addiction in patients suffering from chronic pain? Incidentally, high rates of tobacco smoking are observed in heroin-dependent and methadone-maintained patients (Guydish et al. 2011; Miller and Sigmon 2015; Talka et al. 2015b). Both methadone and heroin act by stimulating the mu opioid receptors. Again, it is not clear if high rates of smoking in opioid-dependent patients is due to stimulation of the endogenous opioid system or due to an independent vulnerability to nicotine addiction. However, it has been reported that opioid-dependent smokers respond poorly to smoking cessation medications and may be at higher risk of relapse (Miller and Sigmon 2015). Therefore, opioid-dependent smokers may represent a unique subset of smokers. Overall, the above findings suggest prevalence of nicotine addiction in disease states that involve the endogenous opioid system. Further, patients suffering from chronic pain and opioid-dependent patients provide significant opportunities to promote smoking cessation.
Support for a role of the endogenous opioid system in nicotine dependence comes from a large body of preclinical and clinical studies. In fact, several studies have reported changes in levels of opioid peptides and/or expression of opioid receptors after exposure to acute and chronic nicotine (for review, see Berrendero et al. 2010; Drews and Zimmer 2010; Hadjiconstantinou et al. 2011; Kishioka et al. 2014). There is also evidence to suggest that genetic polymorphisms in opioid receptors may increase vulnerability to development of nicotine addiction and influence response to smoking cessation medications (Crist and Berrettini 2014). Taken together, the above studies suggest a possible role for the endogenous opioid system in development and treatment of nicotine dependence.
This review discusses our current state of knowledge with respect to the role of the endogenous opioid system in nicotine dependence using animal models. Additionally, the review discusses relevant human studies that further our understanding of the role of the opioid system in nicotine dependence. Importantly, the review identifies several gaps in the literature and challenges that exist and must be addressed for promoting smoking cessation via manipulation of the endogenous opioid system. The review thus adds to the above described erudite reviews on the subject.
Development of nicotine dependence
Research in nicotine dependence largely focuses on three important phases. First is the acquisition and maintenance phase, which is mediated by the reinforcing effects of nicotine. Tobacco smoking produces a pleasurable rush, mild euphoria, increased arousal, decreased fatigue, and relaxation in humans (Henningfield et al. 1985). Similarly, nicotine administration in animals maintains nicotine-seeking behavior (Markou 2008; Watkins et al. 2000a). The second phase of nicotine dependence is characterized by withdrawal symptoms upon abstinence from smoking, which occur due to alteration of brain reward systems following chronic nicotine exposure. Withdrawal symptoms in nicotine-dependent smokers include depressed mood, anxiety, irritability, craving, insomnia, and weight gain (Hughes et al. 1991; Shiffman and Jarvik 1976). Similarly, withdrawal of nicotine in nicotine-dependent animals results in increased rearing, jumping, shakes, abdominal constrictions, chewing, scratching, facial tremors, aversion demonstrated by conditioned place aversion (CPA), and depression-like state characterized by elevation of brain reward thresholds (Epping-Jordan et al. 1998; Jackson et al. 2015; Jonkman et al. 2008; Malin et al. 2006; Watkins et al. 2000a). Nicotine withdrawal in animals can be either spontaneous when administration of nicotine is stopped or precipitated when nicotine withdrawal is induced by administrating a nicotinic acetylcholine receptor (nAChR) antagonist. Finally, the last phase in nicotine dependence is relapse amongst abstinent smokers. Relapse can occur on exposure to stressful situations, nicotine, or cues associated with tobacco smoking (Brigham et al. 1990). Learned associations between nicotine and environmental stimuli largely contribute to relapse amongst abstinent smokers (Crombag et al. 2008). In animals, presentation of environmental cues and contexts associated with nicotine facilitates either preference for nicotine-associated chambers or reinitiation of nicotine-seeking behavior after a period of abstinence and/or extinction training (Caggiula et al. 2002; Paterson et al. 2005; Stoker and Markou 2015). Further, stress or priming with nicotine can also produce reinstatement of nicotine-seeking behavior in animals (Buczek et al. 1999; Chiamulera et al. 1996; Shaham et al. 2003). Below, we will discuss the role of endogenous opioids in the above described phases of nicotine dependence.
Endogenous opioid system and nAChR systems
Neuronal nAChRs
The actions of nicotine in the brain are mediated by neuronal nAChRs, which also serve as receptors for the endogenous ligand acetylcholine. The activation of the neuronal nAChRs opens an ion channel that allows for entry of sodium and calcium ions and results in excitation of the neuron. Neuronal nAChRs are composed of five subunits of either only alpha type (i.e., homomeric) or mixture of alpha and beta type subunits (i.e., heteromeric) (Dani and Bertrand 2007). Based on the type of alpha (α2-α9) and beta (β1-β4) subunits that come together to form the heteromeric nAChRs, these receptors generally have a lot of diversity and vary in pharmacological response to nicotine and acetylcholine. In contrast, homomeric nAChRs are most commonly formed of five α7 subunits and are frequently located on glutamatergic neurons (Mansvelder and McGehee 2000, 2002; McGehee et al. 1995). Neuronal nAChRs are widely distributed throughout the CNS and can be found in several brain regions such as the nucleus accumbens (NAcc), amygdala, hippocampus, cortex, globus pallidus, thalamus, hypothalamus, and ventral tegmental area (VTA).
Opioid receptors and peptides
Opioid receptors were first discovered in the 1970s and are broadly classified into three types: mu opioid receptors (MORs), kappa opioid receptors (KORs), and delta opioid receptors (DORs) (Kieffer and Evans 2009; Pert and Snyder 1973). Several endogenous peptides activate these opioid receptors including β-endorphin, met- and leu-enkephalin, and dynorphins (Berrendero et al. 2010). These endogenous peptides have differing affinity for the opioid receptors. The β-endorphin has the highest affinity for the MORs, the met- and leu-enkephalin preferentially bind to the DORs, and dynorphins are endogenous ligands for the KORs (Lutz and Pfister 1992). The opioid receptors are coupled to inhibitory-type G-proteins (Gi/G0), and therefore, activation of these receptors by endogenous ligands or exogenous agonists have an inhibitory effect on neuronal activation or neurochemical release (Kieffer and Evans 2009). These inhibitory actions are mediated by a number of intracellular events and can include the following: inhibition of adenyl cyclase, reduction in the opening of voltage-gated calcium channels, and stimulation of potassium current through several channels including G-protein inwardly rectifying potassium channels (GIRKs). Opioid receptors are located at both pre- and post-synaptic sites and are associated with modulation of neurotransmitter release. Further, repeated agonist stimulation results in changes in functional activity and/or expression of opioid receptors (Dang and Christie 2012). Finally, heterodimerization has been reported between the MORs and KORs and also between the MORs and DORs (Chakrabarti et al. 2010; Pradhan et al. 2011).
Opioid receptors and their endogenous ligands are distributed throughout the CNS and peripheral tissues (Le Merrer et al. 2009; Mansour et al. 1995). Specifically, opioid receptors are found in several limbic and cortical nuclei such as the NAcc, amygdala, hippocampus, prefrontal cortex, globus pallidus, thalamus, hypothalamus, and VTA. These brain regions form parts of circuitries mediating reward, pain, mood, anxiety, and emotional responses. In summary, there is significant overlap in distribution of neuronal nACh and opioid receptors in the brain. Additionally, while nAChRs are excitatory in nature, the opioid receptors are inhibitory in nature.
Interaction between opioid and nACh receptor systems
Nicotine does not bind directly to opioid receptors. Instead, nicotine binds to nAChRs located on presynaptic terminals of neurons containing opioid peptides. Thus, the effect of nicotine on opioid peptide release is largely indirect. Additionally, nAChRs are also located on somatodendritic regions and can influence excitability of opioid-containing neurons (Barik and Wonnacott 2009). The nicotine-induced release of different opioid peptides is regulated differently depending on the peptide. Nicotine-induced increase in met-enkephalin is glutamate-dependent and occurs via activation of α7-containing nAChRs (Isola et al. 2000). Thus, blockade of the ionotropic glutamate receptors (i.e., N-methyl-D-aspartate (NMDA) and amino-3-hydroxy-5-methyl-4-isoxazolepropionate/kainate (AMPA)) located on opioid-containing neurons can attenuate nicotine-induced met-enkephalin release. In contrast to met-enkephalin, nicotine-induced increase in β-endorphin is dependent on nicotine-induced dopamine release and D2 dopamine receptors (Hadjiconstantinou and Neff 2011). Finally, nicotine-induced increase in dynorphin release was blocked by nAChR antagonists, D2 receptor antagonists, and NMDA receptor antagonists (Isola et al. 2009). Therefore, nicotine-induced dynorphin release is regulated by multiple neurotransmitter systems. Repeated exposure to nicotine can alter expression and/or functioning of the different opioid receptors, which are discussed in detail below.
It must be mentioned here that endogenous acetylcholine release can be altered by presynaptic opioid receptors located on cholinergic neurons. For example, pharmacological activation of opioid receptors located on striatal, cortical, and hippocampal brain slices resulted in inhibition of acetylcholine release (Lapchak et al. 1989). It is not known if these in vitro findings also occur under in vivo conditions. Theoretically, inhibition of ACh release in vivo by endogenous opioids can result in upregulation of the corresponding nAChRs. This will make the nAChRs more sensitive to nicotine and can in turn influence release of endogenous opioids. Together, these data suggest reciprocal regulation of nACh and opioid receptors by nicotine and endogenous opioid peptides. In summary, the data suggest complex interactions between the neuronal nACh and opioid receptors, which requires further exploration.
MORs and nicotine-induced effects
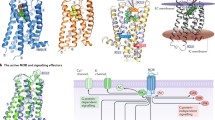
MORs are involved in drug reward and several drugs of abuse such as morphine and heroin act primarily by stimulating the MORs (Charbogne et al. 2014; Le Merrer et al. 2009). In this section, we focus on the role of MORs in the reinforcing effects of nicotine, nicotine withdrawal, and reinstatement of nicotine seeking (see Fig. 1). Effects of pharmacological and genetic manipulations of MORs on nicotine-induced behaviors are summarized in Tables 1 and 2, respectively.
The figure represents effects of pharmacological and genetic manipulations of the endogenous opioid system on nicotine-dependent behaviors such as nicotine reward, nicotine withdrawal-induced effects, and reinstatement of nicotine seeking. As shown in the figure, it appears that mu opioid receptors (MORs) and delta opioid receptors (DORs) play a similar role in nicotine-dependent behaviors. Knockout of the MORs and DORs decreased the rewarding effects of nicotine. These findings are consistent with pharmacological studies, which suggest that blockade of the MORs and DORs decreased the rewarding effects of nicotine. Further, activation of the MORs and DORs decreased the aversive effects associated with nicotine withdrawal. In comparison to the MORs and DORs, the kappa opioid receptors (KORs) appear to play an opposite role in nicotine reward and nicotine withdrawal. Pharmacological activation of the KORs decreased the rewarding effects of nicotine, while blockade of the KORs blocked the aversive effects associated with nicotine withdrawal. Interestingly, blockade of the MORs and KORs attenuated reinstatement of nicotine seeking in animals suggesting that such compounds may help to prevent relapse in humans. The effects of knockout of the KORs in nicotine-dependent behaviors has not been investigated
MORs and reinforcing effects of nicotine
Based on the role of MORs in reward, it is hypothesized that indirect nicotine-induced stimulation of the MORs will facilitate the rewarding effects of nicotine, while blockade of the MORs will attenuate the rewarding effects of nicotine. Consistent with this hypothesis, MOR antagonists naloxanazine and naloxone attenuated intravenous nicotine self-administration in rats (Ismayilova and Shoaib 2010; Liu and Jernigan 2011, but see also DeNoble and Mele 2006) (see Table 1)]. Interestingly, naltrexone, another MOR antagonist did not attenuate nicotine self-administration in Sprague-Dawley rats (Corrigall and Coen 1991; Liu et al. 2009). It is not clear if differences in pharmacology of naltrexone vs. naloxanazine contributed to differences in the above findings. Naloxanazine specifically targets type 1 MORs, while naltrexone targets both type 1 and type 2 MORs. Further, nicotine self-administration protocols and rat strains were different between the studies and could have contributed to the reported findings. Additionally, MOR antagonists naloxone and glycyl-glutamine attenuated nicotine-induced conditioned place preference (CPP) (Goktalay et al. 2006; Walters et al. 2005). Further, naloxone-mediated decrease in nicotine-induced CPP was shown to be mediated via a decrease in cAMP response element binding protein (CREB) phosphorylation (Walters et al. 2005). Even though these pharmacological studies reveal the role of MORs in the reinforcing effects of nicotine, the role of MORs in animal models with extended access to nicotine have not been demonstrated. Additionally, it remains to be determined if effects of repeated administration of the MOR antagonists such as naloxone and naltrexone on nicotine self-administration are similar to effects of acute administration of these compounds as described above. The latter studies will help in determining development of tolerance to the effects of MOR receptor antagonists on the reinforcing effects of nicotine.
Consistent with the role of MORs in nicotine reward, both nicotine-induced behavioral sensitization and nicotine-induced CPP was attenuated in mice lacking the MORs compared to the wild-type mice (Berrendero et al. 2002; Walters et al. 2005; Yoo et al. 2004). Additionally, mice lacking β-endorphin, the endogenous ligand for the MORs, showed attenuated nicotine-induced CPP (Trigo et al. 2009). There is indirect evidence to suggest that nicotine increases release of β-endorphin and activates the MORs (Davenport et al. 1990). However, direct evidence is lacking, and in fact, an in vivo microdialysis study showed no increase in β-endorphin levels in the NAcc after acute nicotine administration (Olive and Becker 2008). Taken together, these data suggest that MORs and its endogenous ligand β-endorphin mediate the reinforcing effects of nicotine.
The rewarding effects of nicotine like other drugs of abuse are mediated by mesolimbic dopaminergic neurons, which originate in the VTA and project to different brain regions including the NAcc and amygdala (Koob and Volkow 2010). MORs are extensively distributed in both the NAcc and VTA (Mansour et al. 1988). Activation of MORs in the VTA increased dopamine levels in the NAcc and inhibited VTA dopaminergic neurons projecting to the amygdala (Devine et al. 1993; Ford et al. 2006). Similarly, nicotine administration also increased dopamine levels in the NAcc and VTA (D’Souza et al. 2011; Di Chiara and Imperato 1988a; Rahman et al. 2004). Future studies need to explore the effects of pharmacological and/or genetic manipulation of MORs in specific brain regions such as the NAcc and VTA on nicotine self-administration and/or nicotine-induced CPP.
High rates of smoking have been reported in both heroin-dependent and methadone-maintained patients (Guydish et al. 2011; Mello et al. 1980). Additionally, an increase in cigarette consumption has been reported in patients using buprenorphine, a partial agonist of the MORs (Mello et al. 1985; Mutschler et al. 2002). Interestingly, activation of MORs in the frontal cortices was associated with reports of cigarette liking and wanting in human smokers (Kuwabara et al. 2014). It is not clear if the high rates of smoking in methadone-maintained patients are because of stimulation of MORs or blockade of nAChRs. Studies suggest that methadone blocks α3β4-containing nAChRs and activates α7-containing nAChRs (Talka et al. 2015a; Xiao et al. 2001). It is possible that the high rates of smoking in opioid-dependent abusers may be independent of opioid abuse and could be due to concurrent increased vulnerability to addiction in these individuals, a hypothesis that needs further exploration. To understand this phenomenon better, there is a need to develop animal models assessing effects of both nicotine and heroin in the same model. This could be done by exposing animals to heroin prior to nicotine self-administration. Alternately, the effects of nicotine exposure on heroin self-administration also need to be assessed. Here, it must be mentioned that repeated administration of nicotine sensitized animals to the rewarding effects of morphine (Vihavainen et al. 2008). Such models will facilitate our understanding of neurobiological changes occurring with coabuse of heroin and nicotine. Further, these models will potentially help in the development of better treatments for heroin abusers with high rates of smoking. As a caveat, it must be mentioned here that high rates of smoking are not exclusive to opioid-dependent subjects and is also reported amongst abusers of other drugs of abuse such as stimulants and alcohol (Dawson 2000; Weinberger and Sofuoglu 2009).
In human smokers, MOR antagonists attenuated the rewarding effects of smoking. A decrease in the number of cigarettes smoked has been reported in patients taking MOR antagonists naltrexone and naloxone compared to placebo (Epstein and King 2004; Gorelick et al. 1988; Karras and Kane 1980; King et al. 2013a; King and Meyer 2000; Lee et al. 2005, but see also Wong et al. 1999). This decrease in cigarettes smoked amongst smokers after administration of MOR antagonists reflects a decrease in the rewarding effects of nicotine. Additionally, smokers reported decreased satisfaction from smoking after naltrexone administration (Wewers et al. 1998). Overall, both experimental studies in animals and humans suggest a role for MORs in nicotine reward and blockade of these receptors decreased the rewarding effects of nicotine.
MORs and chronic nicotine exposure
Chronic nicotine exposure resulted in alteration in MOR expression/functioning depending on the protocol of nicotine treatment, time of collection of tissue, biomarker measured, and species/strain of animals used (see Table 3). For example, MORs were upregulated in the striatum after chronic nicotine treatment (14 days) in Sprague-Dawley rats, when measured using [3H]-[D-Ala2,N-Me-Phe4,Gly-ol5]-enkephalin (DAMGO) binding (Wewers et al. 1999). Interestingly, this upregulation of MORs was sex-dependent with more prominent effects in females than males. Additionally, MOR mRNA expression was increased in the VTA, but not in the NAcc, after chronic nicotine administration (8 days) in C57BL/6 mice (Walters et al. 2005). The dose of nicotine that produced this increase in MOR mRNA expression also produced nicotine-induced CPP, thus suggesting that increased MOR expression in the VTA expression possibly mediates the reinforcing effects of nicotine. Together, these findings suggest increased MOR-mediated transmission after chronic nicotine exposure. In contrast to the above findings, chronic nicotine exposure via the oral route (7 weeks) in NMRI mice resulted in no changes in MOR functioning or DAMGO binding in the striatum and VTA (Vihavainen et al. 2008). Finally, a decrease in MOR density was reported in the striatum (dorsal and ventral) in C57BL/6 mice after chronic nicotine treatment (Galeote et al. 2006). Overall, the above studies suggest that MOR expression/function is altered by chronic nicotine treatment, but these alterations depended on the nicotine administration protocol, biomarker measured, time of collection of brain tissue, and species/strain used in the study.
In addition to changes in MORs expression/functioning, decreases in hypothalamic β-endorphin levels and proopiomelanocortin (POMC) mRNA levels have been reported following chronic nicotine administration (Rasmussen 1998; Rosecrans et al. 1985). The hypothalamic-pituitary axis plays an important role in the aversive effects associated with nicotine withdrawal (Koob and Volkow 2010). The hypothalamus also plays an important role in regulating appetite, and changes in appetite and weight gain are frequently associated with nicotine withdrawal. Therefore, dysregulation in the β-endorphin-MOR system in the hypothalamus-pituitary axis possibly suggests a role for MOR signaling in nicotine withdrawal. In current smokers, increases in plasma levels of β-endorphin have been reported (Gilbert et al. 1992; Pomerleau et al. 1983). The increase in plasma β-endorphin was seen after smoking high doses of nicotine and was accompanied by aversive symptoms such as nausea, malaise, and general discomfort (Gilbert et al. 1992). This suggests that the increase in peripheral β-endorphin levels is more a reflection of aversive effects associated with smoking. However, it is not fully understood if this increase in plasma β-endorphin reflects a brain region-specific increase or generalized increase in brain β-endorphin levels.
The role of MORs in nicotine withdrawal has also been investigated. Administration of MOR antagonist naloxone in nicotine-dependent animals resulted in expression of somatic signs of nicotine withdrawal (Biala et al. 2005; Malin et al. 1993). It is hypothesized that this naloxone-precipitated nicotine withdrawal syndrome could be mediated by binding of naloxone to nAChRs. In fact, in vitro studies have demonstrated that naloxone can block nicotine-induced effects in cell systems that lack opioid receptors (Tome et al. 2001). As a caveat, it must be mentioned here that interaction of naloxone with the nAChRs occurs at very high doses (Watkins et al. 2000b). Additionally, MOR agonist morphine attenuated aversive somatic signs associated with spontaneous nicotine withdrawal (Malin et al. 1993). In addition to precipitation of somatic signs of nicotine withdrawal, low doses of the MOR antagonist naloxone induced CPA in nicotine-dependent rats (Ise et al. 2000; Watkins et al. 2000b). Administration of MOR agonist morphine reversed mecamylamine-induced CPA in nicotine-dependent animals (Ise et al. 2000). Watkins et al. (2000b) reported elevation of brain reward thresholds in nicotine-dependent rats only after administration of high doses of naloxone. Interestingly, this high dose naloxone-induced elevation of brain reward thresholds was also observed in saline controls, suggesting that the effect of naloxone at high doses on brain reward thresholds was not due to chronic nicotine exposure. Together, these pharmacological studies suggest increased MOR-mediated transmission following chronic nicotine exposure. Importantly, the data suggest that decrease in MOR-mediated neurotransmission via blockade of MORs in nicotine-dependent animals precipitated aversive affective and somatic withdrawal signs. Further, activation of MORs in nicotine-dependent animals attenuated aversive somatic and affective signs associated with nicotine withdrawal. Thus, MORs are involved in nicotine withdrawal and can serve as targets to alleviate nicotine withdrawal-associated symptoms.
Intriguingly, somatic signs associated with mecamylamine-precipitated nicotine withdrawal in nicotine-dependent mice were attenuated in MOR knockout mice compared to their respective wild-type controls (Berrendero et al. 2002). Somatic manifestation of nicotine withdrawal are largely mediated by peripherally located receptors, and thus these findings suggest that peripherally located MORs play an important role in mediating the effects of nicotine withdrawal. The apparent discrepancy between findings of this study using a genetic approach and pharmacological studies discussed above could be explained by compensatory neuroadaptations that can occur following congenital knockout of a specific gene in knockout animals. Further, as discussed above knockout of the MORs makes the animal less sensitive to the rewarding effects of nicotine (Berrendero et al. 2002). Together, these findings suggest that knockout of the MORs makes animals less sensitive to the effects of nicotine. Future studies should focus on compensatory changes occurring following genetic knockout of MORs as such studies will help identify factors that decrease sensitivity to nicotine. Such factors can possibly be used in the future as targets for promoting smoking cessation.
The role of MORs has also been investigated in human smokers after a period of overnight abstinence (early withdrawal). Using [11C]-carfentanil, clinical studies have reported lower availability of MORs in several brain regions such as the thalamus, amygdala, and basal ganglia in abstinent smokers compared to non-smoking controls (Scott et al. 2007; Weerts et al. 2014, but see also Falcone et al. 2012). Lower availability of MORs is suggestive of decreased MOR-mediated neurotransmission. Importantly, the availability of MORs during early abstinence was inversely proportional to the severity of nicotine dependence. Additionally, lower availability of MORs in several mesolimbic brain regions (globus pallidus, thalamus, ventral striatum, and amygdala) was associated with higher craving for nicotine in nicotine-dependent smokers (Weerts et al. 2014). In summary, these neuroimaging studies suggest that decreased MOR-mediated neurotransmission is associated with aversive effects of smoking cessation. However, it is not known if these changes in availability of MORs are sustained or restricted to the time point used in the above studies. Further, the above described findings were observed after smoking of denicotinized cigarettes and could reflect a response to cues associated with smoking. Nevertheless, findings from animals studies discussed above are consistent with neuroimaging findings in abstinent smokers.
Clinical pharmacological studies evaluating the effects of MOR antagonists on nicotine withdrawal symptoms suggest either no effect or attenuation of nicotine withdrawal symptoms. For example, administration of naloxone had no effect on nicotine withdrawal symptoms in nicotine-dependent human subjects (Gorelick et al. 1988, but see also Krishnan-Sarin et al. 1999). Similarly, naltrexone administration had no effect on withdrawal symptom scores (Covey et al. 1999; Knott and Fisher 2007; Rohsenow et al. 2007; Sutherland et al. 1995; Wong et al. 1999). Importantly, naltrexone reduced craving in nicotine-dependent smokers (King et al. 2013a; King et al. 2006; King et al. 2012). Studies also support use of naltrexone prior to quit date in smokers wanting to quit. In fact, initiation of the MOR antagonist naltrexone prior to the quit date in nicotine-dependent smokers may help predict response to naltrexone during abstinence from smoking (King et al. 2013a). In other words, patients who are sensitive to naltrexone prior to quitting may continue to respond to naltrexone after smoking cessation. In summary, blockade of MOR-mediated neurotransmission in nicotine-dependent patients does not exacerbate nicotine withdrawal symptoms and infact may help in decreasing craving for nicotine. These findings from clinical pharmacological studies in humans thus appear to be contradictory to findings from neuroimaging studies in human abstinent smokers and animal studies described above. The reasons for this discrepancy are not entirely clear. One possible reason could be due to assessment at a specific time-point closer to withdrawal from nicotine in human neuroimaging and animal studies.
MORs and nicotine-seeking behaviors
MORs also play a role in reinstatement of nicotine seeking. Blockade of MORs using naltrexone attenuated cue-induced reinstatement of nicotine seeking (Liu et al. 2009). The role of MORs in nicotine seeking is consistent with its role in reinstatement of other drugs such as alcohol and cocaine (Giuliano et al. 2015; Gutierrez-Cuesta et al. 2014; Simmons and Self 2009). Additionally, MORs play a role in learning and memory recall, which plays a role in reinstatement of drug-seeking behavior (Bianchi et al. 2013; Farahmandfar et al. 2012). Interestingly, direct injections of the MOR agonist DAMGO in the VTA did not reinstate extinguished responding for nicotine, which implies that MORs in the VTA may not have a role in reinstatement of nicotine seeking (Corrigall et al. 2000). However, MORs are extensively found in brain regions such as the NAcc, anterior cingulate, hippocampus, and amygdala, which play a role in reinstatement of drug seeking including nicotine seeking (Koob and Volkow 2010; Le Merrer et al. 2009). The role of MORs in reinstatement of nicotine seeking in the above mentioned brain regions has not been investigated. Future studies will need to address this gap in knowledge.
Differential regulation of the MORs in the brain has been reported after cocaine-induced and cue-induced reinstatement of cocaine seeking (Georgiou et al. 2015). Using quantitative radiography, cocaine-induced but not cue-induced reinstatement of cocaine seeking upregulated MOR binding in the basolateral amygdala. In contrast, cue-induced but not cocaine-induced reinstatement of cocaine seeking upregulated MOR binding in the caudate putamen and NAcc core. It is not clear if these findings are restricted to cocaine or will extend to nicotine. Future work will need to determine if MOR functioning is differentially regulated by nicotine-induced vs. cue-induced reinstatement of nicotine seeking.
The effect of nicotine-associated cues on MORs has also been investigated in humans. PET imaging and [11C]-carfentanil displacement studies have reported a decrease in availability of MORs in the anterior cingulate, thalamus, NAcc, and amygdala following smoking of denicotinized cigarette in overnight abstinent smokers (Nuechterlein et al. 2016). These brain regions play an important role in relapse. Therefore, the decrease in MOR availability in these brain regions following smoking of denicotinized cigarettes could be due to activation of MORs by nicotine-associated environmental and sensory cues, which can act as powerful substitutes during nicotine withdrawal-induced craving (Rose et al. 2010). In summary, MORs play a role in relapse to smoking and could serve as potential targets to prevent relapse in abstinent smokers.
MORs: therapeutic potential and future directions
Based on the preclinical data described above, one can conclude that blockade of MORs may help in attenuating the rewarding effects of nicotine and in preventing reinstatement of nicotine-seeking behavior. Further, the studies described above suggest that stimulation of MORs may help in attenuating nicotine withdrawal effects. To date, the MOR antagonists naltrexone and naloxone have had limited success in promoting long-term cessation in human smokers. Several studies report no significant effect on long-term abstinence with naltrexone compared to placebo either alone or in combination with nicotine replacement therapy (Baltieri et al. 2009; Covey et al. 1999; King et al. 2006; King et al. 2012; Toll et al. 2010; Wong et al. 1999, see also reviews David et al. 2014; David et al. 2013).
Although naltrexone has not been very effective in improving long-term smoking cessation, administration of naltrexone was more efficacious compared to placebo in limiting smoking cessation-induced weight gain in women, but not in men (King et al. 2012; King et al. 2013b). These findings suggest that MOR antagonists may have gender-based effects, the mechanisms of which are not fully understood. These data are consistent with other studies discussed below that suggest gender-based differences in functioning of the endogenous opioid system. Addition of naltrexone to nicotine replacement therapy improved short-term abstinence rates amongst smokers and reduced smoking cessation-induced weight gain (Krishnan-Sarin et al. 2003; O’Malley et al. 2006). Overall, the data support the use of naltrexone for limiting negative consequences associated with smoking cessation such as weight gain, especially amongst women smokers.
Naltrexone may also be of use in certain subpopulations of smokers desiring to quit, such as those suffering from comorbid mental disorders including depression and alcohol dependence. Addition of naltrexone to nicotine replacement treatment improved quit rates in smokers suffering from depression (Walsh et al. 2008). Naltrexone, which has been approved by the FDA for treatment of alcohol, was also effective in smokers with heavy alcohol consumption habits. Smokers with high alcohol consumption constitute approximately 20–25% of smokers and have a distinct clinical profile compared to smokers who do not drink heavily (Dani and Harris 2005; Toll et al. 2012). In fact, administration of naltrexone was shown to improve quit rates in heavy drinking smokers (King et al. 2009). Further, combining varenicline with low-dose naltrexone (25 mg/day) attenuated consumption of both alcohol and cigarettes compared to placebo in smokers who were classified as heavy drinkers (Ray et al. 2014). Additionally, the study reported decreased craving for cigarettes and attenuation of “high” associated with smoking and alcohol consumption. Moreover, naltrexone in combination with varenicline compared to placebo attenuated activation of anterior cingulate cortex, a brain region involved in cigarette craving, in response to cigarette-related cues (Ray et al. 2015). Together, these data suggest that naltrexone may have potential for improving efficacy of currently approved smoking cessation medications in smokers with high alcohol consumption. As a caveat, the treatment period in both studies was relatively short (only 9–12 days), and therefore, these findings must at best be considered preliminary in nature. However, smokers with significant alcohol consumption history are generally less responsive to currently approved smoking cessation medications and are more likely to relapse (Kahler et al. 2010). Thus, combining smoking cessation treatments with naltrexone may be beneficial in this subgroup of smokers and further work is warranted. In summary, although MOR antagonists such as naltrexone have not shown efficacy in maintaining long-term abstinence in smokers to date, identifying subpopulation of smokers based on gender, comorbid alcohol abuse, and psychiatric comorbidity (e.g., depression) may improve response to naltrexone in smokers. Also, MOR antagonists may have greater success in promoting smoking cessation when combined with currently approved smoking cessation medications such as nicotine replacement and varenicline.
Polymorphism of the OPRM1 gene is associated with differences in subjective experiences of nicotine reward and susceptibility to nicotine dependence. In humans, a single nucleotide polymorphism in the OPRM1 gene, OPRM1 A118G, has been extensively investigated (Crist and Berrettini 2014). This polymorphism is associated with substitution of adenine by guanine in exon1 of the OPRM1 gene. Lower brain MOR mRNA and protein has been reported in individuals possessing the OPRM1 A118G polymorphism (Zhang et al. 2005). Although not exactly similar, G allele carriers are like MOR receptor knockout mice which have been shown to be less sensitive to nicotine. Importantly, women carrying the low-activity G allele (A/G and G/G) have reported reduced reinforcing value of nicotine and were less likely to differentiate between nicotine vs. denicotinized cigarettes (Ray et al. 2006). In contrast, there was no association of nicotine reinforcement with this genotype amongst male smokers. Additionally, smokers homozygous for the A allele (i.e., OPRM1 A118A) were more susceptible to nicotine dependence compared to smokers with G allele (i.e., OPRM1 A118G) (Verhagen et al. 2012). Consistent with these findings, neuroimaging studies have reported increased availability of free MORs in smokers homozygous for the A allele compared to smokers carrying the G allele in the brain regions such as the amygdala and NAcc (Domino et al. 2015; Ray et al. 2011). Further, these neuroimaging studies reported blunted release of endogenous opioids after nicotine smoking in smokers carrying the G allele compared to smokers homozygous for the A allele. In nicotine-dependent smokers, overnight abstinence from smoking resulted in increased blood flow to regions associated with craving in individuals who were homozygous for A allele (OPRM1 A118A) (Wang et al. 2008). However, OPRM1 A118G polymorphism has not been strongly associated with smoking initiation or response to smoking cessation treatment (Munafo et al. 2013; Verhagen et al. 2012). In summary, the above studies suggest that polymorphism in the OPRM1 gene determines susceptibility to nicotine dependence and A allele carriers may be more susceptible to craving upon abstinence from smoking and are more likely to relapse. The discovery of the OPRM1 A118G polymorphism spurs the need to look for other polymorphisms in the MORs that may be involved in development of nicotine dependence and/or response to smoking cessation treatment.
Other MOR gene polymorphisms have been reported that determine opioid withdrawal severity in humans (Jones et al. 2016). It remains to be seen if these polymorphisms influence other nicotine-dependent behaviors such as nicotine reward and nicotine withdrawal-induced craving. Future studies must also focus on understanding the interaction effects of OPRM1 polymorphism with polymorphisms of other genes, such as those regulating other neurotransmitters (dopamine and glutamate) involved in nicotine reward, on nicotine dependence.
It must be mentioned here that OPRM1 A118G polymorphism was initially reported to predispose individuals to the risk of developing alcoholism and also determine response to naltrexone in alcoholic patients (Kim et al. 2009; Ray and Hutchison 2004, 2007). More recent studies have not supported a role for OPRM1 A118G polymorphism in predicting susceptibility to development of alcohol dependence or response to naltrexone (Anton et al. 2012; Arias et al. 2006; Schacht et al. 2013).
Future work must explore the influence of gender and age on the role of MORs in nicotine-dependent behaviors. Gender-dependent differences in response to opioid analgesics that act via MORs have been reported (Bodnar and Kest 2010; Cicero et al. 1997; Kepler et al. 1989). As discussed above, Wewers et al. (1999) reported gender-dependent effects on MOR functioning after chronic nicotine exposure. Also, King et al. (2012) reported greater effect of naltrexone compared to placebo on smoking cessation-induced weight gain in women and not in men. Finally, as described above, gene-gender interaction effects have been associated with polymorphism of OPRM1 A118G gene. However, the interaction of gender and MORs on nicotine-induced behaviors such as nicotine self-administration, cue-induced nicotine seeking, and nicotine withdrawal has not been explored in detail and further work is warranted. Another factor that needs further investigation is the influence of age on MOR function and nicotine-dependent behaviors. Chronic exposure to nicotine in adolescent animals compared to adult animals resulted in downregulation of MORs in the hippocampus and striatum (Marco et al. 2007). Importantly, the effects of chronic nicotine exposure during adolescence on MOR function in the striatum were more marked in males than in females. In summary, the interaction of age (adolescents vs. adults) and gender on the role of MORs in nicotine-dependent behaviors needs to be further explored.
The role of MORs in specific brain regions on nicotine reward and other nicotine-dependent behaviors also needs further investigation. Based on their location in the NAcc, MORs play a differential role in hedonia (pleasure) experienced after food reward compared to motivation to consume food reward. It was reported that stimulation of MORs in a small (1 mm3) rostrodorsal region of the NAcc shell enhanced hedonic reactions as assessed by measuring the orofacial reaction to sucrose taste (Castro and Berridge 2014). In contrast to this hedonic hotspot in the rostrodorsal region of the NAcc shell, stimulation of MORs in the caudal region of the NAcc shell inhibited hedonic reactions for sucrose. However, irrespective of their location in the NAcc shell, stimulation of MORs increased motivation to consume food rewards. Together, these data suggest a differential role for MORs based on their distribution in different parts of the NAcc shell in hedonia experienced after consumption of food vs. motivation to consume food rewards. It is not clear if this heterogeneity in the role of MORs based on their localization is restricted to food reward or if it influences other types of reward such as nicotine reward. Future studies must explore the role of MORs in the different parts of the NAcc shell (rostrodorsal vs. caudal) in hedonic reaction to nicotine vs. motivation to consume nicotine. In summary, more work is required to understand the role of MORs in nicotine dependence and to fully exploit the MORs as a target for smoking cessation treatment.
DORs and nicotine-induced effects
DORs are widely distributed in the mesolimbic brain regions that play a role in reward, emotional processing, and drug addiction (Cahill et al. 2001; Mansour et al. 1996). Direct intracerebroventricular injection of DOR agonists induced CPP on its own (Shippenberg et al. 1987; Suzuki et al. 1997). Additionally, direct injection of a DOR agonist in the NAcc reduced brain reward thresholds, suggesting that activation of DORs was rewarding (Duvauchelle et al. 1997). Pharmacological blockade or genetic elimination of the DORs induced anxiety- and depression-like behaviors (Filliol et al. 2000; Perrine et al. 2006; Saitoh et al. 2005). Further, knockout of pro-enkephalin (PENK) gene, which is responsible for met- and leu-enkephalin synthesis, resulted in increased anxiety in mice compared to wild-type mice (Konig et al. 1996). Moreover, activation of DORs has also been shown to have anxiolytic and antidepressant-like actions (Broom et al. 2002; Saitoh et al. 2004; Vergura et al. 2008). Finally, activation of DORs via inhibition of enkephalin breakdown resulted in anxiolytic and antidepressant-like effects (Nieto et al. 2005). In summary, the above studies suggest a role for DORs in reward and emotional processing. Effects of pharmacological and genetic manipulation of the DORs on nicotine-induced behaviors are summarized in Tables 1 and 2, respectively (see also Fig. 1).
DORs and reinforcing effects of nicotine
Several studies have explored the role of DORs in the reinforcing effects of nicotine. Nicotine increased the levels of endogenous DOR agonist met-enkephalin in the ventral and dorsal striatum in mice (Dhatt et al. 1995). Importantly, DOR antagonist natrindole attenuated nicotine-self-administration in mice (Berrendero et al. 2012). Additionally, nicotine-induced CPP was attenuated in DOR knockout mice compared to wild-type counterparts. Moreover, fewer DOR knockout mice acquired nicotine self-administration compared to wild-type mice and the DOR knockout mice showed lower levels of nicotine-induced increase in NAcc dopamine compared to wild-type mice (Berrendero et al. 2012). In contrast to studies in mice, DOR antagonists did not influence nicotine self-administration in rats (Ismayilova and Shoaib 2010; Liu and Jernigan 2011). It is not clear if these differences in findings were due to use of different species in the above studies. Interestingly, blockade of DORs, using DOR antagonist natrindole, did not have any effect on nicotine-induced locomotor sensitization (Heidbreder et al. 1996). To date, the role of DORs in specific brain regions such as the NAcc and VTA in the reinforcing effects of nicotine have not been evaluated. Overall, the above studies suggest that the DORs play a role in mediating the rewarding effects of nicotine. There are similarities between the role played by MORs and DORs in nicotine reward. Further like MOR knockout mice, DOR knockout mice appear to be less sensitive to the rewarding effects of nicotine. However, more work is required to understand the role of DORs in nicotine reward in specific brain regions such as the NAcc and VTA. There is also a need to explore the role of DORs in animals with extended access to nicotine.
DORs and chronic nicotine exposure
Chronic nicotine administration altered met-enkephalin levels and DOR signaling (see Table 3). The nicotine-induced changes in preproenkephalin mRNA and met-enkephalin levels were time sensitive. For example, striatal met-enkephalin levels were decreased in rats after chronic nicotine when brain tissue was collected 1 h after the last nicotine dose (Wewers et al. 1999). Interestingly, striatal and hippocampal preproenkephalin mRNA levels were increased 24 h after withdrawal from nicotine in rats with chronic nicotine exposure (Houdi et al. 1998). Together, these findings suggest a compensatory increase in preproenkephalin mRNA in response to decrease in met-enkephalin levels. Consistent with these findings, both preproenkephalin mRNA and met-enkephalin levels were increased in the ventral striatum, 24 h after last dose of nicotine, in mice with chronic nicotine treatment (Isola et al. 2002).
Withdrawal from nicotine did not alter DOR binding in the NAcc and caudate/putamen (McCarthy et al. 2011). However, DOR mRNA was significantly elevated in the NAcc, prefrontal cortex, and hippocampus during nicotine withdrawal and this increase in DOR mRNA was sensitive to time since withdrawal from nicotine (see Table 3). Additionally DOR signaling, which was measured via uncoupling of the DOR-associated G-protein, was decreased in the NAcc during nicotine withdrawal. In summary, even though there may be compensatory increase in PENK mRNA and met-enkephalin levels, the decrease in DOR signaling suggest that there is an overall decrease in DOR-mediated functioning during nicotine withdrawal.
Temporal changes in DOR signaling during nicotine withdrawal overlaps with some of the affective aversive effects associated with nicotine withdrawal. Nicotine withdrawal-induced anhedonia and anxiety usually peaks between 4 and 24 h, and this coincides with DOR dysregulation (Costall et al. 1989; Epping-Jordan et al. 1998; Jackson et al. 2009; Jonkman et al. 2005; Stoker et al. 2008). As described above, decreased DOR-mediated transmission has anxiogenic and depression-like effects. Together, these data suggest that DOR dysregulation possibly mediates some of the aversive effects associated with nicotine withdrawal. In fact, activation of DORs attenuated mecamylamine precipitated nicotine withdrawal-induced CPA (Ise et al. 2000). In contrast, DORs are not involved in nicotine withdrawal-induced somatic aversive effects. There was no difference in mecamylamine-precipitated nicotine withdrawal-associated somatic signs between DOR wild-type and knockout mice (Berrendero et al. 2012). These findings are unlike findings in MOR knockout mice, which showed decreased aversive somatic signs during mecamylamine-precipitated nicotine withdrawal. Together, the above studies suggest that DORs are involved in aversive affective symptoms associated with nicotine withdrawal, which are mediated by receptors located in the brain. However, DORs are not involved in somatic effects associated with nicotine withdrawal, which are predominantly mediated by peripherally located receptors. In summary, central but not peripheral DORs play a role in nicotine withdrawal.
DORs and nicotine-seeking behavior
The role of DORs in reinstatement of nicotine seeking has not been explored. However, cue-induced reinstatement of cocaine seeking was attenuated in DOR knockout mice compared to their wild-type littermates (Gutierrez-Cuesta et al. 2014). Consistent with this finding, activation of DORs using DOR-specific peptides facilitated cocaine-induced reinstatement of CPP (Kotlinska et al. 2010). Similarly, DORs have been shown to play a role in reinstatement of morphine-induced CPP and expression of ethanol-induced CPP (Bie et al. 2012; Bie et al. 2009). Further behavioral, genetic, and electrophysiological evidence suggests that DORs play an important role in learning and memory, which support their possible role in reinstatement of drug-seeking behavior (Chavkin et al. 1985; Klenowski et al. 2015; Le Merrer et al. 2013). Based on the data described above, we hypothesize that blockade of DORs will attenuate cue-induced nicotine seeking. Further work is required to fully understand the role of DORs in nicotine seeking.
DORs: therapeutic potential and future directions
Currently, there are no FDA-approved medications available that exclusively target the DORs. However, clinical studies using compounds selectively targeting the DORs have been conducted (Richards et al. 2016). The DORs have been considered as a therapeutic target for the treatment of pain for a couple of decades (Gendron et al. 2015; Pradhan et al. 2011). There has been a growing interest in targeting the DORs for the treatment of addiction (Charbogne et al. 2014; Klenowski et al. 2015; Lutz and Kieffer 2013). Studies discussed above have shown that blockade of DORs attenuated the rewarding effects of nicotine and can potentially attenuate reinstatement of nicotine seeking. Further, activation of DORs has been shown to alleviate the aversive effects associated with nicotine withdrawal. DORs can be activated by both peptide- and non-peptide-based agonists and/or by inhibiting the enzyme that is responsible for breakdown of enkephalin (Gendron et al. 2015). It is hypothesized that DOR agonists are less likely to produce dependence compared to MOR agonists, which can be advantageous for using DOR agonists therapeutically. Taken together, both DOR antagonists and agonist have the potential to promote smoking cessation.
Several challenges exist in targeting the DORs. Over the last few years, significant advances have been made in understanding the structure and downstream effects of DOR activation. Using pharmacological agonist and antagonists, the DORs have been classified into at least two subtypes (e.g., DOR1 and DOR2) (Gendron et al. 2015). Additionally, several different types of secondary messenger systems have been found to be associated with the DORs. However, the DOR gene codes for only one type of protein and no alternative splicing of the gene has been reported. In humans, polymorphisms in the DOR gene have been reported (Simonin et al. 1994; Wei and Loh 2011). Future studies will need to assess if differences in DOR subtypes, DOR secondary messenger signaling, and/or polymorphisms in the genes coding for the DORs affect development of nicotine dependence.
Gender-based differences in DOR-mediated analgesia have been reported. For example, DOR agonists produced greater analgesic effects in male rats compared to female rats (Bartok and Craft 1997). Further, the effects of age (adolescent vs. adults) on DOR-mediated effects have not been investigated as has been for KORs (see below). More recently, a study reported increased ethanol consumption during adolescence in animals with a history of prenatal ethanol exposure (Fabio et al. 2015). As a caveat, this study reported only an increase in MOR mRNA in the VTA, but no change in DOR mRNA, suggesting no role for DORs in the VTA in the observed findings. Nevertheless, future studies must assess the impact of gender and age on DOR-mediated effects in nicotine dependence.
KORs and nicotine-induced effects
KORs and their endogenous ligand dynorphin are widely distributed throughout the brain, including the mesolimbic dopaminergic system in both humans and rodents (Mansour et al. 1996; Schmidt et al. 1994). Activation of KORs increased brain reward thresholds during the intracranial self-stimulation procedure, suggesting development of a depression-like state (Todtenkopf et al. 2004). Further, activation of KORs decreased the rewarding effects of drugs of abuse (Bruijnzeel 2009; Shippenberg et al. 2007; Wee and Koob 2010). In contrast, blockade of KORs decreased immobility time in animals in the forced swim test, suggesting antidepressant-like effects (Mague et al. 2003). Together, the data suggest that KORs mediate negative motivational and affective states. Below we discuss the role of KORs in nicotine-induced behaviors. The effects of pharmacological manipulation of KORs on nicotine-induced behaviors are summarized in Table 1 and Fig. 1.
KORs and reinforcing effects of nicotine
Acute nicotine administration changes dynorphin-KOR signaling. Increase in striatal dynorphin levels and prodynorphin mRNA have been reported following acute subcutaneous administration of high doses of nicotine (>0.5 mg/kg, base) (Isola et al. 2009). The nicotine-induced increase in dynorphin was temporally phasic in nature with an initial increase at 1 h followed by a decrease at 2 h and then again an increase between 6 and 24 h. Further, Isola et al. (2009) showed that the nicotine-induced increase in synthesis and release of dynorphin was mediated via other neurotransmitters such as glutamate and dopamine. In contrast, low doses of nicotine (<0.5 mg/kg) decreased or had no effect on preprodynorphin mRNA levels (Isola et al. 2009; Le Foll et al. 2003). Overall, the data suggest temporal dose-dependent changes in dynorphin and prodynorphin mRNA levels after acute nicotine administration.
Studies have also evaluated the role of KORs in the reinforcing effects of nicotine. Consistent with the role of KORs in negative emotional states described above, administration of low doses of the KOR agonist (±)U-50,488H (1–3 mg/kg) decreased nicotine self-administration in Lister hooded rats, suggesting that KOR activation attenuated the reinforcing effects of nicotine (Ismayilova and Shoaib 2010). Further, KOR agonists attenuated nicotine-induced locomotor activity (Hahn et al. 2000). Additionally, mice lacking the prodynorphin gene compared to wild-type mice self-administered nicotine at lower doses, suggesting that the dynorphin-KOR system plays an inhibitory role in the reinforcing effects of nicotine (Galeote et al. 2009). Interestingly, activation of the KORs, using the KOR agonist GNTI, did not affect the reinforcing effects of nicotine as assessed using intravenous nicotine self-administration in male Sprague-Dawley rats (Liu and Jernigan 2011). As compared to Ismayilova and Shoaib (2010) described above, Liu and Jernigan used a different strain of rats (Lister hooded vs. Sprague-Dawley rats) and a different KOR agonist [(±)U-50,488H vs. GNTI]. It is possible that the difference in findings between the above described studies could be due to the above described differences. In summary, the data suggest that activation of KORs has an inhibitory effect on reinforcing effects of nicotine. Thus, the role of the KORs appears to be opposite to the role played by other opioid receptors such as the MORs and DORs in the rewarding effects of nicotine.
Although dynorphin blocks nAChR-mediated effects in PC12 cells, the precise mechanism by which KOR activation decreases the rewarding effects of nicotine is not fully understood (Itoh et al. 2000). KOR activation on its own has been shown to decrease both firing of VTA dopaminergic neurons and release of dopamine in the NAcc (Di Chiara and Imperato 1988b; Margolis et al. 2003). However, the effects of KOR activation on nicotine-induced increases in NAcc dopamine have not yet been addressed. Further, future studies may need to explore the effects of direct injections of KOR agonists and antagonists in specific brain regions such as the NAcc and VTA on nicotine self-administration and nicotine-induced increases in dopamine in these brain regions.
First-time smokers often report unpleasant experiences such as vomiting, headache, tachycardia, and gastric discomfort (DiFranza et al. 2004). Based on the role of KORs and dynorphin in dysphoria, it is hypothesized that the dynorphin-KOR system is potentially involved in mediating the aversive effects of nicotine experienced by first-time smokers (Fowler and Kenny 2014). However, the role of KORs in the aversive effects of nicotine has not been directly explored. The rewarding and aversive effects of nicotine are mediated by distinct but overlapping circuits (D’Souza and Markou 2011; Laviolette and van der Kooy 2004). Therefore, future studies must focus on identifying the role of KORs in specific brain regions in the aversive and rewarding effects of nicotine. In summary, activation of KORs using low doses of a KOR agonist can decrease the reinforcing effects of nicotine either by decreasing the rewarding effects of nicotine and/or by increasing the aversive effects of nicotine.
Activation of KORs is also associated with a stress-like state and can thus increase motivation for drugs of abuse. Consistent with this hypothesis, activation of KORs using high doses of the KOR agonist (±)U-50,488H (5 or 10 mg/kg, i.p.) increased expression of nicotine-induced CPP compared to controls (Smith et al. 2012). This increase in nicotine-induced CPP was mediated by activation of the KORs in the amygdala. Smith et al. (2012) administered the KOR agonist only once, and this administration was done 1 h prior to testing for CPP (i.e., on the test day) and not during conditioning with nicotine. Compared to Ismayilova and Shoaib (2010) (described above), Smith et al. (2012) not only used a different model and protocol (self-administration vs. CPP) to assess the effects of KOR activation on the reinforcing effects of nicotine but also used higher doses of the KOR agonist (±)U-50,488H (1–3 vs. 5–10 mg/kg). The dose of KOR agonist used by Smith et al. produces a stress-like state (Wee and Koob 2010). Based on the above findings, one can conclude that increased recruitment of KORs using high doses of a KOR agonist can induce stress and indirectly increase motivation for nicotine. Further studies are required to determine the specific brain regions that are involved during the excessive recruitment of KORs by high doses of a KOR agonist.
KORs and chronic nicotine exposure
Chronic nicotine treatment in adult rats decreased prodynorphin mRNA levels in the NAcc, but increased prodynorphin mRNA levels in the prefrontal cortex and caudate putamen (Carboni et al. 2016) (see Table 3). Importantly, these changes in prodynorphin mRNA levels were accompanied by nicotine-induced locomotor sensitization. However, no changes in KOR mRNA were observed after chronic nicotine treatment in any of the above described regions. Taken together, these data suggest that chronic nicotine treatment differentially affects dynorphin synthesis in the different brain regions. The precise role of these changes in dynorphin levels and/or prodynorphin mRNA in specific brain regions on nicotine-induced behaviors is not fully understood. Future studies need to identify the role of dynorphin in specific brain regions on nicotine-induced behavioral effects by altering dynorphin levels in specific brain regions using viral-mediated overexpression and siRNA techniques.
Age of the animals influenced KOR-mediated behavioral and neurochemical effects after chronic nicotine treatment using osmotic minipumps. For example, administration of KOR agonist (±)U-50,488H after chronic nicotine treatment increased anxiety-like behavior, induced CPA, and decreased NAcc dopamine levels in adult, but not adolescent rats compared to respective controls (Tejeda et al. 2012). Together, the data suggest that KORs are more sensitive after chronic nicotine treatment in adult, but not in adolescent rats. This could be protective mechanism to limit nicotine intake in adults. These data also suggest that the KOR-dependent protective mechanism is absent in adolescents making them more vulnerable to nicotine addiction.
Withdrawal from nicotine after chronic nicotine treatment altered dynorphin and prodynorphin mRNA levels, and these molecular changes were dependent on the time elapsed since the last dose of nicotine. For example, a decrease in striatal dynorphin levels was reported after nicotine withdrawal when brain tissue was collected between from 4 to 72 h. Additionally, there was an increase in striatal prodynorphin mRNA between 8 and 96 h (Isola et al. 2008) (see Table 3). These data suggest that nicotine withdrawal-induced decrease in dynorphin levels was followed by a compensatory increase in dynorphin synthesis as suggested by an increase in prodynorphin mRNA. Interestingly, withdrawal from nicotine after chronic nicotine administration was accompanied by desensitization of KORs in the NAcc (McCarthy et al. 2010). The desensitization of KORs was first observed at 24-48 h after withdrawal of nicotine and lasted for over 72 h. This decrease in KOR signaling coincides with aversive affective effects associated with nicotine withdrawal as described above in the section on DORs. It is not clear if this decrease in KOR signaling is directly due to nicotine withdrawal or a compensatory mechanism to counter the increase in dynorphin synthesis described above.
Pharmacological manipulation of KORs influenced both somatic and affective aversive effects associated with nicotine withdrawal. Administration of high doses of the KOR agonist (±)U-50,488H (5 mg/kg, s.c.) enhanced spontaneous nicotine withdrawal-induced aversive somatic effects in adult rats, suggesting increased sensitivity of the KORs during nicotine withdrawal (Tejeda et al. 2012). In this study, somatic signs of nicotine withdrawal were assessed 24 h after removal of nicotine pumps, while the KOR agonist was administered 25 min before recording of somatic withdrawal signs. Interestingly, the same study reported that the increased sensitivity of the dynorphin-KOR system during nicotine withdrawal was observed in adult rats and not in adolescent rats despite receiving similar nicotine treatment as adult rats. This suggests that the KORs do not mediate nicotine withdrawal-induced aversive effects in adolescent rats. In contrast, activation of the KORs using low doses of the KOR agonist (±)U-50,488H (0.01–1 mg/kg) 30 min prior to induction of mecamylamine-precipitated nicotine withdrawal, attenuated nicotine withdrawal-induced CPA (Ise et al. 2002). These data suggest that KOR activation attenuated nicotine withdrawal-associated aversive affective effects. It is not clear if the differences in the role of the KORs between those reported by Tejeda et al. (2012) vs. Ise et al. (2002) are due to the fact that different withdrawal effects were measured in the two studies (somatic vs. affective) or because nicotine withdrawal was induced differently in the two studies (spontaneous vs. precipitated). Besides, there were also methodological differences between Tejeda et al. (2012) and Ise et al. (2002) such as the dose of the KOR agonist used (5 vs. 0.01–1 mg/kg), dose of nicotine used (3.2 mg/kg/day, base vs. 10 mg/kg, base) and duration of nicotine treatment (14 vs. 7 days). Further investigation is required to determine if KORs are differentially regulated by differences in nicotine treatment and/or by spontaneous vs. precipitated nicotine withdrawal. Finally, activation of peripheral KORs using a compound (ICI204, 448) that does not cross the blood-brain barrier inhibited nicotine withdrawal induced increases in feeding, metabolism, and locomotor activity in rats (Sudakov et al. 2014). These data suggest that peripheral and central KORs may be mediating different aspects of nicotine withdrawal and possibly play a differential role in these effects. In summary, the above data support a role for the KORs in somatic and affective aversive effects associated with nicotine withdrawal.
Long-acting KOR antagonist nor-BNI (5 and 15 mg/kg, s.c.) attenuated aversive somatic effects associated with spontaneous nicotine withdrawal in adult rats (Tejeda et al. 2012). Consistent with these findings, KOR antagonists nor-BNI and JDTic attenuated nicotine withdrawal-induced aversive somatic and affective symptoms in mice (Jackson et al. 2010). However, KOR antagonists such as nor-BNI and JDTic have several pharmacokinetic limitations such as that they are both long-acting (approx. 21 days) and have delayed onset of action (Munro et al. 2012). This complicates study design and to counter these limitations, studies have evaluated the effect of a short acting KOR antagonist LY2456302 on nicotine withdrawal-induced affective and somatic effects (Jackson et al. 2015). LY2456302 alleviated nicotine withdrawal-induced aversive somatic and affective effects in a manner similar to JDTic and nor-BNI. In summary, although further work is required to understand the role of KORs in nicotine withdrawal, to date a majority of the literature supports the hypothesis that activation of KORs worsens the aversive somatic and affective effects associated with nicotine withdrawal. Further, blockade of KORs attenuated nicotine withdrawal-induced aversive effects and can be utilized to facilitate smoking cessation in smokers.
KORs and nicotine-seeking behavior
A few studies have investigated the role of KORs in reinstatement of nicotine seeking. For example, it is known that footshock-induced stress facilitates reinstatement of nicotine seeking (Buczek et al. 1999). Notably, blockade of the KORs with the KOR antagonist nor-BNI attenuated stress-induced, but not cue-induced reinstatement of nicotine seeking (Grella et al. 2014). Further, KOR antagonist nor-BNI attenuated reinstatement of stress-induced nicotine CPP, but did not have any effect on nicotine-induced reinstatement of CPP (Jackson et al. 2013). Consistent with these findings, KOR antagonists attenuated stress-induced reinstatement of cocaine-seeking behavior (Redila and Chavkin 2008).
Exposure to chronic mild stress can lead to tolerance-like effects of KOR activation. For example, exposure to chronic stress attenuated KOR activation-induced reinstatement of nicotine-CPP, possibly due to either downregulation or desensitization of KORs (Al-Hasani et al. 2013). Future studies will need to explore alterations in the dynorphin-KOR system after repeated stress and its possible impact on reinstatement of drug seeking including nicotine seeking. In summary, data to date demonstrate that blockade of KOR-mediated neurotransmission attenuated reinstatement of nicotine seeking and that KORs may be useful targets to prevent relapse amongst abstinent smokers.
KORs: therapeutic potential and future directions
Currently, there are no FDA approved medications that exclusively target the KORs. KOR antagonists have recently been tested in humans (Buda et al. 2015; Lowe et al. 2014). Based on the studies described above, we hypothesize that KOR antagonists will promote smoking cessation by attenuating both nicotine withdrawal-induced aversive effects and reinstatement of nicotine seeking amongst abstinent smokers.
Further, we hypothesize that low doses of the KOR agonists may have a role in promoting smoking cessation by increasing the aversive effects of nicotine or decreasing the rewarding effects of nicotine. A major challenge in utilizing KOR agonists clinically has been the occurrence of dysphoria upon activation of KORs. In the future, it may be possible to dissociate dysphoric effects from activation of KORs. Currently, efforts are on to develop novel KOR agonists/positive allosteric modulators with minimal dysphoric effects (Burford et al. 2013; Zhou et al. 2013). Such KOR ligands may have greater clinical utility and allow for a more complete exploitation of the KORs as a possible therapeutic target for smoking cessation treatment.
Several single nucleotide polymorphisms in dynorphin-KOR system genes have been reported in humans (Clarke et al. 2012; Huang et al. 2008; Wang et al. 2014). Some of these polymorphisms in the KOR genes increase vulnerability to drug dependence in humans (Li and Zhang 2013). Exactly how these polymorphisms alter dynorphin release or dynorphin-KOR signaling is unknown. Do some of these genetic variations in dynorphin-KOR system genes alter aversive effects of nicotine and increase potential to develop nicotine addiction? Moreover, do these genetic variations in the dynorphin-KOR system alter aversive effects associated with nicotine withdrawal? In summary, further work is required to understand the influence polymorphisms in genes related to the KORs in the development of nicotine dependence.
Both animal and human studies have reported gender-dependent differences following pharmacological manipulation of the dynorphin-KOR system (Gear et al. 1996, 1999; Russell et al. 2014; Zacny and Beckman 2004). To date, the focus of these gender-dependent differences in the dynorphin-KOR system has largely been restricted to the role of dynorphin-KOR system in pain (Rasakham and Liu-Chen 2011). However, considering the distribution of KORs in several brain regions involved in addiction, there is growing interest in understanding the interaction of gender and the dynorphin-KOR system in drug addiction (Chartoff and Mavrikaki 2015). In keeping with the focus of this review, it will be of great interest to see if gender and dynorphin-KOR system interactions seen in pain extend to acute and chronic effects of nicotine. Furthermore, if there are gender-dependent differential effects of nicotine on the dynorphin-KOR system, it will be interesting to investigate if these differential effects impact manifestations of nicotine withdrawal and/or response to currently available smoking cessation treatments?
It is well known that activation of KORs produces a negative aversive state in both animals and humans (Bruijnzeel 2009). However, the cellular mechanism of this KOR-mediated negative aversive effect is not completely understood. Increased expression of serotonin reuptake transporters in mesolimbic brain regions such as the NAcc, resulting in decreased synaptic serotonin levels, has been suggested as one possible mechanism (Schindler et al. 2012). Another possible cellular mechanism that may mediate KOR-mediated aversive effects is the activation of the p38 mitogen-activated protein kinase (MAPK) pathway (Bruchas et al. 2007). In support of this mechanism, phosphorylation of the p38 MAPK pathway in the VTA dopaminergic neurons was shown to mediate the aversive effects of the KOR agonist (±)U-50,488H (Ehrich et al. 2015). Interestingly, microglial p38 MAPK pathway plays a role in nicotine withdrawal-induced hyperalgesia (Ding et al. 2015). Future studies must identify exactly what downstream KOR-associated pathways are activated by acute and chronic nicotine exposure. Understanding these downstream pathways will help in effectively manipulating the KORs and make them a more viable therapeutic target for future smoking cessation medications.
Conclusions
The endogenous opioid system, which was originally conceived as an endogenous response system to painful stimuli, is now seen as an important mediator in drug addiction. Several FDA-approved medications currently used to treat alcohol and heroin addiction target the MORs. In this review, we focused on the effects of nicotine on the endogenous opioid system and the possible role of the different opioid receptors as potential targets to promote smoking cessation. Amongst the three opioid receptors discussed in this review, the role of the MORs in nicotine-dependent behaviors has been extensively investigated in both animals and humans. This is primarily because of a better understanding of the MOR structure and the availability of clinically viable medications targeting the MORs. Moreover, identification of the OPRM1 A118G polymorphism continues to make the MORs an exciting target with the highest potential for the development of smoking cessation medications. MOR antagonists attenuated nicotine seeking in animal models and reduced craving in human smokers. Thus, MOR antagonists and/or negative allosteric modulators would help promote smoking cessation. However, future studies need to identify patient subpopulations that may be more responsive to MOR-based smoking cessation medications.
The role of the DORs in nicotine dependence has been explored the least amongst the three opioid receptors. This is partly because of incomplete understanding of the structure of the DORs and the limited availability of clinically viable medications/compounds selectively targeting the DORs. Preclinical research has shown that DOR antagonists decreased nicotine seeking, while the DOR agonists attenuated the aversive effects associated with nicotine withdrawal. Thus, both DOR agonists and antagonists have the potential to promote smoking cessation. However, based on the literature reviewed above, the likelihood of seeing a successful smoking cessation medication targeting the DORs in the near future appears to be comparatively low. The development of clinically viable selective compounds targeting the DORs will go a long way in facilitating the process of exploiting the DORs as a potential target for future smoking cessation medications.
In contrast to the MORs and DORs, the KORs appear to have an inhibitory role in the rewarding effects of drugs of abuse including nicotine. Interesting work conducted in adolescent animals suggests a limited role for KORs in nicotine-dependent behaviors in adolescents, which possibly makes them more vulnerable to nicotine addiction. Preclinical research has shown that KOR antagonists attenuated both the aversive effects of nicotine withdrawal and reinstatement of nicotine seeking. Therefore, KOR antagonists and/or negative allosteric modulators would be useful as smoking cessation medications. The availability of clinically viable KOR antagonists has greatly increased the potential of the KORs as a target for future smoking cessation medications.
However, despite the extensive investigation of the endogenous opioid system in nicotine dependence over the last two decades, much work still needs to be accomplished. For example, little is known about the role of the different opioid receptors in specific brain regions in nicotine seeking and nicotine withdrawal. Therefore, future work must focus on identifying the role of the different opioid receptors in specific brain regions and neural circuits in nicotine-dependent behaviors using a combination of pharmacological, molecular, and genetic approaches. Additionally, the influence of age and gender on the role of opioid receptors in nicotine-dependent behaviors needs to be evaluated. Finally, genetic studies in humans must continue to assess the influence of polymorphisms in opioid receptor-linked genes in nicotine dependence. Identification of such polymorphisms will help in both identifying individuals vulnerable to develop nicotine addiction and patient subpopulations who may be more responsive to opioid-based smoking cessation medications. In conclusion, the opioid receptors are promising targets and it is our belief that continued focus on the endogenous opioid system will provide efficacious smoking cessation medications in the near future.
Abbreviations
- AMPA:
-
Amino-3-hydroxy-5-methyl-4-isoxazolepropionate/kainate
- CPP:
-
Conditioned place preference
- CPA:
-
Conditioned place version
- DAMGO:
-
[D-Ala2,N-Me-Phe4,Gly-ol5]-enkephalin
- DORs:
-
Delta opioid receptors
- GNTI:
-
Guanidinonaltrindole
- ICSS:
-
Intracranial self-stimulation
- KORs:
-
Kappa opioid receptors
- MK-801:
-
(5R,10S)-(−)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cylcohepten-5,10-imine
- MORs:
-
Mu opioid receptors
- NAcc:
-
Nucleus accumbens
- NMDA:
-
N-methyl-D-aspartate
- VTA:
-
Ventral tegmental area
References
Al-Hasani R, McCall JG, Bruchas MR (2013) Exposure to chronic mild stress prevents kappa opioid-mediated reinstatement of cocaine and nicotine place preference. Front Pharmacol 4:96
Andersson H, Ejlertsson G, Leden I (1998) Widespread musculoskeletal chronic pain associated with smoking. An epidemiological study in a general rural population. Scand J Rehabil Med 30:185–191
Anton RF, Voronin KK, Randall PK, Myrick H, Tiffany A (2012) Naltrexone modification of drinking effects in a subacute treatment and bar-lab paradigm: influence of OPRM1 and dopamine transporter (SLC6A3) genes. Alcohol Clin Exp Res 36:2000–2007
Arias A, Feinn R, Kranzler HR (2006) Association of an Asn40Asp (A118G) polymorphism in the mu-opioid receptor gene with substance dependence: a meta-analysis. Drug Alcohol Depend 83:262–268
Baltieri DA, Daro FR, Ribeiro PL, Andrade AG (2009) Effects of topiramate or naltrexone on tobacco use among male alcohol-dependent outpatients. Drug Alcohol Depend 105:33–41
Barik J, Wonnacott S (2009) Molecular and cellular mechanisms of action of nicotine in the CNS. Handb Exp Pharmacol 173–207. doi:10.1007/978-3-540-69248-5_7
Bartok RE, Craft RM (1997) Sex differences in opioid antinociception. J Pharmacol Exp Ther 282:769–778
Berrendero F, Kieffer BL, Maldonado R (2002) Attenuation of nicotine-induced antinociception, rewarding effects, and dependence in mu-opioid receptor knock-out mice. J Neurosci 22:10935–10940
Berrendero F, Plaza-Zabala A, Galeote L, Flores A, Bura SA, Kieffer BL, Maldonado R (2012) Influence of delta-opioid receptors in the behavioral effects of nicotine. Neuropsychopharmacology 37:2332–2344
Berrendero F, Robledo P, Trigo JM, Martin-Garcia E, Maldonado R (2010) Neurobiological mechanisms involved in nicotine dependence and reward: participation of the endogenous opioid system. Neurosci Biobehav Rev 35:220–231
Biala G, Budzynska B, Kruk M (2005) Naloxone precipitates nicotine abstinence syndrome and attenuates nicotine-induced antinociception in mice. Pharmacol Rep 57:755–760
Bianchi E, Menicacci C, Ghelardini C (2013) Dual effect of morphine in long-term social memory in rat. Br J Pharmacol 168:1786–1793
Bie B, Wang Y, Cai YQ, Zhang Z, Hou YY, Pan ZZ (2012) Upregulation of nerve growth factor in central amygdala increases sensitivity to opioid reward. Neuropsychopharmacology 37:2780–2788
Bie B, Zhu W, Pan ZZ (2009) Ethanol-induced delta-opioid receptor modulation of glutamate synaptic transmission and conditioned place preference in central amygdala. Neuroscience 160:348–358
Bodnar RJ, Kest B (2010) Sex differences in opioid analgesia, hyperalgesia, tolerance and withdrawal: central mechanisms of action and roles of gonadal hormones. Horm Behav 58:72–81
Brigham J, Henningfield JE, Stitzer ML (1990) Smoking relapse: a review. Int J Addict 25:1239–1255
Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH (2002) Nonpeptidic delta-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacology 26:744–755
Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C (2007) Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci 27:11614–11623
Bruijnzeel AW (2009) Kappa-opioid receptor signaling and brain reward function. Brain Res Rev 62:127–146
Buczek Y, Le AD, Wang A, Stewart J, Shaham Y (1999) Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology 144:183–188
Buda JJ, Carroll FI, Kosten TR, Swearingen D, Walters BB (2015) A double-blind, placebo-controlled trial to evaluate the safety, tolerability, and pharmacokinetics of single, escalating oral doses of JDTic. Neuropsychopharmacology 40:2059–2065
Burford NT, Clark MJ, Wehrman TS, Gerritz SW, Banks M, O’Connell J, Traynor JR, Alt A (2013) Discovery of positive allosteric modulators and silent allosteric modulators of the mu-opioid receptor. Proc Natl Acad Sci U S A 110:10830–10835
Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF (2002) Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology 163:230–237
Cahill CM, McClellan KA, Morinville A, Hoffert C, Hubatsch D, O’Donnell D, Beaudet A (2001) Immunohistochemical distribution of delta opioid receptors in the rat central nervous system: evidence for somatodendritic labeling and antigen-specific cellular compartmentalization. J Comp Neurol 440:65–84
Callahan-Lyon P (2014) Electronic cigarettes: human health effects. Tob Control 23(Suppl 2):ii36–ii40
Carboni L, Romoli B, Romualdi P, Zoli M (2016) Repeated nicotine exposure modulates prodynorphin and pronociceptin levels in the reward pathway. Drug Alcohol Depend 166:150–158
Castro DC, Berridge KC (2014) Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness "liking" and "wanting". J Neurosci 34:4239–4250
Chakrabarti S, Liu NJ, Gintzler AR (2010) Formation of mu−/kappa-opioid receptor heterodimer is sex-dependent and mediates female-specific opioid analgesia. Proc Natl Acad Sci U S A 107:20115–20119
Charbogne P, Kieffer BL, Befort K (2014) 15 years of genetic approaches in vivo for addiction research: opioid receptor and peptide gene knockout in mouse models of drug abuse. Neuropharmacology 76(Pt B):204–217
Chartoff EH, Mavrikaki M (2015) Sex differences in kappa opioid receptor function and their potential impact on addiction. Front Neurosci 9:466
Chavkin C, Shoemaker WJ, McGinty JF, Bayon A, Bloom FE (1985) Characterization of the prodynorphin and proenkephalin neuropeptide systems in rat hippocampus. J Neurosci 5:808–816
Chiamulera C, Borgo C, Falchetto S, Valerio E, Tessari M (1996) Nicotine reinstatement of nicotine self-administration after long-term extinction. Psychopharmacology 127:102–107
Cicero TJ, Nock B, Meyer ER (1997) Sex-related differences in morphine’s antinociceptive activity: relationship to serum and brain morphine concentrations. J Pharmacol Exp Ther 282:939–944
Clarke TK, Ambrose-Lanci L, Ferraro TN, Berrettini WH, Kampman KM, Dackis CA, Pettinati HM, O’Brien CP, Oslin DW, Lohoff FW (2012) Genetic association analyses of PDYN polymorphisms with heroin and cocaine addiction. Genes Brain Behav 11:415–423
Corrigall WA, Coen KM (1991) Opiate antagonists reduce cocaine but not nicotine self-administration. Psychopharmacology 104:167–170
Corrigall WA, Coen KM, Adamson KL, Chow BL, Zhang J (2000) Response of nicotine self-administration in the rat to manipulations of mu-opioid and gamma-aminobutyric acid receptors in the ventral tegmental area. Psychopharmacology 149:107–114
Costall B, Kelly ME, Naylor RJ, Onaivi ES (1989) The actions of nicotine and cocaine in a mouse model of anxiety. Pharmacol Biochem Behav 33:197–203
Covey LS, Glassman AH, Stetner F (1999) Naltrexone effects on short-term and long-term smoking cessation. J Addict Dis 18:31–40
Crist RC, Berrettini WH (2014) Pharmacogenetics of OPRM1. Pharmacol Biochem Behav 123:25–33
Crombag HS, Bossert JM, Koya E, Shaham Y (2008) Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond Ser B Biol Sci 363:3233–3243
D’Souza MS, Liechti ME, Ramirez-Nino AM, Kuczenski R, Markou A (2011) The metabotropic glutamate 2/3 receptor agonist LY379268 blocked nicotine-induced increases in nucleus accumbens shell dopamine only in the presence of a nicotine-associated context in rats. Neuropsychopharmacology 36:2111–2124
D’Souza MS, Markou A (2011) Neuronal mechanisms underlying development of nicotine dependence: implications for novel smoking-cessation treatments. Addict Sci Clin Pract 6:4–16
Dang VC, Christie MJ (2012) Mechanisms of rapid opioid receptor desensitization, resensitization and tolerance in brain neurons. Br J Pharmacol 165:1704–1716
Dani JA, Bertrand D (2007) Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol 47:699–729
Dani JA, Harris RA (2005) Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci 8:1465–1470
Davenport KE, Houdi AA, Van Loon GR (1990) Nicotine protects against mu-opioid receptor antagonism by beta-funaltrexamine: evidence for nicotine-induced release of endogenous opioids in brain. Neurosci Lett 113:40–46
David SP, Chu IM, Lancaster T, Stead LF, Evins AE, Prochaska JJ (2014) Systematic review and meta-analysis of opioid antagonists for smoking cessation. BMJ Open 4:e004393
David SP, Lancaster T, Stead LF, Evins AE, Prochaska JJ (2013) Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. doi:10.1002/14651858.CD003086.pub3
Dawson DA (2000) Drinking as a risk factor for sustained smoking. Drug Alcohol Depend 59:235–249
DeNoble VJ, Mele PC (2006) Intravenous nicotine self-administration in rats: effects of mecamylamine, hexamethonium and naloxone. Psychopharmacology 184:266–272
Devine DP, Leone P, Carlezon WA Jr, Wise RA (1993) Ventral mesencephalic delta opioid receptors are involved in modulation of basal mesolimbic dopamine neurotransmission: an anatomical localization study. Brain Res 622:348–352
Dhatt RK, Gudehithlu KP, Wemlinger TA, Tejwani GA, Neff NH, Hadjiconstantinou M (1995) Preproenkephalin mRNA and methionine-enkephalin content are increased in mouse striatum after treatment with nicotine. J Neurochem 64:1878–1883
Di Chiara G, Imperato A (1988a) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 85:5274–5278
Di Chiara G, Imperato A (1988b) Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther 244:1067–1080
DiFranza JR, Savageau JA, Fletcher K, Ockene JK, Rigotti NA, McNeill AD, Coleman M, Wood C (2004) Recollections and repercussions of the first inhaled cigarette. Addict Behav 29:261–272
Ding Y, Shi W, Xie G, Yu A, Wang Q, Zhang Z (2015) CX3CR1 mediates nicotine withdrawal-induced hyperalgesia via microglial P38 MAPK signaling. Neurochem Res 40:2252–2261
Ditre JW, Brandon TH, Zale EL, Meagher MM (2011) Pain, nicotine, and smoking: research findings and mechanistic considerations. Psychol Bull 137:1065–1093
Domino EF, Hirasawa-Fujita M, Ni L, Guthrie SK, Zubieta JK (2015) Regional brain [(11)C]carfentanil binding following tobacco smoking. Prog Neuro-Psychopharmacol Biol Psychiatry 59:100–104
Drews E, Zimmer A (2010) Modulation of alcohol and nicotine responses through the endogenous opioid system. Prog Neurobiol 90:1–15
Duvauchelle CL, Fleming SM, Kornetsky C (1997) DAMGO and DPDPE facilitation of brain stimulation reward thresholds is blocked by the dopamine antagonist cis-flupenthixol. Neuropharmacology 36:1109–1114
Ehrich JM, Messinger DI, Knakal CR, Kuhar JR, Schattauer SS, Bruchas MR, Zweifel LS, Kieffer BL, Phillips PE, Chavkin C (2015) Kappa opioid receptor-induced aversion requires p38 MAPK activation in VTA dopamine neurons. J Neurosci 35:12917–12931
Epping-Jordan MP, Watkins SS, Koob GF, Markou A (1998) Dramatic decreases in brain reward function during nicotine withdrawal. Nature 393:76–79
Epstein AM, King AC (2004) Naltrexone attenuates acute cigarette smoking behavior. Pharmacol Biochem Behav 77:29–37
Fabio MC, Macchione AF, Nizhnikov ME, Pautassi RM (2015) Prenatal ethanol increases ethanol intake throughout adolescence, alters ethanol-mediated aversive learning, and affects mu but not delta or kappa opioid receptor mRNA expression. Eur J Neurosci 41:1569–1579
Falcone M, Gold AB, Wileyto EP, Ray R, Ruparel K, Newberg A, Dubroff J, Logan J, Zubieta JK, Blendy JA, Lerman C (2012) Mu-opioid receptor availability in the amygdala is associated with smoking for negative affect relief. Psychopharmacology 222:701–708
Farahmandfar M, Naghdi N, Karimian SM, Kadivar M, Zarrindast MR (2012) Amnesia induced by morphine in spatial memory retrieval inhibited in morphine-sensitized rats. Eur J Pharmacol 683:132–139
Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL (2000) Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet 25:195–200
Ford CP, Mark GP, Williams JT (2006) Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci 26:2788–2797
Fowler CD, Kenny PJ (2014) Nicotine aversion: neurobiological mechanisms and relevance to tobacco dependence vulnerability. Neuropharmacology 76(Pt B):533–544
Galeote L, Berrendero F, Bura SA, Zimmer A, Maldonado R (2009) Prodynorphin gene disruption increases the sensitivity to nicotine self-administration in mice. Int J Neuropsychopharmacol 12:615–625
Galeote L, Kieffer BL, Maldonado R, Berrendero F (2006) Mu-opioid receptors are involved in the tolerance to nicotine antinociception. J Neurochem 97:416–423
Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD (1996) Kappa-opioids produce significantly greater analgesia in women than in men. Nat Med 2:1248–1250
Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD (1999) The kappa opioid nalbuphine produces gender- and dose-dependent analgesia and antianalgesia in patients with postoperative pain. Pain 83:339–345
Gendron L, Mittal N, Beaudry H, Walwyn W (2015) Recent advances on the delta opioid receptor: from trafficking to function. Br J Pharmacol 172:403–419
Georgiou P, Zanos P, Ehteramyan M, Hourani S, Kitchen I, Maldonado R, Bailey A (2015) Differential regulation of mGlu5 R and MuOPr by priming- and cue-induced reinstatement of cocaine-seeking behaviour in mice. Addict Biol 20:902–912
Gilbert DG, Meliska CJ, Williams CL, Jensen RA (1992) Subjective correlates of cigarette-smoking-induced elevations of peripheral beta-endorphin and cortisol. Psychopharmacology 106:275–281
Giuliano C, Goodlett CR, Economidou D, Garcia-Pardo MP, Belin D, Robbins TW, Bullmore ET, Everitt BJ (2015) The novel mu-opioid receptor antagonist GSK1521498 decreases both alcohol seeking and drinking: evidence from a new preclinical model of alcohol seeking. Neuropsychopharmacology 40:2981–2992
Goktalay G, Cavun S, Levendusky MC, Hamilton JR, Millington WR (2006) Glycyl-glutamine inhibits nicotine conditioned place preference and withdrawal. Eur J Pharmacol 530:95–102
Gorelick DA, Rose J, Jarvik ME (1988) Effect of naloxone on cigarette smoking. J Subst Abus 1:153–159
Grella SL, Funk D, Coen K, Li Z, Le AD (2014) Role of the kappa-opioid receptor system in stress-induced reinstatement of nicotine seeking in rats. Behav Brain Res 265:188–197
Gutierrez-Cuesta J, Burokas A, Mancino S, Kummer S, Martin-Garcia E, Maldonado R (2014) Effects of genetic deletion of endogenous opioid system components on the reinstatement of cocaine-seeking behavior in mice. Neuropsychopharmacology 39:2974–2988
Guydish J, Passalacqua E, Tajima B, Chan M, Chun J, Bostrom A (2011) Smoking prevalence in addiction treatment: a review. Nicotine Tob Res 13:401–411
Hadjiconstantinou M, Duchemin AM, Zhang H, Neff NH (2011) Enhanced dopamine transporter function in striatum during nicotine withdrawal. Synapse 65:91–98
Hadjiconstantinou M, Neff NH (2011) Nicotine and endogenous opioids: neurochemical and pharmacological evidence. Neuropharmacology 60:1209–1220
Hahn B, Stolerman IP, Shoaib M (2000) Kappa-opioid receptor modulation of nicotine-induced behaviour. Neuropharmacology 39:2848–2855
Heidbreder C, Shoaib M, Shippenberg TS (1996) Differential role of delta-opioid receptors in the development and expression of behavioral sensitization to cocaine. Eur J Pharmacol 298:207–216
Henningfield JE, Miyasato K, Jasinski DR (1985) Abuse liability and pharmacodynamic characteristics of intravenous and inhaled nicotine. J Pharmacol Exp Ther 234:1–12
Hooten WM (2016) Chronic pain and mental health disorders: shared neural mechanisms, epidemiology, and treatment. Mayo Clin Proc 91:955–970
Houdi AA, Dasgupta R, Kindy MS (1998) Effect of nicotine use and withdrawal on brain preproenkephalin a mRNA. Brain Res 799:257–263
Huang CJ, Liu HF, Su NY, Hsu YW, Yang CH, Chen CC, Tsai PS (2008) Association between human opioid receptor genes polymorphisms and pressure pain sensitivity in females*. Anaesthesia 63:1288–1295
Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW (1991) Symptoms of tobacco withdrawal. A replication and extension. Arch Gen Psychiatry 48:52–59
Hutchinson D, Shepstone L, Moots R, Lear JT, Lynch MP (2001) Heavy cigarette smoking is strongly associated with rheumatoid arthritis (RA), particularly in patients without a family history of RA. Ann Rheum Dis 60:223–227
Ise Y, Narita M, Nagase H, Suzuki T (2000) Modulation of opioidergic system on mecamylamine-precipitated nicotine-withdrawal aversion in rats. Psychopharmacology 151:49–54
Ise Y, Narita M, Nagase H, Suzuki T (2002) Modulation of kappa-opioidergic systems on mecamylamine-precipitated nicotine-withdrawal aversion in rats. Neurosci Lett 323:164–166
Ismayilova N, Shoaib M (2010) Alteration of intravenous nicotine self-administration by opioid receptor agonist and antagonists in rats. Psychopharmacology 210:211–220
Isola R, Duchemin AM, Tejwani GA, Neff NH, Hadjiconstantinou M (2000) Glutamate receptors participate in the nicotine-induced changes of met-enkephalin in striatum. Brain Res 878:72–78
Isola R, Zhang H, Duchemin AM, Tejwani GA, Neff NH, Hadjiconstantinou M (2002) Met-enkephalin and preproenkephalin mRNA changes in the striatum of the nicotine abstinence mouse. Neurosci Lett 325:67–71
Isola R, Zhang H, Tejwani GA, Neff NH, Hadjiconstantinou M (2008) Dynorphin and prodynorphin mRNA changes in the striatum during nicotine withdrawal. Synapse 62:448–455
Isola R, Zhang H, Tejwani GA, Neff NH, Hadjiconstantinou M (2009) Acute nicotine changes dynorphin and prodynorphin mRNA in the striatum. Psychopharmacology 201:507–516
Itoh H, Andoh T, Watanabe I, Sasaki T, Kamiya Y, Okumura F (2000) Dynorphins directly inhibit neuronal nicotinic acetylcholine receptors in PC12 cells. Eur J Neurosci 12:1253–1262
Jackson KJ, Carroll FI, Negus SS, Damaj MI (2010) Effect of the selective kappa-opioid receptor antagonist JDTic on nicotine antinociception, reward, and withdrawal in the mouse. Psychopharmacology 210:285–294
Jackson KJ, Jackson A, Carroll FI, Damaj MI (2015) Effects of orally-bioavailable short-acting kappa opioid receptor-selective antagonist LY2456302 on nicotine withdrawal in mice. Neuropharmacology 97:270–274
Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, Damaj MI (2009) The role of alpha6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. J Pharmacol Exp Ther 331:547–554
Jackson KJ, McLaughlin JP, Carroll FI, Damaj MI (2013) Effects of the kappa opioid receptor antagonist, norbinaltorphimine, on stress and drug-induced reinstatement of nicotine-conditioned place preference in mice. Psychopharmacology 226:763–768
Jackson KJ, Muldoon PP, De Biasi M, Damaj MI (2015) New mechanisms and perspectives in nicotine withdrawal. Neuropharmacology 96:223–234
Jamison RN, Stetson BA, Parris WC (1991) The relationship between cigarette smoking and chronic low back pain. Addict Behav 16:103–110
Jones JD, Luba RR, Vogelman JL, Comer SD (2016) Searching for evidence of genetic mediation of opioid withdrawal by opioid receptor gene polymorphisms. Am J Addict 25:41–48
Jonkman S, Henry B, Semenova S, Markou A (2005) Mild anxiogenic effects of nicotine withdrawal in mice. Eur J Pharmacol 516:40–45
Jonkman S, Risbrough VB, Geyer MA, Markou A (2008) Spontaneous nicotine withdrawal potentiates the effects of stress in rats. Neuropsychopharmacology 33:2131–2138
Kahler CW, Spillane NS, Metrik J (2010) Alcohol use and initial smoking lapses among heavy drinkers in smoking cessation treatment. Nicotine Tob Res 12:781–785
Karras A, Kane JM (1980) Naloxone reduces cigarette smoking. Life Sci 27:1541–1545
Kepler KL, Kest B, Kiefel JM, Cooper ML, Bodnar RJ (1989) Roles of gender, gonadectomy and estrous phase in the analgesic effects of intracerebroventricular morphine in rats. Pharmacol Biochem Behav 34:119–127
Kieffer BL, Evans CJ (2009) Opioid receptors: from binding sites to visible molecules in vivo. Neuropharmacology 56(Suppl 1):205–212
Kim SG, Kim CM, Choi SW, Jae YM, Lee HG, Son BK, Kim JG, Choi YS, Kim HO, Kim SY, Oslin DW (2009) A micro opioid receptor gene polymorphism (A118G) and naltrexone treatment response in adherent Korean alcohol-dependent patients. Psychopharmacology 201:611–618
King A, Cao D, Vanier C, Wilcox T (2009) Naltrexone decreases heavy drinking rates in smoking cessation treatment: an exploratory study. Alcohol Clin Exp Res 33:1044–1050
King A, Cao D, Zhang L, Rueger SY (2013a) Effects of the opioid receptor antagonist naltrexone on smoking and related behaviors in smokers preparing to quit: a randomized controlled trial. Addiction 108:1836–1844
King A, de Wit H, Riley RC, Cao D, Niaura R, Hatsukami D (2006) Efficacy of naltrexone in smoking cessation: a preliminary study and an examination of sex differences. Nicotine Tob Res 8:671–682
King AC, Cao D, O’Malley SS, Kranzler HR, Cai X, deWit H, Matthews AK, Stachoviak RJ (2012) Effects of naltrexone on smoking cessation outcomes and weight gain in nicotine-dependent men and women. J Clin Psychopharmacol 32:630–636
King AC, Cao D, Zhang L, O’Malley SS (2013b) Naltrexone reduction of long-term smoking cessation weight gain in women but not men: a randomized controlled trial. Biol Psychiatry 73:924–930
King AC, Meyer PJ (2000) Naltrexone alteration of acute smoking response in nicotine-dependent subjects. Pharmacol Biochem Behav 66:563–572
Kirkpatrick DR, McEntire DM, Hambsch ZJ, Kerfeld MJ, Smith TA, Reisbig MD, Youngblood CF, Agrawal DK (2015) Therapeutic basis of clinical pain modulation. Clinical and translational science 8:848–856
Kishioka S, Kiguchi N, Kobayashi Y, Saika F (2014) Nicotine effects and the endogenous opioid system. J Pharmacol Sci 125:117–124
Klenowski P, Morgan M, Bartlett SE (2015) The role of delta-opioid receptors in learning and memory underlying the development of addiction. Br J Pharmacol 172:297–310
Knott VJ, Fisher DJ (2007) Naltrexone alteration of the nicotine-induced EEG and mood activation response in tobacco-deprived cigarette smokers. Exp Clin Psychopharmacol 15:368–381
Konig M, Zimmer AM, Steiner H, Holmes PV, Crawley JN, Brownstein MJ, Zimmer A (1996) Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature 383:535–538
Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238
Kotlinska JH, Gibula-Bruzda E, Pachuta A, Kunce D, Witkowska E, Chung NN, Schiller PW, Izdebski J (2010) Influence of new deltorphin analogues on reinstatement of cocaine-induced conditioned place preference in rats. Behav Pharmacol 21:638–648
Krishnan-Sarin S, Meandzija B, O’Malley S (2003) Naltrexone and nicotine patch smoking cessation: a preliminary study. Nicotine Tob Res 5:851–857
Krishnan-Sarin S, Rosen MI, O’Malley SS (1999) Naloxone challenge in smokers. Preliminary evidence of an opioid component in nicotine dependence. Arch Gen Psychiatry 56:663–668
Kuwabara H, Heishman SJ, Brasic JR, Contoreggi C, Cascella N, Mackowick KM, Taylor R, Rousset O, Willis W, Huestis MA, Concheiro M, Wand G, Wong DF, Volkow ND (2014) Mu opioid receptor binding correlates with nicotine dependence and reward in smokers. PLoS One 9:e113694
Lapchak PA, Araujo DM, Collier B (1989) Regulation of endogenous acetylcholine release from mammalian brain slices by opiate receptors: hippocampus, striatum and cerebral cortex of guinea-pig and rat. Neuroscience 31:313–325
Laviolette SR, van der Kooy D (2004) The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci 5:55–65
Le Foll B, Diaz J, Sokoloff P (2003) Increased dopamine D3 receptor expression accompanying behavioral sensitization to nicotine in rats. Synapse 47:176–183
Le Merrer J, Becker JA, Befort K, Kieffer BL (2009) Reward processing by the opioid system in the brain. Physiol Rev 89:1379–1412
Le Merrer J, Rezai X, Scherrer G, Becker JA, Kieffer BL (2013) Impaired hippocampus-dependent and facilitated striatum-dependent behaviors in mice lacking the delta opioid receptor. Neuropsychopharmacology 38:1050–1059
Lee YS, Joe KH, Sohn IK, Na C, Kee BS, Chae SL (2005) Changes of smoking behavior and serum adrenocorticotropic hormone, cortisol, prolactin, and endogenous opioids levels in nicotine dependence after naltrexone treatment. Prog Neuro-Psychopharmacol Biol Psychiatry 29:639–647
Li Z, Zhang H (2013) Analyzing interaction of mu-, delta- and kappa-opioid receptor Gene variants on alcohol or drug dependence using a pattern discovery-based method. J Addict Res Ther Suppl 7:007
Liu X, Jernigan C (2011) Activation of the opioid mu1, but not delta or kappa, receptors is required for nicotine reinforcement in a rat model of drug self-administration. Prog Neuro-Psychopharmacol Biol Psychiatry 35:146–153
Liu X, Palmatier MI, Caggiula AR, Sved AF, Donny EC, Gharib M, Booth S (2009) Naltrexone attenuation of conditioned but not primary reinforcement of nicotine in rats. Psychopharmacology 202:589–598
Lowe SL, Wong CJ, Witcher J, Gonzales CR, Dickinson GL, Bell RL, Rorick-Kehn L, Weller M, Stoltz RR, Royalty J, Tauscher-Wisniewski S (2014) Safety, tolerability, and pharmacokinetic evaluation of single- and multiple-ascending doses of a novel kappa opioid receptor antagonist LY2456302 and drug interaction with ethanol in healthy subjects. J Clin Pharmacol 54:968–978
Lutz PE, Kieffer BL (2013) The multiple facets of opioid receptor function: implications for addiction. Curr Opin Neurobiol 23:473–479
Lutz RA, Pfister HP (1992) Opioid receptors and their pharmacological profiles. J Recept Res 12:267–286
Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC Jr, Jones RM, Portoghese PS, Carlezon WA Jr (2003) Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther 305:323–330
Malin DH, Lake JR, Carter VA, Cunningham JS, Wilson OB (1993) Naloxone precipitates nicotine abstinence syndrome in the rat. Psychopharmacology 112:339–342
Malin DH, Lake JR, Smith TD, Khambati HN, Meyers-Paal RL, Montellano AL, Jennings RE, Erwin DS, Presley SE, Perales BA (2006) Bupropion attenuates nicotine abstinence syndrome in the rat. Psychopharmacology 184:494–503
Mansour A, Burke S, Pavlic RJ, Akil H, Watson SJ (1996) Immunohistochemical localization of the cloned kappa 1 receptor in the rat CNS and pituitary. Neuroscience 71:671–690
Mansour A, Fox CA, Akil H, Watson SJ (1995) Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci 18:22–29
Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ (1988) Anatomy of CNS opioid receptors. Trends Neurosci 11:308–314
Mansvelder HD, McGehee DS (2000) Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron 27:349–357
Mansvelder HD, McGehee DS (2002) Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol 53:606–617
Marco EM, Granstrem O, Moreno E, Llorente R, Adriani W, Laviola G, Viveros MP (2007) Subchronic nicotine exposure in adolescence induces long-term effects on hippocampal and striatal cannabinoid-CB1 and mu-opioid receptors in rats. Eur J Pharmacol 557:37–43
Margolis EB, Hjelmstad GO, Bonci A, Fields HL (2003) Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci 23:9981–9986
Markou A (2008) Review. Neurobiology of nicotine dependence. Philos Trans R Soc Lond B Biol Sci 363:3159–3168
McCarthy MJ, Zhang H, Neff NH, Hadjiconstantinou M (2010) Nicotine withdrawal and kappa-opioid receptors. Psychopharmacology 210:221–229
McCarthy MJ, Zhang H, Neff NH, Hadjiconstantinou M (2011) Desensitization of delta-opioid receptors in nucleus accumbens during nicotine withdrawal. Psychopharmacology 213:735–744
McGehee DS, Heath MJ, Gelber S, Devay P, Role LW (1995) Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science 269:1692–1696
Mello NK, Lukas SE, Mendelson JH (1985) Buprenorphine effects on cigarette smoking. Psychopharmacology 86:417–425
Mello NK, Mendelson JH, Sellers ML, Kuehnle JC (1980) Effects of heroin self-administration on cigarette smoking. Psychopharmacology 67:45–52
Miller ME, Sigmon SC (2015) Are pharmacotherapies ineffective in opioid-dependent smokers? Reflections on the scientific literature and future directions. Nicotine Tob Res 17:955–959
Munafo MR, Johnstone EC, Aveyard P, Marteau T (2013) Lack of association of OPRM1 genotype and smoking cessation. Nicotine Tob Res 15:739–744
Munro TA, Berry LM, Van’t Veer A, Beguin C, Carroll FI, Zhao Z, Carlezon WA Jr, Cohen BM (2012) Long-acting kappa opioid antagonists nor-BNI, GNTI and JDTic: pharmacokinetics in mice and lipophilicity. BMC Pharmacol 12:5
Mutschler NH, Stephen BJ, Teoh SK, Mendelson JH, Mello NK (2002) An inpatient study of the effects of buprenorphine on cigarette smoking in men concurrently dependent on cocaine and opioids. Nicotine Tob Res 4:223–228
Nieto MM, Guen SL, Kieffer BL, Roques BP, Noble F (2005) Physiological control of emotion-related behaviors by endogenous enkephalins involves essentially the delta opioid receptors. Neuroscience 135:305–313
Nuechterlein EB, Ni L, Domino EF, Zubieta JK (2016) Nicotine-specific and non-specific effects of cigarette smoking on endogenous opioid mechanisms. Prog Neuro-Psychopharmacol Biol Psychiatry 69:69–77
O’Malley SS, Cooney JL, Krishnan-Sarin S, Dubin JA, McKee SA, Cooney NL, Blakeslee A, Meandzija B, Romano-Dahlgard D, Wu R, Makuch R, Jatlow P (2006) A controlled trial of naltrexone augmentation of nicotine replacement therapy for smoking cessation. Arch Intern Med 166:667–674
Olive MF, Becker HC (2008) Effects of the mGluR2/3 agonist LY379268 and the mGluR5 antagonist MPEP on handling-induced convulsions during ethanol withdrawal in mice. Alcohol 42:191–197
Paterson NE, Froestl W, Markou A (2005) Repeated administration of the GABAB receptor agonist CGP44532 decreased nicotine self-administration, and acute administration decreased cue-induced reinstatement of nicotine-seeking in rats. Neuropsychopharmacology 30:119–128
Perrine SA, Hoshaw BA, Unterwald EM (2006) Delta opioid receptor ligands modulate anxiety-like behaviors in the rat. Br J Pharmacol 147:864–872
Pert CB, Snyder SH (1973) Opiate receptor: demonstration in nervous tissue. Science 179:1011–1014
Pomerleau OF, Fertig JB, Seyler LE, Jaffe J (1983) Neuroendocrine reactivity to nicotine in smokers. Psychopharmacology 81:61–67
Pradhan AA, Befort K, Nozaki C, Gaveriaux-Ruff C, Kieffer BL (2011) The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci 32:581–590
Rahman S, Zhang J, Corrigall WA (2004) Local perfusion of nicotine differentially modulates somatodendritic dopamine release in the rat ventral tegmental area after nicotine preexposure. Neurochem Res 29:1687–1693
Rasakham K, Liu-Chen LY (2011) Sex differences in kappa opioid pharmacology. Life Sci 88:2–16
Rasmussen DD (1998) Effects of chronic nicotine treatment and withdrawal on hypothalamic proopiomelanocortin gene expression and neuroendocrine regulation. Psychoneuroendocrinology 23:245–259
Ray LA, Courtney KE, Ghahremani DG, Miotto K, Brody A, London ED (2014) Varenicline, low dose naltrexone, and their combination for heavy-drinking smokers: human laboratory findings. Psychopharmacology 231:3843–3853
Ray LA, Courtney KE, Ghahremani DG, Miotto K, Brody A, London ED (2015) Varenicline, naltrexone, and their combination for heavy-drinking smokers: preliminary neuroimaging findings. Am J Drug Alcohol Abuse 41:35–44
Ray LA, Hutchison KE (2004) A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res 28:1789–1795
Ray LA, Hutchison KE (2007) Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch Gen Psychiatry 64:1069–1077
Ray R, Jepson C, Patterson F, Strasser A, Rukstalis M, Perkins K, Lynch KG, O’Malley S, Berrettini WH, Lerman C (2006) Association of OPRM1 A118G variant with the relative reinforcing value of nicotine. Psychopharmacology 188:355–363
Ray R, Ruparel K, Newberg A, Wileyto EP, Loughead JW, Divgi C, Blendy JA, Logan J, Zubieta JK, Lerman C (2011) Human mu opioid receptor (OPRM1 A118G) polymorphism is associated with brain mu-opioid receptor binding potential in smokers. Proc Natl Acad Sci U S A 108:9268–9273
Redila VA, Chavkin C (2008) Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology 200:59–70
Richards EM, Mathews DC, Luckenbaugh DA, Ionescu DF, Machado-Vieira R, Niciu MJ, Duncan WC, Nolan NM, Franco-Chaves JA, Hudzik T, Maciag C, Li S, Cross A, Smith MA, Zarate CA Jr (2016) A randomized, placebo-controlled pilot trial of the delta opioid receptor agonist AZD2327 in anxious depression. Psychopharmacology 233:1119–1130
Rohsenow DJ, Monti PM, Hutchison KE, Swift RM, MacKinnon SV, Sirota AD, Kaplan GB (2007) High-dose transdermal nicotine and naltrexone: effects on nicotine withdrawal, urges, smoking, and effects of smoking. Exp Clin Psychopharmacol 15:81–92
Rose JE, Salley A, Behm FM, Bates JE, Westman EC (2010) Reinforcing effects of nicotine and non-nicotine components of cigarette smoke. Psychopharmacology 210:1–12
Rosecrans JA, Hendry JS, Hong JS (1985) Biphasic effects of chronic nicotine treatment on hypothalamic immunoreactive beta-endorphin in the mouse. Pharmacol Biochem Behav 23:141–143
Russell SE, Rachlin AB, Smith KL, Muschamp J, Berry L, Zhao Z, Chartoff EH (2014) Sex differences in sensitivity to the depressive-like effects of the kappa opioid receptor agonist U-50488 in rats. Biol Psychiatry 76:213–222
Saitoh A, Kimura Y, Suzuki T, Kawai K, Nagase H, Kamei J (2004) Potential anxiolytic and antidepressant-like activities of SNC80, a selective delta-opioid agonist, in behavioral models in rodents. J Pharmacol Sci 95:374–380
Saitoh A, Yoshikawa Y, Onodera K, Kamei J (2005) Role of delta-opioid receptor subtypes in anxiety-related behaviors in the elevated plus-maze in rats. Psychopharmacology 182:327–334
Schacht JP, Anton RF, Voronin KE, Randall PK, Li X, Henderson S, Myrick H (2013) Interacting effects of naltrexone and OPRM1 and DAT1 variation on the neural response to alcohol cues. Neuropsychopharmacology 38:414–422
Schindler AG, Messinger DI, Smith JS, Shankar H, Gustin RM, Schattauer SS, Lemos JC, Chavkin NW, Hagan CE, Neumaier JF, Chavkin C (2012) Stress produces aversion and potentiates cocaine reward by releasing endogenous dynorphins in the ventral striatum to locally stimulate serotonin reuptake. J Neurosci 32:17582–17596
Schmidt P, Schroder H, Maderspach K, Staak M (1994) Immunohistochemical localization of kappa opioid receptors in the human frontal cortex. Brain Res 654:223–233
Scott DJ, Domino EF, Heitzeg MM, Koeppe RA, Ni L, Guthrie S, Zubieta JK (2007) Smoking modulation of mu-opioid and dopamine D2 receptor-mediated neurotransmission in humans. Neuropsychopharmacology 32:450–457
Shaham Y, Shalev U, Lu L, De Wit H, Stewart J (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology 168:3–20
Shiffman SM, Jarvik ME (1976) Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology 50:35–39
Shippenberg TS, Bals-Kubik R, Herz A (1987) Motivational properties of opioids: evidence that an activation of delta-receptors mediates reinforcement processes. Brain Res 436:234–239
Shippenberg TS, Zapata A, Chefer VI (2007) Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther 116:306–321
Simmons D, Self DW (2009) Role of mu- and delta-opioid receptors in the nucleus accumbens in cocaine-seeking behavior. Neuropsychopharmacology 34:1946–1957
Simonin F, Befort K, Gaveriaux-Ruff C, Matthes H, Nappey V, Lannes B, Micheletti G, Kieffer B (1994) The human delta-opioid receptor: genomic organization, cDNA cloning, functional expression, and distribution in human brain. Mol Pharmacol 46:1015–1021
Smith JS, Schindler AG, Martinelli E, Gustin RM, Bruchas MR, Chavkin C (2012) Stress-induced activation of the dynorphin/kappa-opioid receptor system in the amygdala potentiates nicotine conditioned place preference. J Neurosci 32:1488–1495
Stoker AK, Markou A (2015) Neurobiological bases of cue- and nicotine-induced reinstatement of nicotine seeking: implications for the development of smoking cessation medications. Curr Top Behav Neurosci 24:125–154
Stoker AK, Semenova S, Markou A (2008) Affective and somatic aspects of spontaneous and precipitated nicotine withdrawal in C57BL/6J and BALB/cByJ mice. Neuropharmacology 54:1223–1232
Stolerman IP, Jarvis MJ (1995) The scientific case that nicotine is addictive. Psychopharmacology 117:2–10 discussion 14-20
Sudakov SK, Nazarova GA, Alekseeva EV, Kolpakov AA (2014) Effect of activated peripheral kappa-opioid receptors on the action of nicotine and its withdrawal in nicotine-dependent rats. Bull Exp Biol Med 156:609–611
Sutherland G, Stapleton JA, Russell MA, Feyerabend C (1995) Naltrexone, smoking behaviour and cigarette withdrawal. Psychopharmacology 120:418–425
Suzuki T, Tsuji M, Mori T, Ikeda H, Misawa M, Nagase H (1997) Involvement of dopamine-dependent and -independent mechanisms in the rewarding effects mediated by delta opioid receptor subtypes in mice. Brain Res 744:327–334
Talka R, Salminen O, Tuominen RK (2015a) Methadone is a non-competitive antagonist at the alpha4beta2 and alpha3* nicotinic acetylcholine receptors and an agonist at the alpha7 nicotinic acetylcholine receptor. Basic Clin Pharmacol Toxicol 116:321–328
Talka R, Tuominen RK, Salminen O (2015b) Methadone’s effect on nAChRs--a link between methadone use and smoking? Biochem Pharmacol 97:542–549
Tejeda HA, Natividad LA, Orfila JE, Torres OV, O’Dell LE (2012) Dysregulation of kappa-opioid receptor systems by chronic nicotine modulate the nicotine withdrawal syndrome in an age-dependent manner. Psychopharmacology 224:289–301
Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA Jr (2004) Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology 172:463–470
Toll BA, Cummings KM, O’Malley SS, Carlin-Menter S, McKee SA, Hyland A, Wu R, Hopkins J, Celestino P (2012) Tobacco quitlines need to assess and intervene with callers’ hazardous drinking. Alcohol Clin Exp Res 36:1653–1658
Toll BA, White M, Wu R, Meandzija B, Jatlow P, Makuch R, O’Malley SS (2010) Low-dose naltrexone augmentation of nicotine replacement for smoking cessation with reduced weight gain: a randomized trial. Drug Alcohol Depend 111:200–206
Tome AR, Izaguirre V, Rosario LM, Cena V, Gonzalez-Garcia C (2001) Naloxone inhibits nicotine-induced receptor current and catecholamine secretion in bovine chromaffin cells. Brain Res 903:62–65
Trigo JM, Zimmer A, Maldonado R (2009) Nicotine anxiogenic and rewarding effects are decreased in mice lacking beta-endorphin. Neuropharmacology 56:1147–1153
USDHHS (2014) The health consequences of smoking: 50 years of progress. A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2010., Atlanta, GA: US
Vergura R, Balboni G, Spagnolo B, Gavioli E, Lambert DG, McDonald J, Trapella C, Lazarus LH, Regoli D, Guerrini R, Salvadori S, Calo G (2008) Anxiolytic- and antidepressant-like activities of H-Dmt-tic-NH-CH(CH2-COOH)-bid (UFP-512), a novel selective delta opioid receptor agonist. Peptides 29:93–103
Verhagen M, Kleinjan M, Engels RC (2012) A systematic review of the A118G (Asn40Asp) variant of OPRM1 in relation to smoking initiation, nicotine dependence and smoking cessation. Pharmacogenomics 13:917–933
Vihavainen T, Piltonen M, Tuominen RK, Korpi ER, Ahtee L (2008) Morphine-nicotine interaction in conditioned place preference in mice after chronic nicotine exposure. Eur J Pharmacol 587:169–174
Walsh Z, Epstein A, Munisamy G, King A (2008) The impact of depressive symptoms on the efficacy of naltrexone in smoking cessation. J Addict Dis 27:65–72
Walters CL, Cleck JN, Kuo YC, Blendy JA (2005) Mu-opioid receptor and CREB activation are required for nicotine reward. Neuron 46:933–943
Wang SC, Tsou HH, Chung RH, Chang YS, Fang CP, Chen CH, Ho IK, Kuo HW, Liu SC, Shih YH, Wu HY, Huang BH, Lin KM, Chen AC, Hsiao CF, Liu YL (2014) The association of genetic polymorphisms in the kappa-opioid receptor 1 gene with body weight, alcohol use, and withdrawal symptoms in patients with methadone maintenance. J Clin Psychopharmacol 34:205–211
Wang Z, Ray R, Faith M, Tang K, Wileyto EP, Detre JA, Lerman C (2008) Nicotine abstinence-induced cerebral blood flow changes by genotype. Neurosci Lett 438:275–280
Watkins SS, Koob GF, Markou A (2000a) Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res 2:19–37
Watkins SS, Stinus L, Koob GF, Markou A (2000b) Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J Pharmacol Exp Ther 292:1053–1064
Wee S, Koob GF (2010) The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology 210:121–135
Weerts EM, Wand GS, Kuwabara H, Xu X, Frost JJ, Wong DF, McCaul ME (2014) Association of smoking with mu-opioid receptor availability before and during naltrexone blockade in alcohol-dependent subjects. Addict Biol 19:733–742
Wei LN, Loh HH (2011) Transcriptional and epigenetic regulation of opioid receptor genes: present and future. Annu Rev Pharmacol Toxicol 51:75–97
Weinberger AH, Sofuoglu M (2009) The impact of cigarette smoking on stimulant addiction. Am J Drug Alcohol Abuse 35:12–17
Wewers ME, Dhatt R, Tejwani GA (1998) Naltrexone administration affects ad libitum smoking behavior. Psychopharmacology 140:185–190
Wewers ME, Dhatt RK, Snively TA, Tejwani GA (1999) The effect of chronic administration of nicotine on antinociception, opioid receptor binding and met-enkelphalin levels in rats. Brain Res 822:107–113
Wong GY, Wolter TD, Croghan GA, Croghan IT, Offord KP, Hurt RD (1999) A randomized trial of naltrexone for smoking cessation. Addiction 94:1227–1237
Xiao Y, Smith RD, Caruso FS, Kellar KJ (2001) Blockade of rat alpha3beta4 nicotinic receptor function by methadone, its metabolites, and structural analogs. J Pharmacol Exp Ther 299:366–371
Yoo JH, Lee SY, Loh HH, Ho IK, Jang CG (2004) Loss of nicotine-induced behavioral sensitization in micro-opioid receptor knockout mice. Synapse 51:219–223
Zacny JP, Beckman NJ (2004) The effects of a cold-water stimulus on butorphanol effects in males and females. Pharmacol Biochem Behav 78:653–659
Zhang Y, Wang D, Johnson AD, Papp AC, Sadee W (2005) Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem 280:32618–32624
Zhou L, Lovell KM, Frankowski KJ, Slauson SR, Phillips AM, Streicher JM, Stahl E, Schmid CL, Hodder P, Madoux F, Cameron MD, Prisinzano TE, Aube J, Bohn LM (2013) Development of functionally selective, small molecule agonists at kappa opioid receptors. J Biol Chem 288:36703–36716
Zvolensky MJ, McMillan KA, Gonzalez A, Asmundson GJ (2010) Chronic musculoskeletal pain and cigarette smoking among a representative sample of Canadian adolescents and adults. Addict Behav 35:1008–1012
Acknowledgements
This work was supported by a New Investigator grant from the American Association of Colleges of Pharmacy to Dr. Manoranjan S. D’Souza. This article is dedicated to the memory of Dr. Athina Markou, an excellent researcher and a wonderful mentor, who continues to inspire all of her mentees and the scientific community even though she is no more with us.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Norman, H., D’Souza, M.S. Endogenous opioid system: a promising target for future smoking cessation medications. Psychopharmacology 234, 1371–1394 (2017). https://doi.org/10.1007/s00213-017-4582-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-017-4582-0