Abstract
Rationale
Bupropion reduces discomfort and craving associated with smoking cessation. This study determined whether a rat model of nicotine dependence could detect such nicotine abstinence-alleviating effects.
Objectives
Experiments determined whether the abstinence-alleviating effects of bupropion were detectable by (1) behavioral abstinence signs precipitated by the nicotinic antagonist mecamylamine, (2) place aversion conditioned to mecamylamine-precipitated nicotine abstinence, and (3) spontaneous behavioral abstinence signs after abrupt nicotine withdrawal.
Methods
In experiments 1 and 2, nicotine-dependent rats were coinfused for 7 days with 3.15 mg/kg/day nicotine and 20 mg/kg/day bupropion or with nicotine alone. They were then challenged with 1 mg/kg mecamylamine and observed for behavioral abstinence signs (experiment 1) or place aversion conditioned to precipitated abstinence (experiment 2). In experiment 3, rats were nicotine-infused for 7 days as above. A day after termination of nicotine infusion, rats were observed for spontaneous nicotine abstinence signs before and after injection with saline or bupropion.
Results
In experiment 1, rats coinfused with nicotine and bupropion had significantly fewer mecamylamine-precipitated abstinence signs than rats infused with nicotine alone but similar numbers to rats infused with saline alone. In experiment 2, bupropion pretreatment significantly reduced the aversiveness of mecamylamine-precipitated nicotine abstinence. In experiment 3, a single bupropion injection dose-dependently alleviated spontaneous nicotine abstinence syndrome.
Conclusions
These results suggest that these rat models of nicotine dependence and abstinence syndrome may be useful in detecting nicotine abstinence-alleviating effects of potential medications for smoking cessation. The effects of acute bupropion administration raise interesting questions regarding bupropion's mechanism of action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A rodent model of nicotine physical dependence and abstinence syndrome has been introduced and validated (Malin 2001; Malin et al. 1992, 1994). In this model, dependence is induced by continuous subcutaneous infusion of nicotine bitartrate via an Alzet osmotic minipump, and abstinence is initiated by removal of the pump or rapidly precipitated by injection of nicotinic antagonists such as mecamylamine (Malin et al. 1994). The severity of the abstinence syndrome is assessed by counting occurrences of behavioral abstinence signs on a standard checklist. This model has met a variety of validity criteria (Malin 2001), and the abstinence syndrome has generally been quantitatively reproducible across studies (Malin et al. 1994, 1998). Several independent research groups have confirmed the basic phenomena of the continuous infusion model (Adams and Cicero 1998; Carboni et al. 2000; Epping-Jordan et al. 1998; Hildebrand et al. 1997, 1998; Suzuki et al. 1996; Watkins et al. 2000). Nicotine abstinence precipitated in rats rendered nicotine dependent by this method is clearly aversive, as assessed by the conditioned place aversion procedure (Suzuki et al. 1996).
One objective in developing the rat model of nicotine dependence was to provide a means to rapidly screen compounds for potential alleviation of nicotine abstinence syndrome, which would suggest potential usefulness in certain stages of smoking cessation. This gives rise to the question: would the model be capable of detecting the benefits of a drug already known to be useful in smoking cessation? Bupropion (Zyban and Wellbutrin) is such a drug. In addition to its use as an antidepressant (Ascher et al. 1995; Horst and Preskorn 1998), bupropion has been used clinically to aid smoking cessation and has produced significant enhancement of quit rates (Gonzales et al. 2001; Hayford et al. 1999; Hays et al. 2001; Hurt et al. 1997; Jamerson et al. 2001; Johnston et al. 2001; Jorenby et al. 1999) as well as decreased cue-induced cigarette craving (Brody et al. 2004). Providing a possible explanation for these findings, animal studies found that bupropion, possibly acting as a noncompetitive nicotinic receptor antagonist, can alter nicotine reinforcement (Bruijnzeel and Markou 2003; Glick et al. 2002) as well as nicotine locomotor, hypothermic, convulsive, and antinociceptive actions (Slemmer et al. 2000).
Suggesting an additional explanation for clinical efficacy, bupropion significantly reduced the severity of withdrawal syndrome in smoking cessation (Shiffman et al. 2000). The main purpose of the present study was to determine whether bupropion would have a similar effect on nicotine abstinence syndrome in the rat continuous infusion model of nicotine dependence. This was assessed in three different ways. First, mecamylamine-precipitated behavioral abstinence signs were observed in already-nicotine-dependent rats that were then coinfused with nicotine and bupropion prior to nicotine antagonist challenge. Secondly, the aversiveness of mecamylamine-precipitated nicotine abstinence was evaluated by conditioned place aversion in rats treated as above. Lastly, an experiment attempted to confirm the ability of acutely administered bupropion to alleviate ongoing spontaneous nicotine abstinence syndrome (initiated by termination of nicotine infusion), as reported by Cryan et al. (2003).
Experiment 1: chronic bupropion attenuates mecamylamine-precipitated nicotine abstinence syndrome in the rat
Bupropion treatment for smoking cessation is generally initiated at least 7 days before the target quit date and continues during the withdrawal period following smoking cessation (Hayford et al. 1999; Jorenby et al. 1999). To model this general treatment approach, rats already rendered nicotine dependent were subsequently continuously infused with bupropion together with further nicotine infusion, followed by challenge with a nicotine antagonist at a dose previously shown to precipitate a vigorous abstinence syndrome only in nicotine-dependent rats.
Experiment 1 methods
Subjects
The subjects were 24 male Sprague–Dawley rats, weighing 389–454 g. They were individually housed and maintained on ad libitum food and water and a 12-h light/dark cycle. Animal care and use in all three experiments conformed to standards established by the National Institute of Health.
Dependence induction
The research design is shown in Fig. 1 (top). A nondependent control group (n=8) was subcutaneously implanted with a 2ML1 Alzet osmotic minipump (Durect, Cupertino, CA, USA) filled with saline. After 7 days of saline infusion, the pump was replaced with another saline-filled minipump, and the rats were infused with saline for 7 more days. A nicotine-dependent control group (n=8) was subcutaneously implanted with a minipump filled with nicotine bitartrate [−] isomer (Sigma, St. Louis, MO, USA) in saline. These rats were rendered nicotine dependent by 7-day infusion of 9 mg/kg/day nicotine bitartrate (3.15 mg/kg/day expressed as the base). This treatment has been shown to induce significant nicotine dependence, as assessed by both spontaneous and mecamylamine-precipitated abstinence syndromes (Malin et al. 1992, 1994). The pump was then replaced with another minipump filled with the same concentration of nicotine bitartrate, and the rats were infused for 7 more days with nicotine to maintain dependence.
Diagrams of three experimental designs. Experiment 1 (top). The effect of nicotine bupropion coinfusion during the last 7 days of nicotine infusion on somatic behavioral signs precipitated by 1 mg/kg mecamylamine. Experiment 2 (middle). The effect of bupropion coinfusion on place aversion conditioned to mecamylamine-precipitated nicotine abstinence syndrome. Experiment 3 (bottom). The effect of 7.5 or 11.5 mg/kg burpropion or saline s.c. on somatic behavioral signs during spontaneous nicotine abstinence
A nicotine-dependent, bupropion-treated experimental group (n=8) received the same nicotine infusion as the nicotine-dependent control group for the first 7 days. The minipump was then replaced with another minipump filled with nicotine bitartrate together with bupropion HCl (Sigma). These rats were coinfused for the next 7 days with 9 mg/kg/day nicotine bitartrate and 20 mg/kg/day bupropion HCl. This infusion rate of bupropion was selected because it resulted in an antidepressant-like effect in the rat Forced Swim Test (West and Weiss 1998). All minipump implants were carried out under halothane anesthesia.
Observations
Rats were habituated to a clear rectangular observation chamber (48×38×20 cm) for 30 min on day 7 of infusion, immediately prior to removal of the first minipump and implantation of the second pump. Rats were observed for abstinence signs over 30 min on day 13 to confirm that they were not undergoing nicotine abstinence syndrome prior to mecamylamine challenge. On day 14, each rat was injected subcutaneously with 1 mg/kg mecamylamine HCl (Sigma). This dose of mecamylamine has been shown previously to induce a vigorous nicotine abstinence syndrome in rats infused for 1 week with 9 mg/kg/day nicotine bitartrate but not in nondependent rats infused with saline alone (Malin et al. 1994). Immediately following the mecamylamine injection, rats were observed over 30 min for nicotine abstinence signs. Observers counted the number of signs based on a checklist of nicotine abstinence signs developed and validated by Malin et al. (1992). Categories included “wet dog” shakes and tremors, gasps and writhes, teeth chatters and chews, ptosis, and miscellaneous, less frequent signs (seminal ejaculations, scratches, yawns, and hops). Ptosis was counted no more frequently than once each minute, and any continuous teeth chatter, chewing, or tremors were counted no more than once every 15 s. All observations were carried out under “blind” conditions.
Experiment 1 results
As shown in Fig. 2, rats infused with nicotine for 2 weeks had far more overall mecamylamine-precipitated abstinence signs (total occurrences of all categories of sign) than rats coinfused with bupropion during their second week of nicotine infusion or rats infused for 2 weeks with saline alone. Analysis of variance (ANOVA) revealed a significant effect of drug infusion [F(2,21)=4.92, p<0.05]. Post hoc analysis [Fisher's least significant difference (LSD)] revealed that the group infused for 2 weeks with nicotine alone had significantly more abstinence signs than the saline-infused group and the group coinfused with nicotine and bupropion, p<0.01 and p<0.05, respectively. There was no significant difference between the saline-infused rats and those coinfused with nicotine and bupropion.
Overall nicotine abstinence signs/30 min (M±SEM) precipitated by 1 mg/kg mecamylamine HCl s.c. in rats infused with saline for the preceding 14 days (white bar), infused with 9 mg/kg/day nicotine bitartrate for 14 days (single hatched bar), or infused with nicotine bitartrate for 7 days and nicotine bitartrate plus 20 mg/kg/day bupropion for the next 7 days (double hatched bar). **p<0.01 vs the saline-infused group and p<0.05 vs. the group coinfused with nicotine and bupropion
Figure 3 shows the occurrences of various categories of abstinence signs exhibited by the three groups. In every category except shakes/tremors, the rats infused with nicotine alone exhibited more precipitated abstinence signs than the other two groups. According to Fisher's LSD test, the group infused with saline and the group coinfused with nicotine and bupropion both had significantly fewer (p<0.05) gasps/writhes and instances of ptosis than the group infused with nicotine alone.
Occurrences of various categories of nicotine abstinence signs/30 min (M±SEM) precipitated by 1 mg/kg mecamylamine HCl s.c. in rats infused with saline for the preceding 14 days (white bar), infused with 9 mg/kg/day nicotine bitartrate for 14 days (single hatched bar) or infused with nicotine bitartrate for 7 days, and nicotine bitartrate plus 20 mg/kg/day bupropion for the next 7 days (double hatched bar). *p<0.05 vs the group infused for 14 days with nicotine alone
Observation of the rats on day 13 (a day prior to mecamylamine challenge) revealed an unusually fine and persistent head tremor in one rat coinfused with nicotine and bupropion, qualitatively different from any previously observed nicotine abstinence signs. Since this atypical tremor preceded nicotine abstinence, it might tentatively be attributed to bupropion treatment rather than nicotine abstinence. On day 14, two of the rats in the group coinfused with nicotine and bupropion exhibited the atypical tremor, resulting in this group having an elevated number of shakes/tremors.
Experiment 2: bupropion attenuates the aversiveness of mecamylamine-precipitated nicotine withdrawal in the rat
This experiment determined whether the ability of bupropion to reduce the behavioral signs of antagonist-precipitated nicotine abstinence syndrome would correspond to an ability to reduce the aversiveness of the syndrome. The dose of mecamylamine employed has been previously shown to be nonaversive in nonnicotine-dependent (saline infused) rats in the conditioned place aversion procedure while producing a marked aversion in nicotine-dependent rats (Suzuki et al. 1996).
Experiment 2 methods
Subjects
The subjects were 21 male Sprague–Dawley rats weighing 423–468 g. The rats were housed individually and maintained as above.
Dependence induction
The research design is shown in Fig. 1 (middle). All rats were implanted subcutaneously with one 2ML1 Alzet osmotic minipump under halothane anesthesia. Nicotine dependence was induced by 7 days of continuous infusion of 9 mg/kg/day nicotine bitartrate (3.15 mg/kg/day expressed as the base). One week later, the pumps were removed under halothane anesthesia, and another pump was implanted. Ten of the rats received 7 more days of 9 mg/kg/day nicotine tartrate infusion, whereas the other 11 rats received 7 additional days of 9 mg/kg/day nicotine bitartrate together with 20 mg/kg/day bupropion HCl as described above.
Conditioned place preference apparatus
An automated, custom-designed conditioned place preference apparatus was constructed by Columbus Instruments, Columbus, OH, USA. It consisted of two plexiglas boxes, 30×30×30-cm high each and separated by a guillotine door that could either be closed or raised 12 cm to permit free passage between chambers. The walls and floor of the two chambers were covered with plastic sheeting. One box was checkered in black and white with a textured transparent plastic floor over the sheeting. The other box was speckled in shades of gray, white, and black with a smooth transparent plastic floor. A transparent plexiglas start box, gated by a guillotine door, opened onto the center of the apparatus at the boundary of the checkered and speckled sides. The two chambers had transparent tops, and they were dimly illuminated by 13-W red darkroom bulbs 55 cm above the top of each chamber. Switches mounted under the floor were triggered by a weight difference of 80 g or more between the two sides. The switches were connected to two timers that displayed the cumulative time the rat spent in each side.
Testing procedure
Beginning on day 10 of nicotine infusion, each rat was habituated to the apparatus on 3 successive days. On each day, the door between the chambers was raised, and the rat was allowed to explore freely for 15 min. The third habituation session served as a pretest to determine each rat's preferred side and the number of seconds spent on that side. On the subsequent day (the conditioning day), each rat was injected with saline and locked for 30 min in its initially nonpreferred side. It was then injected subcutaneously with 1 mg/kg mecamylamine to precipitate nicotine abstinence and was locked for 30 min in its initially preferred side. Twenty-four hours after the saline injection, each rat was returned for a 15-min free choice trial with the door between the chambers left in the open position. The number of seconds spent in the previously preferred chamber was recorded. Each rat's aversion score was the change from pretest to postconditioning test in the seconds spent in the initially preferred chamber that had been associated with nicotine abstinence. A negative score was thus indicative of conditioned aversion. This measure was chosen to be consistent with the earlier study (Suzuki et al. 1996) that established the aversiveness of the rat model of precipitated nicotine abstinence syndrome.
Experiment 2 results
Coinfusion of bupropion did not have a clear-cut effect on preference between the two chambers during the pretest (prior to conditioning). Of the time that they committed to either chamber, rats infused with nicotine alone spent 71.9±8.2% (mean±SEM) in the speckled chamber. Rats coinfused with nicotine plus bupropion after the first 7 days of nicotine spent 86.3±8.1% of the time in the speckled chamber. This difference was not significant [NS; t(19)=1.25].
Following the conditioning session, the rats infused only with nicotine demonstrated marked aversion to their initially preferred chamber, which had been associated with nicotine abstinence (Fig. 4). They reduced by 200.3±61.3 s (mean±SEM), the time spent in the conditioned chamber. In contrast, the rats coinfused with bupropion reduced their time in the initially preferred, abstinence-associated chamber by only 13.9±16.6 s. This 93% difference in the aversion scores was significant [t(19)=3.06, p<0.01].
Aversion to a previously preferred chamber paired with mecamylamine-precipitated nicotine abstinence, as measured by decrease from pretest in time spent in that chamber (M±SEM). Rats had been infused with nicotine for 14 days (open bar) or with nicotine for 7 days and nicotine plus bupropion for the next 7 days (hatched bar). **p<0.01 vs the nicotine only group
Experiment 3: acute bupropion dose-dependently reduces nicotine abstinence syndrome in the rat
This experiment determined whether prolonged continuous bupropion administration was essential to the drug's abstinence alleviating effects or whether acutely administered bupropion might also be effective. In addition, the experiment evaluated the effect of bupropion on spontaneous, rather than precipitated nicotine withdrawal syndrome. This has more direct preclinical relevance, since the discomfort experienced in the days following smoking cessation (Shiffman and Jarvik 1976; Hatsukami et al. 1984) constitutes a spontaneous abstinence syndrome.
Experiment 3 methods
Subjects
The subjects were 21 male Sprague–Dawley rats, weighing 375–430 g, maintained as described in experiment 1.
Dependence induction
The research design is shown in Fig. 1 (bottom). All rats were implanted with an Alzet 2ML1 osmotic minipump and were infused for 7 days with 9 mg/kg/day nicotine bitartrate (3.15 mg/kg/day expressed as the base) as described in experiment 1. Seven days (164 h) after implantation, nicotine infusion was abruptly terminated by pump removal under halothane anesthesia.
Observations
Rats were habituated to the observation chamber for 30 min on days 5, 6, and 7 of nicotine infusion. Eighteen (preinjection) and twenty-two hours (postinjection) following termination of nicotine infusion, rats were observed under “blind” conditions over 30 min for spontaneous behavioral abstinence signs as described in experiment 1. These time points were chosen because numbers of abstinence signs are maximal between 17 and 23 h following termination of 9 mg/kg/day nicotine bitartrate infusion (Malin et al. 1996, 1998).
Ten minutes prior to the 22-h observation, groups of seven rats each were injected subcutaneously with 11.5 or 7.5 mg/kg bupropion HCl or with saline alone. Subchronically administered injections at doses ranging from 5–15 mg/kg bupropion have been shown to decrease immobility in the Forced Swim Test, an indicator of antidepressant activity (Cooper et al. 1980, 1994; Reneric and Lucki 1998). Final selection of doses as well as the interval between bupropion injection and behavioral observation was based on small pilot experiments. Abstinence reversal scores for each rat were the number of signs postinjection (22-h observation) as a percentage of signs preinjection (18-h observation). Thus, complete reversal of abstinence syndrome would be indicated by a score of 0%, while no reversal would be indicated by a score of 100%. For each individual category of abstinence sign, only postinjection scores were analyzed to avoid dividing by zero in cases where a subject had no preinjection signs in a particular category.
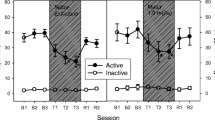
Locomotor immobility was also assessed for each rat during both behavioral observations. Low activity is a correlate of nicotine abstinence syndrome (Malin et al. 1992). The stationary behavior score for each rat was the cumulative duration of episodes of immobility (complete lack of ambulatory and exploratory behavior) during each 30-min observation. Each rat's immobility reversal score was its postinjection immobility score as a percentage of its preinjection immobility score.
Experiment 3 results
As shown in Fig. 5, bupropion dose-dependently reduced abstinence reversal scores (overall postinjection nicotine abstinence signs as a percentage of preinjection signs). ANOVA revealed a significant dose effect [F(2,18)=23.51, p<0.001]. There was a significant negative linear trend of abstinence reversal scores as a function of dose [F(1,18)=46.35, p<0.001]. Post hoc comparisons (Fisher's LSD test) revealed significant differences between each of the three groups. Nicotine-dependent rats injected with either 7.5 or 11.5 mg/kg bupropion had significantly reduced abstinence reversal scores compared with rats injected with saline alone (p<0.001). In addition, the group receiving the higher dose of bupropion had a significantly greater reduction in signs than the group receiving the lower dose of bupropion (p<0.01). These results were not attributable to any significant preinjection differences in overall abstinence signs among the three treatment groups. Preinjection abstinence scores (M±SEM) were 46.14±7.56, 43.71±6.72, and 45.86±3.63 for the saline, low dose and high dose groups, respectively. ANOVA revealed no significant effect of treatment group on preinjection scores [F(2,18)=0.04, NS].
Figure 6 shows the occurrences of various categories of abstinence signs exhibited by the three groups. Abstinence signs were lower in bupropion-treated than in saline-treated rats in all categories except miscellaneous, less frequent signs. There were significant negative linear trends as a function of dose for gasps/writhes [F(1,18)=18.47, p<0.001] and ptosis [F(1,18)=19.39, p<0.001]. In both of these categories, post hoc comparisons (Fisher's LSD test) indicated that both bupropion dose groups had significantly fewer signs than saline-treated rats (p<0.001 and p<0.01, respectively).
Stationary behavior during nicotine abstinence was significantly reduced by bupropion in a dose-dependent manner. Saline-injected rats averaged 100.61% of their preinjection stationary behavior, whereas animals injected with 7.5 and 11.5 mg/kg bupropion averaged 89.17 and 62.45%, respectively, of their preinjection immobility scores. ANOVA revealed a significant dose effect [F(2,18)=5.09, p<0.05]. There was also a significant negative linear trend of immobility percentage scores as a function of dose [F(1,18)=8.56, p<0.01]. According to Fisher's LSD test, subjects receiving the higher dose of bupropion had significantly lower immobility percentage scores than the rats receiving saline alone (p<0.01) or the lower dose of bupropion (p<0.05).
Discussion
The ability of bupropion to alleviate nicotine abstinence syndrome was readily detected by three different variations of the rat model of nicotine dependence (Malin et al. 1992; Malin et al. 1994; Suzuki et al. 1996). Bupropion's abstinence-alleviating effect in the rat was extremely robust, since it resulted from both acutely administered bupropion and bupropion chronically coadministered along with nicotine and since it affected both spontaneous or mecamylamine-precipitated nicotine abstinence. It was also detectable by somatically expressed behavioral signs, duration of immobility, and conditioned place aversion. This is in addition to a previous report (Cryan et al. 2003) of bupropion reversing the elevated intracranial self-stimulation (ICSS) thresholds that result from nicotine abstinence, an indicator of impaired reinforcement mechanisms (Epping-Jordan et al. 1998). The Cryan et al. (2003) study also reported dose-dependent bupropion alleviation of spontaneous somatic behavioral nicotine abstinence signs. The results of experiment 3 are quite consistent with results of that study. The differences appear to be rather minor. In the present study, bupropion alleviation of nicotine abstinence syndrome was evaluated at 22 h after termination of nicotine infusion, as compared with 12 h in the Cryan et al. (2003) study, and significant alleviation of signs was observed with 7.5 and 11.5 mg/kg bupropion, as compared with 20 and 40 mg/kg bupropion. In both studies, the bupropion-induced decrease in gasps and writhes reached significance, while the decrease in ptosis also reached significance in the present study. The effects of coinfusing bupropion along with nicotine in experiments 1 and 2 are most readily interpreted as interference with nicotine dependence, since 1 mg/kg mecamylamine precipitates neither abstinence signs nor conditioned place aversion in rats not exposed to nicotine (Malin et al. 1994; Suzuki et al. 1996). Thus, there would be virtually no room to demonstrate an alleviation of mecamylamine effects by bupropion infusion alone in the absence of nicotine infusion.
These results have implications for the validity and usefulness of rat models of nicotine dependence and abstinence. As reviewed by Malin (2001), the basic model involving somatically expressed behavioral signs has met many criteria of internal validity; that is, several experiments have established that the signs result from chronic nicotine exposure in the rat, followed by decreased nicotine exposure. However, the model's degree of external validity and, thus, its degree of preclinical usefulness, will be indicated by the number of phenomena that are observed both in the rat model and in human smokers. For instance, the opiate antagonist naloxone induces abstinence signs selectively in nicotine-dependent rats (Malin et al. 1993) and also in smokers as opposed to nonsmokers (Krishnan-Sarin et al. 1999). The finding that bupropion alleviates nicotine abstinence syndrome in rats as well as in smokers (Shiffman et al. 2000) adds to the rat model's apparent degree of external validity. It is particularly encouraging that bupropion had this effect in rats when employed in a manner that models the common clinical practice of beginning chronic bupropion treatment during ongoing smoking in tobacco-dependent individuals prior to the target quit date (Hayford et al. 1999; Jorenby et al. 1999). It is also encouraging that the rat model detected a major bupropion-induced decrease in the aversiveness of nicotine abstinence, since negative affect during smoking cessation appears to contribute to early relapse (Laje et al. 2001). Taken as a whole, the results suggest that the model in its several variations may be useful in preliminary screening for other compounds with similar potentially useful effects.
It is also interesting that an entire pattern of the measures (somatic behavioral signs, immobility, ICSS thresholds, and conditioned aversion) that increase as a group in nicotine abstinence should decrease as a group when nicotine-abstinent rats are treated with bupropion. This is consistent with the hypothesis that these measures are somewhat coherent and reflect, to some extent, a common state of the organism induced by nicotine dependence followed by nicotine abstinence.
The neurobiological mechanisms of bupropion's efficacy in smoking cessation are complex and somewhat unclear. Since depression is a risk factor for nicotine dependence (Glassman et al. 1990) and since bupropion has long been used as an antidepressant, it might be assumed that bupropion aids smoking cessation through its antidepressant action. However, bupropion and most other antidepressants require chronic administration before achieving antidepressant effects (Stahl et al. 2004), while acute administration immediately attenuated nicotine abstinence syndrome in this study and that of Cryan et al. (2003). In clinical studies, bupropion efficacy in smoking cessation was not mediated through depressive symptoms (Lerman et al. 2004) and was unrelated to a history of depression (Hayford et al. 1999), casting further doubt on the antidepressant hypothesis. Bupropion is also a noncompetitive antagonist of several subtypes of nicotinic cholinergic receptors, reversing a variety of nicotine actions in animal models (Fryer and Lukas 1999; Miller et al. 2002; Slemmer et al. 2000). Bupropion can alter nicotine self-administration in the rat, although the direction and extent of this effect depend on such factors as the bupropion dose and the nicotine reinforcement schedule (Bruijnzeel and Markou 2003; Glick et al. 2002; Rauhut et al. 2003; Shoaib et al. 2002). Bupropion's nicotinic antagonist action might explain interference with the reinforcing effect of smoking or with the dependence-inducing action of nicotine in experiments 1 and 2. However, nicotine receptor antagonism would not explain the ability of a single bupropion injection to reverse nicotine abstinence syndrome in experiment 3 in rats that are already nicotine dependent and deprived of nicotine. If anything, nicotinic receptor antagonism would be expected to enhance the effects of reduced nicotinic receptor stimulation and intensify the resulting abstinence syndrome.
Bupropion and its hydroxybupropion metabolite bind respectively to dopamine and norepinephrine transporters and interfere with dopamine and norepinephrine reuptake, resulting in higher extracellular catecholamine levels (Ascher et al. 1995; Damaj et al. 2004; Ferris and Cooper 1993; Miller et al. 2002). Nicotine potently induces dopamine release in the mesolimbic pathway, an action essential to its reinforcing effect (Balfour et al. 2000; Corrigall and Coen 1991). In studies using the same rat model of nicotine dependence (Malin et al. 1992, 1994) employed in the current study, nicotine abstinence resulted in decreased dopamine release in several brain regions associated with the mesolimbic dopamine pathway (Carboni et al. 2000; Fung et al. 1996; Hildebrand et al. 1998; Panagis et al. 2000). Therefore, it is possible that bupropion may alleviate nicotine abstinence syndrome, at least in part, by restoring levels of extracellular dopamine available at the synapse. On the other hand, nicotine also facilitates norpeinephrine turnover (Singer et al. 2004) and, based on studies with genetically modified mice, the noradrenergic actions of the hydroxybupropion metabolite appear to be essential for the antidepressant-like actions of bupropion in the tail suspension and forced swimming tests (Cryan et al. 2004). The catecholaminergic actions of bupropion might also explain its ability to substitute for nicotine in the drug discrimination procedure in a manner that is not mediated by nicotinic receptors, since it is not blocked by the nicotinic receptor antagonist mecamylamine (Wiley et al. 2002; Young and Glennon 2002). Among antidepressant and transmitter reuptake inhibitor drugs, bupropion has nearly unique efficacy in smoking cessation (Kotlyar et al. 2001). This might result from its dual effects on nicotinic and catecholaminergic mechanisms as well as its dual effects on nicotine reinforcement and nicotine abstinence syndrome. In the search for further smoking cessation treatments, it is desirable to identify other compounds or combinations of compounds with such dual activities.
References
Adams ML, Cicero TJ (1998) Nitric oxide mediates mecamylamine- and naloxone-precipitated nicotine withdrawal. Eur J Pharmacol 345:R1–R2
Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, Golden RN, Martin P, Potter WZ, Richelson E et al (1995) Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry 56:395–401
Balfour DJ, Wright AE, Benwell ME, Birrell CE (2000) The putative role of extra-synaptic mesolimbic dopamine in the neurobiology of nicotine dependence. Behav Brain Res 113:73–83
Brody AL, Mandelkern MA, Lee G, Smith E, Sadeghi M, Saxena S, Jarvik ME, London ED (2004) Attenuation of cue-induced cigarette craving and anterior cingulate cortex activation in bupropion-treated smokers: a preliminary study. Psychiatry Res 130(3):269–281
Bruijnzeel AW, Markou A (2003) Characterization of the effects of bupropion on the reinforcing properties of nicotine and food in rats. Synapse 501:20–28
Carboni E, Bortone L, Giua C, Di Chiara G (2000) Dissociation of physical abstinence signs from changes in extracellular dopamine in the nucleus accumbens and in the prefrontal cortex of nicotine dependent rats. Drug Alcohol Depend 58:93–102
Cooper BR, Hester TJ, Maxwell RA (1980) Behavioral and biochemical effects of the antidepressant bupropion (Wellbutrin): evidence for selective blockade of dopamine uptake in vivo. J Pharmacol Exp Ther 215:127–134
Cooper BR, Wang CM, Cox RF, Norton R, Shea V, Ferris RM (1994) Evidence that the acute behavioral and electrophysiological effects of bupropion (Wellbutrin) are mediated by a noradrenergic mechanism. Neuropsychopharmacology 11:133–141
Corrigall WA, Coen KM (1991) Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology 104:171–176
Cryan JF, Bruijnzeel AW, Skjei KL, Markou A (2003) Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology 168:347–358
Cryan JF, O'Leary OF, Jin SH, Friedland JC, Ouyang M, Hirsch BR, Page ME, Dalvi A, Thomas SA, Lucki I (2004) Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proc Natl Acad Sci U S A 101(21):8186–8191
Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, Lukas RJ, Martin BR (2004) Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Mol Pharmacol 66(3):675–682
Epping-Jordan MP, Watkins SS, Koob GF, Markou A (1998) Dramatic decreases in brain reward function during nicotine withdrawal. Nature 393:76–79
Ferris RM, Cooper BR (1993) Mechanism of antidepressant activity of bupropion. J Clin Psychiatry Monograph 11:2–14
Fryer JD, Lukas RJ (1999) Noncompetitive functional inhibition at diverse, human nicotinic acetylcholine receptor subtypes by bupropion, phencyclidine, and ibogaine. J Pharmacol Exp Ther 288:88–92
Fung YK, Schmid MJ, Anderson TM, Lau YS (1996) Effects of nicotine withdrawal on central dopaminergic systems. Pharmacol Biochem Behav 53:635–640
Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, Johnson J (1990) Smoking, smoking cessation, and major depression. JAMA 264:1546–1549
Glick SD, Maisonneuve IM, Kitchen BA (2002) Modulation of nicotine self-administration in rats by combination therapy with agents blocking alpha3beta4 nicotinic receptors. Eur J Pharmacol 448:185–191
Gonzales DH, Nides MA, Ferry LH, Kustra RP, Jamerson BD, Segall N, Herrero LA, Krishen A, Sweeney A, Buaron K, Metz A (2001) Bupropion SR as an aid to smoking cessation in smokers treated previously with bupropion: a randomized placebo-controlled study. Clin Pharmacol Ther 69:438–444
Hatsukami DK, Hughes JR, Pickens RW, Svikis D (1984) Tobacco withdrawal symptoms: an experimental analysis. Psychopharmacology 84:231–236
Hayford KE, Patten CA, Rummans TA, Schroeder DR, Offord KP, Croghan IT, Glover ED, Sachs DP, Hurt RD (1999) Efficacy of bupropion for smoking cessation in smokers with a former history of major depression or alcoholism. Br J Psychiatry 174:173–178
Hays JT, Hurt RD, Rigotti NA, Niaura R, Gonzales D, Durcan MJ, Sachs DP, Wolter TD, Buist AS, Johnston JA, White JD (2001) Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation. A randomized, controlled trial. Ann Intern Med 135:423–433
Hildebrand BE, Nomikos GG, Bondjers C, Nisell M, Svensson TH (1997) Behavioral manifestations of the nicotine abstinence syndrome in the rat: peripheral versus central mechanisms. Psychopharmacology 129:348–356
Hildebrand BE, Nomikos GG, Hertel P, Schilstrom B, Svensson TH (1998) Reduced dopamine output in the nucleus accumbens but not in the medial prefrontal cortex in rats displaying a mecamylamine-precipitated nicotine withdrawal syndrome. Brain Res 779:214–225
Horst WD, Preskorn SH (1998) Mechanisms of action and clinical characteristics of three atypical antidepressants: venlafaxine, nefazodone, bupropion. J Affect Disord 51:237–254
Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, Khayrallah MA, Schroeder DR, Glover PN, Sullivan CR (1997) A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med 337:1195–1202
Jamerson BD, Nides M, Jorenby DE, Donahue R, Garrett P, Johnston JA, Fiore MC, Rennard SI, Leischow SJ (2001) Late-term smoking cessation despite initial failure: an evaluation of bupropion sustained release, nicotine patch, combination therapy, and placebo. Clin Ther 23:744–752
Johnston JA, Fiedler-Kelly J, Glover ED, Sachs DP, Grasela TH, DeVeaugh-Geiss J (2001) Relationship between drug exposure and the efficacy and safety of bupropion sustained release for smoking cessation. Nicotine Tob Res 3:131–140
Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, Fiore MC, Baker TB (1999) A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med 340:685–691
Kotlyar M, Golding M, Hatsukami DK, Jamerson BD (2001) Effect of non-nicotine pharmacotherapy on smoking behavior. Pharmacotherapy 21:1530–1548
Krishnan-Sarin S, Rosen MI, O'Malley SS (1999) Naloxone challenge in smokers: preliminary evidence of an opioid component in nicotine dependence. Arch Gen Psychiatry 56:663–668
Laje RP, Berman JA, Glassman AH (2001) Depression and nicotine: preclinical and clinical evidence for common mechanisms. Curr Psychiatry Rep 3:470–474
Lerman C, Niaura R, Collins BN, Wileyto P, Audrain-McGovern J, Pinto A, Hawk L, Epstein LH (2004) Effect of bupropion on depression symptoms in a smoking cessation clinical trial. Psychol Addict Behav 18(4):362–366
Malin DH (2001) Nicotine dependence: studies with a laboratory model. Pharmacol Biochem Behav 70:551–559
Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, Carter VA, Cunningham JS, Wilson OB (1992) Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav 43:779–784
Malin DH, Lake JR, Carter VA, Cunningham JS, Wilson OB (1993) Naloxone precipitates nicotine abstinence syndrome in the rat. Psychopharmacology 112:339–342
Malin DH, Lake JR, Carter VA, Cunningham JS, Hebert KM, Conrad DL, Wilson OB (1994) The nicotinic antagonist mecamylamine precipitates nicotine abstinence syndrome in the rat. Psychopharmacology 115:180–184
Malin DH, Lake JR, Payne MC, Short PE, Carter VA, Cunningham JS, Wilson OB (1996) Nicotine alleviation of nicotine abstinence syndrome is naloxone-reversible. Pharmacol Biochem Behav 53:81–85
Malin DH, Lake JR, Shenoi M, Upchurch TP, Johnson SC, Schweinle WE, Cadle CD (1998) The nitric oxide synthesis inhibitor nitro-L-arginine (L-NNA) attenuates nicotine abstinence syndrome in the rat. Psychopharmacology 140:371–377
Miller DK, Sumithran SP, Dwoskin LP (2002) Bupropion inhibits nicotine-evoked [(3)H]overflow from rat striatal slices preloaded with [(3)H]dopamine and from rat hippocampal slices preloaded with [(3)H]norepinephrine. J Pharmacol Exp Ther 302:1113–1122
Panagis G, Hildebrand BE, Svensson TH, Nomikos GG (2000) Selective c-fos induction and decreased dopamine release in the central nucleus of amygdala in rats displaying a mecamylamine-precipitated nicotine withdrawal syndrome. Synapse 35:15–25
Rauhut AS, Neugebauer N, Dwoskin LP, Bardo MT (2003) Effect of bupropion on nicotine self-administration in rats. Psychopharmacology 169:1–9
Reneric JP, Lucki I (1998) Antidepressant behavioral effects by dual inhibition of monoamine reuptake in the rat forced swimming test. Psychopharmacology 136:190–197
Shiffman SM, Jarvik ME (1976) Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology 50:35–39
Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwaltney CJ, Paty JA, Gnys M, Evoniuk G, DeVeaugh-Geiss J (2000) The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology 148:33–40
Shoaib M, Sidhpura N, Shafait S (2002) Investigating the actions of bupropion on dependence-related effects of nicotine in rats. Psychopharmacology 165:405–412
Singer S, Rossi S, Verzosa S, Hashim A, Lonow R, Cooper T, Sershen H, Lajtha A (2004) Nicotine-induced changes in neurotransmitter levels in brain areas associated with cognitive function. Neurochem Res 29(9):1779–1792
Slemmer JE, Martin BR, Damaj MI (2000) Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther 295:321–327
Stahl SM, Pradko JF, Haight BR, Modell JG, Rockett CB, Learned-Coughlin S (2004) A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiat 6:159–166
Suzuki T, Ise Y, Tsuda M, Maeda J, Misawa M (1996) Mecamylamine-precipitated nicotine-withdrawal aversion in rats. Eur J Pharmacol 314:281–284
Watkins SS, Stinus L, Koob GF, Markou A (2000) Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J Pharmacol Exp Ther 292:1053–1064
West CHK, Weiss JM (1998) Effects of antidepressant drugs on rats bred for low activity in the swim test. Pharmacol Biochem Behav 61:67–79
Wiley JL, Lavecchia KL, Martin BR, Damaj MI (2002) Nicotine-like discriminative stimulus effects of bupropion in rats. Exp Clin Psychopharmacol 10:129–135
Young R, Glennon RA (2002) Nicotine and bupropion share a similar discriminative stimulus effect. Eur J Pharmacol 443:113–118
Acknowledgements
This study was supported by grants from GlaxoSmithKline.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malin, D.H., Lake, J.R., Smith, T.D. et al. Bupropion attenuates nicotine abstinence syndrome in the rat. Psychopharmacology 184, 494–503 (2006). https://doi.org/10.1007/s00213-005-0135-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0135-z