Abstract
Rationale
The role played by endogenous opioids in mediating the reinforcing properties of nicotine is unclear. As with preclinical studies, clinical trials with naloxone, a prototypic opioid receptor antagonist have yielded equivocal findings with regard to its efficacy in reducing cigarette smoking.
Objective
The aim of the present study was to examine the effects of three opioids that exhibit relative selectivity at μ-, κ- and δ-opioid receptors on nicotine self-administration in male hooded Lister rats.
Methods
Graded doses (0.3, 1.0, and 3.0 mg/kg IP) of each opioid agonist or antagonist were tested in different groups of rats repeatedly over three consecutive nicotine intravenous nicotine-self administration (0.03 mg/kg/infusion) sessions. The same treatments were tested in parallel groups of rats trained to respond for food reinforcement.
Results
Naloxone was very effective in attenuating the levels of nicotine self-administered across all doses tested. The selective κ-opioid receptor agonist U50,488, reduced nicotine self-administration in doses of 1 and 3 mg/kg, while the 0.3 mg/kg dose produced a small increase in nicotine intake. Finally, the specific δ-opioid receptor antagonist, naltrindole did not significantly modify nicotine self-administration behaviour. In contrast, all three opioids failed to modify behaviour maintained by food reinforcement.
Conclusions
These findings suggest endogenous opioids are crucial in mediating the reinforcing effects of nicotine and that the μ-opioid receptor subtype may represent a potential target for selectively reducing nicotine-taking behaviour as part of a pharmacological approach to develop smoking cessation aids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tobacco smoking is one of the most prevalent addictions impacting health and behaviour (George and O'Malley 2004; Giovino 2002). Dependence to tobacco has been attributed to nicotine, which is considered to be the major psychoactive ingredient in cigarettes (Stolerman and Jarvis 1995), and nicotine is recognised for its ability to maintain self-administration behaviour in humans and laboratory animals (Caggiula et al. 2001; Chaudhri et al. 2006; Goldberg and Henningfield 1988; Stolerman and Shoaib 1991).
Animal models offer the potential to study nicotine dependence and the underlying neurobiological mechanisms that may facilitate development of new smoking cessation treatments. As in humans (Goldberg and Henningfield 1988), laboratory animals can be shown to self-administer infusions of nicotine (Corrigall 1999; Corrigall and Coen 1989; Corrigall et al. 1992; Goldberg and Henningfield 1988; Sannerud et al. 1994; Shoaib et al. 1997a). Evidence on the role of endogenous opioids in nicotine reinforcement processes is limited relative to other neurotransmitter systems, such as dopamine and GABA (Corrigall 1999). Before preclinical studies on nicotine dependence were published, a couple of early clinical trials examined the opioid receptor antagonist naloxone in tobacco smokers. A key paper by Karras and Kane (1980) reported a one-third decrease in smoking after naloxone administration. However, a later study by Nemeth-Coslett and Griffiths (1986) failed to replicate this finding with tests using a wider range of naloxone doses. In a more elaborate trial to replicate Karras and Kane (1980) result, Gorelick et al. (1988), using a double blind cross-over design, obtained a 16% reduction in number of cigarettes smoked. In this study, naloxone did not have any effect on subjective and physiological measures and more significantly did not elicit any signs of a tobacco withdrawal syndrome (Gorelick et al. 1988). In contrast, a recent trial reported naloxone to dose-dependently increase withdrawal signs and symptoms in smokers (Krishnan-Sarin et al. 1999). Similarly, the long-acting opioid receptor antagonist naltrexone has also been found to significantly reduce desire to smoke, craving, and total number of cigarettes smoked (King and Meyer 2000), although previous studies did not show any similar reduction in smoking behaviour (Sutherland et al. 1995; Wong et al. 1999). The four cessation trials with naltrexone have been reviewed by David et al. (2009) extensively in the Cochrane review on opioid antagonists for smoking cessation, concluding that ‘it was not possible to confirm or refute whether naltrexone helps smokers quit’ and urging the need for larger trials with naltrexone (David et al. 2009). Further insight into which endogenous opioids are involved came from a clinical trial with cyclazocine, a κ-opioid agonist/μ-opioid partial agonist, which was found to attenuate cigarette smoking behaviour (Pickworth et al. 2004). Thus, the literature on the role of endogenous opioids in tobacco dependence remains unclear.
Data from laboratory animals on the role of endogenous opioids in nicotine dependence has also provided equivocal findings. Microinfusion of the μ-opioid agonist, DAMGO, into the rat ventral tegmental area suppressed intravenous nicotine self-administration behaviour (Corrigall et al. 2000), while systemic naloxone pretreatment failed to show any reduction in responding maintained by intravenous nicotine infusions in rats (Corrigall and Coen 1991). A recent study reports on naltrexone having no effect on the reinforcing effects of nicotine in rats (Liu et al. 2009). Results have been more consistent from nicotine withdrawal paradigms based largely on measuring somatic signs. A relatively large dose of naloxone precipitated physical withdrawal effects in rats made dependent to nicotine administered chronically via an osmotic minipump, while pretreatment with κ-opioid agonists, U50,488 and TRK-820 diminished mecamylamine-precipitated nicotine withdrawal symptoms in rats (Ise et al. 2002).
Given the increased availability of opioid ligands commercially and their pharmacological characterisation, the aim of the present study was to examine the effects of naloxone, a broad spectrum opioid antagonist along with two selective compounds, U50,488 that shows agonist properties at the κ-opioid receptor subtype (Suarez-Roca and Maixner 1983) and naltrindole that is a selective antagonist at δ-opioid receptor subtype (Portoghese et al. 1990) on the reinforcing properties of nicotine. Re-examination of naloxone within a refined model of nicotine self-administration may help to resolve the controversy on the potential utility of naltrexone for smoking cessation. Furthermore, since opioid neurotransmission is involved in appetitive behaviours, to control for non-specific suppression of operant responding, the same treatments will be examined in parallel groups of rats trained to respond for food pellets under similar schedules of reinforcement. Findings from this study will improve the understanding on the role of opioids in the primary reinforcing effects of nicotine and thus inform on their potential as targets for developing more effective smoking cessation aids.
Materials and methods
Animals
Male hooded Lister rats (Harlan, Bicester, UK) initially weighing 200–250 g were housed individually in a room maintained at 20–22°C with a light–dark cycle (light from 8 a.m. to 8 p.m.). Once surgically implanted with an intravenous catheter, rats received their daily diet (20–24 g) approximately 1–2 h following the end of the self-administration session. No food restriction was applied to the nicotine self-administration experiments. For experiments involving food reinforcement, access to food was restricted to maintain body weights at 85% of those under free-feeding conditions. Water was available ad libitum. All these studies complied with all local and national ethical requirements and were carried out according to the Animals (Experimental Procedures) Act, 1986 under licence from the UK Home Office.
Nicotine self-administration procedure
Surgery
For self-administration studies, under surgical anaesthesia (a mixture of medetomidine 0.3 mg/kg and ketamine 70 mg/kg, i.p.), rats were implanted with a chronic Silastic catheter into the external jugular vein as described previously by Shoaib (2008). The catheter was connected to an L-shaped connector (Plastics-One, Roanoke, VA) that was mounted on the rat's skull embedded in dental acrylate being held in place by stainless steel screws. Daily flushing with 0.9% physiological saline containing Baytril (enthrofloxacin) (0.16 mg/kg/day) and diluted heparin (0.01 units/kg/day) maintained the patency of the intravenous catheter throughout the duration of the experiment. Once animals regained body weights above pre-operative weights, the self-administration sessions started.
Apparatus
Twelve standard operant conditioning chambers (Med-Associates, VT, US) were used that consisted of a Plexiglas™ enclosure with one house light, one visual stimulus light, two levers, one tether and a fluid swivel. One lever was defined as active and presses on it resulted in fluid infusions; presses on the other lever were recorded but had no programmed consequence. Catheters were connected to a syringe containing nicotine solution held in an infusion pump (Razal, MED-Associates, VT, US). The operant chambers were controlled by a Dell microcomputer (Dell, Ireland).
Self-administration procedure
In 1-h limited access sessions, rats were given the opportunity to lever press for intravenous infusions of nicotine (0.03 mg/kg/infusion) delivered in 90 µl volume as described previously (Shoaib 2008; Shoaib et al. 1997a). A visual stimulus light was utilised to signal availability of nicotine, which was turned off for 20 s during the time-out period. No other visual stimuli were employed for training.
Tests on nicotine self-administration
Once rats showed response accuracy with at least 80% of the responses on the active lever and with stable intake of nicotine (±2 infusions) over 2 days, the number of responses required to produce an infusion was increased progressively up to three (fixed ratio, FR-3). Once rats were on a schedule of FR-3 and met a stability criterion (less than 20% variability from the mean number of infusions self-administered over three sessions), extinction tests were conducted during which nicotine was replaced with saline for three consecutive sessions. Under these conditions, three consecutive sessions are necessary to observe changes on nicotine-maintained responding (Shoaib 2008). Following the extinction test, self-administration behaviour was reinstated by replacing syringes with nicotine and tests with the opioid compounds began once stability criteria were met by each group. As conducted before (Shoaib 2008), each group of rats was tested repeatedly for three consecutive sessions following tests with a range of doses of the opioid compound or vehicle administered 30 min prior to the start of the nicotine self-administration session. Separate groups of rats (n = 8) were used to test each compound in which each dose was tested repeatedly for three consecutive sessions. The order of the doses presented was randomly selected. At least 3 days of nicotine self-administration was allowed to re-establish baseline responding between each dose.
Responding maintained by food presentation procedure
Apparatus
Experiments on food reinforcement were performed in six standard experimental chambers (Campden Instruments, UK) each containing two response levers and a device for delivering 45 mg grain-based food pellets (Bio-Serv, Frenchtown, NJ, US) into food hoppers placed in between the levers. The experiments were controlled by a Dell microcomputer (Dell, Ireland) located in the same room.
Procedure
Rats were initially shaped to consume food pellets delivered every 10 s during a 30-min session. Following each pellet delivery, the stimulus light was turned off. Approximately 12 pellets were placed in the hopper before the start of these shaping sessions. As the rats learned to lever press for food, a timeout was introduced and was progressively increased to the maximum of 240 s. This increased timeout was employed to maintain the response rates approximately the same for the two reinforcers. The schedule of reinforcement was also increased progressively to FR-3 (dependent on response accuracy at least 80% of the responses on the active lever and with stable pellets dispensed (±2 food pellets) over 2 days). The visual stimulus light was used to signal availability of the food pellet and was turned off during the time out period.
Tests on responding maintained by food
Tests with the opioids were conducted using a similar design as those as with tests on nicotine self-administration; rats were only tested if they exhibited stable levels of responding on a FR-3 schedule (less than 20% variability from the mean number of responses over three sessions). Extinction tests were conducted during which food was not presented over three consecutive sessions. Separate groups of rats (n = 6) were used to test each compound in which each dose was tested repeatedly for three consecutive sessions. The opioid antagonist or agonist or vehicle was administered 30 min prior to the start of the food reinforcement session. Following each test, at least 3 days of responding without any treatment were allowed to re-establish baseline responding between each test. The order of the doses and vehicle tested was randomly selected.
Drugs
Nicotine bitartrate (BDH, Poole, Dorset, UK) was dissolved in isotonic saline. The pH of nicotine solution was adjusted to 7 with dilute NaOH. Naloxone hydrochloride (Sigma, Dorset, UK), naltrindole (Sigma, Dorset, UK), and U50,488 (Upjohn, Kalamazoo, US) were all administered SC in saline (1 ml/kg). Doses of drugs are expressed as those of the base. The range of doses for each opioid was based on published literature.
Statistical analyses
Data from self-administration and food reinforcement experiments in the form of total responses, number of infusions or pellets from each session were analysed using multi-factorial ANOVA for repeated measures (SPSS, version 15). Post-hoc pairwise comparisons were made relative to baseline levels of nicotine self-administration prior to the repeated tests. To ascertain how selective the opioid treatments were between the reinforcers, the number of responses made over the three test sessions was averaged per dose before conducting the appropriate multi-factorial ANOVA for repeated measures. Post hoc (LSD test) pairwise comparisons were conducted to identify significant differences from controls.
Results
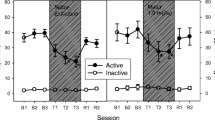
Figure 1 illustrates the total number of responses emitted by groups of rats self-administering intravenous nicotine or responding for food pellets. Both reinforcers maintained steady rates of responding thus satisfying the stability criterion which permitted evaluation of naloxone. The left panels of Figure 1 illustrate the modifications on nicotine self-administration following pre-treatment with graded doses of naloxone, while the right panel in these figures demonstrate the effects of the same dose of naloxone on responding maintained by food.
Pre-treatment with naloxone (0.3, 1.0, and 3.0 mg/kg SC) on responding maintained by intravenous nicotine (left panels) and food pellets (right panels) in rats (n = 6–8). Each point represents mean ± SEM number of active and inactive responses in a 60-min session. Symbols (*) show significant differences (P < 0.05) from session B3 following Tukey's t tests
Repeated treatment with saline over three successive sessions did not alter nicotine self-administration behaviour and nor food-reinforced behaviour. Naloxone pre-treatment produced a dramatic reduction on nicotine-maintained responses across all doses tested (Fig. 1). The left section of Fig. 1 demonstrates the significant decreases on active lever press responses following repeated naloxone administration for three successive days in various doses [0.3 mg/kg, F(8,36) = 11.8; P < 0.001; 1 mg/kg, F(8,36) = 3.97, P < 0.05; 3 mg/kg, F(8,36) = 3.89, P < 0.05]. The largest dose of naloxone (3.0 mg/kg) produced persistent decreases in nicotine intake since reductions were still apparent following termination of naloxone treatment (Fig. 1). These reductions on nicotine self-administration were specific since comparable tests in groups of rats trained to lever press for food reinforcement with the same doses of naloxone over three successive sessions had little effect (Fig. 1). This degree of selectivity by naloxone was confirmed in a two-way ANOVA for repeated measures, which yielded a significant interaction between reinforcer type (nicotine vs food) and naloxone dose as factors [(F(3,33) = 6.56; P < 0.01)].
Results with the selective δ-opioid receptor antagonist naltrindole are illustrated in Fig. 2. Naltrindole produced variable results on nicotine self-administration (see left section of Fig. 2). Across the doses tested, there appeared to be a trend for naltrindole to reduce nicotine-induced behaviour; however, these reductions on nicotine intake were not significant across all doses. Similarly, naltrindole had little effect on responding maintained by food reinforcement (Fig. 2). All three doses were ineffective. No interaction was obtained in a two-way ANOVA for repeated measures with reinforcer type (nicotine vs food) and naltrindole dose as factors [F(3,27) = 0.74; P > 0.05].
Pre-treatment with naltrindole (0.3, 1.0, and 3.0 mg/kg SC) on responding maintained by intravenous nicotine (left panels) and food pellets (right panels) in rats (n = 6–8). Each point represents mean±SEM number of active and inactive responses in a 60-min session. Symbols (*) show significant differences (P < 0.05) from session B3 following Tukey's t tests
The administration of the selective κ-opioid receptor agonist, U50,488 produced opposing effects on nicotine self-administration dependent on dose (Fig. 3). A small increase in nicotine intake was observed following treatment with 0.3 mg/kg of U50,488 [F(8,56) = 2.8, P < 0.05], while larger doses of U50,488 decreased nicotine self-administration [1.0 mg/kg, F(8,64) = 2.83, P < 0.01; 3.0 mg/kg, F(8,56) = 1.84, P = 0.089]. These effects of U50,488 were selective to nicotine since all three doses did not modify responding maintained by food (Fig. 3). This degree of selectivity by U50,488 was confirmed in a two-way ANOVA for repeated measures, which yielded a significant interaction between reinforcer type (nicotine vs food) and U50,488 dose as factors [(F(3,33) = 2.90; P < 0.05)].
Pre-treatment with U50,488 (0.3, 1.0, and 3.0 mg/kg SC) on responding maintained by intravenous nicotine (left panels) and food pellets (right panels) in rats (n = 6–8). Each point represents mean ± SEM number of active and inactive responses in a 60-min session. Symbols (*) show significant differences (P < 0.05) from session B3 following Tukey's t tests
Discussion
The present study demonstrates that nicotine self-administration behaviour may be specifically altered by opioids in doses that do not modify food-reinforced behaviour. Naloxone was the most effective ligand, reducing nicotine intake by approximately 70% across all doses, while smaller reductions were observed with U50,488 and naltrindole; ligands showing specificity for the κ- and δ-opioid receptor subtypes, respectively. It should be noted that the profile of these decreases in response rates appear different to those observed during extinction over consecutive sessions (Shoaib 2008).
The robust decreases produced by naloxone pretreatment contrast with previously published studies by Corrigall and Coen (1991), DeNoble and Mele (2006) and Liu et al. (2009); all three studies were not observing any consistent decreases on nicotine self-administration behaviour following pretreatment with naloxone or naltrexone. There are a number of procedural factors to consider that may account for this discrepancy. The first and most plausible difference is the status of food restriction implemented. In the present study, no food restriction was utilised in maintaining rats on intravenous nicotine reinforcement. In addition to this, these subjects had not been previously shaped to respond for food, which was an additional factor. This may explain why naloxone was selective for nicotine, since similar doses had no effect on food-maintained responding in food-restricted subjects. Another important aspect was how quickly the rats adapted when reinforcement was not available. In previous reports of nicotine self-administration, at least 10 extinction sessions were necessary before levels of responding fell (Corrigall et al. 1988; Donny et al. 1995). These latter studies demonstrate that responding can be maintained by non-pharmacological factors as highlighted by Caggiula's studies (Caggiula et al. 2001). DeNoble and Mele (2006) used multiple visual stimuli to reinforce lever press responding (house lights oscillating at 10 Hz and lights above levers coming on), while Corrigall et al. (1988) utilised stimulus lights discretely paired with the onset of nicotine self-administration. A recent study with naltrexone also failed to attenuate nicotine self-administration in doses that could block cue-induced reinstatement effects of nicotine-seeking behaviour (Liu et al. 2009). In all three of these studies, the common problem was the resistance to extinction suggesting that under their conditions, nicotine was not contributing exclusively as the primary reinforcer.
It is well established that conditioned reinforcing effects play an important role in the maintenance and regulation of smoking behaviour and craving (Butschky et al. 1995; Chiamulera 2005; Hasenfratz et al. 1993). Of the various studies described above, the present training parameters used were based on those previously used in other pharmacological investigations on nicotine reinforcement (Shoaib 2008; Shoaib et al. 1997a, 1997b). A single event consisting of the visual stimulus light was utilised to signal availability of nicotine and was turned off for 20 s following each nicotine infusion which has been shown to favour a more rapid extinction.
Naloxone and the U50,488 selectively reduced nicotine self-administration in doses that did not affect responding maintained by food. These findings suggest that endogenous opioids may not be as important in the motivational processes for food reinforcement. However, it should also be noted that the schedules maintaining nicotine and food reinforcement were set to match the total number of reinforcers earned per session. The extended time out employed in the food studies will have generated higher response rates that may be resistant to opioid treatment. This is a limitation when investigating the nature of suppressant effects on nicotine-maintained behaviour for which many others have used food-maintained behaviour as a comparator (Rauhut et al. 2005; Paterson et al. 2004).
The suppression produced by the opioid ligands on nicotine self-administration is most likely because of their aversive effects, which preferentially oppose the positive reinforcing effects of nicotine. These actions have been shown for naloxone against rewarding effects of opioids (Bechara et al. 1995; Mucha et al. 1985; Shippenberg and Bals-Kubik 1995). Opioid antagonists were reported to produce robust aversive effects in both morphine-dependent (Downs and Woods 1975, 1976; Goldberg et al. 1971; Hand et al. 1988) and naïve rats (Bechara and van der Kooy 1985; Mucha et al. 1985). These aversive effects are thought to be due to decreases in extracellular levels of dopamine in the mesolimbic dopamine system (Di Chiaria and Imperato 1988a) produced by naloxone and kappa agonist (Di Chiara and Imperato 1988b).
The present findings are further substantiated by behavioural studies reporting on preproenkaephalin knock-out mice failing to develop nicotine-induced conditioned place preference (Berrendero et al. 2005) and naloxone blocking the expression of nicotine-induced conditioned place preference (Walters et al. 2005). The variability of effects observed with naltrindole and the lack of significant changes on nicotine self-administration are in line with previous studies in which naltrindole failed to modify nicotine-induced sensitisation in nicotine-treated rats (Heidbreder et al. 1996). Significant effects were seen however with the specific κ-opioid receptor agonist, U50,488, which produced an interesting profile on nicotine self-administration behaviour. The reductions on nicotine-taking behaviour are very much in agreement with previous reports with 1 mg/kg dose of U50,488 decreasing nicotine-stimulated locomotor activity (Hahn et al. 2000). In a smaller dose of 0.3 mg/kg, there was a trend for the kappa agonist to increase nicotine self-administration suggesting a dual role of endogenous dynorphins. Activation of κ-opioid receptors produces a depressant response on extracellular levels of dopamine in mesolimbic brain regions (Manzanares et al. 1991). Since selective κ-opioid antagonists can produce an opposite effect on accumbal dopamine (Maisonneuve et al. 1994; Spanagel et al. 1992), the κ-opioid system has been suggested to exert a tonic control over dopamine release in this area of the brain which may explain U50,488's effects on nicotine reinforcement. Moreover, studies by Heidbreder et al. (1995), suggested that κ-opioid receptor agonists can produce opposing effects from binding to other opioid receptor subtypes with lower affinity.
In summary, the findings from this investigation with an array of opioids demonstrate that nicotine self-administration can be reduced selectively by naloxone pretreatment suggesting an intrinsic role for endogenous opioids. Selective activation at either the κ-opioid or blockade at δ-opioid receptors may not be sufficient to alter nicotine-taking behaviour. These observations suggest that the μ-opioid receptor may be the most prominent subtype, although it cannot be overlooked that the efficacy of naloxone may have involved blockade at all three opioid receptor subtypes, since consistent decreases were not observed following pretreatment with the δ-opioid specific antagonist. Despite the inconsistency in the literature from both preclinical and clinical fields on the therapeutic utility of naltrexone as a smoking cessation aid, David et al (2009) suggests further trials be conducted with naltrexone. Moreover, neuroimaging studies in smokers have also identified interactions with dopaminergic systems (Scott et al. 2007), which provide further clinical evidence for targeting µ-opioid receptors for smoking cessation.
References
Bechara A, van der Kooy D (1985) Opposite motivational effects of endogenous opioids in brain and periphery. Nature 314:533–534
Bechara A, Nader K, van der Kooy D (1995) Neurobiology of withdrawal motivation: evidence for two separate aversive effects produced in morphine-naive versus morphine-dependent rats by both naloxone and spontaneous withdrawal. Behav Neurosci 109:91–105
Berrendero F, Mendizabal V, Robledo P, Galeote L, Bilkei-Gorzo A, Zimmer A, Maldonado R (2005) Nicotine-induced antinociception, rewarding effects, and physical dependence are decreased in mice lacking the preproenkephalin gene. J Neurosci 25:1103–1112
Butschky MF, Bailey D, Henningfield JE, Pickworth WB (1995) Smoking without nicotine delivery decreases withdrawal in 12-hour abstinent smokers. Pharmacol Biochem Behav 50:91–96
Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF (2001) Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav 70:515–530
Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF (2006) Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 184:353–366
Chiamulera C (2005) Cue reactivity in nicotine and tobacco dependence: a “multiple-action” model of nicotine as a primary reinforcement and as a enhancer of the effects of smoking-associated stimuli. Brain Research Reviews 48:74–97
Corrigall WA (1999) Nicotine self-administration in animals as a dependence model. Nicotine Tob Res 1:11–20
Corrigall WA, Coen KM (1989) Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 99:473–478
Corrigall WA, Coen KM (1991) Opiate antagonists reduce cocaine but not nicotine self-administration. Psychopharmacology (Berl) 104:167–170
Corrigall WA, Herling S, Coen KM (1988) Evidence for opioid mechanisms in the behavioral effects of nicotine. Psychopharmacology (Berl) 96:29–35
Corrigall WA, Franklin KB, Coen KM, Clarke PB (1992) The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 107:285–289
Corrigall WA, Coen KM, Adamson KL, Chow BL, Zhang J (2000) Response of nicotine self-administration in the rat to manipulations of mu-opioid and gamma-aminobutyric acid receptors in the ventral tegmental area. Psychopharmacology (Berl) 149:107–114
David SP, Lancaster T, Stead LF, Evins AE, Cahill K (2009) Opioid antagonists for smoking cessation. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No.: CD003086. doi:10.1002/14651858.CD003086.pub2.
DeNoble VJ, Mele PC (2006) Intravenous nicotine self-administration in rats: effects of mecamylamine, hexamethonium and naloxone. Psychopharmacology (Berl) 184:266–272
Di Chiara G, Imperato A (1988a) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. National Academy of Sciences of the United States of America, pp 5274–5278
Di Chiara G, Imperato A (1988b) Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther 244:1067–1080
Donny EC, Caggiula AR, Knopf S, Brown C (1995) Nicotine self-administration in rats. Psychopharmacology (Berl) 122:390–394
Downs DA, Woods JH (1975) Naloxone as a negative reinforcer in rhesus monkeys: effects of dose, schedule, and narcotic regimen. Pharmacol Rev 27:397–406
Downs DA, Woods JH (1976) Morphine, pentazocine and naloxone effects on responding under a multiple schedule of reinforcement in rhesus monkeys and pigeons. J Pharmacol Exp Ther 196:298–306
George TP, O’Malley SS (2004) Current pharmacological treatments for nicotine dependence. Trends Pharmacol Sci 25:42–48
Giovino GA (2002) Epidemiology of tobacco use in the United States. Oncogene 21:7326–7340
Goldberg SR, Henningfield JE (1988) Reinforcing effects of nicotine in humans and experimental animals responding under intermittent schedules of i.v. drug injection. Pharmacol Biochem Behav 30:227–234
Goldberg SR, Hoffmeister F, Schlichting U, Wuttke W (1971) Aversive properties of nalorphine and naloxone in morphine-dependent rhesus monkeys. J Pharmacol Exp Ther 179:268–276
Gorelick DA, Rose J, Jarvik ME (1988) Effect of naloxone on cigarette smoking. J Subst Abuse 1:153–159
Hahn B, Stolerman IP, Shoaib M (2000) Kappa-opioid receptor modulation of nicotine-induced behaviour. Neuropharmacology 39:2848–2855
Hand TH, Koob GF, Stinus L, Le Moal M (1988) Aversive properties of opiate receptor blockade: evidence for exclusively central mediation in naive and morphine-dependent rats. Brain Res 474:364–368
Hasenfratz M, Jacober A, Battig K (1993) Smoking-related subjective and physiological changes: pre- to postpuff and pre- to postcigarette. Pharmacol Biochem Behav 46:527–534
Heidbreder C, Babovic-Vuksanovic D, Shoaib M, Shippenberg TS (1995) Development of behavioral sensitization to cocaine: Influence of kappa opioid receptor agonists. J Pharmacol Exp Ther 275:150–163
Heidbreder C, Shoaib M, Shippenberg TS (1996) Differential role of delta-opioid receptors in the development and expression of behavioral sensitization to cocaine. Eur J Pharmacol 298:207–216
Ise Y, Narita M, Nagase H, Suzuki T (2002) Modulation of kappa-opioidergic systems on mecamylamine-precipitated nicotine-withdrawal aversion in rats. Neurosci Lett 323:164–166
Karras A, Kane JM (1980) Naloxone reduces cigarette smoking. Life Sci 27:1541–1545
King AC, Meyer PJ (2000) Naltrexone alteration of acute smoking response in nicotine-dependent subjects. Pharmacol Biochem Behav 66:563–572
Krishnan-Sarin S, Rosen MI, O’Malley SS (1999) Naloxone challenge in smokers. Preliminary evidence of an opioid component in nicotine dependence. Arch Gen Psychiatry 56:663–668
Liu X, Palmatier MI, Caggiula AR, Sved AF, Donny EC, Gharib M, Booth S (2009) Naltrexone attenuation of conditioned but not primary reinforcement of nicotine in rats. Psychopharmacology (Berl) 202:589–598
Maisonneuve IM, Archer S, Glick SD (1994) U50, 488, a kappa opioid receptor agonist, attenuates cocaine-induced increases in extracellular dopamine in the nucleus accumbens of rats. Neurosci Lett 181:57–60
Manzanares J, Lookingland KJ, Moore KE (1991) Kappa opioid receptor-mediated regulation of dopaminergic neurons in the rat brain. J Pharmacol Exp Ther 256:500–505
Mucha RF, Millan MJ, Herz A (1985) Aversive properties of naloxone in non-dependent (naive) rats may involve blockade of central beta-endorphin. Psychopharmacology (Berl) 86:281–285
Nemeth-Coslett R, Griffiths RR (1986) Naloxone does not affect cigarette smoking. Psychopharmacology (Berl) 89:261–264
Paterson NE, Froestl W, Markou A (2004) The GABAB receptor agonists baclofen and CGP44532 decreased nicotine self-administration in the rat. Psychopharmacology (Berl) 172:179–86
Pickworth WB, Lee EM, Abreu ME, Umbricht A, Preston KL (2004) A laboratory study of hydromorphone and cyclazocine on smoking behavior in residential polydrug users. Pharmacol Biochem Behav 77:711–715
Portoghese et al (1990) Design of peptidomimetic d opioid receptor antagonists using the message-address concept. J Med Chem 33:1714
Rauhut AS, Dwoskin LP, Bardo MT (2005) Tolerance does not develop to the decrease in nicotine self-administration produced by repeated bupropion administration. Nicotine Tob Res 7:901–907
Sannerud CA, Prada J, Goldberg DM, Goldberg SR (1994) The effects of sertraline on nicotine self-administration and food-maintained responding in squirrel monkeys. Eur J Pharmacol 271:461–469
Scott DJ, Domino EF, Heitzeg MM, Koeppe RA, Ni L, Guthrie S, Zubieta JK (2007) Smoking modulation of mu-opioid and dopamine D2 receptor-mediated neurotransmission in humans. Neuropsychopharmacology 32:450–457
Shippenberg TS, Bals-Kubik R (1995) Involvement of the mesolimbic dopamine system in mediating the aversive effects of opioid antagonists in the rat. Behav Pharmacol 6:99–106
Shoaib M (2008) The cannabinoid antagonist AM251 attenuates nicotine self-administration and nicotine-seeking behaviour in rats. Neuropharmacology 54:438–444
Shoaib M, Schindler CW, Goldberg SR (1997a) Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl) 129:35–43
Shoaib M, Thorndike E, Schindler CW, Goldberg SR (1997b) Discriminative stimulus effects of nicotine and chronic tolerance. Pharmacol Biochem Behav 56:167-173
Spanagel R, Herz A, Shippenberg TS (1992) Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci USA 89:2046–2050
Stolerman IP, Jarvis MJ (1995) The scientific case that nicotine is addictive. Psychopharmacology (Berl) 117:2–10, discussion 14–20
Stolerman IP, Shoaib M (1991) The neurobiology of tobacco addiction. Trends Pharmacol Sci 12:467–473
Suarez-Roca, Maixner (1983) Activation of kappa opioid receptors by U-50488H and morphine enhances the release of substance P from rat trigeminal nucleus slices. J Pharmacol Exp Ther 264:648
Sutherland G, Stapleton JA, Russell MA, Feyerabend C (1995) Naltrexone, smoking behaviour and cigarette withdrawal. Psychopharmacology (Berl) 120:418–425
Walters CL, Cleck JN, Kuo YC, Blendy JA (2005) Mu-opioid receptor and CREB activation are required for nicotine reward. Neuron 46:933–943
Wong GY, Wolter TD, Croghan GA, Croghan IT, Offord KP, Hurt RD (1999) A randomized trial of naltrexone for smoking cessation. Addiction 94:1227–1237
Acknowledgments
The research was funded by Newcastle University. We thank Victoria Wing and Andrew Holt for assisting with some of the behavioural experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ismayilova, N., Shoaib, M. Alteration of intravenous nicotine self-administration by opioid receptor agonist and antagonists in rats. Psychopharmacology 210, 211–220 (2010). https://doi.org/10.1007/s00213-010-1845-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-010-1845-4